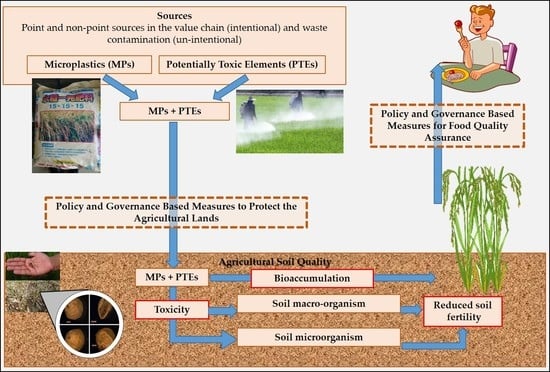
Microplastics and Potentially Toxic Elements: Potential Human Exposure Pathways through Agricultural Lands and Policy Based Countermeasures
Abstract
:1. Introduction
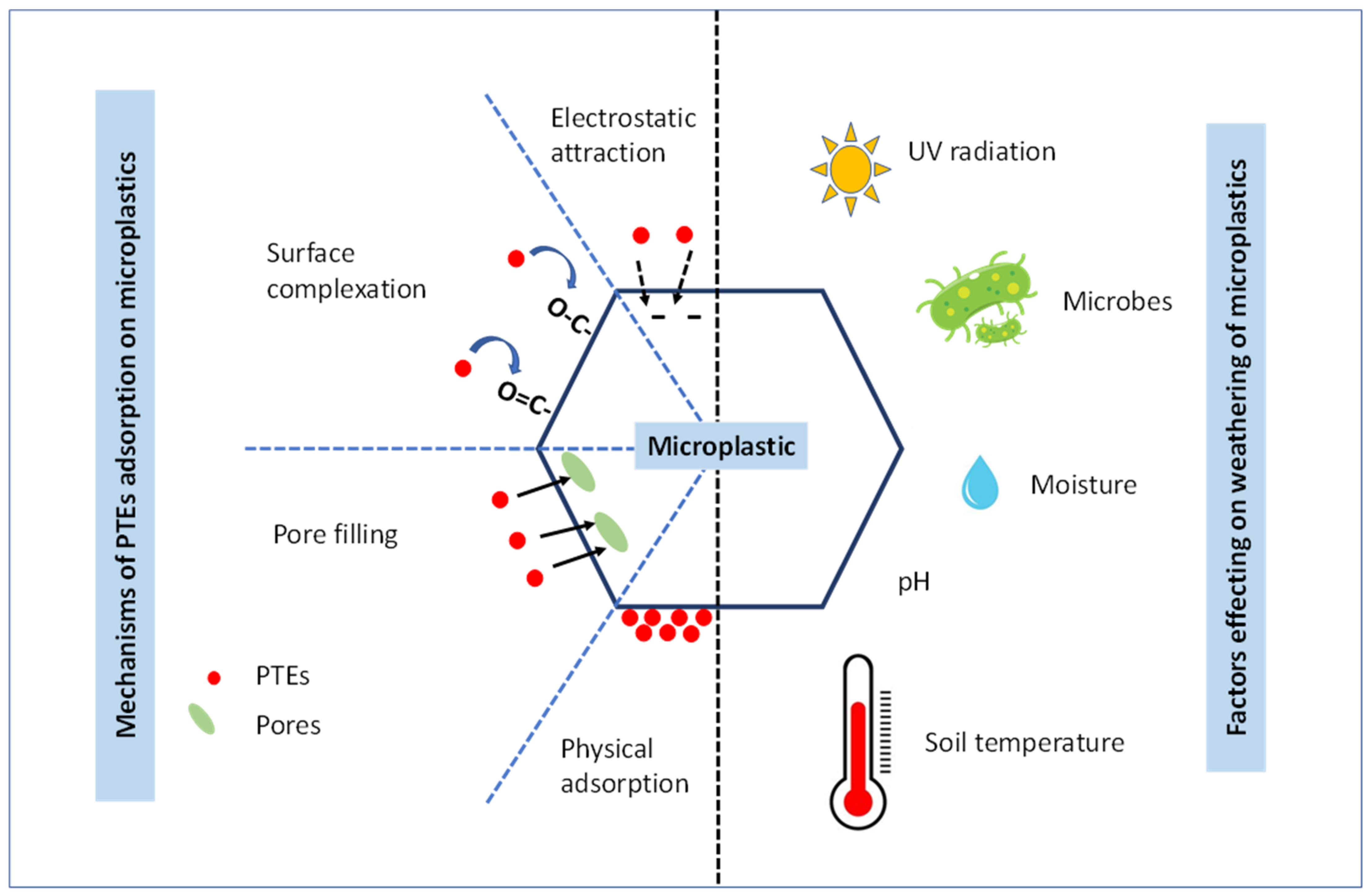
2. Adsorption of Potentially Toxic Elements onto MPs
2.1. Adsorption Mechanisms of Potentially Toxic Elements on MPs
2.2. Transport of Potentially Toxic Elements via MPs
3. The Effect of Environmental Factors on the Adsorption of PTEs onto MPs
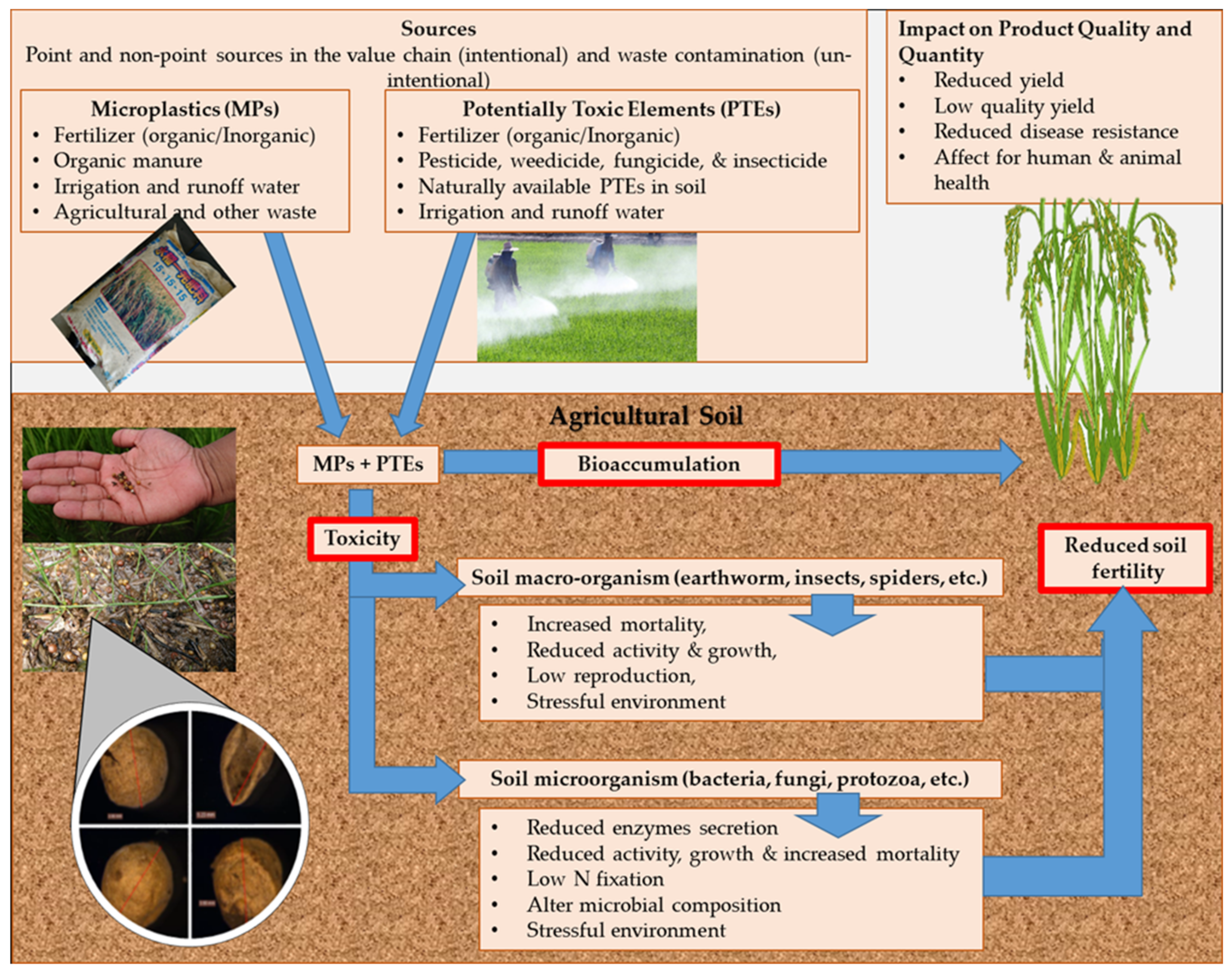
4. Effects of MPs on the Bioaccumulation and Toxicity of PTEs
4.1. Microplastic Uptake by Plants
4.2. Effect of MPs on Soil Animals and Microbial Activity
4.3. MP Accumulation in Plants and Toxicity
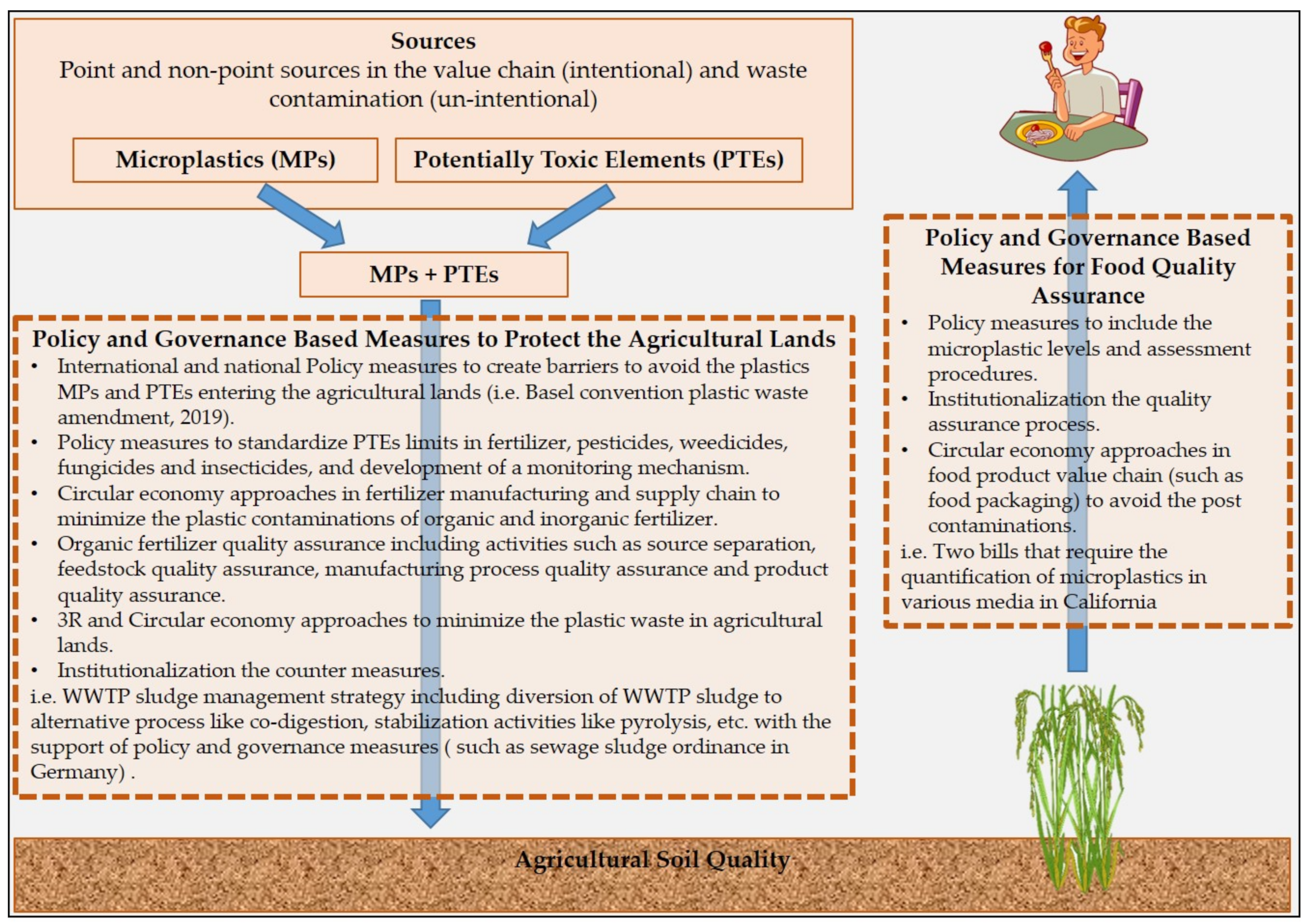
5. Policy and Governance Measures
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adyel, T.M. Accumulation of Plastic Waste during COVID-19. Science 2020, 369, 1314–1315. [Google Scholar] [PubMed]
- Benson, N.U.; Bassey, D.E.; Palanisami, T. COVID Pollution: Impact of COVID-19 Pandemic on Global Plastic Waste Footprint. Heliyon 2021, 7, e06343. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Silva, A.L.P.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. COVID-19 Pandemic Repercussions on the Use and Management of Plastics. Environ. Sci. Technol. 2020, 54, 7760–7765. [Google Scholar] [CrossRef]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and Wonderful? Microplastics Prevail in Snow from the Alps to the Arctic. Sci. Adv. 2019, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Brodhagen, M.; Goldberger, J.R.; Hayes, D.G.; Inglis, D.A.; Marsh, T.L.; Miles, C. Policy Considerations for Limiting Unintended Residual Plastic in Agricultural Soils. Environ. Sci. Policy 2017, 69, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of Microplastic Accumulation in Agricultural Soils from Sewage Sludge Disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic Fibers in Atmospheric Fallout: A Source of Microplastics in the Environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Abeynayaka, A.; Werellagama, I.; Ngoc-Bao, P.; Hengesbaugh, M.; Gajanayake, P.; Nallaperuma, B.; Karkour, S.; Xuan-Thanh, B.; Itsubo, N. Microplastics in Wastewater Treatment Plants; Bui, X.-T., Nguyen, D.D., Nguyen, P.D., Ngo, H.H., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as Contaminants in the Soil Environment: A Mini-Review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, N.; Kusube, T.; Nagao, S.; Okochi, H. Accumulation of Microcapsules Derived from Coated Fertilizer in Paddy Fields. Chemosphere 2021, 267, 129185. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an Emerging Threat to Terrestrial Ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Ruiz, H.; Martin-Closas, L.; Pelacho, A.M. Biodegradable Plastic Mulches: Impact on the Agricultural Biotic Environment. Sci. Total Environ. 2021, 750, 141228. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.W.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, Y.S.; Bolan, N.S. Microplastics as Pollutants in Agricultural Soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef]
- Nizzetto, L.; Langaas, S.; Futter, M. Pollution: Do Microplastics Spill on to Farm Soils? Nature 2016, 537, 488. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Wang, L.; Chen, Q.; Kalogerakis, N.; Ji, R.; Ma, Y. Interactions between Microplastics and Organic Pollutants: Effects on Toxicity, Bioaccumulation, Degradation, and Transport. Sci. Total Environ. 2020, 748, 142427. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The Potential of Microplastics as Carriers of Metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef]
- Richard, H.; Carpenter, E.J.; Komada, T.; Palmer, P.T.; Rochman, C.M. Biofilm Facilitates Metal Accumulation onto Microplastics in Estuarine Waters. Sci. Total Environ. 2019, 683, 600–608. [Google Scholar] [CrossRef]
- Zou, J.; Liu, X.; Zhang, D.; Yuan, X. Adsorption of Three Bivalent Metals by Four Chemical Distinct Microplastics. Chemosphere 2020, 248, 126064. [Google Scholar] [CrossRef]
- Hou, D.; O’Connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.W.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal Contamination and Bioremediation of Agricultural Soils for Food Safety and Sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Kwon, E.E.; Vithanage, M.; Rinklebe, J.; Moon, D.H.; Meers, E.; Tsang, D.C.W.; Ok, Y.S. Soil Lead Immobilization by Biochars in Short-Term Laboratory Incubation Studies. Environ. Int. 2019, 127, 190–198. [Google Scholar] [CrossRef]
- Abbasi, S.; Moore, F.; Keshavarzi, B.; Hopke, P.K.; Naidu, R.; Rahman, M.M.; Oleszczuk, P.; Karimi, J. PET-Microplastics as a Vector for Heavy Metals in a Simulated Plant Rhizosphere Zone. Sci. Total Environ. 2020, 744, 140984. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A. Microplastics Could Be a Threat to Plants in Terrestrial Systems Directly or Indirectly. Environ. Pollut. 2020, 267, 115653. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for Degradation of Plastic Polymers Floating in the Marine Environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Sharma, N. Mechanistic Implications of Plastic Degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Verla, A.W.; Enyoh, C.E.; Verla, E.N.; Nwarnorh, K.O. Microplastic–Toxic Chemical Interaction: A Review Study on Quantified Levels, Mechanism and Implication. SN Appl. Sci. 2019, 1, 1400. [Google Scholar] [CrossRef] [Green Version]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Adsorption of Trace Metals to Plastic Resin Pellets in the Marine Environment. Environ. Pollut. 2012, 160, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.; Wang, Q.; Zhang, L.; Sun, L.; Lin, C.; Yang, H. Heavy Metals in Sediment, Microplastic and Sea Cucumber Apostichopus Japonicus from Farms in China. Mar. Pollut. Bull. 2019, 143, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Chen, C.H.; Gao, X.W.; Kim, J.K.; Xin, Z.X. A Study on the Compatibility and Physical Properties of Chlorinated Polyethylene Rubber/Nitrile Rubber Blends. J. Appl. Polym. Sci. 2011, 120, 1180–1185. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in Air: Are We Breathing It In? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Hassan, I.; Peng, Y.; Huo, S.; Ling, L. Behaviors and Influencing Factors of the Heavy Metals Adsorption onto Microplastics: A Review. J. Clean. Prod. 2021, 319, 128777. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A.; Khan, S.M.; Akhter, N. Interactions and Effects of Microplastics with Heavy Metals in Aquatic and Terrestrial Environments. Environ. Pollut. 2021, 290, 118104. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Y.; Li, J.; Wang, F.; Xia, S.; Zhao, J. Biofilm Alters Tetracycline and Copper Adsorption Behaviors onto Polyethylene Microplastics. Chem. Eng. J. 2020, 392, 123808. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Liu, G.; He, G.; Liu, W. Adsorption Mechanism of Cadmium on Microplastics and Their Desorption Behavior in Sediment and Gut Environments: The Roles of Water PH, Lead Ions, Natural Organic Matter and Phenanthrene. Water Res. 2020, 184, 116209. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Teng, F.; Zhou, C. Surfactant Changes Lead Adsorption Behaviors and Mechanisms on Microplastics. Chem. Eng. J. 2021, 405, 126989. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wangjin, X.; Wang, Y.; Meng, G.; Chen, Y. The Adsorption Behavior of Metals in Aqueous Solution by Microplastics Effected by UV Radiation. J. Environ. Sci. 2020, 87, 272–280. [Google Scholar] [CrossRef]
- Guo, X.; Hu, G.; Fan, X.; Jia, H. Sorption Properties of Cadmium on Microplastics: The Common Practice Experiment and A Two-Dimensional Correlation Spectroscopic Study. Ecotoxicol. Environ. Saf. 2020, 190, 110118. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. As(III) Adsorption onto Different-Sized Polystyrene Microplastic Particles and Its Mechanism. Chemosphere 2020, 239, 124792. [Google Scholar] [CrossRef]
- Jiang, S.; Huang, L.; Nguyen, T.A.H.; Ok, Y.S.; Rudolph, V.; Yang, H.; Zhang, D. Copper and Zinc Adsorption by Softwood and Hardwood Biochars under Elevated Sulphate-Induced Salinity and Acidic PH Conditions. Chemosphere 2016, 142, 64–71. [Google Scholar] [CrossRef]
- Yang, X.; Igalavithana, A.D.; Oh, S.-E.; Nam, H.; Zhang, M.; Wang, C.-H.; Kwon, E.E.; Tsang, D.C.W.; Ok, Y.S. Characterization of Bioenergy Biochar and Its Utilization for Metal/Metalloid Immobilization in Contaminated Soil. Sci. Total Environ. 2018, 640–641, 704–713. [Google Scholar] [CrossRef]
- Rao, Z.; Niu, S.; Zhan, N.; Wang, X.; Song, X. Microplastics in Sediments of River Yongfeng from Maanshan City, Anhui Province, China. Bull. Environ. Contam. Toxicol. 2020, 104, 166–172. [Google Scholar] [CrossRef]
- Torkzaban, S.; Bradford, S.A.; van Genuchten, M.T.; Walker, S.L. Colloid Transport in Unsaturated Porous Media: The Role of Water Content and Ionic Strength on Particle Straining. J. Contam. Hydrol. 2008, 96, 113–127. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, L.; Wang, T.; Chen, Q.; Ji, R. Microplastics as Vectors of Chemicals and Microorganisms in the Environment. In Particulate Plastics in Terrestrial and Aquatic Environments; CRC Press: Boca Raton, FL, USA, 2020; pp. 209–230. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Adams, C.A.; Sun, Y. Effects of Co-Contamination of Microplastics and Cd on Plant Growth and Cd Accumulation. Toxics 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Garçon, M.; Sauzéat, L.; Carlson, R.W.; Shirey, S.B.; Simon, M.; Balter, V.; Boyet, M. Nitrile, Latex, Neoprene and Vinyl Gloves: A Primary Source of Contamination for Trace Element and Zn Isotopic Analyses in Geological and Biological Samples. Geostand. Geoanalytical Res. 2017, 41, 367–380. [Google Scholar] [CrossRef]
- Turner, A.; Holmes, L.; Thompson, R.C.; Fisher, A.S. Metals and Marine Microplastics: Adsorption from the Environment versus Addition during Manufacture, Exemplified with Lead. Water Res. 2020, 173, 115577. [Google Scholar] [CrossRef]
- Fred-Ahmadu, O.H.; Bhagwat, G.; Oluyoye, I.; Benson, N.U.; Ayejuyo, O.O.; Palanisami, T. Interaction of Chemical Contaminants with Microplastics: Principles and Perspectives. Sci. Total Environ. 2020, 706, 135978. [Google Scholar] [CrossRef]
- Do Carmo Ramos, S.N.; Xavier, A.L.P.; Teodoro, F.S.; Gil, L.F.; Gurgel, L.V.A. Removal of Cobalt(II), Copper(II), and Nickel(II) Ions from Aqueous Solutions Using Phthalate-Functionalized Sugarcane Bagasse: Mono- and Multicomponent Adsorption in Batch Mode. Ind. Crops Prod. 2016, 79, 116–130. [Google Scholar] [CrossRef]
- Isobe, A.; Kubo, K.; Tamura, Y.; Kako, S.; Nakashima, E.; Fujii, N. Selective Transport of Microplastics and Mesoplastics by Drifting in Coastal Waters. Mar. Pollut. Bull. 2014, 89, 324–330. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horton, A.A.; Dixon, S.J. Microplastics: An Introduction to Environmental Transport Processes. Wiley Interdiscip. Rev. Water 2018, 5, e1268. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Han, B.; Sun, Y.; Wang, F. Microplastics Influence the Adsorption and Desorption Characteristics of Cd in an Agricultural Soil. J. Hazard. Mater. 2020, 388, 121775. [Google Scholar] [CrossRef]
- Whitacre, D.M. Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2014; Volume 229. [Google Scholar] [CrossRef]
- Jahnke, A.; Arp, H.P.H.; Escher, B.I.; Gewert, B.; Gorokhova, E.; Kühnel, D.; Ogonowski, M.; Potthoff, A.; Rummel, C.; Schmitt-Jansen, M.; et al. Reducing Uncertainty and Confronting Ignorance about the Possible Impacts of Weathering Plastic in the Marine Environment. Environ. Sci. Technol. Lett. 2017, 4, 85–90. [Google Scholar] [CrossRef]
- Liu, P.; Zhan, X.; Wu, X.; Li, J.; Wang, H.; Gao, S. Effect of Weathering on Environmental Behavior of Microplastics: Properties, Sorption and Potential Risks. Chemosphere 2020, 242, 125193. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, Q.; Xing, X.; Chen, W.; She, Z.; Luo, Z. Raman Spectra and Surface Changes of Microplastics Weathered under Natural Environments. Sci. Total Environ. 2020, 739, 139990. [Google Scholar] [CrossRef]
- Palmisano, A.C.; Pettigrew, C.A. Biodegradability of Plastics. Bioscience 1992, 42, 680–685. [Google Scholar] [CrossRef]
- Orhan, Y.; Hrenović, J.; Büyükgüngör, H. Biodegradation of plastic compost bags under controlled soil conditions. Acta Chim. Slov. 2004, 51, 579–588. [Google Scholar]
- Ren, Z.; Gui, X.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. Microplastics in the Soil-Groundwater Environment: Aging, Migration, and Co-Transport of Contaminants—A Critical Review. J. Hazard. Mater. 2021, 419, 126455. [Google Scholar] [CrossRef]
- Binda, G.; Spanu, D.; Monticelli, D.; Pozzi, A.; Bellasi, A.; Bettinetti, R.; Carnati, S.; Nizzetto, L. Unfolding the Interaction between Microplastics and (Trace) Elements in Water: A Critical Review. Water Res. 2021, 204, 117637. [Google Scholar] [CrossRef]
- Wijesekara, H.; Bolan, N.S.; Bradney, L.; Obadamudalige, N.; Seshadri, B.; Kunhikrishnan, A.; Dharmarajan, R.; Ok, Y.S.; Rinklebe, J.; Kirkham, M.B.; et al. Trace Element Dynamics of Biosolids-Derived Microbeads. Chemosphere 2018, 199, 331–339. [Google Scholar] [CrossRef]
- Li, X.; Mei, Q.; Chen, L.; Zhang, H.; Dong, B.; Dai, X.; He, C.; Zhou, J. Enhancement in Adsorption Potential of Microplastics in Sewage Sludge for Metal Pollutants after the Wastewater Treatment Process. Water Res. 2019, 157, 228–237. [Google Scholar] [CrossRef]
- Joshi, P.M.; Juwarkar, A.A. In Vivo Studies to Elucidate the Role of Extracellular Polymeric Substances from Azotobacter in Immobilization of Heavy Metals. Environ. Sci. Technol. 2009, 43, 5884–5889. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, N.; Qu, L.; Wu, G. Heavy Metal Accumulation Characteristics and Physiological Response of Sabina Chinensis and Platycladus Orientalis to Atmospheric Pollution. J. Environ. Sci. 2022, 112, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Guo, L.; Deng, Z.; Wang, D.; Liu, L. Assessment of Heavy Metal Pollution and Water Quality Characteristics of the Reservoir Control Reaches in the Middle Han River, China. Sci. Total Environ. 2021, 799, 149472. [Google Scholar] [CrossRef] [PubMed]
- El Alouani, M.; Saufi, H.; Moutaoukil, G.; Alehyen, S.; Nematollahi, B.; Belmaghraoui, W.; Taibi, M. Application of Geopolymers for Treatment of Water Contaminated with Organic and Inorganic Pollutants: State-of-the-Art Review. J. Environ. Chem. Eng. 2021, 9, 105095. [Google Scholar] [CrossRef]
- Gadore, V.; Ahmaruzzaman, M. Tailored Fly Ash Materials: A Recent Progress of Their Properties and Applications for Remediation of Organic and Inorganic Contaminants from Water. J. Water Process Eng. 2021, 41, 101910. [Google Scholar] [CrossRef]
- Taherlou, A.; Asadollahfardi, G.; Salehi, A.M.; Katebi, A. Sustainable Use of Municipal Solid Waste Incinerator Bottom Ash and the Treated Industrial Wastewater in Self-Compacting Concrete. Constr. Build. Mater. 2021, 297, 123814. [Google Scholar] [CrossRef]
- Szopińska, M.; Luczkiewicz, A.; Jankowska, K.; Fudala-Ksiazek, S.; Potapowicz, J.; Kalinowska, A.; Bialik, R.J.; Chmiel, S.; Polkowska, Ż. First Evaluation of Wastewater Discharge Influence on Marine Water Contamination in the Vicinity of Arctowski Station (Maritime Antarctica). Sci. Total Environ. 2021, 789, 147912. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghachtouli, N.; El Arroussi, H. Phycoremediation Mechanisms of Heavy Metals Using Living Green Microalgae: Physicochemical and Molecular Approaches for Enhancing Selectivity and Removal Capacity. Heliyon 2021, 5, e02477. [Google Scholar] [CrossRef]
- Rahaman, M.H.; Islam, M.A.; Islam, M.M.; Rahman, M.A.; Alam, S.M.N. Biodegradable Composite Adsorbent of Modified Cellulose and Chitosan to Remove Heavy Metal Ions from Aqueous Solution. Curr. Res. Green Sustain. Chem. 2021, 4, 100119. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Guibal, E. A Novel Algal-Based Sorbent for Heavy Metal Removal. Chem. Eng. J. 2018, 332, 582–595. [Google Scholar] [CrossRef]
- Feng, Z.; Ji, S.; Ping, J.; Cui, D. Recent Advances in Metabolomics for Studying Heavy Metal Stress in Plants. TrAC-Trends Anal. Chem. 2021, 143, 116402. [Google Scholar] [CrossRef]
- Yang, D.; Liu, Y.; Liu, S.; Li, C.; Zhao, Y.; Li, L.; Lu, S. Exposure to Heavy Metals and Its Association with DNA Oxidative Damage in Municipal Waste Incinerator Workers in Shenzhen, China. Chemosphere 2020, 250, 126289. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Khan, F.A.; Noureldeen, A.; Rinklebe, J.; Sonne, C.; Rajakaruna, N.; Ahmad, P. Biotransfer, Bioaccumulation and Detoxification of Nickel along the Soil-Faba Bean-Aphid-Ladybird Food Chain. Sci. Total Environ. 2021, 785, 147226. [Google Scholar] [CrossRef]
- Vieira, K.S.; Baptista Neto, J.A.; Crapez, M.A.C.; Gaylarde, C.; da Silva Pierri, B.; Saldaña-Serrano, M.; Bainy, A.C.D.; Nogueira, D.J.; Fonseca, E.M. Occurrence of Microplastics and Heavy Metals Accumulation in Native Oysters Crassostrea Gasar in the Paranaguá Estuarine System, Brazil. Mar. Pollut. Bull. 2021, 166, 112225. [Google Scholar] [CrossRef]
- Xiang, Y.; Jiang, L.; Zhou, Y.; Luo, Z.; Zhi, D.; Yang, J.; Lam, S.S. Microplastics and Environmental Pollutants: Key Interaction and Toxicology in Aquatic and Soil Environments. J. Hazard. Mater. 2022, 422, 126843. [Google Scholar] [CrossRef]
- Capriotti, M.; Cocci, P.; Bracchetti, L.; Cottone, E.; Scandiffio, R.; Caprioli, G.; Sagratini, G.; Mosconi, G.; Bovolin, P.; Palermo, F.A. Microplastics and Their Associated Organic Pollutants from the Coastal Waters of the Central Adriatic Sea (Italy): Investigation of Adipogenic Effects in Vitro. Chemosphere 2021, 263, 128090. [Google Scholar] [CrossRef]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption Behavior of Organic Pollutants on Microplastics. Ecotoxicol. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef]
- Ricardo, I.A.; Alberto, E.A.; Silva Júnior, A.H.; Macuvele, D.L.P.; Padoin, N.; Soares, C.; Gracher Riella, H.; Starling, M.C.V.M.; Trovó, A.G. A Critical Review on Microplastics, Interaction with Organic and Inorganic Pollutants, Impacts and Effectiveness of Advanced Oxidation Processes Applied for Their Removal from Aqueous Matrices. Chem. Eng. J. 2021, 424, 130282. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Vaid, M.; Sarma, K.; Gupta, A. Microplastic Pollution in Aquatic Environments with Special Emphasis on Riverine Systems: Current Understanding and Way Forward. J. Environ. Manag. 2021, 293, 112860. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, R.; Zhang, S.; Sun, Y.; Wang, F. Uptake and Translocation of Nano/Microplastics by Rice Seedlings: Evidence from a Hydroponic Experiment. J. Hazard. Mater. 2022, 421, 126700. [Google Scholar] [CrossRef] [PubMed]
- Watteau, F.; Dignac, M.F.; Bouchard, A.; Revallier, A.; Houot, S. Microplastic Detection in Soil Amended With Municipal Solid Waste Composts as Revealed by Transmission Electronic Microscopy and Pyrolysis/GC/MS. Front. Sustain. Food Syst. 2018, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, D.; Pan, S.; Shen, Z.; Song, Y.; Jin, Y.; Wu, W.M.; Hou, D. Microplastics Undergo Accelerated Vertical Migration in Sand Soil Due to Small Size and Wet-Dry Cycles. Environ. Pollut. 2019, 249, 527–534. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics Accumulate on Pores in Seed Capsule and Delay Germination and Root Growth of the Terrestrial Vascular Plant Lepidium Sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and Genotoxicity of Polystyrene Microplastics on Higher Plant Vicia Faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Qiu, W.; Song, Z. Effect of Microplastics and Arsenic on Nutrients and Microorganisms in Rice Rhizosphere Soil. Ecotoxicol. Environ. Saf. 2021, 211, 111899. [Google Scholar] [CrossRef]
- Meng, F.; Yang, X.; Riksen, M.; Xu, M.; Geissen, V. Response of Common Bean (Phaseolus vulgaris L.) Growth to Soil Contaminated with Microplastics. Sci. Total Environ. 2021, 755, 142516. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, D.; Chae, Y.; Kim, D.; An, Y.J. Crop-Dependent Changes in Water Absorption of Expanded Polystyrene in Soil Environments. Chemosphere 2019, 219, 345–350. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Song, N. Polyethylene Microplastics Increase Cadmium Uptake in Lettuce (Lactuca sativa L.) by Altering the Soil Microenvironment. Sci. Total Environ. 2021, 784, 147133. [Google Scholar] [CrossRef]
- Giorgetti, L.; Spanò, C.; Muccifora, S.; Bottega, S.; Barbieri, F.; Bellani, L.; Ruffini Castiglione, M. Exploring the Interaction between Polystyrene Nanoplastics and Allium Cepa during Germination: Internalization in Root Cells, Induction of Toxicity and Oxidative Stress. Plant Physiol. Biochem. 2020, 149, 170–177. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.G.M.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective Uptake of Submicrometre Plastics by Crop Plants via a Crack-Entry Mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of Polystyrene Nanoplastics (PSNPs) on Seed Germination and Seedling Growth of Wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385, 121620. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, H.; Zhang, J.; Saleem, M.; He, Y.; Zhong, J.; Ma, R. Seasonality Regulates the Effects of Acid Rain on Microbial Community in a Subtropical Agricultural Soil of Southern China. Ecotoxicol. Environ. Saf. 2021, 224, 112681. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Yin, S.; Xiao, K.; Xiong, Q.; Bian, S.; Liang, S.; Hou, H.; Hu, J.; Yang, J. Metabolomics Revealing the Response of Rice (Oryza sativa L.) Exposed to Polystyrene Microplastics. Environ. Pollut. 2020, 266, 115159. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. Microplastic Particles Increase Arsenic Toxicity to Rice Seedlings. Environ. Pollut. 2020, 259, 113892. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Q.; Yin, N.; Tu, C.; Luo, Y. Uptake and Accumulation of Microplastics in an Edible Plant. Kexue Tongbao/Chin. Sci. Bull. 2019, 64, 928–934. [Google Scholar] [CrossRef] [Green Version]
- Zequan, Z.; Changsheng, S.H.U.; Xuefang, D.; Yunping, L.; Jun, L.; Huiting, W.; Zhengsong, P.; Wuyun, Y. Effect of Low Temperature on Seed Germination and Seedling Growth in Wheat. Southwest China J. Agric. Sci. 2010, 23, 22–25. [Google Scholar]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and Micro- Plastics in Soil-Plant System: Effects of Plastic Mulch Film Residues on Wheat (Triticum aestivum) Growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic Shape, Polymer Type, and Concentration Affect Soil Properties and Plant Biomass. Front. Plant Sci. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Zhu, J.; Wang, J.; Wang, H.; Zhan, X. The Joint Toxicity of Polyethylene Microplastic and Phenanthrene to Wheat Seedlings. Chemosphere 2021, 282, 130967. [Google Scholar] [CrossRef]
- Urbina, M.A.; Correa, F.; Aburto, F.; Ferrio, J.P. Adsorption of Polyethylene Microbeads and Physiological Effects on Hydroponic Maize. Sci. Total Environ. 2020, 741, 140216. [Google Scholar] [CrossRef]
- Pignattelli, S.; Broccoli, A.; Renzi, M. Physiological Responses of Garden Cress (L. sativum) to Different Types of Microplastics. Sci. Total Environ. 2020, 727, 138609. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Rillig, M.C. Effects of Microplastic Fibers and Drought on Plant Communities. Environ. Sci. Technol. 2020, 54, 6166–6173. [Google Scholar] [CrossRef]
- Rychter, P.; Rogacz, D.; Lewicka, K.; Kollár, J.; Kawalec, M.; Mosnáiek, J. Ecotoxicological Properties of Tulipalin A-Based Superabsorbents versus Conventional Superabsorbent Hydrogels. Adv. Polym. Technol. 2019, 2019, 2947152. [Google Scholar] [CrossRef]
- Rong, L.; Zhao, L.; Zhao, L.; Cheng, Z.; Yao, Y.; Yuan, C.; Wang, L.; Sun, H. LDPE Microplastics Affect Soil Microbial Communities and Nitrogen Cycling. Sci. Total Environ. 2021, 773, 145640. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The Microplastisphere: Biodegradable Microplastics Addition Alters Soil Microbial Community Structure and Function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Qi, R.; Jones, D.L.; Li, Z.; Liu, Q.; Yan, C. Behavior of Microplastics and Plastic Film Residues in the Soil Environment: A Critical Review. Sci. Total Environ. 2020, 703, 134722. [Google Scholar] [CrossRef]
- Fu, Q.; Lai, J.L.; Ji, X.H.; Luo, Z.X.; Wu, G.; Luo, X.G. Alterations of the Rhizosphere Soil Microbial Community Composition and Metabolite Profiles of Zea Mays by Polyethylene-Particles of Different Molecular Weights. J. Hazard. Mater. 2022, 423, 127062. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Xu, Y.; Lei, F.; Yu, X.; Ouyang, Z.; Chen, Y.; Jia, H.; Guo, X. Degradation of Polyethylene Plastic in Soil and Effects on Microbial Community Composition. J. Hazard. Mater. 2021, 416, 126173. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Du, W.; Ai, F.; Xu, F.; Zhu, J.; Yin, Y.; Ji, R.; Guo, H. Polystyrene Microplastics Alleviate the Effects of Sulfamethazine on Soil Microbial Communities at Different CO2 Concentrations. J. Hazard. Mater. 2021, 413, 125286. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.G.; Lv, J.; Fu, T.; Ma, Q.; Song, W.; Wang, Y.P.; Li, F.M. Continuous Plastic-Film Mulching Increases Soil Aggregation but Decreases Soil PH in Semiarid Areas of China. Soil Tillage Res. 2017, 167, 46–53. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of Soil Dissolved Organic Matter to Microplastic Addition in Chinese Loess Soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Sun, X.; Peng, Y.; Xiao, L. Mixing Effect of Polylactic Acid Microplastic and Straw Residue on Soil Property and Ecological Function. Chemosphere 2020, 243, 125271. [Google Scholar] [CrossRef]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of Soil Enzyme Activities and Bacterial Communities to the Accumulation of Microplastics in an Acid Cropped Soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE Microplastic Films Alter Microbial Community Composition and Enzymatic Activities in Soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef]
- Seeley, M.E.; Song, B.; Passie, R.; Hale, R.C. Microplastics Affect Sedimentary Microbial Communities and Nitrogen Cycling. Nat. Commun. 2020, 11, 2372. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef]
- Ricciardi, M.; Pironti, C.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Aquatic Environment: Occurrence, Persistence, Analysis, and Human Exposure. Water 2021, 13, 973. [Google Scholar] [CrossRef]
- Chae, Y.; An, Y.J. Nanoplastic Ingestion Induces Behavioral Disorders in Terrestrial Snails: Trophic Transfer Effects: Via Vascular Plants. Environ. Sci. Nano 2020, 7, 975–983. [Google Scholar] [CrossRef]
- Rainieri, S.; Conlledo, N.; Larsen, B.K.; Granby, K.; Barranco, A. Combined Effects of Microplastics and Chemical Contaminants on the Organ Toxicity of Zebrafish (Danio rerio). Environ. Res. 2018, 162, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Yuan, X.Z.; Jia, Y.; Feng, L.J.; Zhu, F.P.; Dong, S.S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.L.; et al. Differentially Charged Nanoplastics Demonstrate Distinct Accumulation in Arabidopsis Thaliana. Nat. Nanotechnol. 2020, 15, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, Y.; Song, Z. Effects of Polyethylene Microplastic on the Phytotoxicity of Di-n-Butyl Phthalate in Lettuce (Lactuca sativa L. var. ramosa Hort). Chemosphere 2019, 237, 124482. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Li, X.; Gonçalves, J.M.; Shi, H.; Tian, T.; Chen, N. Effects of Residual Plastic-Film Mulch on Field Corn Growth and Productivity. Sci. Total Environ. 2020, 729, 138901. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ingraffia, R.; De Souza Machado, A.A. Microplastic Incorporation into Soil in Agroecosystems. Front. Plant Sci. 2017, 8, 1805. [Google Scholar] [CrossRef] [Green Version]
- Rochman, C.M.; Cook, A.-M.; Koelmans, A.A. Plastic Debris and Policy: Using Current Scientific Understanding to Invoke Positive Change. Environ. Toxicol. Chem. 2016, 35, 1617–1626. [Google Scholar] [CrossRef]
- Harris, L.S.T.; Fennell, J.; Fales, R.J.; Carrington, E. Spatial–Temporal Growth, Distribution, and Diffusion of Marine Microplastic Research and National Plastic Policies. Water Air Soil Pollut. 2021, 232, 400. [Google Scholar] [CrossRef]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in Cosmetics: Environmental Issues and Needs for Global Bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef]
- Xanthos, D.; Walker, T.R. International Policies to Reduce Plastic Marine Pollution from Single-Use Plastics (Plastic Bags and Microbeads): A Review. Mar. Pollut. Bull. 2017, 118, 17–26. [Google Scholar] [CrossRef]
- Galarpe, V.R.K.R.; Jaraula, C.M.B.; Paler, M.K.O. The Nexus of Macroplastic and Microplastic Research and Plastic Regulation Policies in the Philippines Marine Coastal Environments. Mar. Pollut. Bull. 2021, 167, 112343. [Google Scholar] [CrossRef]
- Liang, Y.; Tan, Q.; Song, Q.; Li, J. An Analysis of the Plastic Waste Trade and Management in Asia. Waste Manag. 2021, 119, 242–253. [Google Scholar] [CrossRef]
- Rochman, C.M.; Polhemus, D.; Wyer, H.; Coffin, S.; Moore, S.; Weisberg, S.B. Steps Scientists Can Take to Inform Aquatic Microplastics Management: A Perspective Informed by the California Experience. Appl. Spectrosc. 2020, 74, 971–975. [Google Scholar]
- Milojevic, N.; Cydzik-Kwiatkowska, A. Agricultural Use of Sewage Sludge as a Threat of Microplastic (MP) Spread in the Environment and the Role of Governance. Energies 2021, 14, 6293. [Google Scholar] [CrossRef]
- Christodoulou, A.; Stamatelatou, K. Overview of Legislation on Sewage Sludge Management in Developed Countries Worldwide. Water Sci. Technol. 2016, 73, 453–462. [Google Scholar] [CrossRef]
- Deme, G.G.; Ewusi-Mensah, D.; Olagbaju, O.A.; Okeke, E.S.; Okoye, C.O.; Odii, E.C.; Ejeromedoghene, O.; Igun, E.; Onyekwere, J.O.; Oderinde, O.K.; et al. Macro Problems from Microplastics: Toward a Sustainable Policy Framework for Managing Microplastic Waste in Africa. Sci. Total Environ. 2022, 804, 150170. [Google Scholar] [CrossRef]
- Gil-Ortiz, R.; Naranjo, M.Á.; Ruiz-Navarro, A.; Atares, S.; García, C.; Zotarelli, L.; Bautista, A.S.; Vicente, O. Enhanced Agronomic Efficiency Using a New Controlled-Released, Polymeric-Coated Nitrogen Fertilizer in Rice. Plants 2020, 9, 1183. [Google Scholar] [CrossRef]
- Deng, L.; Cai, L.; Sun, F.; Li, G.; Che, Y. Public Attitudes towards Microplastics: Perceptions, Behaviors and Policy Implications. Resour. Conserv. Recycl. 2020, 163, 105096. [Google Scholar] [CrossRef]
- Barcelo, D.; Pico, Y. Case Studies of Macro- and Microplastics Pollution in Coastal Waters and Rivers: Is There a Solution with New Removal Technologies and Policy Actions? Case Stud. Chem. Environ. Eng. 2020, 2, 100019. [Google Scholar] [CrossRef]
| Microplastic/Plastic | Plant | Effect | Reference | ||
|---|---|---|---|---|---|
| Types | Size (mm) | Concentration (% w/w) | |||
| Polystyrene (PS) | 0.55~56 | 2 | Onion (Allium fistulosum) | Increased root biomass, total root length, and mean diameter | [50] |
| <0.00005 | - | Garden onion (Allium cepa) | The root length was inhibited | [94] | |
| 0.1–0.15 | 0.1–10 | Corn (Zea mays) | Plant root biomass decreased | [44] | |
| D = 0.001 | - | Garden lettuce (Lactuaca sativa) | The PS is transported through the vascular system to the stem and leaves | [95] | |
| 0.0001 | 5 | Wheat (Triticum aestivum) | The root length increased, the root/shoot ratio decreased, and the biomass increased | [96] | |
| 5 | - | Broad bean (Vicia faba) | Decreased biomass and catalase enzyme activity in addition to blocking cell connections or cell wall pores for the transport of nutrients in roots | [89] | |
| D = <0.001 | - | Rice (Oryza sativa) | PS was mostly aggregated in the vascular systems of the roots, stems, and leaves, with a high possibility of entering the food chain | [97] | |
| <50 | - | Rice (Oryza sativa) | Higher doses of PS caused a ≈40% decrease in shoot biomass | [98] | |
| 0.01 | - | Rice (Oryza sativa) | Affected the transpiration and stomata of rice seedlings primarily via inhibiting their root vigor | [99] | |
| <0.048 | - | Garden cress (Lepidium sativum) | Significantly declined germination rate and inhibited plant growth | [88] | |
| <0.001 | - | Carrot (Daucus carota L.) | Entered the roots and accumulated in the intercellular layer; particles were able to translocate to the leaves | [90] | |
| 0.001 | - | Lettuce (Lactuca sativa L., Rosa) | Adherence, uptake, accumulation, and translocation of PS in the vascular tissue | [100] | |
| Low density polyethylene (LDPE) | L: 4–10 | 1 | Garden lettuce (Lactuaca sativa) | The total biomass decreased and the composition of the rhizosphere bacterial community changed | [95] |
| L = 6.9; W = 6.1 | 1 | Wheat (Triticum aestivum) | The fruit biomass and leaf number decreased | [101] | |
| - | 1 | Wheat (Triticum aestivum) | Affected vegetative and reproductive growth | [102] | |
| 0.053–1 | 0.2–2.5 | Bean (Phaseolus vulgaris) | Aboveground and root biomass affected but the effect was not significant | [91] | |
| L = 5, W = 5 | 0.1–0.4 | Carrot (Daucus carota) | Aboveground biomass and root mass decreased with increasing concentration | [103] | |
| Poly lactic acid (PLA) | 0.1–0.15 | 0.1–10 | Corn (Zea mays) | High concentration of PLA significantly reduced plant biomass | [15] |
| 0.065 | 0.1–0.001 | Perennial ryegrass (Lolium perenne) | Reduced shoot height and biomass | [16] | |
| - | - | Bean (Phaseolus vulgaris) | Root and aboveground biomass reduced | [91] | |
| Polyethylene (PE) | D = 0.2–0.25 | 0.5–8.0 | Wheat (Triticum aestivum L.) | A high concentration of PE damaged the antioxidant system in wheat roots | [104] |
| 0.003 | - | Corn (Zea mays) | PE reduced or blocked water and nutrient uptake as well as the growth of the maize plant | [105] | |
| 0.5 | 0.1–10 | Lettuce (Lactuca sativa L.) | Increased the toxicity, uptake, accumulation, and bioavailability of heavy metals | [93] | |
| High density polyethylene (HDPE) | - | 0.1–0.001 | Carrot (Daucus carota) | Shoot height and biomass reduced, fewer seeds germinated | [16] |
| 0.01–0.15 | 0.1–10 | There was no significant change in plant biomass | [103] | ||
| Polyamide (PA) | 0.015–0.02 | 2 | Onion (Allium fistulosum) | Significantly affected plant biomass, root traits, tissue elemental composition, and soil microbial activity | [50] |
| 0.015–0.02 | 2 | Wheat (Triticum aestivum) | The total biomass increased as did the total root length and mean diameter | [96] | |
| Polypropylene (PP) | - | 0.02 | Garden cress (Lepidium sativum) | Occurrence of oxidative burst | [106] |
| L = 5, W = 5 | 0.1–0.4 | Carrot (Daucus carota) | Aboveground biomass and root mass decreased with increasing concentration | [103] | |
| Polyester fibers (PFs) | L = 5, D = 0.008 | 0.2 | Onion (Allium fistulosum) | Significantly changed plant biomass, root traits, tissue elemental composition, and soil microbial activity | [50] |
| L = 1.3, D = 0.03 | - | Grasses (Festuca brevipila) and herbs (Achillea millefolium) | Decreased biomass | [107] | |
| Polyether sulfone (PES) | L = 5, D = 0.008 | 0.2 | Onion (Allium fistulosum) | The total biomass and root biomass increased, the total root length and mean diameter increased, and the root microbial activity increased | [50] |
| L = 1.3, D = 0.03 | 0.4 | Wood small-reed (Calamagrostis epigejos) | The root biomass increased | [107] | |
| Expandable polystyrene (EPS) | 8.3 | - | Mung bean (Phaseolus radiates), lettuce (Lactuca sativa), and rice (Oryza sativa) | Low levels of interaction with the crop dependent and water absorption rate | [92] |
| Polyvinyl chloride (PVC) | 0.018–0.15 | 0.5–2 | Lettuce (Lactuva sativa L.) | PCV-a promoted carotenoid synthesis whereas PVC-b inhibited it | [95] |
| Melamine phenolic (MP) | 0.0048 | - | Garden cress (Lepidium sativum) | Accumulated on the root hairs, the germination rate was significantly reduced, and pores in the seed capsule were physically blocked | [88] |
| Polyetherimide (PEIs) | - | 0.01–0.1 | Oat (Avena sativa) and radish (Raphanus sativus) | Nitrogen released from the tested PEIs but no harmful effect; harmful to plants only at high concentrations | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igalavithana, A.D.; Mahagamage, M.G.Y.L.; Gajanayake, P.; Abeynayaka, A.; Gamaralalage, P.J.D.; Ohgaki, M.; Takenaka, M.; Fukai, T.; Itsubo, N. Microplastics and Potentially Toxic Elements: Potential Human Exposure Pathways through Agricultural Lands and Policy Based Countermeasures. Microplastics 2022, 1, 102-120. https://doi.org/10.3390/microplastics1010007
Igalavithana AD, Mahagamage MGYL, Gajanayake P, Abeynayaka A, Gamaralalage PJD, Ohgaki M, Takenaka M, Fukai T, Itsubo N. Microplastics and Potentially Toxic Elements: Potential Human Exposure Pathways through Agricultural Lands and Policy Based Countermeasures. Microplastics. 2022; 1(1):102-120. https://doi.org/10.3390/microplastics1010007
Chicago/Turabian StyleIgalavithana, Avanthi Deshani, Mahagama Gedara Y. L. Mahagamage, Pradeep Gajanayake, Amila Abeynayaka, Premakumara Jagath Dickella Gamaralalage, Masataka Ohgaki, Miyuki Takenaka, Takayuki Fukai, and Norihiro Itsubo. 2022. "Microplastics and Potentially Toxic Elements: Potential Human Exposure Pathways through Agricultural Lands and Policy Based Countermeasures" Microplastics 1, no. 1: 102-120. https://doi.org/10.3390/microplastics1010007