Regulation of DNA Methylation Through EBP1 Interaction with NLRP2 and NLRP7
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid Constructs
2.2. Yeast Two-Hybrid Screen
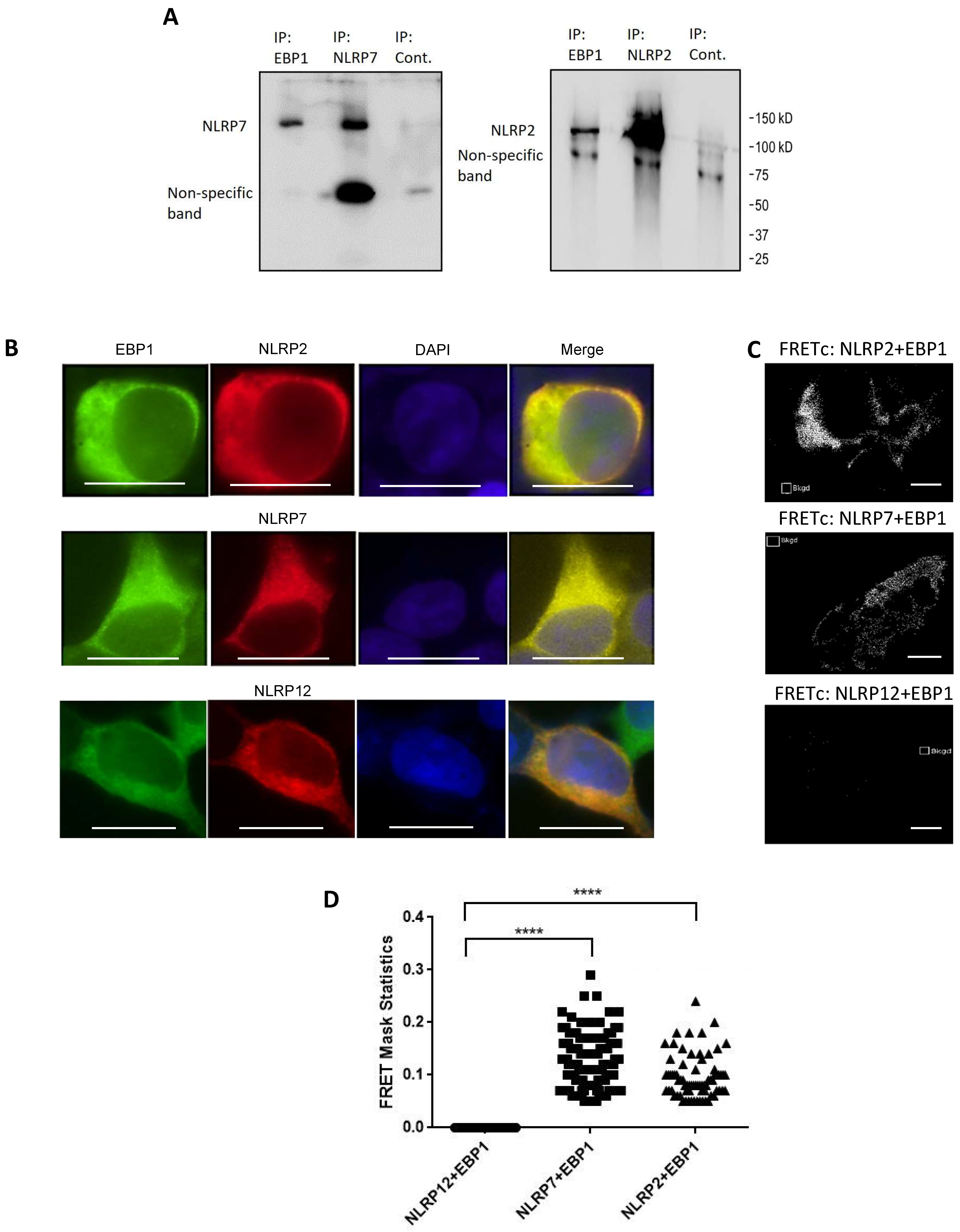
2.3. Co-Immunoprecipitation
2.4. Confocal Microscopy and Immunostaining
2.5. Three-Channel Corrected FRET(c) Analysis
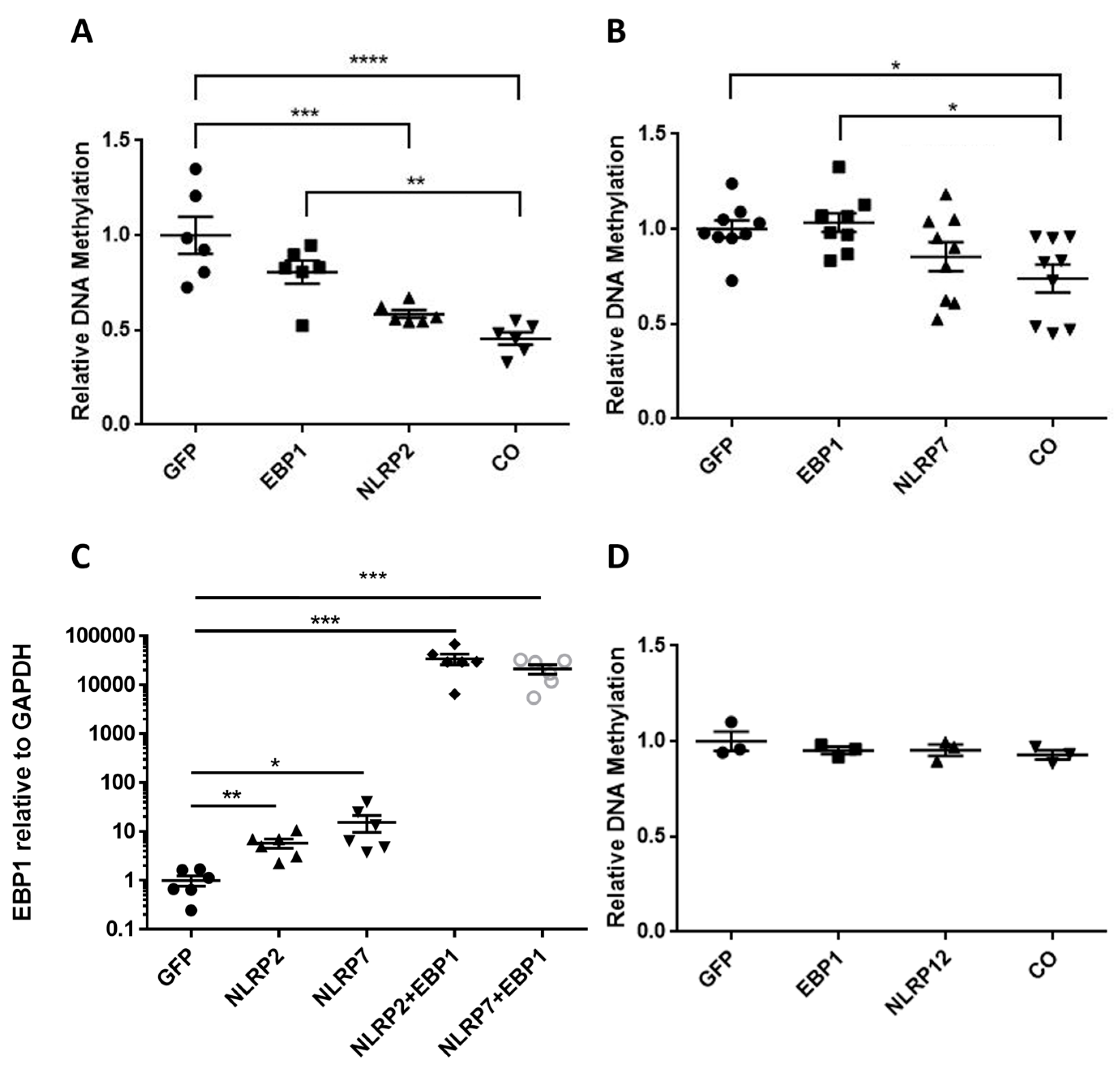
2.6. Global DNA Methylation Analysis
2.7. RNA Isolation and q-PCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Identification of Potential Proteins That Interact with NLRP2 and NLRP7 Using Y2H Screening
3.2. Confirmation of NLRP2/7 and EBP1 Interactions in Human Cells
3.3. Combinatorial Effect of NLRP2/7 and EBP1 on DNA Methylation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCR | Polymerase Chain Reaction |
| SDS-PAGE | Sodium dodecyl sulphate-polyacrylamide gel electrophoresis |
| FRET | Förster’s Resonance Energy Transfer |
| ELISA | Enzyme Linked Immunosorbent Assay |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal Bovine Serum |
References
- Huang, J.Y.; Su, M.; Lin, S.H.; Kuo, P.L. A genetic association study of NLRP2 and NLRP7 genes in idiopathic recurrent miscarriage. Hum. Reprod. 2013, 28, 1127–1134. [Google Scholar] [CrossRef]
- Baek, K.H.; Lee, E.J.; Kim, Y.S. Recurrent pregnancy loss: The key potential mechanisms. Trends Mol. Med. 2007, 13, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Aruna, M.; Nagaraja, T.; Andal, S.; Tarakeswari, S.; Sirisha, P.V.; Reddy, A.G.; Thangaraj, K.; Singh, L.; Reddy, B.M. Role of progesterone receptor polymorphisms in the recurrent spontaneous abortions: Indian case. PLoS ONE 2010, 5, e8712. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.K.; Wen, H.; Ting, J.P. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 2011, 29, 707–735. [Google Scholar] [CrossRef] [PubMed]
- Yeretssian, G. Effector functions of NLRs in the intestine: Innate sensing, cell death, and disease. Immunol. Res. 2012, 54, 25–36. [Google Scholar] [CrossRef]
- Huang, T.C.; Chang, K.C.; Chang, J.Y.; Tsai, Y.S.; Yang, Y.J.; Chang, W.C.; Mo, C.F.; Yu, P.H.; Chiang, C.T.; Lin, S.P.; et al. Variants in Maternal Effect Genes and Relaxed Imprinting Control in a Special Placental Mesenchymal Dysplasia Case with Mild Trophoblast Hyperplasia. Biomedicines 2021, 9, 544. [Google Scholar] [CrossRef]
- Begemann, M.; Rezwan, F.I.; Beygo, J.; Docherty, L.E.; Kolarova, J.; Schroeder, C.; Buiting, K.; Chokkalingam, K.; Degenhardt, F.; Wakeling, E.L.; et al. Maternal variants in NLRP and other maternal effect proteins are associated with multilocus imprinting disturbance in offspring. J. Med. Genet. 2018, 55, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Soellner, L.; Begemann, M.; Degenhardt, F.; Geipel, A.; Eggermann, T.; Mangold, E. Maternal heterozygous NLRP7 variant results in recurrent reproductive failure and imprinting disturbances in the offspring. Eur. J. Hum. Genet. 2017, 25, 924–929. [Google Scholar] [CrossRef]
- Rubio, C.; Pehlivan, T.; Rodrigo, L.; Simón, C.; Remohí, J.; Pellicer, A. Embryo aneuploidy screening for unexplained recurrent miscarriage: A minireview. Am. J. Reprod. Immunol. 2005, 53, 159–165. [Google Scholar] [CrossRef]
- Meyer, E.; Lim, D.; Pasha, S.; Tee, L.J.; Rahman, F.; Yates, J.R.; Woods, C.G.; Reik, W.; Maher, E.R. Germline mutation in NLRP2 (NALP2) in a familial imprinting disorder (Beckwith-Wiedemann Syndrome). PLoS Genet. 2009, 5, e1000423. [Google Scholar] [CrossRef]
- Van Gorp, H.; Kuchmiy, A.; Van Hauwermeiren, F.; Lamkanfi, M. NOD-like receptors interfacing the immune and reproductive systems. FEBS J. 2014, 281, 4568–4582. [Google Scholar] [CrossRef]
- Zhong, Y.; Kinio, A.; Saleh, M. Functions of NOD-Like Receptors in Human Diseases. Front. Immunol. 2013, 4, 333. [Google Scholar] [CrossRef]
- Zhang, P.; Dixon, M.; Zucchelli, M.; Hambiliki, F.; Levkov, L.; Hovatta, O.; Kere, J. Expression analysis of the NLRP gene family suggests a role in human preimplantation development. PLoS ONE 2008, 3, e2755. [Google Scholar] [CrossRef] [PubMed]
- Bracey, N.A.; Gershkovich, B.; Chun, J.; Vilaysane, A.; Meijndert, H.C.; Wright, J.R., Jr.; Fedak, P.W.; Beck, P.L.; Muruve, D.A.; Duff, H.J. Mitochondrial NLRP3 protein induces reactive oxygen species to promote Smad protein signaling and fibrosis independent from the inflammasome. J. Biol. Chem. 2014, 289, 19571–19584. [Google Scholar] [CrossRef]
- Stevenson, B.W.; Gorman, M.A.; Koach, J.; Cheung, B.B.; Marshall, G.M.; Parker, M.W.; Holien, J.K. A structural view of PA2G4 isoforms with opposing functions in cancer. J. Biol. Chem. 2020, 295, 16100–16112. [Google Scholar] [CrossRef]
- Hwang, I.; Ko, H.R.; Ahn, J.Y. The roles of multifunctional protein ErbB3 binding protein 1 (EBP1) isoforms from development to disease. Exp. Mol. Med. 2020, 52, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Z.; Wolf, D.; Goff, S.P. EBP1, a novel host factor involved in primer binding site-dependent restriction of moloney murine leukemia virus in embryonic cells. J. Virol. 2014, 88, 1825–1829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peng, H.; Chang, B.; Lu, C.; Su, J.; Wu, Y.; Lv, P.; Wang, Y.; Liu, J.; Zhang, B.; Quan, F.; et al. Nlrp2, a maternal effect gene required for early embryonic development in the mouse. PLoS ONE 2012, 7, e30344. [Google Scholar] [CrossRef]
- Fallahi, J.; Fallahi, J.; Razban, V.; Momtahan, M.; Akbarzadeh-Jahromi, M.; Namavar-Jahromi, B.; Anvar, Z.; Fardaei, M. A Novel Mutation in NLRP7 Related to Recurrent Hydatidiform Mole and Reproductive Failure. Int. J. Fertil. Steril. 2019, 13, 135–138. [Google Scholar]
- Djuric, U.; El-Maarri, O.; Lamb, B.; Kuick, R.; Seoud, M.; Coullin, P.; Oldenburg, J.; Hanash, S.; Slim, R. Familial molar tissues due to mutations in the inflammatory gene, NALP7, have normal postzygotic DNA methylation. Hum. Genet. 2006, 120, 390–395. [Google Scholar] [CrossRef]
- Singer, H.; Biswas, A.; Nuesgen, N.; Oldenburg, J.; El-Maarri, O. NLRP7, Involved in Hydatidiform Molar Pregnancy (HYDM1), Interacts with the Transcriptional Repressor ZBTB16. PLoS ONE 2015, 10, e0130416. [Google Scholar] [CrossRef]
- Li, G.; Tian, X.; Lv, D.; Zhang, L.; Zhang, Z.; Wang, J.; Yang, M.; Tao, J.; Ma, T.; Wu, H.; et al. NLRP7 is expressed in the ovine ovary and associated with in vitro pre-implantation embryo development. Reproduction 2019, 158, 415–427. [Google Scholar] [CrossRef]
- Peng, H.; Liu, H.; Liu, F.; Gao, Y.; Chen, J.; Huo, J.; Han, J.; Xiao, T.; Zhang, W. NLRP2 and FAF1 deficiency blocks early embryogenesis in the mouse. Reproduction 2017, 154, 245–251. [Google Scholar] [CrossRef]
- Andreasen, L.; Christiansen, O.B.; Niemann, I.; Bolund, L.; Sunde, L. NLRP7 or KHDC3L genes and the etiology of molar pregnancies and recurrent miscarriage. Mol. Hum. Reprod. 2013, 19, 773–781. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, L.; Su, M.; Zeng, Q.; Luo, P.; Chu, L. NLRP7 maintains genomic stability during early human embryogenesis via mediating alternative splicing. Commun. Biol. 2025, 8, 125. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, J.; Zhao, F.; Sun, Y.; Chen, Z. NLRP2 in health and disease: Emerging roles in epigenetic regulation. Immunology 2024, 171, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.R.; Hwang, I.; Jin, E.J.; Yun, T.; Ryu, D.; Kang, J.S.; Park, K.W.; Shin, J.H.; Cho, S.W.; Lee, K.H.; et al. Roles of ErbB3-binding protein 1 (EBP1) in embryonic development and gene-silencing control. Proc. Natl. Acad. Sci. USA 2019, 116, 24852–24860. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, S.; Sathappan, V.; Utama, B.; Lorenzo, I.; Kaskar, K.; Van den Veyver, I.B. Maternally expressed NLRP2 links the subcortical maternal complex (SCMC) to fertility, embryogenesis and epigenetic reprogramming. Sci. Rep. 2017, 7, 44667. [Google Scholar]
- Kim, Y.; Ko, H.R.; Hwang, I.; Ahn, J.Y. ErbB3 binding protein 1 contributes to adult hippocampal neurogenesis by modulating Bmp4 and Ascl1 signaling. BMB Rep. 2024, 57, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, S.; Wen, S.; Wan, Y.W.; Peng, H.H.; Otta, S.; Liu, Z.; Iacovino, M.; Mahen, E.M.; Kyba, M.; Sadikovic, B.; et al. NLRP7 affects trophoblast lineage differentiation, binds to overexpressed YY1 and alters CpG methylation. Hum. Mol. Genet. 2014, 23, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.C.; Shao, L.; Peng, H.H.; Rosetta, R.; del Gaudio, D.; Wagner, A.F.; Al-Hussaini, T.K.; Van den Veyver, I.B. A recurrent intragenic genomic duplication, other novel mutations in NLRP7 and imprinting defects in recurrent biparental hydatidiform moles. Mol. Hum. Reprod. 2008, 14, 33–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Maarri, O.; Seoud, M.; Coullin, P.; Herbiniaux, U.; Oldenburg, J.; Rouleau, G.; Slim, R. Maternal alleles acquiring paternal methylation patterns in biparental complete hydatidiform moles. Hum. Mol. Genet. 2003, 12, 1405–1413. [Google Scholar] [CrossRef] [PubMed][Green Version]
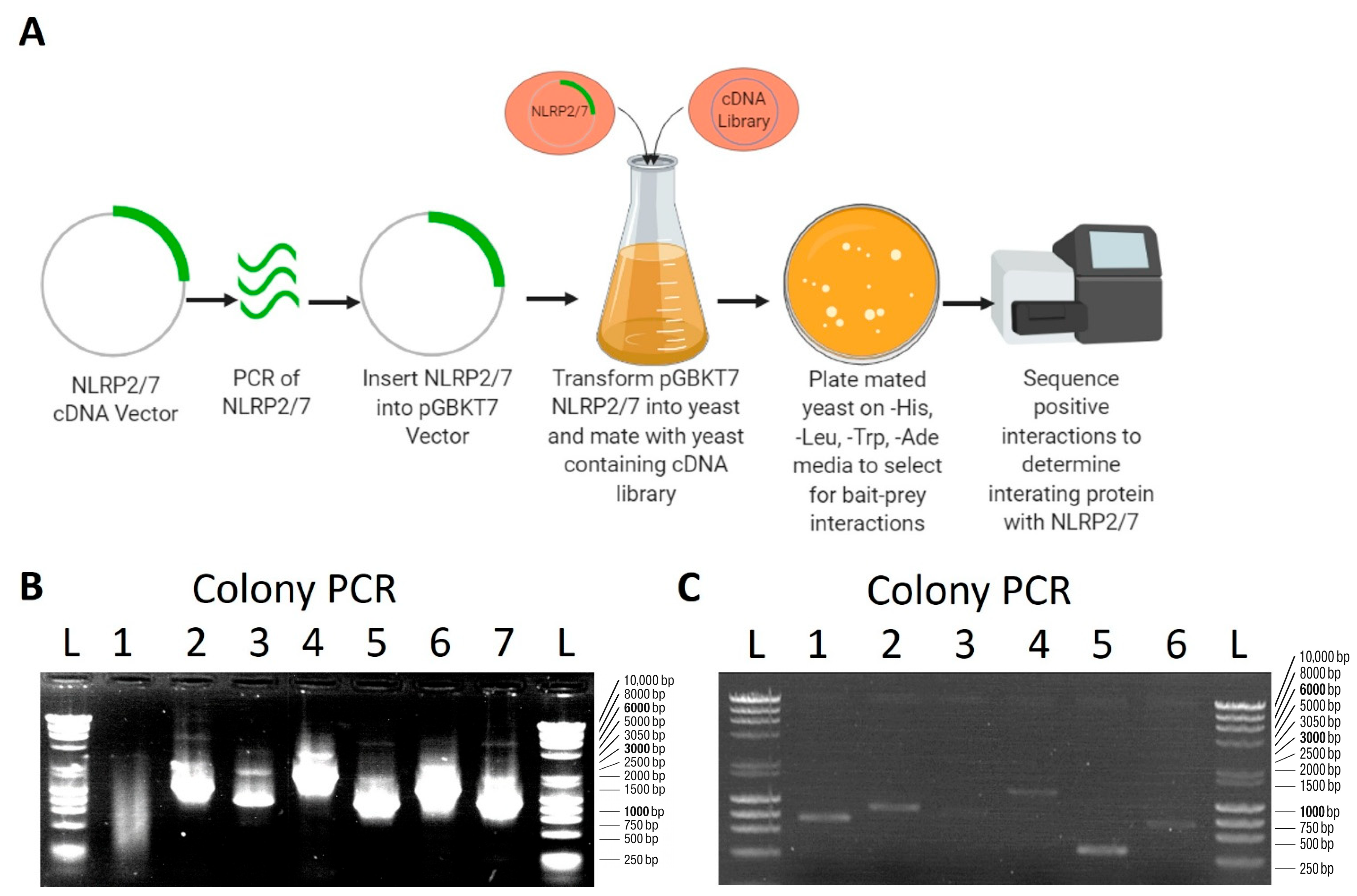
| Primer | Sequence |
|---|---|
| Yeast Amplimer Forward | CTA TTC GAT GAT GAA GAT ACC CCA CCA AAC C |
| Yeast Amplimer Reverse | GTG AAC TTG CGG GGT TTT TCA GTA TCT ACG AT |
| hEBP1 Forward | AGA GCA TTT GAA GAT GAG |
| hEBP1 Reverse | TCAGTCCCCAGCTTCATT |
| hGAPDH Forward | ACCACCCTGTTGCTGTAGCCAA |
| hGAPDH Reverse | GTCTCCTCTGACTTCAACAGCG |
| Protein Name | Abbreviation | Description |
|---|---|---|
| ErbB3-binding protein 1 | EBP1 (PA2G4) | RNA-binding protein involved in growth regulation |
| Mannan binding lectin serine peptidase 1 | MASP1 | Synthesis of proteins involved in the lectin complement pathway |
| Kinesin family member 20A | KIF20A | Kinesin-like protein |
| Protein tyrosine phosphatase receptor type M | PTPRM | Involved in cell growth, differentiation, and oncogenic transformation |
| Ring finger protein 170 | RNF170 | RING domain-containing protein involved in ubiquitination. |
| Charged multivesicular body protein 3 | CHMP3 | Protein sorting through the multivesicular body |
| Protein Name | Abbreviation | Description |
|---|---|---|
| ErbB3-binding protein 1 | EBP1 (PA2G4) | RNA-binding protein involved in growth regulation. |
| SNAP Associated Protein | SNAPIN | SNARE complex involved in protein and membrane trafficking |
| ArfGAP with SH3 domain, ankyrin repeat and PH domain 1 | ASAP1 | Membrane trafficking and cytoskeleton remodeling |
| Cannabinoid receptor interacting protein 1 | CNRIP1 | Cannabinoid receptor 1 interacting protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, N.H.; So, M.; Lupfer, C.R. Regulation of DNA Methylation Through EBP1 Interaction with NLRP2 and NLRP7. DNA 2025, 5, 49. https://doi.org/10.3390/dna5040049
Son NH, So M, Lupfer CR. Regulation of DNA Methylation Through EBP1 Interaction with NLRP2 and NLRP7. DNA. 2025; 5(4):49. https://doi.org/10.3390/dna5040049
Chicago/Turabian StyleSon, Nayeon Hannah, Matthew So, and Christopher R. Lupfer. 2025. "Regulation of DNA Methylation Through EBP1 Interaction with NLRP2 and NLRP7" DNA 5, no. 4: 49. https://doi.org/10.3390/dna5040049
APA StyleSon, N. H., So, M., & Lupfer, C. R. (2025). Regulation of DNA Methylation Through EBP1 Interaction with NLRP2 and NLRP7. DNA, 5(4), 49. https://doi.org/10.3390/dna5040049







