Small RNA and Epigenetic Control of Plant Immunity
Abstract
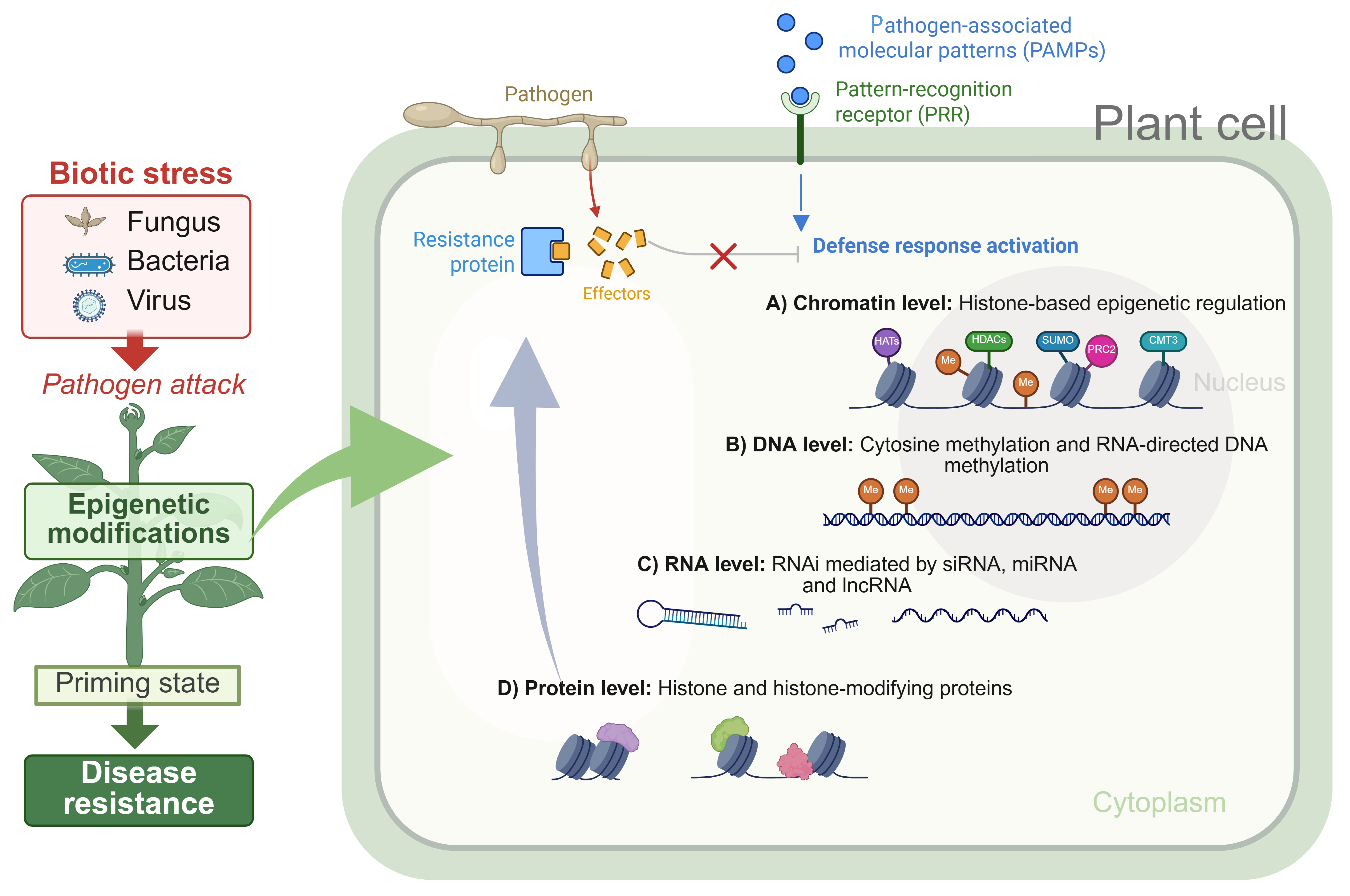
1. Introduction
2. Epigenetic Regulation of Plant Immunity
2.1. DNA Methylation and Plant Defense
2.2. Histone Modifications in Defense Signaling
2.3. Chromatin Remodeling in Plant Immunity
Priming and Epigenetic Memory Mechanisms
3. Small RNA-Mediated Gene Silencing in Plant Immunity
3.1. Small RNAs in Plant Immunity
3.2. Role in Transcriptional Gene Silencing
3.3. Role in Post-Transcriptional Gene Silencing
3.4. Crosstalk Between Small RNAs and Chromatin Remodeling
4. Epigenetic Memory and Transgenerational Immunity
4.1. Priming and Immune Memory
4.2. Epigenetic Memory and Transgenerational Priming in Plant Immunity
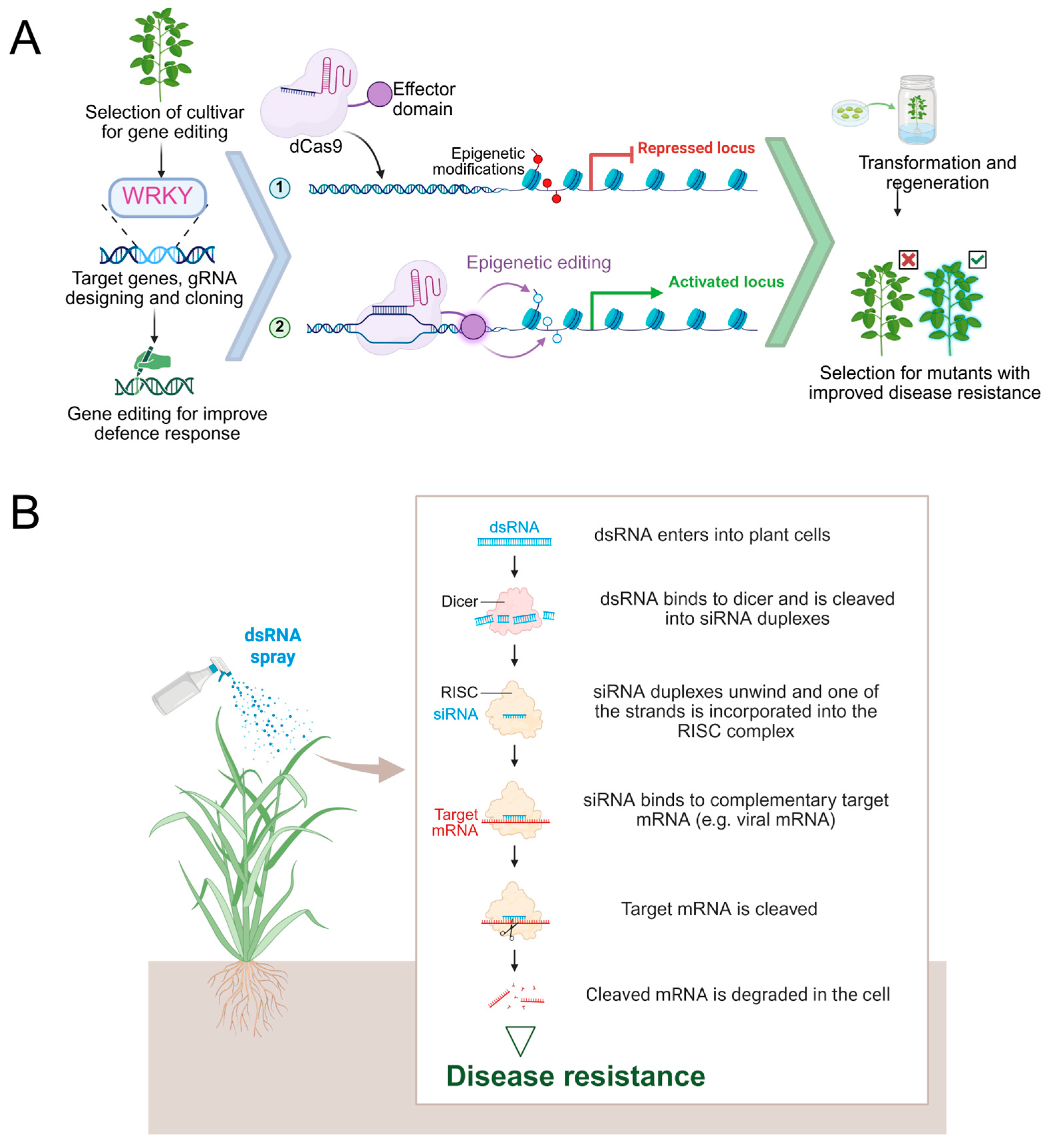
4.3. Implications for Epigenetic Breeding and Crop Improvement
4.4. Natural and Engineered Epigenetic Mechanisms in Plant Immune Regulation
4.5. Technological Advances and Challenges in Epigenomic Immunity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RdDM | RNA-directed DNA Methylation |
| siRNA | Small Interfering RNA |
| miRNA | MicroRNA |
| phasiRNA | Phased Small Interfering RNA |
| PTGS | Post-Transcriptional Gene Silencing |
| TGS | Transcriptional Gene Silencing |
| PR | Pathogenesis-Related |
| NLR | Nucleotide-binding Leucine-rich Repeat |
| H3K4me3 | Histone 3 Lysine 4 Trimethylation |
| H3K27me3 | Histone 3 Lysine 27 Trimethylation |
| CHH | Cytosine methylation in the CHH sequence context |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| dCas9 | Catalytically Dead Cas9 |
| ATAC-seq | Assay for Transposase-Accessible Chromatin Sequencing |
| ChIP-seq | Chromatin Immunoprecipitation Sequencing |
| ta-siRNA | Trans-acting Small Interfering RNA |
| DRM2 | Domains Rearranged Methyltransferase 2 |
| AGO | Argonaute |
| DCL | Dicer-Like Protein |
| RDR | RNA-Dependent RNA Polymerase |
| SGS3 | Suppressor of Gene Silencing 3 |
References
- Zipfel, C. Plant pattern-recognition receptors. Trends Immunol. 2014, 35, 345–351. [Google Scholar] [CrossRef]
- Nabi, Z.; Manzoor, S.; Nabi, S.U.; Wani, T.A.; Gulzar, H.; Farooq, M.; Arya, V.M.; Baloch, F.S.; Vlădulescu, C.; Popescu, S.M.; et al. Pattern-triggered immunity and effector-triggered immunity: Crosstalk and cooperation of PRR and NLR-mediated plant defense pathways during host–pathogen interactions. Physiol. Mol. Biol. Plants 2024, 30, 587–604. [Google Scholar] [CrossRef]
- Yu, X.; Niu, H.; Liu, C.; Wang, H.; Yin, W.; Xia, X. Synergistic signal mechanisms in plant immunity. Plant Biotechnol. J. 2024, 22, 2113–2128. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Ahn, H.-K.; Ding, P.; Jones, J.D.G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 2021, 592, 110–115. [Google Scholar] [CrossRef]
- Qiao, S.; Song, W.; Hu, W.; Wang, F.; Liao, A.; Tan, W.; Yang, S. The role of plant DNA methylation in development, stress response, and crop breeding. Agronomy 2024, 15, 94. [Google Scholar] [CrossRef]
- Halder, K.; Chaudhuri, A.; Abdin, M.Z.; Majee, M.; Datta, A. Chromatin-based transcriptional reprogramming in plants under abiotic stresses. Plants 2022, 11, 1449. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Pati, D.; Mohapatra, R.; Sahu, B.B.; Singh, P. The impact of microbes in plant immunity and priming induced inheritance: Sustainable approach for crop protection. Plant Stress 2022, 4, 100072. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Priming crops for the future: Rewiring stress memory. Trends Plant Sci. 2022, 27, 699–716. [Google Scholar] [CrossRef]
- Villagómez-Aranda, A.L.; Feregrino-Pérez, A.A.; García-Ortega, L.F.; González-Chavira, M.M.; Torres-Pacheco, I.; Guevara-González, R.G. Activating stress memory: Eustressors as potential tools for plant breeding. Plant Cell Rep. 2022, 41, 1481–1498. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Vu, N.T.; Cheong, J.-J. Transcriptional stress memory and transgenerational inheritance of drought tolerance in plants. Int. J. Mol. Sci. 2022, 23, 12918. [Google Scholar] [CrossRef] [PubMed]
- Bieluszewski, T.; Prakash, S.; Roulé, T.; Wagner, D. The role and activity of SWI/SNF chromatin remodelers. Annu. Rev. Plant Biol. 2023, 74, 139–163. [Google Scholar] [CrossRef]
- English, M.A.; Gayet, R.V.; Collins, J.J. Designing biological circuits: Synthetic biology within the operon model and beyond. Annu. Rev. Biochem. 2021, 90, 221–244. [Google Scholar] [CrossRef]
- Vaschetto, L.M. DNA methylation, histone modifications, and non-coding RNA pathways. In Epigenetics in Crop Improvement Safeguarding Food Security in an Ever-Changing Climate; Vaschetto, L.M., Ed.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- López Sánchez, A.; Stassen, J.H.M.; Furci, L.; Smith, L.M.; Ton, J. The role of DNA (de)methylation in immune responsiveness of Arabidopsis. Plant J. 2016, 88, 361–374. [Google Scholar] [CrossRef]
- Filipowicz, W.; Jaskiewicz, L.; Kolb, F.A.; Pillai, R.S. Post-transcriptional gene silencing by SiRNAs and MiRNAs. Curr. Opin. Struct. Biol. 2005, 15, 331–341. [Google Scholar] [CrossRef]
- van der Vlugt, C.J.B. Overview of sixteen scientific opinions on genetically modified plants obtained by new genomic techniques. EFSA Support. Publ. 2021, 18, 1973E. [Google Scholar] [CrossRef]
- Wakasa, Y.; Kawakatsu, T.; Takaiwa, F. Theoretical and applied epigenetics in plants. In Applied RNA Bioscience; Masuda, S., Izawa, S., Eds.; Springer: Singapore, 2018; pp. 265–286. [Google Scholar] [CrossRef]
- Lim, P.S.; Li, J.; Holloway, A.F.; Rao, S. Epigenetic regulation of inducible gene expression in the immune system. Immunology 2013, 139, 285–293. [Google Scholar] [CrossRef]
- Abreha, K.B.; Ortiz, R.; Carlsson, A.S.; Geleta, M. Understanding the Sorghum–Colletotrichum sublineola interactions for enhanced host resistance. Front. Plant Sci. 2021, 12, 641969. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Sakai, T.; Krishna Shrestha, R.; Manosalva Perez, N.; Guo, W.; Ngou, B.P.M.; He, S.; Liu, C.; Feng, X.; Zhang, R.; et al. Chromatin accessibility landscapes activated by cell-surface and intracellular immune receptors. J. Exp. Bot. 2021, 72, 7927–7941. [Google Scholar] [CrossRef] [PubMed]
- Igolkina, A.A.; Zinkevich, A.; Karandasheva, K.O.; Popov, A.A.; Selifanova, M.V.; Nikolaeva, D.; Tkachev, V.; Penzar, D.; Nikitin, D.M.; Buzdin, A. H3K4me3, H3K9ac, H3K27ac, H3K27me3 and H3K9me3 histone tags suggest distinct regulatory evolution of open and condensed chromatin landmarks. Cells 2019, 8, 1034. [Google Scholar] [CrossRef]
- Thatcher, L.; Gao, L.-L.; Singh, K. Jasmonate signalling and defence responses in the model legume Medicago truncatula—A focus on responses to Fusarium wilt disease. Plants 2016, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, L.; Wang, L.; Liu, L.; Li, L.; Sun, L.; Rao, Q.; Zhang, J.; Huang, S. JMJ704 positively regulates rice defense response against Xanthomonas oryzae pv. oryzae infection via reducing H3K4me2/3 associated with negative disease resistance regulators. BMC Plant Biol. 2015, 15, 286. [Google Scholar] [CrossRef]
- Erdmann, R.M.; Picard, C.L. RNA-directed DNA methylation. PLoS Genet. 2020, 16, e1009034. [Google Scholar] [CrossRef] [PubMed]
- Boden, S.A.; Kavanová, M.; Finnegan, E.J.; Wigge, P.A. Thermal stress effects on grain yield in Brachypodium distachyon occur via H2A.Z-nucleosomes. Genome Biol. 2013, 14, R65. [Google Scholar] [CrossRef]
- Hauser, M.-T.; Aufsatz, W.; Jonak, C.; Luschnig, C. Transgenerational epigenetic inheritance in plants. Biochim. Et Biophys. Acta (BBA)—Gene Regul. Mech. 2011, 1809, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Bilichak, A.; Kovalchuk, I. Transgenerational response to stress in plants and its application for breeding. J. Exp. Bot. 2016, 67, 2081–2092. [Google Scholar] [CrossRef]
- Du, K.; Wu, J.; Wang, J.; Xie, W.; Yin, L.; Li, X.; Li, C.; Dong, A. the chromatin remodeling factor OsINO80 promotes H3K27me3 and H3K9me2 deposition and maintains TE silencing in rice. Nat. Commun. 2024, 15, 10919. [Google Scholar] [CrossRef]
- Bencke-Malato, M.; Cabreira, C.; Wiebke-Strohm, B.; Bücker-Neto, L.; Mancini, E.; Osorio, M.B.; Homrich, M.S.; Turchetto-Zolet, A.C.; De Carvalho, M.C.; Stolf, R.; et al. Genome-wide annotation of the soybean WRKY family and functional characterization of genes involved in response to Phakopsora pachyrhiziinfection. BMC Plant Biol. 2014, 14, 236. [Google Scholar] [CrossRef] [PubMed]
- Heimann, L. Signal Integration on Photosynthetic Promoters in the C4 Grass Species Zea mays, Sorghum bicolor, and Setaria italica. Ph.D. Thesis, Gottfried Wilhelm Leibniz Universität Hannover, Hannover, Germany, 2013. [Google Scholar]
- Mishra, S.; Roychowdhury, R.; Ray, S.; Hada, A.; Kumar, A.; Sarker, U.; Aftab, T.; Das, R. Salicylic acid (SA)-mediated plant immunity against biotic stresses: An insight on molecular components and signaling mechanism. Plant Stress 2024, 11, 100427. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Zhu, F.; Fan, T.; Zhang, J.; Lo, C. Alternative splicing is a Sorghum bicolor defense response to fungal infection. Planta 2020, 251, 14. [Google Scholar] [CrossRef]
- Mudunkothge, J.S.; Krizek, B.A. Three Arabidopsis AIL/PLT genes act in combination to regulate shoot apical meristem function. Plant J. 2012, 71, 108–121. [Google Scholar] [CrossRef]
- Gao, S.; Wang, F.; Niran, J.; Li, N.; Yin, Y.; Yu, C.; Jiao, C.; Yao, M. Transcriptome analysis reveals defense-related genes and pathways against Xanthomonas campestris pv. vesicatoria in pepper (Capsicum annuum L.). PLoS ONE 2021, 16, e0240279. [Google Scholar] [CrossRef] [PubMed]
- Kawa, D.; Julkowska, M.; Montero Sommerfeld, H.; ter Horst, A.; Haring, M.A.; Testerink, C. Phosphate-dependent root system architecture responses to salt stress. Plant Physiol. 2016, 172, 690–706. [Google Scholar] [CrossRef]
- Li, G.; Zhang, S.; Li, F.; Zhang, H.; Zhang, S.; Zhao, J.; Sun, R. Variability in the viral protein linked to the genome of turnip mosaic virus influences interactions with eif(iso)4Es in Brassica rapa. Plant Pathol. J. 2021, 37, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Jiang, J.; Leichter, S.M.; Liu, J.; Biswal, M.; Khudaverdyan, N.; Zhong, X.; Song, J. Mechanistic basis for maintenance of CHG DNA methylation in plants. Nat. Commun. 2022, 13, 3877. [Google Scholar] [CrossRef] [PubMed]
- Halter, T.; Wang, J.; Amesefe, D.; Lastrucci, E.; Charvin, M.; Singla Rastogi, M.; Navarro, L. The Arabidopsis active demethylase ROS1 Cis-regulates defence genes by erasing DNA methylation at promoter-regulatory regions. Elife 2021, 10, e62994. [Google Scholar] [CrossRef]
- Ling, Y.; Xiong, X.; Yang, W.; Liu, B.; Shen, Y.; Xu, L.; Lu, F.; Li, M.; Guo, Y.; Zhang, X. Comparative analysis of transcriptomics and metabolomics reveals defense mechanisms in melon cultivars against Pseudoperonospora cubensis infection. Int. J. Mol. Sci. 2023, 24, 17552. [Google Scholar] [CrossRef]
- Chang, X.; Liu, L.; Liu, Z.; Qiao, L.; Shi, R.; Lu, L. Chemical induction of DNA demethylation by 5-azacytidine enhances tomato fruit defense against gray mold through dicer-like protein DCL2c. Hortic. Res. 2024, 11, uhae164. [Google Scholar] [CrossRef]
- Zhong, Q.; Xu, Y.; Rao, Y. Mechanism of rice resistance to bacterial leaf blight via phytohormones. Plants 2024, 13, 2541. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, Z.; Zhu, J. Active DNA demethylation in plants: 20 Years of discovery and beyond. J. Integr. Plant Biol. 2022, 64, 2217–2239. [Google Scholar] [CrossRef]
- Tirnaz, S.; Merce, C.; Bayer, P.E.; Severn-Ellis, A.A.; Edwards, D.; Batley, J. Effect of Leptosphaeria maculans infection on promoter DNA methylation of defence genes in Brassica napus. Agronomy 2020, 10, 1072. [Google Scholar] [CrossRef]
- Tirnaz, S.; Batley, J. DNA methylation: Toward crop disease resistance improvement. Trends Plant Sci. 2019, 24, 1137–1150. [Google Scholar] [CrossRef]
- Xie, S.S.; Duan, C.G. Epigenetic regulation of plant immunity: From chromatin codes to plant disease resistance. Abiotech 2023, 4, 124–139. [Google Scholar] [CrossRef]
- Mierziak, J.; Wojtasik, W. Epigenetic weapons of plants against fungal pathogens. BMC Plant Biol. 2024, 24, 175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bishop, B.; Ringenberg, W.; Muir, W.M.; Ogas, J. The CHD3 remodeler PICKLE associates with genes enriched for trimethylation of histone H3 lysine 27. Plant Physiol. 2012, 159, 418–432. [Google Scholar] [CrossRef]
- Bhadouriya, S.L.; Mehrotra, S.; Basantani, M.K.; Loake, G.J.; Mehrotra, R. Role of chromatin architecture in plant stress responses: An update. Front. Plant Sci. 2021, 11, 603380. [Google Scholar] [CrossRef] [PubMed]
- Jaskiewicz, M.; Conrath, U.; Peterhänsel, C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 2011, 12, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Cui, X.; Shen, Y. The roles of histone methylation in the regulation of abiotic stress responses in plants. Plant Stress 2024, 11, 100303. [Google Scholar] [CrossRef]
- Luna, E.; Bruce, T.J.A.; Roberts, M.R.; Flors, V.; Ton, J. Next-generation systemic acquired resistance. Plant Physiol. 2012, 158, 844–853. [Google Scholar] [CrossRef]
- Wang, S.; He, J.; Hu, B.; Deng, M.; Li, W.; Guo, J.; Song, Y.; Zheng, Q.; Song, X.; Ma, F.; et al. An integrative multi-omics analysis of histone modifications and DNA methylation reveals the epigenomic landscape in apple under drought stress. Plant Biotechnol. J. 2025. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Zhou, J.-X.; Huang, H.-W.; Li, Y.-Q.; Shao, C.-R.; Li, L.; Cai, T.; Chen, S.; He, X.-J. Two components of the RNA-directed DNA methylation pathway associate with MORC6 and silence loci targeted by MORC6 in Arabidopsis. PLoS Genet. 2016, 12, e1006026. [Google Scholar] [CrossRef]
- Fukudome, A.; Fukuhara, T. Plant dicer-like proteins: Double-stranded RNA-cleaving enzymes for small RNA biogenesis. J. Plant Res. 2017, 130, 33–44. [Google Scholar] [CrossRef]
- Shi, G.; Wang, S.; Wang, P.; Zhan, J.; Tang, Y.; Zhao, G.; Li, F.; Ge, X.; Wu, J. Cotton miR393-TIR1 module regulates plant defense against Verticillium dahliae via auxin perception and signaling. Front. Plant Sci. 2022, 13, 888703. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, T.; Zhu, G.; Wu, B.; Zhang, C.; Zhu, H. LncRNAs exert indispensable roles in orchestrating the interaction among diverse noncoding RNAs and enrich the regulatory network of plant growth and its adaptive environmental stress response. Hortic. Res. 2023, 10, uhad234. [Google Scholar] [CrossRef]
- Tang, J.; Gu, X.; Liu, J.; He, Z. Roles of small RNAs in crop disease resistance. Stress Biol. 2021, 1, 6. [Google Scholar] [CrossRef]
- Bravo-Vázquez, L.A.; Castro-Pacheco, A.M.; Pérez-Vargas, R.; Velázquez-Jiménez, J.F.; Paul, S. The emerging applications of artificial microRNA-mediated gene silencing in plant biotechnology. Non-Coding RNA 2025, 11, 19. [Google Scholar] [CrossRef]
- Mittal, U.; Kumar, V.; Kukreja, S.; Singh, B.; Goutam, U. Scope of small RNA technology to develop biotic stress tolerant food crops. In Plant Small RNA in Food Crops; Praveen Guleria, P., Kumar, V., Mo, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 545–569. [Google Scholar]
- Zhu, Q.-H.; Fan, L.; Liu, Y.; Xu, H.; Llewellyn, D.; Wilson, I. miR482 regulation of NBS-LRR defense genes during fungal pathogen infection in cotton. PLoS ONE 2013, 8, e84390. [Google Scholar] [CrossRef]
- Wang, M.; Weiberg, A.; Dellota, E.; Yamane, D.; Jin, H. Botrytis small RNA Bc-siR37 suppresses plant defense genes by cross-kingdom RNAi. RNA Biol. 2017, 14, 421–428. [Google Scholar] [CrossRef]
- Katiyar-Agarwal, S.; Morgan, R.; Dahlbeck, D.; Borsani, O.; Villegas, A.; Zhu, J.-K.; Staskawicz, B.J.; Jin, H. A Pathogen-inducible endogenous siRNA in plant immunity. Proc. Natl. Acad. Sci. USA 2006, 103, 18002–18007. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Filho, L.A.A.; Laubinger, S. Role of small RNAs in regulation of plant responses to stress. In Molecular Mechanisms in Plant Adaptation; Laitinen, R.A.E., Ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 147–169. [Google Scholar]
- Jeyaraj, A.; Liu, S.; Han, R.; Zhao, Y.; Elango, T.; Wang, Y.; Chen, X.; Zhuang, J.; Li, X. The regulation of auxin receptor gene CsAFB2 by csn-miR393a confers resistance against Colletotrichum gloeosporioides in tea plants. Mol. Plant Pathol. 2025, 26, e13499. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.P.; Wang, P.; Zhu, X.; Li, X.; Zheng, T.; Shangguan, L.; Fang, J. Ectopic expression of CSD1 and CSD2 targeting genes of miR398 in grapevine is associated with oxidative stress tolerance. Funct. Integr. Genom. 2017, 17, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Lu, Q.; Wang, J.; Wang, L.; Xiang, F.; Liu, Z. miR160 and its target genes ARF10, ARF16 and ARF17 modulate hypocotyl elongation in a light, BRZ, or PAC-dependent manner in Arabidopsis. Plant Sci. 2021, 303, 110686. [Google Scholar] [CrossRef]
- Dey, S.; Sarkar, A.; Chowdhury, S.; Singh, R.; Mukherjee, A.; Ghosh, Z.; Kundu, P. Heightened miR6024-NLR interactions facilitate necrotrophic pathogenesis in tomato. Plant Mol. Biol. 2022, 109, 717–739. [Google Scholar] [CrossRef]
- Ramesh, S.V.; Govindasamy, V.; Rajesh, M.K.; Sabana, A.A.; Praveen, S. Stress-responsive miRNAome of Glycine max (L.) Merrill: Molecular insights and way forward. Planta 2019, 249, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Di, D.; Wu, L.; Yang, J.; Lu, Y.; Shi, W. MicroRNAs are involved in regulating plant development and stress response through fine-tuning of TIR1/AFB-dependent auxin signaling. Int. J. Mol. Sci. 2022, 23, 510. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-I.; Chiang, S.-F.; Lin, W.-Y.; Chen, J.-W.; Tseng, C.-Y.; Wu, P.-C.; Chiou, T.-J. Regulatory network of microRNA399 and PHO2 by systemic signaling. Plant Physiol. 2008, 147, 732–746. [Google Scholar] [CrossRef]
- Xie, K.; Wu, C.; Xiong, L. Genomic organization, differential expression, and interaction of SQUAMOSA promoter-binding-like transcription factors and microRNA156 in rice. Plant Physiol. 2006, 142, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yan, S.; Yang, T.; Zhang, S.; Chen, Y.; Liu, B. Small RNAs in regulating temperature stress response in plants. J. Integr. Plant Biol. 2017, 59, 774–791. [Google Scholar] [CrossRef]
- Aydinoglu, F.; Akgul, B. Mısır (Zea mays L.) Bitkisinin üşüme stresine toleransı sırasında yaprak büyüme bölgelerinde mikroRNA aracılıklı redoks regülasyonunun incelenmesi. Anadolu J. Agric. Sci. 2019, 34, 172–183. [Google Scholar] [CrossRef]
- Millar, A.A.; Lohe, A.; Wong, G. Biology and function of miR159 in plants. Plants 2019, 8, 255. [Google Scholar] [CrossRef]
- Kumar, K.; Mandal, S.N.; Neelam, K.; de los Reyes, B.G. MicroRNA-mediated host defense mechanisms against pathogens and herbivores in rice: Balancing gains from genetic resistance with trade-offs to productivity potential. BMC Plant Biol. 2022, 22, 351. [Google Scholar] [CrossRef]
- Zhao, M.; Cai, C.; Zhai, J.; Lin, F.; Li, L.; Shreve, J.; Thimmapuram, J.; Hughes, T.J.; Meyers, B.C.; Ma, J. Coordination of microRNAs, PhasiRNAs, and NB-LRR Genes in response to a plant pathogen: Insights from analyses of a set of soybean Rps gene near-isogenic lines. Plant Genome 2015, 14, plantgenome2014.09.0044. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-P.; Lederer, B.; Oberkofler, L.; Huang, L.; Johnson, N.R.; Platten, F.; Dunker, F.; Tisserant, C.; Weiberg, A. A Fungal RNA-dependent RNA polymerase is a novel player in plant infection and cross-kingdom RNA interference. PLoS Pathog. 2023, 19, e1011885. [Google Scholar] [CrossRef]
- Zhang, B.-S.; Li, Y.-C.; Guo, H.-S.; Zhao, J.-H. Verticillium dahliae secretes small RNA to target host MIR157d and retard plant floral transition during infection. Front. Plant Sci. 2022, 13, 847086. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Tahir, A.; Cui, X.; Xu, J.; Sun, J.; Zhang, N.; Sun, R.; Deng, S.; Xing, H.; Zhao, J. Genome-Wide Identification of Phytophthora sojae-associated microRNAs and network in a resistant and a susceptible soybean germplasm. Agronomy 2022, 12, 2922. [Google Scholar] [CrossRef]
- Asha, S.; Soniya, E.V. Transfer RNA derived small RNAs targeting defense responsive genes are induced during Phytophthora capsici infection in black pepper (Piper nigrum L.). Front. Plant Sci. 2016, 7, 767. [Google Scholar] [CrossRef]
- Pertermann, R.; Tamilarasan, S.; Gursinsky, T.; Gambino, G.; Schuck, J.; Weinholdt, C.; Lilie, H.; Grosse, I.; Golbik, R.P.; Pantaleo, V.; et al. A viral suppressor modulates the plant immune response early in infection by regulating microRNA activity. mBio 2018, 9, 10-1128. [Google Scholar] [CrossRef]
- Movahedi, A.; Zhang, J.; Sun, W.; Kadkhodaei, S.; Mohammadi, K.; Almasizadehyaghuti, A.; Yin, T.; Zhuge, Q. Plant small RNAs: Definition, classification and response against stresses. Biologia 2018, 73, 285–294. [Google Scholar] [CrossRef]
- Misra, V.; Mall, A.K. Genome-wide profiling of abiotic stress-responsive non-coding RNAs. In Decoding non-coding RNA: Enhancing plant abiotic stress tolerance; Springer Nature: Singapore, 2025; pp. 141–195. [Google Scholar] [CrossRef]
- Wagh, S.G.; Bhor, S.A.; Miyao, A.; Hirochika, H.; Toriba, T.; Hirano, H.-Y.; Kobayashi, K.; Yaeno, T.; Nishiguchi, M. Synergy between virus and three kingdom pathogens, fungus, bacterium and virus is lost in rice mutant lines of OsRDR1/6. Plant Sci. 2024, 349, 112244. [Google Scholar] [CrossRef]
- Cui, J.; Luan, Y.; Jiang, N.; Bao, H.; Meng, J. Comparative transcriptome analysis between resistant and susceptible tomato allows the identification of LncRNA16397 conferring resistance to phytophthora infestans by co-expressing glutaredoxin. Plant J. 2017, 89, 577–589. [Google Scholar] [CrossRef]
- Jha, U.C.; Nayyar, H.; Jha, R.; Khurshid, M.; Zhou, M.; Mantri, N.; Siddique, K.H.M. Long non-coding RNAs: Emerging players regulating plant abiotic stress response and adaptation. BMC Plant Biol. 2020, 20, 466. [Google Scholar] [CrossRef]
- Waseem, M.; Liu, Y.; Xia, R. Long non-coding RNAs, the dark matter: An emerging regulatory component in plants. Int. J. Mol. Sci. 2021, 22, 86. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, N.; Meng, J.; Yang, G.; Liu, W.; Zhou, X.; Ma, N.; Hou, X.; Luan, Y. LncRNA33732-respiratory burst oxidase module associated with WRKY1 in tomato- Phytophthora infestans interactions. Plant J. 2019, 97, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xu, Y.; Li, Q.; Cao, Y.; Yang, D.; Liu, S.; Wang, X.; Mi, Y.; Liu, Y.; Ding, C.; et al. A lncRNA fine-tunes salicylic acid biosynthesis to balance plant immunity and growth. Cell Host Microbe 2022, 30, 1124–1138.e8. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Tang, Y. Noncoding RNA-mediated regulation of DNA methylation: Insights into plant epigenetic mechanisms. J. Plant Growth Regul. 2024, 44, 373–388. [Google Scholar] [CrossRef]
- Wu, H.; Li, B.; Iwakawa, H.O.; Pan, Y.; Tang, X.; Ling-hu, Q.; Liu, Y.; Sheng, S.; Feng, L.; Zhang, H.; et al. Plant 22-nt siRNAs mediate translational repression and stress adaptation. Nature 2020, 581, 89–93. [Google Scholar] [CrossRef]
- Sigman, M.J.; Panda, K.; Kirchner, R.; McLain, L.L.; Payne, H.; Peasari, J.R.; Husbands, A.Y.; Slotkin, R.K.; McCue, A.D. An siRNA-guided ARGONAUTE protein directs RNA polymerase V to initiate DNA methylation. Nat. Plants 2021, 7, 1461–1474. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, J.; Liu, J.; Fang, J.; Zhong, X.; Song, J. DNA conformational dynamics in the context-dependent Non-CG CHH methylation by plant methyltransferase DRM2. J. Biol. Chem. 2023, 299, 105433. [Google Scholar] [CrossRef] [PubMed]
- Dangwal, M.; Malik, G.; Kapoor, S.; Kapoor, M. De Novo Methyltransferase, OsDRM2, Interacts with the ATP-Dependent RNA Helicase, OseIF4A, in Rice. J. Mol. Biol. 2013, 425, 2853–2866. [Google Scholar] [CrossRef]
- Popova, O.V.; Dinh, H.Q.; Aufsatz, W.; Jonak, C. The RdDM pathway is required for basal heat tolerance in Arabidopsis. Mol. Plant 2013, 6, 396–410. [Google Scholar] [CrossRef]
- Wang, J.; Meng, X.; Yuan, C.; Harrison, A.P.; Chen, M. The Roles of Cross-Talk Epigenetic Patterns in Arabidopsis thaliana. Brief. Funct. Genom. 2016, 15, 278–287. [Google Scholar] [CrossRef]
- Kim, J.H. Multifaceted chromatin structure and transcription changes in plant stress response. Int. J. Mol. Sci. 2021, 22, 2013. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, P.; Obrępalska-Stęplowska, A. Suppress to survive—Implication of plant viruses in PTGS. Plant Mol. Biol. Rep. 2015, 33, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Raja, P.; Wolf, J.N.; Bisaro, D.M. RNA silencing directed against gemini viruses: Post-transcriptional and epigenetic components. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2010, 1799, 337–351. [Google Scholar] [CrossRef]
- Miloro, F.; Kis, A.; Havelda, Z.; Dalmadi, Á. Barley AGO4 proteins show overlapping functionality with distinct small RNA-binding properties in heterologous complementation. Plant Cell Rep. 2024, 43, 96. [Google Scholar] [CrossRef]
- Liao, L.; Xie, B.; Guan, P.; Jiang, N.; Cui, J. New insight into the molecular mechanism of miR482/2118 during plant resistance to pathogens. Front. Plant Sci. 2022, 13, 1026762. [Google Scholar] [CrossRef]
- Leonetti, P.; Stuttmann, J.; Pantaleo, V. Regulation of plant antiviral defense genes via host RNA-silencing mechanisms. Virol. J. 2021, 18, 194. [Google Scholar] [CrossRef]
- Csorba, T.; Kontra, L.; Burgyán, J. Viral silencing suppressors: Tools forged to fine-tune host-pathogen coexistence. Virology 2015, 479–480, 85–103. [Google Scholar] [CrossRef]
- Petrov, N.M.; Stoyanova, M.I.; Gaur, R.K. siRNAs-based gene silencing of potato virus Y by simultaneous blocking of HC-Pro and NIa. Biotechnol. Biotechnol. Equip. 2023, 37, 1–6. [Google Scholar] [CrossRef]
- Thiébeauld, O.; Charvin, M.; Singla-Rastogi, M.; Perez-Quintero, A.L.; Yang, F.; Pontier, D.; Barraud, P.; Pouzet, C.; Bapaume, L.; Amesefe, D.; et al. A bacterial effector directly targets Arabidopsis Argonaute 1 to suppress pattern-triggered immunity and cause disease. bioRxiv 2017, 215590. [Google Scholar] [CrossRef]
- Nasfi, S.; Kogel, K.H. Packaged or unpackaged: Appearance and transport of extracellular noncoding RNAs in the plant apoplast. ExRNA 2022, 4, 13. [Google Scholar] [CrossRef]
- Wagh, S.G.; Alam, M.M.; Kobayashi, K.; Yaeno, T.; Yamaoka, N.; Toriba, T.; Hirano, H.Y.; Nishiguchi, M. Analysis of rice RNA-dependent RNA polymerase 6 (OsRDR6) gene in response to viral, bacterial and fungal pathogens. J. Gen. Plant Pathol. 2016, 82, 12–17. [Google Scholar] [CrossRef]
- Dasgupta, I.; Chatterjee, A. Recent advances in miRNA delivery systems. Methods Protoc. 2021, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Krzyszton, M.; Kufel, J.; Zakrzewska-Placzek, M. RNA interference and turnover in plants—A complex partnership. Front. Plant Sci. 2025, 16, 1608888. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, Y.; Ma, W.; Zhao, Y.; Jiang, H.; Zhang, M. Identification and characterization of NBS-encoding disease resistance genes in Lotus japonicus. Plant Syst. Evol. 2010, 289, 101–110. [Google Scholar] [CrossRef]
- Park, S.Y.; Cho, J.; Jeong, D.-H. Small regulatory RNAs in rice epigenetic regulation. Biochem. Soc. Trans. 2022, 50, 1215–1225. [Google Scholar] [CrossRef]
- Olmo, R.; Quijada, N.M.; Morán-Diez, M.E.; Hermosa, R.; Monte, E. Identification of tomato microRNAs in late response to Trichoderma atroviride. Int. J. Mol. Sci. 2024, 25, 1617. [Google Scholar] [CrossRef]
- Kurihara, Y.; Matsui, A.; Hanada, K.; Kawashima, M.; Ishida, J.; Morosawa, T.; Tanaka, M.; Kaminuma, E.; Mochizuki, Y.; Matsushima, A.; et al. Genome-wide suppression of aberrant mRNA-like noncoding RNAs by NMD in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 2453–2458. [Google Scholar] [CrossRef]
- Mourrain, P.; Bé, C.; Elmayan, T.; Feuerbach, F.; Godon, C.; Morel, J.-B.; Jouette, D.; Lacombe, A.-M.; Nikic, S.; Picault, N.; et al. Arabidopsis SGS2 and SGS3 genes are required for post-transcriptional gene silencing and virus resistance. Cell 2000, 101, 539–550. [Google Scholar] [CrossRef]
- Peragine, A.; Yoshikawa, M.; Wu, G.; Albrecht, H.L.; Poethig, R.S. SGS3 and SGS2/SDE1/RDR6 are required for juvenile development and the production of trans-acting siRNAs in Arabidopsis. Genes. Dev. 2004, 18, 2368–2379. [Google Scholar] [CrossRef]
- Tanaka, T.; Wagh, S.G.; Alam, M.M.; Chen, H.; Miyao, A.; Hirochika, H.; Kobayashi, K.; Yaeno, T.; Yamaoka, N.; Nishiguchi, M. Functional analysis of OsSGS3, a rice gene involved in RNA silencing. Jpn. J. Phytopathol. 2015, 81, 213. (in Japanese). [Google Scholar]
- Gu, X.; Si, F.; Feng, Z.; Li, S.; Liang, D.; Yang, P.; Yang, C.; Yan, B.; Tang, J.; Yang, Y.; et al. The OsSGS3-TasiRNA-OsARF3 module orchestrates abiotic-biotic stress response trade-off in rice. Nat. Commun. 2023, 14, 4441. [Google Scholar] [CrossRef]
- Elmayan, T.; Blein, T.; Elvira-Matelot, E.; Le Masson, I.; Christ, A.; Bouteiller, N.; Crespi, M.D.; Vaucheret, H. Arabidopsis SGS3 is recruited to chromatin by CHR11 to select RNA that initiate siRNA production. Nat. Commun. 2025, 16, 2978. [Google Scholar] [CrossRef]
- Pavet, V.; Quintero, C.; Cecchini, N.M.; Rosa, A.L.; Alvarez, M.E. Arabidopsis displays centromeric DNA hypomethylation and cytological alterations of heterochromatin upon attack by Pseudomonas syringae. Mol. Plant-Microbe Interact. 2006, 19, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Kourani, M.; Mohareb, F.; Rezwan, F.I.; Anastasiadi, M.; Hammond, J.P. Genetic and physiological responses to heat stress in Brassica napus. Front. Plant Sci. 2022, 13, 832147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-T.; Wang, M.; Wang, Z.-M.; Fang, R.-X.; Wang, X.-J.; Jia, Y.-T. Dynamic and coordinated expression changes of rice small RNAs in response to Xanthomonas oryzae pv. oryzae. J. Genet. Genom. 2015, 42, 625–637. [Google Scholar] [CrossRef]
- Stare, T.; Stare, K.; Weckwerth, W.; Wienkoop, S.; Gruden, K. Comparison between proteome and transcriptome response in potato (Solanum tuberosum L.) leaves following potato virus Y (PVY) infection. Proteomes 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Deleris, A.; Stroud, H.; Bernatavichute, Y.; Johnson, E.; Klein, G.; Schubert, D.; Jacobsen, S.E. Loss of the DNA methyltransferase MET1 induces H3K9 hypermethylation at PcG target genes and redistribution of H3K27 trimethylation to transposons in Arabidopsis thaliana. PLoS Genet. 2012, 8, e1003062. [Google Scholar] [CrossRef]
- Borrelli, V.; Lanubile, A.; Marocco, A. Plant hormones and plant defense response against pathogens: In Hormones and Plant Response; Gupta, D.K., Corpas, F.J., Eds.; Plants in Challenging Environments; Springer: Cham, Switzerland, 2021; Volume 2, pp. 1–21. [Google Scholar] [CrossRef]
- Roychowdhury, R.; Mishra, S.; Anand, G.; Dalal, D.; Gupta, R.; Kumar, A.; Gupta, R. Decoding the molecular mechanism underlying salicylic acid (SA)-mediated plant immunity: An integrated overview from its biosynthesis to the mode of action. Physiol. Plant. 2024, 176, e14399. [Google Scholar] [CrossRef]
- Kamle, M.; Borah, R.; Bora, H.; Jaiswal, A.K.; Singh, R.K.; Kumar, P. Systemic acquired resistance (SAR) and induced systemic resistance (ISR): Role and mechanism of action against phytopathogens. In Fungal Biotechnology and Bioengineering; Hesham, A.L., Upadhyay, R., Sharma, G., Manoharachary, C., Gupta, V., Eds.; Fungal Biology; Springer: Cham, Switzerland, 2020; pp. 457–470. [Google Scholar] [CrossRef]
- Morán-Diez, M.E.; Martínez de Alba, Á.E.; Rubio, M.B.; Hermosa, R.; Monte, E. Trichoderma and the plant heritable priming responses. J. Fungi 2021, 7, 318. [Google Scholar] [CrossRef]
- Hussain, A.; Noman, A.; Khan, M.I.; Zaynab, M.; Aqeel, M.; Anwar, M.; Ashraf, M.F.; Liu, Z.; Raza, A.; Mahpara, S.; et al. Molecular regulation of pepper innate immunity and stress tolerance: An overview of WRKY TFs. Microb. Pathog. 2019, 135, 103610. [Google Scholar] [CrossRef]
- Catoni, M.; Alvarez-Venegas, R.; Worrall, D.; Holroyd, G.; Barraza, A.; Luna, E.; Ton, J.; Roberts, M.R. Long-lasting defence priming by β-aminobutyric acid in tomato is marked by genome-wide changes in DNA methylation. Front. Plant Sci. 2022, 13, 836326. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, C.; Lei, C.; Zou, Y.; Li, Y.; Zheng, Y.; Fang, Y. Dual function of VvWRKY18 transcription factor in the β-aminobutyric acid-activated priming defense in grapes. Physiol. Plant. 2021, 172, 1477–1492. [Google Scholar] [CrossRef]
- Yang, Z.; Zhi, P.; Chang, C. Priming seeds for the future: Plant immune memory and application in crop protection. Front. Plant Sci. 2022, 13, 961840. [Google Scholar] [CrossRef]
- Viggiano, L.; de Pinto, M.C. Dynamic DNA methylation patterns in stress response. In Plant Epigenetics; Rajewsky, N., Jurga, S., Barciszewski, J., Eds.; RNA Technologies; Springer: Cham, Switzerland, 2017; pp. 281–302. [Google Scholar] [CrossRef]
- Lanubile, A.; Ferrarini, A.; Maschietto, V.; Delledonne, M.; Marocco, A.; Bellin, D. Functional genomic analysis of constitutive and inducible defense responses to Fusarium verticillioides infection in maize genotypes with contrasting ear rot resistance. BMC Genom. 2014, 15, 710. [Google Scholar] [CrossRef]
- Zierold, U.; Scholz, U.; Schweizer, P. Transcriptome analysis of MLO-mediated resistance in the epidermis of barley. Mol. Plant Pathol. 2005, 6, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Beacon, T.H.; Delcuve, G.P.; López, C.; Nardocci, G.; Kovalchuk, I.; van Wijnen, A.J.; Davie, J.R. The dynamic broad epigenetic (H3K4me3, H3K27ac) Domain as a mark of essential genes. Clin. Epigenetics 2021, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Espinas, N.A.; Saze, H.; Saijo, Y. Epigenetic control of defense signaling and priming in plants. Front. Plant Sci. 2016, 7, 1201. [Google Scholar] [CrossRef]
- Parrilla-Doblas, J.T.; Roldán-Arjona, T.; Ariza, R.R.; Córdoba-Cañero, D. Active DNA demethylation in plants. Int. J. Mol. Sci. 2019, 20, 4683. [Google Scholar] [CrossRef]
- López-Márquez, D.; Del-Espino, Á.; Ruiz-Albert, J.; Bejarano, E.R.; Brodersen, P.; Beuzón, C.R. Regulation of plant immunity via small RNA-mediated control of NLR expression. J. Exp. Bot. 2023, 74, 6052–6068. [Google Scholar] [CrossRef]
- Slaughter, A.; Daniel, X.; Flors, V.; Luna, E.; Hohn, B.; Mauch-Mani, B. Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol. 2012, 158, 835–843. [Google Scholar] [CrossRef]
- López-Galiano, M.J.; González-Hernández, A.I.; Crespo-Salvador, O.; Rausell, C.; Real, M.D.; Escamilla, M.; Camañes, G.; García-Agustín, P.; González-Bosch, C.; García-Robles, I. Epigenetic regulation of the expression of WRKY75 Transcription factor in response to biotic and abiotic stresses in Solanaceae plants. Plant Cell Rep. 2018, 37, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Yu, K.; Stringlis, I.A.; van Bentum, S.; de Jonge, R.; Snoek, B.L.; Pieterse, C.M.J.; Bakker, P.A.H.M.; Berendsen, R.L. Transcriptome signatures in Pseudomonas simiae WCS417 shed light on role of root-secreted coumarins in Arabidopsis-mutualist communication. Microorganisms 2021, 9, 575. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing plant defense priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Pohare, M.B.; Wagh, S.G.; Udayasuriyan, V. Bacillus thuringiensis as potential biocontrol agent for sustainable agriculture. In Current Trends in Microbial Biotechnology for Sustainable Agriculture; Yadav, A.N., Singh, J., Singh, C., Yadav, N., Eds.; Springer: Singapore, 2021; pp. 439–468. ISBN 978-981-15-6949-4. [Google Scholar]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef]
- Salunke, T.R.; Sontakke, O.P.; Chavan, S.C.; Bhosale, K.S.; Wayase, U.R.; Barmukh, R.B.; Ahire, M.L.; Shelar, P.V.; Nikalje, G.C.; Mankar, G.D. Microbial modulation of plant epigenetics: The role of miRNA and lncRNA in enhancing salt tolerance. Discov. Plants 2025, 2, 166. [Google Scholar] [CrossRef]
- Rasmann, S.; Agrawal, A.A. Plant defense against herbivory: Progress in identifying synergism, redundancy, and antagonism between resistance traits. Curr. Opin. Plant Biol. 2009, 12, 473–478. [Google Scholar] [CrossRef]
- Papikian, A.; Liu, W.; Gallego-Bartolomé, J.; Jacobsen, S.E. Site-specific manipulation of Arabidopsis loci using CRISPR-Cas9 SunTag systems. Nat. Commun. 2019, 10, 729. [Google Scholar] [CrossRef]
- Patil, A.M.; Pawar, B.D.; Wagh, S.G.; Shinde, H.; Shelake, R.M.; Markad, N.R.; Bhute, N.K.; Červený, J.; Wagh, R.S. Abiotic stress in cotton: Insights into plant responses and biotechnological solutions. Agriculture 2024, 14, 1638. [Google Scholar] [CrossRef]
- Ayyappan, V.; Kalavacharla, V.; Thimmapuram, J.; Bhide, K.P.; Sripathi, V.R.; Smolinski, T.G.; Manoharan, M.; Thurston, Y.; Todd, A.; Kingham, B. Genome-wide profiling of histone modifications (H3K9me2 and H4K12ac) and gene expression in rust (Uromyces appendiculatus) inoculated common bean (Phaseolus vulgaris L.). PLoS ONE 2015, 10, e0132176. [Google Scholar] [CrossRef]
- Banihashemian, S.N.; Mirmajlessi, S.M. Epigenetic modifications, immune control processes, and plant responses to nematodes. Agriculture 2025, 15, 742. [Google Scholar] [CrossRef]
- Yu, A.; Lepère, G.; Jay, F.; Wang, J.; Bapaume, L.; Wang, Y.; Abraham, A.L.; Penterman, J.; Fischer, R.L.; Voinnet, O.; et al. Dynamics and biological relevance of DNA demethylation in Arabidopsis antibacterial defense. Proc. Natl. Acad. Sci. USA 2013, 110, 2389–2394. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Yang, Y.; Schmid, M.; Wang, Y. miRNA mediated regulation and interaction between plants and pathogens. Int. J. Mol. Sci. 2021, 22, 2913. [Google Scholar] [CrossRef] [PubMed]
- Hilton, I.B.; D’Ippolito, A.M.; Vockley, C.M.; Thakore, P.I.; Crawford, G.E.; Reddy, T.E.; Gersbach, C.A. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat. Biotechnol. 2015, 33, 510–517. [Google Scholar] [CrossRef]
- Ghoshal, B.; Gardiner, J. CRISPR-dCas9-based targeted manipulation of DNA methylation in plants. In CRISPR-Cas Methods; Islam, M.T., Molla, K.A., Eds.; Springer Protocols Handbooks; Humana: New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- López, A.; Ramírez, V.; García-Andrade, J.; Flors, V.; Vera, P. The RNA silencing enzyme RNA polymerase V is required for plant immunity. PLoS Genet. 2011, 7, e1002434. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, M.; He, Y. DNA methylation analysis of the Citrullus lanatus response to Cucumber green mottle mosaic virus infection by whole-genome bisulfite sequencing. Genes 2019, 10, 344. [Google Scholar] [CrossRef]
- Ramirez-Prado, J.S.; Abulfaraj, A.A.; Rayapuram, N.; Benhamed, M.; Hirt, H. Plant immunity: From signaling to epigenetic control of defense. Trends Plant Sci. 2018, 23, 833–844. [Google Scholar] [CrossRef]
- Kang, H.; Fan, T.; Wu, J.; Zhu, Y.; Shen, W.-H. Histone modification and chromatin remodeling in plant response to pathogens. Front. Plant Sci. 2022, 13, 986940. [Google Scholar] [CrossRef]
- Navarro, L.; Dunoyer, P.; Jay, F.; Arnold, B.; Dharmasiri, N.; Estelle, M.; Voinnet, O.; Jones, J.D.G. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 2006, 312, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, H.; Li, N.; Batley, J.; Wang, Y. The miR393-target module regulates plant development and responses to biotic and abiotic stresses. Int. J. Mol. Sci. 2022, 23, 9477. [Google Scholar] [CrossRef] [PubMed]
- Gayathiri, E.; Viswanathan, D.; Prakash, P.; Pandiaraj, S.; Kondapavuluri, B.K.; Singh, C.D.; Thiruvengadam, R.; Balu, S.; Anantharaman, R.; Gaur, A.; et al. A critical review on epigenetics and epigenomics in plant development and stress resilience. Braz. J. Bot. 2025, 48, 51. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, Y.; Wang, Y.; Gao, D.; King, J.; Xu, Y.; Liang, F.-S. Investigating crosstalk between H3K27 acetylation and H3K4 trimethylation in CRISPR/DCas-based epigenome editing and gene activation. Sci. Rep. 2021, 11, 15912. [Google Scholar] [CrossRef]
- Pan, C.; Sretenovic, S.; Qi, Y. CRISPR/DCas-mediated transcriptional and epigenetic regulation in plants. Curr. Opin. Plant Biol. 2021, 60, 101980. [Google Scholar] [CrossRef] [PubMed]
- Selma, S.; Orzáez, D. Perspectives for epigenetic editing in crops. Transgenic Res. 2021, 30, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.M.; Wagh, S.G.; Janvale, G.B.; Pawar, B.D.; Daspute, A.A. Viral delivery of CRISPR/Cas9 genome editing for rapid crop improvement: A promising approach to enhance crop resilience against biotic and abiotic stresses. Int. J. Adv. Biochem. Res. 2024, 8, 782–796. [Google Scholar] [CrossRef]
- Cai, R.; Lv, R.; Shi, X.; Yang, G.; Jin, J. CRISPR/DCas9 Tools: Epigenetic mechanism and application in gene transcriptional regulation. Int. J. Mol. Sci. 2023, 24, 14865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, L.; Chen, H.; Ye, W.; Dong, S.; Zheng, X.; Wang, Y. ATAC-Seq reveals the landscape of open chromatin and cis-regulatory elements in the Phytophthora sojae genome. Mol. Plant-Microbe Interact. 2022, 35, 301–310. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Y. Profiling chromatin regulatory landscape: Insights into the development of ChIP-Seq and ATAC-Seq. Mol. Biomed. 2020, 1, 9. [Google Scholar] [CrossRef]
- Arora, H.; Singh, R.K.; Sharma, S.; Sharma, N.; Panchal, A.; Das, T.; Prasad, A.; Prasad, M. DNA Methylation dynamics in response to abiotic and pathogen stress in plants. Plant Cell Rep. 2022, 41, 1931–1944. [Google Scholar] [CrossRef]
- Srivastava, U.; Kanchan, S.; Kesheri, M.; Sharma, A. Single-cell technology for improvement in significant crops. In Guide to Plant Single-Cell Technology; Elsevier: Amsterdam, The Netherlands, 2025; pp. 299–320. [Google Scholar] [CrossRef]
- Fan, B.-L.; Chen, L.-H.; Chen, L.-L.; Guo, H. Integrative multi-omics approaches for identifying and characterizing biological elements in crop traits: Current progress and future prospects. Int. J. Mol. Sci. 2025, 26, 1466. [Google Scholar] [CrossRef]
- Kakoulidou, I.; Avramidou, E.V.; Baránek, M.; Brunel-Muguet, S.; Farrona, S.; Johannes, F.; Kaiserli, E.; Lieberman-Lazarovich, M.; Martinelli, F.; Mladenov, V.; et al. Epigenetics for crop improvement in times of global change. Biology 2021, 10, 766. [Google Scholar] [CrossRef] [PubMed]
| sRNA Name/Type | Origin | Class and Biogenesis | Molecular Target(s) | Defense Role | Reference |
|---|---|---|---|---|---|
| miR393 | Arabidopsis | miRNA | TIR1 (auxin receptor) | Enhances PTI via auxin suppression | [64] |
| miR398 | Arabidopsis | miRNA | CSD1, CSD2, CCS | Enhances ROS detox during stress | [65] |
| miR160 | Arabidopsis | miRNA | ARF10/16/17 | Regulates auxin–immune crosstalk | [66] |
| miR482 | Tomato | miRNA/phasiRNA | NBS-LRR | Controls immune receptor expression | [67] |
| miR2118 | Glycine max | miRNA/phasiRNA | R-genes | Triggers phasiRNA production | [68] |
| miR167 | Arabidopsis | miRNA | ARF6/8 | Regulates auxin in stress responses | [69] |
| miR399 | Arabidopsis | miRNA | PHO2 | Controls phosphate signaling and defense | [70] |
| miR156 | Rice | miRNA | SPL transcription factors | Modulates development and immunity | [71] |
| TAS1-derived siRNAs | Arabidopsis | ta-siRNA | F-box proteins | Controls auxin signaling | [72] |
| nat-siRNA (PPRL) | Arabidopsis | nat-siRNA | PPR-like proteins | Suppresses immunity repressors | [72] |
| miR528 | Maize | miRNA | L-ascorbate oxidase | Regulates oxidative stress and defense | [73] |
| miR159 | Wheat | miRNA | MYB33/MYB65 | Involved in Fusarium resistance | [74] |
| miR444 | Rice | miRNA | MADS-box TFs | Implicated in defense against pathogens | [75] |
| phasiRNA (NLRs) | Soybean | phasiRNA | NLRs | Controls immune gene bursts | [76] |
| Bc-siR37 | B. cinerea | sRNA | MAPKs, WRKYs | Silences host defense genes | [61,77] |
| Bc-sRNA3 | B. cinerea | sRNA | Host AGO1 pathway | Hijacks RNA silencing | [78] |
| Vd-sRNA1 | V. dahliae | sRNA mimic | PR1, defense genes | Suppresses host immunity | [79] |
| Vd-sRNA2 | V. dahliae | sRNA mimic | ETI-related genes | Promotes virulence | [79] |
| gma-miR1508 | Phytophthora sojae | miRNA mimic | NBS-LRR class of R gen | ETI | [80] |
| 5′AlaCGC tRF | Piper nigrum | tRF (tRNA-derived fragment) | NPR1 | Mediates cleavage of NPR1 mRNA, downregulating the SAR pathway | [81] |
| 5′MetCAT tRF | P. nigrum | tRF | E3 ubiquitin ligase | Targets the mRNA of ubiquitin ligase, affecting PTI/ETI signaling | [81] |
| miR162 | Arabidopsis/Nicotiana benthamiana | miRNA | DCL1 mRNA | p19 VSR binds miR162, upregulates DCL1, and alters miRNA biogenesis to aid early infection | [82] |
| miR168 | Arabidopsis/N. benthamiana | miRNA | AGO1 mRNA | Weak p19 binding; feedback on AGO1; minor role in early infection | [82] |
| miR403 | Arabidopsis/N. benthamiana | miRNA | AGO2 mRNA | Strong p19/2b binding; blocks AGO2 defense early in infection. | [82] |
| Approach | Mechanism | Outcome | Example Genes or Targets | No. of Genes Affected | Regulation Status | Reference |
|---|---|---|---|---|---|---|
| RdDM (RNA-directed DNA Methylation) | siRNA-guided DNA methylation at promoter regions | Transcriptional gene silencing of defense regulators | WRKYs, PR1, R genes | 10–100 | Regulated as non-GMO (varies by region) | [151,152,156,157] |
| Histone Modifications | Acetylation/methylation (e.g., H3K4me3, H3K27me3) | Chromatin remodeling and immune gene activation | SA/JA pathway genes, TFs | Dozens to hundreds | Not regulated as GMO | [49,158,159] |
| Small RNA-Mediated PTGS | miRNA/phasiRNA-mediated silencing of target transcripts | Post-transcriptional silencing; immune homeostasis | miR393, miR482, phasiRNA–NLRs | 1–50 | Generally unregulated (endogenous pathways) | [64,138,160,161] |
| Epigenetic Priming | Long-term chromatin state changes via stress or immune cues | Faster and stronger immune response upon re-exposure | PR1, WRKY6, DML2 loci | Context dependent | No regulation (natural induction) | [45,162] |
| Epigenome Editing (CRISPR/dCas9) | Site-specific modulation of DNA methylation or histone marks | Targeted immune gene activation/repression with heritable outcomes | PR promoters, NLR enhancers | 1–10 | Mixed (regulated or non-GMO by delivery) | [155,163,164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagh, S.G.; Patil, A.M.; Patil, G.B.; Mankar, S.P.; Rastogi, K.; Nishiguchi, M. Small RNA and Epigenetic Control of Plant Immunity. DNA 2025, 5, 47. https://doi.org/10.3390/dna5040047
Wagh SG, Patil AM, Patil GB, Mankar SP, Rastogi K, Nishiguchi M. Small RNA and Epigenetic Control of Plant Immunity. DNA. 2025; 5(4):47. https://doi.org/10.3390/dna5040047
Chicago/Turabian StyleWagh, Sopan Ganpatrao, Akshay Milind Patil, Ghanshyam Bhaurao Patil, Sumeet Prabhakar Mankar, Khushboo Rastogi, and Masamichi Nishiguchi. 2025. "Small RNA and Epigenetic Control of Plant Immunity" DNA 5, no. 4: 47. https://doi.org/10.3390/dna5040047
APA StyleWagh, S. G., Patil, A. M., Patil, G. B., Mankar, S. P., Rastogi, K., & Nishiguchi, M. (2025). Small RNA and Epigenetic Control of Plant Immunity. DNA, 5(4), 47. https://doi.org/10.3390/dna5040047









