Abstract
Mammalian cell lines devoid of mitochondrial DNA (mtDNA) are indispensable in studies aimed at elucidating the contribution of mtDNA to various cellular processes or interactions between nuclear and mitochondrial genomes. However, the repertoire of tools for generating such cells (also known as rho-0 or ρ0 cells) remains limited, and approaches remain time- and labor-intensive, ultimately limiting their availability. Ethidium bromide (EtBr), which is most commonly used to induce mtDNA loss in mammalian cells, is cytostatic and mutagenic as it affects both nuclear and mitochondrial genomes. Therefore, there is growing interest in new tools for generating ρ0 cell lines. Here, we examined the utility of 2′,3′-dideoxycytidine (ddC, zalcitabine) alone or in combination with EtBr for generating ρ0 cell lines of mouse and human origin as well as inducing the ρ0 state in mouse/human somatic cell hybrids. We report that ddC is superior to EtBr in both immortalized mouse fibroblasts and human 143B cells. Also, unlike EtBr, ddC exhibits no cytostatic effects at the highest concentration tested (200 μM), making it more suitable for general use. We conclude that ddC is a promising new tool for generating mammalian ρ0 cell lines.
1. Introduction
Mitochondrial DNA (mtDNA) plays a central role in the biology of mitochondria and, indeed, in all cellular processes, which are affected, either directly or indirectly, by its presence or absence. In cultured cells, knockouts (KOs) of genes essential for mtDNA replication result in the loss of mtDNA [1], whereas the whole-body KOs of the same genes in experimental animals are usually embryonically lethal [2,3,4,5,6,7]. To our knowledge, there are no reports of multicellular organisms retaining their viability after complete mtDNA loss. However, derivatives of cultured cells lacking mtDNA can be obtained. The resulting cells (called rho-0 (ρ0) cells) lack respiratory function and are auxotrophic for uridine and pyruvate [8,9,10].
ρ0 cells are useful as recipients in cybrid technology, which aims to study the effects of mtDNA mutations in a uniform genetic background [11,12,13,14,15,16,17,18,19]. Indeed, phenotypic manifestations of mtDNA mutation depend on complex and as yet incompletely understood interactions of nuclear and mitochondrial genomes. One prominent example of the importance of taking into account these interactions is the case of the G13997A mtDNA mutation in mouse cells. Initially, this mutation was described as promoting metastasis [20]. Transmitochondrial mice carrying this mutation had an increased incidence of lymphoma [21]. However, this increase was only observed in the B6 nuclear background, not in other mouse strains [22]. Therefore, ρ0 cells are an important tool in deciphering the physiological consequences of mtDNA mutations.
Historically, ρ0 cells were obtained by a variety of techniques, including treating cells with inhibitors of mtDNA replication, such as ethidium bromide (EtBr) [8,9,23,24,25,26] or ditercalinium [27,28,29,30], or by targeting enzymatic damage to mtDNA [31,32,33,34]. Each of these techniques has its limitation(s), and therefore, there is ongoing interest in new tools for mtDNA elimination from cultured cells.
Zalcitabine (2′,3′-dideoxycytidine, ddC) was initially developed as an antiretroviral drug to combat HIV infections. ddC is an analog of the cellular DNA precursor 2′-deoxycytidine-5′-triphosphate (dCTP) and is converted inside the cells into 2′,3′-dideoxycytidine-5′-triphosphate (ddCTP) regardless of their infection status [35]. ddCTP suppresses viral integration into the host genome by inhibiting nascent DNA chain elongation by viral reverse transcriptase due to the absence of a hydroxyl group at the 3′ position. Zalcitabine’s side effects, including peripheral neuropathy, stomatitis, and mouth ulcers, limit its dosage and have been linked to the inhibition of mitochondrial DNA polymerase gamma (POLG), which results in mtDNA depletion [36,37,38,39,40,41]. Other so-called “D-drugs” (didanosine (ddI) and stavudine (d4T)) also induce mtDNA depletion [36]. Perplexingly, “non-D-drugs” can also induce mtDNA depletion even though they do not inhibit POLG [37,38,42]. However, no complete loss of mtDNA was reported in patients undergoing antiretroviral therapy; the average mtDNA loss was only about 53% [36]. Therefore, whether zalcitabine or any other nucleoside reverse transcriptase inhibitors can induce complete mtDNA loss in cells in vivo or in culture remains unclear.
In this study, we examined the effects of chronic ddC treatment on mtDNA maintenance in various cell lines. We concluded that ddC alone, or in combination with EtBr, is an efficient means of inducing the ρ0 state, which is superior to that achieved by the broadly used EtBr.
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
MEF#5 is a clone of spontaneously immortalized mouse embryonic fibroblasts with genotype TFAMloxP/loxP, Gt(ROSA)26Sor+/lox-Stop-lox-mito-YFP. Primary embryonic fibroblasts were kindly provided by Nils-Goran Larsson [43]. 143B, HepG2, and HeLa cells were from ATCC (CRL-8303, HB-8065, and CCL-2, respectively). HT1080 cells were from the University of California, Berkeley cell culture core. A549 cells were from the laboratory collection. JCRB2201, 2202, 2207, 2214, and 2222 and human/mouse somatic cell hybrids containing single human chromosomes (Chr1, Chr2, Chr7, Chr14, or Chr22, respectively) were from the cell line repository at the National Institutes of Biomedical Innovation, Health, and Nutrition, Japan and were obtained through Sekisui XenoTech, Kansas City, KS via an agreement with Tottori University [44]. The 143B#6 and 5460#5 cell lines have been described previously [1].
All cells were propagated in Dulbecco’s modified Eagle’s medium (DMEM) containing 4.5 g/L glucose, 10% fetal bovine serum, 50 µg/mL gentamycin, 50 µg/mL uridine, and 1 mM sodium pyruvate in a humidified atmosphere containing 5% CO2 at 37 °C. This modification of the medium (+UP) is permissive for the growth of cells devoid of mtDNA (ρ0 cells). For cultivation of JCRB cells, media were additionally supplemented with 1 mg/mL G418 (JCRB2201, 2202, and 2207) or 1 mg/mL hygromycin (JCRB2214 and 2222) to select for the presence of human chromosomes.
The identity of the cell lines used in this study was confirmed by STR analysis (LabCorp, Burlington, NC, USA). The cell lines were quarterly tested for mycoplasma contamination by PCR.
2.2. Determination of mtDNA Copy Number (mtCN) by Direct Digital Droplet PCR
mtCN was determined as described previously [45]. Briefly, cells were collected by trypsinization and counted, and ~106-cell pellets were generated and frozen at −80 °C. Pellets were resuspended in PBS at ~10,000 cells/μL, and 10 μL aliquots were removed and mixed with 90 μL of solution containing 50 μg proteinase K, 40 µL of H2O, and 50 µL of the DirectPCR solution (Genprice Inc., San Jose, CA, USA, Cat# 388-302-C). The mix was incubated at 50 °C for 30 min and then at 95 °C for another 30 min. The solution was adjusted to 500 μL with H2O, and 3 μL of the resulting solution was used as a template in 20 μL ddPCR to determine nuclear DNA (nDNA) content using the primers and probes listed in Table 1. For mtDNA quantification, nDNA samples were diluted 500-fold, and 3 μL of the resulting dilution was used in 20 μL ddPCRs with the primers and probes listed in Table 1. dddPCRs contained 0.9 μM of each forward and reverse primer, 0.25 μM probe, 10 μL of the 2× ddPCR Supermix for Probes (No dUTP, Bio-Rad, Hercules, CA, USA Cat#1863023), 10 units of EcoRI HF restriction enzyme (New England Biolabs, Beverly, MA, USA, Cat# R3101S), and the balance of water. The cycling parameters were as follows: initial denaturation for 10 min at 95 °C, followed by 40 cycles of 20 s at 94 °C + 1 min at 60 °C, 10 min at 98 °C, and held at 4 °C. Each sample was measured in 2 technical replicates. To calculate mtCN per cell, the concentration of mtDNA targets was multiplied by the dilution factor and divided by the 0.5× concentration of nDNA targets. Each mtDNA template concentration was combined with each nDNA template concentration, generating four mtCN values for each sample.

Table 1.
Oligonucleotides used in this study.
2.3. Clonal Isolation of ρ0 Cells and Confirmation of the ρ0 Status
Upon mtCN in the treated cells dropping below 1, as judged by dddPCR, the cells were serially diluted, plated onto 150 mm tissue culture plates, and allowed to grow until the colonies reached 1–2 mm in diameter (2–3 weeks, for different cell lines). Using cloning disks, the smallest colonies were identified and picked for transfer into 24-well tissue culture plates. Upon expansion in DMEM+UP media containing appropriate antibiotics, cells were dissociated with 100 μL of trypsin, and 10 μL was removed for the detection of mtDNA using DirectPCR reagent as recommended by the manufacturer with primers listed in Table 1 [33].
3. Results
3.1. ddC Is More Effective than EtBr in mtDNA Elimination from Immortalized Mouse Embryonic Fibroblasts
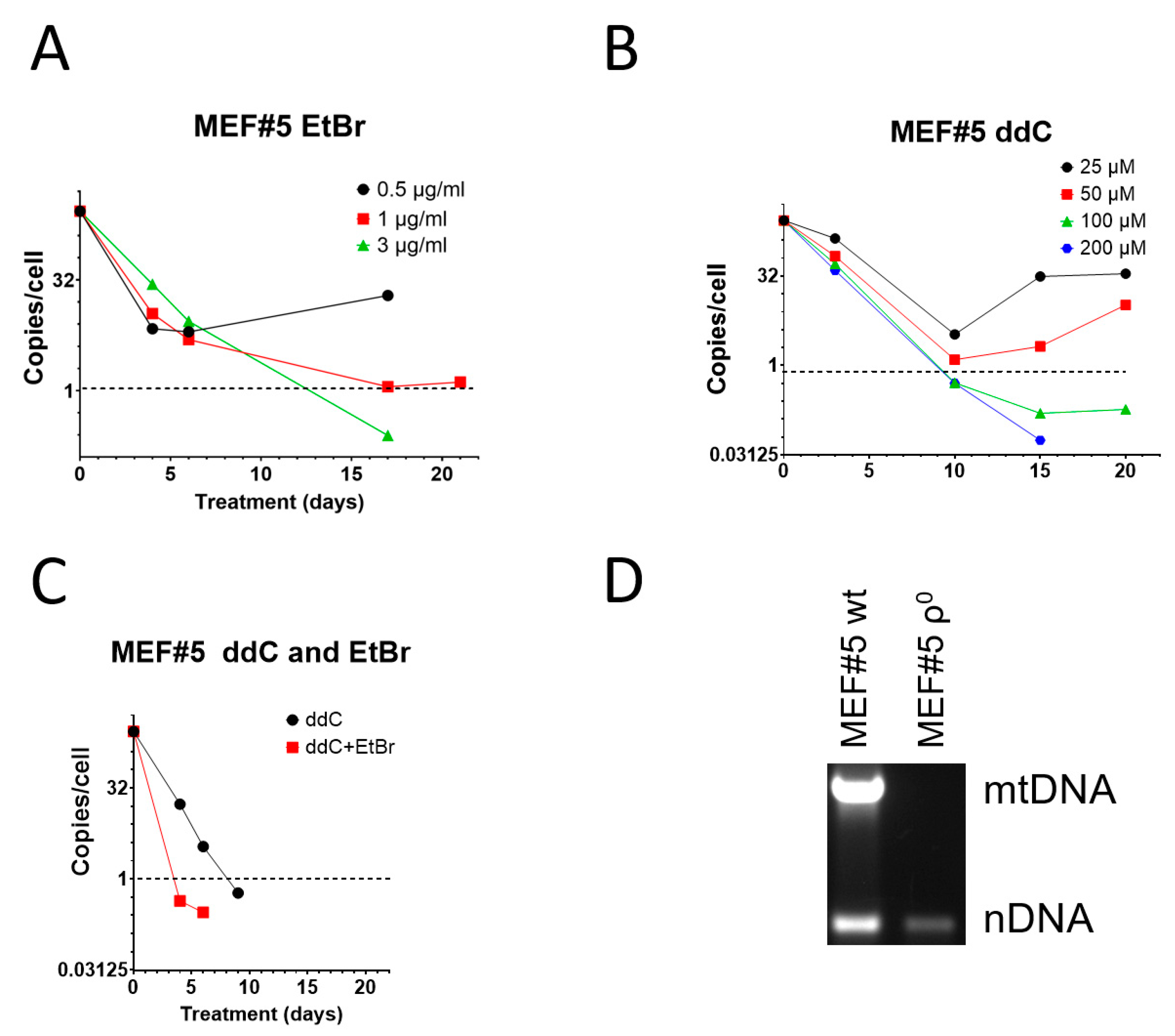
EtBr remains the most popular mtDNA elimination agent [8,9]. However, it has been suggested that in mouse cells, EtBr induces resistance, making this reagent unsuitable for the isolation of mouse ρ0 cell lines [27,28,46]. This assertion does not align with our previous experience with 3T3 cells, which were susceptible to EtBr treatment [47]. Nevertheless, 3T3 cells required higher EtBr concentrations to be effective. Therefore, we compared the effectiveness of EtBr and ddC for mtDNA elimination from spontaneously immortalized mouse embryonic fibroblasts MEF#5. Consistent with our previous observations, low concentrations (0.5 and 1 µg/mL) of EtBr were ineffective in MEF#5 cells and produced resistant cells. However, at 3 µg/mL, EtBr resulted in mtDNA depletion below an arbitrary threshold of an average of 1 mtDNA copy per cell in the population (Figure 1A). It was chosen because, at least in theory, at this threshold, more than 1/3 of cells in the population would be expected to contain no mtDNA at all, and ρ0 cells could be isolated by analyzing random clones from serially diluted cells. At 10 µg/mL EtBr, MEF#5 cells ceased to proliferate after several days in culture.
Figure 1.
mtDNA depletion in MEF#5 with EtBr, ddC, and their combination. (A) Low concentrations of EtBr are ineffective in mtDNA depletion. (B) Low concentrations of ddC are ineffective in mouse cells, whereas higher concentrations are more effective than EtBr. (C) A combination of 200 μM ddC and 0.5 µg/mL EtBr is more effective than either drug alone. (D) MEF#5 cells, cloned after ddC treatment and expanded in the media without ddC, contain no mtDNA.
When compared to EtBr, zalcitabine was more effective in mtDNA depletion, and the 1 mtDNA copy per cell threshold was achieved in less than 10 days (compared to ~12 days with EtBr) at ddC concentrations of 100 and 200 µM (Figure 1B). Importantly, low concentrations of ddC were ineffective in MEF#5 cells and resulted in the growth of resistant cells (Figure 1B). This behavior is similar to that of low concentrations of EtBr (Figure 1A).
The effects of EtBr and ddC were additive. Treatment with a low EtBr concentration that induced resistance (0.5 µg/mL) with ddC at 200 µM was more efficient than treatment with ddC alone in that the threshold was reached in 4 days rather than 8 days (Figure 1C). At the same time, ddC did not exacerbate the cytostatic effects of EtBr. We believe this observation might help combat spontaneous resistance to each of these compounds used individually.
To confirm that ddC can be used to generate murine cell lines permanently depleted of mtDNA, we cloned MEF#5 after depletion with ddC and verified that the resulting cells, after expansion in media without ddC, contained no mtDNA (Figure 1D).
3.2. ddC Is More Effective than EtBr in mtDNA Elimination from Human Cells
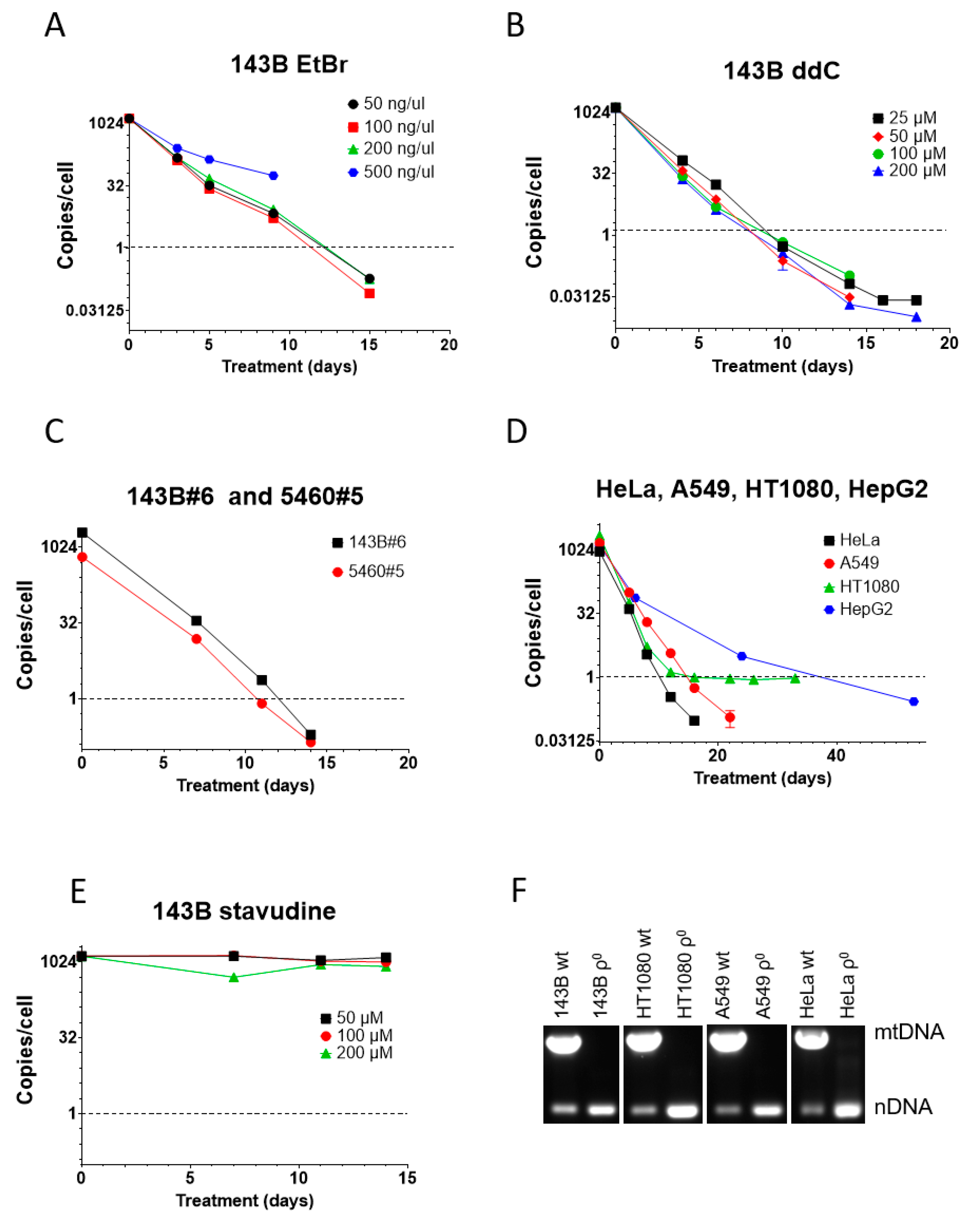
We also compared ddC performance to that of EtBr in human osteosarcoma 143B cells. Here, too, a higher concentration of EtBr (500 ng/mL) was cytostatic and not only resulted in growth arrest and cell death but also in a slower mtDNA depletion, presumably because of the loss of the benefit of mtDNA dilution by cell division (Figure 2A). Importantly, cytostatic EtBr concentration in 143B cells was 20 times lower than in MEF#5 cells, suggesting greater sensitivity of human cells to EtBr as compared to mouse cells. This suggestion agrees with our observations in other cell lines. Other concentrations of EtBr (50, 100, and 200 ng/mL) were equally effective and reduced mtCN to an average 1 mtDNA copy per cell threshold in 12–13 days (Figure 2A).
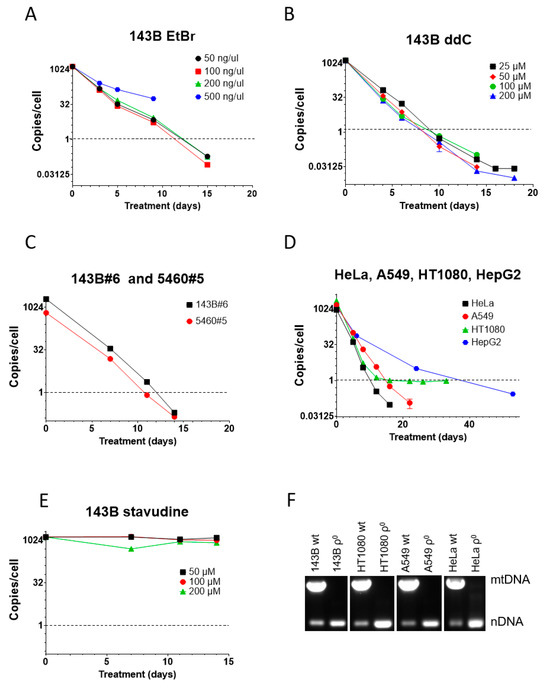
Figure 2.
mtDNA depletion in human cells with EtBr and ddC. (A) High concentrations of EtBr are cytostatic and resulted in the termination of the experiment at 9 days, whereas lower concentrations of EtBr were equally effective. (B) ddC was almost equally effective at all tested concentrations. No cytostatic effect was observed, even at the highest tested concentration. (C) An amount of 200 µM ddC is effective for mtDNA depletion in GeneSwapped derivatives of 143B cells. (D) An amount of 200 µM ddC is effective for reducing mtCN below the 1 mtDNA copy per cell threshold in HeLa, A549, HT1080, and HepG2 cells. (E) Unlike ddC, stavudine (d4T) is ineffective for mtDNA depletion in 143B cells at all tested concentrations. (F) Analysis of mtDNA content in clonal cells isolated after ddC treatment by duplex PCR, with two pairs of primers specific for nuclear DNA and mtDNA.
In contrast to mouse MEF#5 cells, in 143B cells, ddC’s effectiveness was comparable at all concentrations tested, reducing mtCN to the threshold in 8-10 days, which is faster than with EtBr (Figure 2B). This, again, suggests a higher sensitivity of human cells to mtDNA-depleting agents.
143B#6 and 5460#5 cells are derivatives of 143B cells in which the TFAM gene was knocked out and rescued with retrovirally encoded wt TFAM cDNA [1]. Like parental 143B cells, these cell lines responded well to 200 µM ddC, which reduced their mtCN to the threshold level in 11-12 days (Figure 2C).
ddC at 200 µM also worked well in other cell lines of human origin (HeLa, A549, HT1080, and HepG2), reducing their mtCN down to the threshold in 10–39 days (Figure 2D). Curiously, mtCN in HT1080 cells dropped rapidly to 1 and then stayed there for an extended period. We established that this phenomenon resulted from the presence in the nuclear genome of these cells of a mitochondrial pseudogene (NUMT) as mtCN was below 1 when using an alternative mtDNA probe.
We tested the efficiency of a related dideoxy compound, stavudine (d4T, 2′,3′-Didehydro-3′-deoxythymidine). This compound failed to affect mtCN in 143B cells at all tested concentrations, which starkly contrasts previous reports [36,41,48] (Figure 2E).
Finally, to validate the utility of the ddC treatment for the isolation of ρ0 cell lines, we cloned some cell lines after ddC treatment and analyzed their mtDNA content by duplex PCR. In every attempted case, ρ0 clones were isolated (Figure 2F).
3.3. A Combination of EtBr and ddC Is Effective for mtDNA Elimination from Mouse/Human Somatic Cell Hybrids
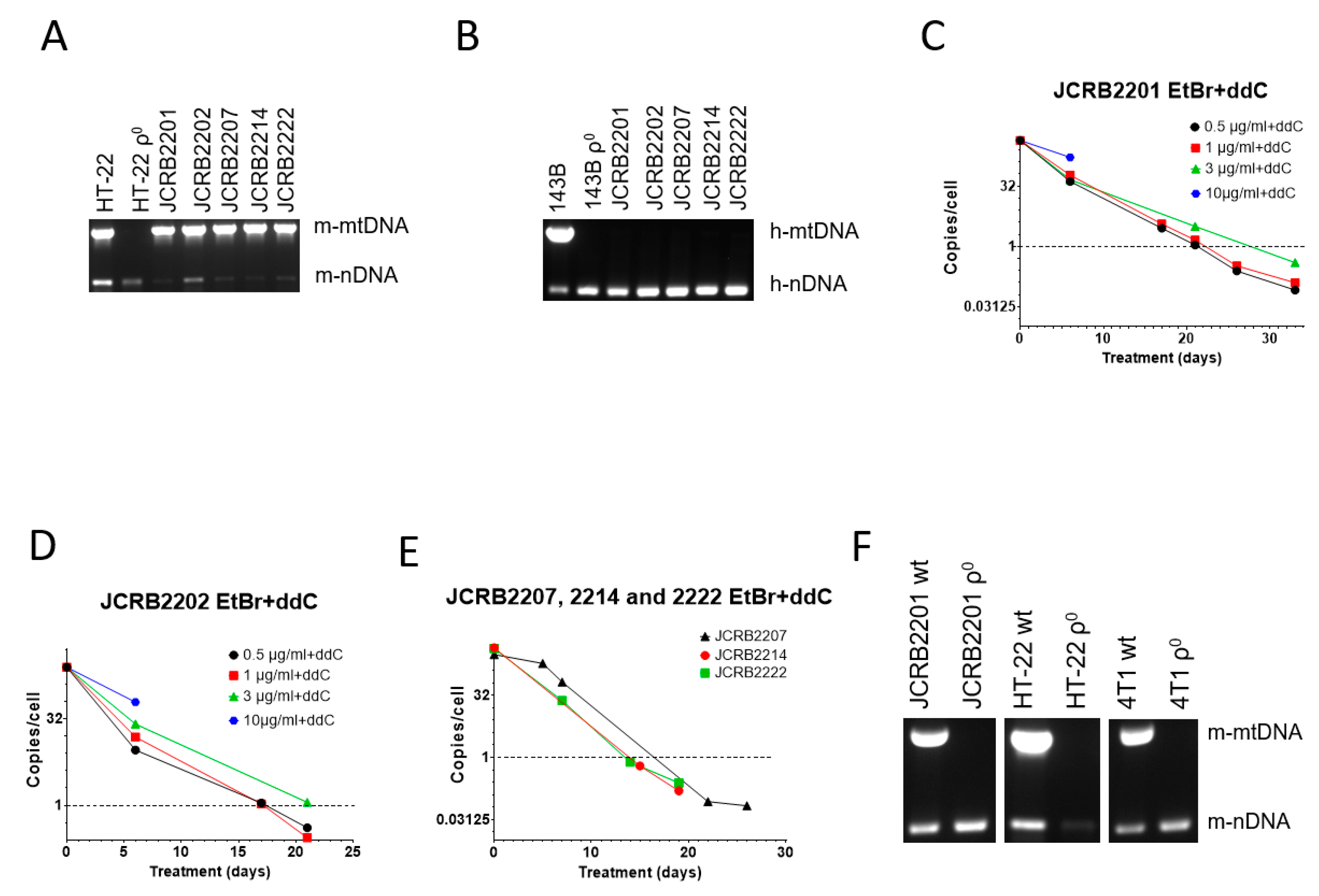
We were also interested in mtDNA elimination from JCRB human/mouse somatic cell hybrids. These hybrids each contain a single human chromosome and retain mouse but not human mtDNA (Figure 3A,B). This is consistent with previous observations that mouse/human somatic cell hybrids rapidly segregate mtDNA and retain mtDNA from a single species, most commonly mouse [49,50]. In our preliminary studies, we established that mouse/human somatic cell hybrids JCRB2201 and JCRB2202 were resistant to mtDNA depletion with EtBr. Therefore, we decided to test the utility of the combined treatment with 200 µM ddC and a range of EtBr concentrations, which has shown great promise in MEF#5 cells (Figure 1C), for mtDNA elimination from these hybrids.
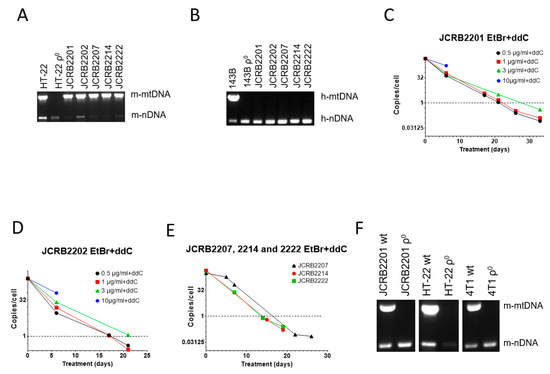
Figure 3.
mtDNA depletion in JCRB mouse/human somatic cell hybrids with a combination of EtBr and ddC. A and B, Analysis of JCRB clones for the presence of mouse (A) and human (B) mtDNA. These hybrids only retain mouse mtDNA. (C,D) JCRB2201 and JCRB2202 responded similarly to 200 µM ddC plus various concentrations of EtBr in that 10 µg/mL EtBr was cytostatic, 3 µg/mL EtBr had intermediate efficiency, presumably due to residual cytostatic effects, and 0.5 and 1 µg/mL EtBr were similarly effective in mtDNA depletion. (E) A combination of 200 µM ddC and 0.5 µg/mL EtBr is effective in mtDNA depletion in JCRB2207, JCRB2214, and JCRB2222 cell lines. (F) mtDNA analysis in clones of JCRB2201, HT-22, and 4T1 cells isolated after treatment with 0.5 µg/mL EtBr plus 200 µM ddC. Prior to analysis, clones were expanded in media without either EtBr or ddC.
Similar to MEF#5 and 143B cells, a combination of ddC with a high concentration of EtBr (10 µg/mL) was cytostatic (Figure 3C,D). At 3 µg/mL EtBr+ddC, mtDNA depletion in both cell lines was delayed compared to lower EtBr concentrations (0.5 and 1 µg/mL). This again suggests the negative effects of EtBr intercalation into nuclear DNA, leading to slower proliferation since these effects are also observed without ddC (e.g., Figure 1A).
As a result, we chose the lowest EtBr concentration (0.5 µg/mL) in combination with 200 µM ddC for mtDNA elimination in the remaining JCRB cell lines (JCRB2207, 2214, and 2222). The treatment was effective in all three cell lines (Figure 3E). We also subjected mouse mammary carcinoma 4T1 cells and the HT-22 immortalized mouse hippocampal neuronal cell line to the combined treatment with EtBr and ddC, which resulted in the successful isolation of ρ0 clones in both cases (Figure 3F).
4. Discussion
With the ever-growing interest in mtDNA biology, the interest in cells devoid of mtDNA also grows. However, their supply remains limited. The methods for generating ρ0 cells are time-consuming, and some techniques only apply to a limited subset of cell lines. Therefore, there is continuing interest in developing new techniques for inducing mtDNA loss in cultured cells.
Here, we examined the suitability of zddC/zalcitabine for generating ρ0 cells. While in patients zalcitabine only induces moderate mtDNA depletion [36], in our study, the application of this drug to many human and mouse cells in culture resulted in a gradual loss of mtDNA, which enabled the isolation of ρ0 derivatives of popular human cell lines, HeLa, A549, 143B, and HT1080, as well as spontaneously immortalized mouse embryonic fibroblasts and single human chromosome-containing mouse/human somatic cell hybrid cell line JCRB2201. We did not pursue the isolation of ρ0 derivatives of other JCRB cell lines because of the lack of interest or availability of the ρ0 derivatives obtained by other techniques. In contrast, another antiretroviral drug with a similar mechanism of action, stavudine, did not induce mtDNA depletion in 143B cells, although it does cause mtDNA depletion in patients [36], experimental animals [41], and cultured mesenchymal stem cells [48].
Our study and previous experience allowed us to make several generalizations. First, EtBr still remains an effective means of inducing the ρ0 state, although the susceptibility of cell lines to this drug varies dramatically. At 500 ng/mL (~1.27 µM), EtBr blocked proliferation and induced cell death in 143B cells, whereas this concentration was too low to induce complete loss of mtDNA in MEFs and resulted in the development of resistance. This observation highlights the need to carefully calibrate the EtBr concentration for each cell line, which is undesirable from a practical standpoint. Moreover, EtBr is mutagenic [51,52], and treatment with this compound induces nuclear DNA (nDNA) rearrangements [53]. Also, EtBr affects nuclear transcription [54] and can block cell proliferation, as shown in this study.
Second, unlike EtBr, ddC was tolerated well by all cell lines tested in this study at 200 µM, the highest concentration used.
Third, cell lines of murine origin tend to be more resistant to the action of mtDNA-depleting agents (in our study, both EtBr and ddC). This may result from the reduced permeability of the plasma membrane, mitochondria, nucleoids, the higher tolerance of mtDNA replication apparatus, or any combination of these factors. However, it is remarkable that the trend is the same in two chemically distinct drugs that act by different mechanisms.
Fourth, the combination of lower EtBr concentrations with ddC prevents the cytostatic effects of EtBr on cellular proliferation while achieving the goal of mtDNA elimination. This is important for two reasons. First, active proliferation helps to achieve mtDNA depletion through dilution in actively proliferating cells upon the blockage of mtDNA replication. Second, nonproliferating cells eventually die.
5. Conclusions
In conclusion, we believe that ddC will become a useful addition to the arsenal of tools for inducing the ρ0 state in cultured cells.
Author Contributions
M.F.A. designed the experiments, N.K. executed the experiments, M.F.A. analyzed the data, and M.F.A. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NATIONAL INSTITUTES OF HEALTH, grant numbers OD010944, S10OD025089, and HL66299, and the OFFICE OF THE ASSISTANT SECRETARY OF DEFENSE FOR HEALTH AFFAIRS, grant numbers W81XWH2110161 and W81XWH2110669. The APC was funded by W81XWH2110669.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained in the article.
Acknowledgments
The authors wish to acknowledge N.G. Larsson for TFAMloxP/LoxP, Gt(ROSA)26Sor+/lox-Stop-lox-mito-YFP MEFs.
Conflicts of Interest
The University of South Alabama entered an agreement with Kerafast, Inc. to distribute ρ0 derivatives of the HeLa, HT-22, and 4T1 cell lines generated in this study. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kozhukhar, N.; Spadafora, D.; Rodriguez, Y.A.R.; Alexeyev, M.F. A Method for In Situ Reverse Genetic Analysis of Proteins Involved mtDNA Replication. Cells 2022, 11, 2168. [Google Scholar] [CrossRef]
- Larsson, N.G.; Wang, J.; Wilhelmsson, H.; Oldfors, A.; Rustin, P.; Lewandoski, M.; Barsh, G.S.; Clayton, D.A. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 1998, 18, 231–236. [Google Scholar] [CrossRef]
- Humble, M.M.; Young, M.J.; Foley, J.F.; Pandiri, A.R.; Travlos, G.S.; Copeland, W.C. Polg2 is essential for mammalian embryogenesis and is required for mtDNA maintenance. Hum. Mol. Genet. 2012, 22, 1017–1025. [Google Scholar] [CrossRef]
- Hance, N.; Ekstrand, M.I.; Trifunovic, A. Mitochondrial DNA polymerase gamma is essential for mammalian embryogenesis. Hum. Mol. Genet. 2005, 14, 1775–1783. [Google Scholar] [CrossRef]
- Kuhl, I.; Miranda, M.; Posse, V.; Milenkovic, D.; Mourier, A.; Siira, S.J.; Bonekamp, N.A.; Neumann, U.; Filipovska, A.; Polosa, P.L.; et al. POLRMT regulates the switch between replication primer formation and gene expression of mammalian mtDNA. Sci. Adv. 2016, 2, e1600963. [Google Scholar] [CrossRef]
- Jiang, M.; Xie, X.; Zhu, X.; Jiang, S.; Milenkovic, D.; Misic, J.; Shi, Y.; Tandukar, N.; Li, X.; Atanassov, I.; et al. The mitochondrial single-stranded DNA binding protein is essential for initiation of mtDNA replication. Sci. Adv. 2021, 7, eabf8631. [Google Scholar] [CrossRef]
- Milenkovic, D.; Matic, S.; Kuhl, I.; Ruzzenente, B.; Freyer, C.; Jemt, E.; Park, C.B.; Falkenberg, M.; Larsson, N.G. TWINKLE is an essential mitochondrial helicase required for synthesis of nascent D-loop strands and complete mtDNA replication. Hum. Mol. Genet. 2013, 22, 1983–1993. [Google Scholar] [CrossRef]
- King, M.P.; Attardi, G. Human cells lacking mtDNA: Repopulation with exogenous mitochondria by complementation. Science 1989, 246, 500–503. [Google Scholar] [CrossRef]
- King, M.P.; Attardi, G. Isolation of human cell lines lacking mitochondrial DNA. Methods Enzymol. 1996, 264, 304–313. [Google Scholar]
- Morais, R.; Gregoire, M.; Jeannotte, L.; Gravel, D. Chick embryo cells rendered respiration-deficient by chloramphenicol and ethidium bromide are auxotrophic for pyrimidines. Biochem. Biophys. Res. Commun. 1980, 94, 71–77. [Google Scholar] [CrossRef]
- Wilkins, H.M.; Carl, S.M.; Swerdlow, R.H. Cytoplasmic hybrid (cybrid) cell lines as a practical model for mitochondriopathies. Redox Biol. 2014, 2C, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.L. Phenotypic expression of malignancy in hybrid and cybrid mouse cells. Somatic Cell Genet. 1978, 4, 477–489. [Google Scholar] [CrossRef]
- Giles, R.E.; Stroynowski, I.; Wallace, D.C. Characterization of mitochondrial DNA in chloramphenicol-resistant interspecific hybrids and a cybrid. Somatic Cell Genet. 1980, 6, 543–554. [Google Scholar] [CrossRef]
- Hayashi, J.; Gotoh, O.; Tagashira, Y.; Tosu, M.; Sekiguchi, T. Identification of mitochondrial DNA species in interspecific cybrids and reconstituted cells using restriction endonuclease. FEBS Lett. 1980, 117, 59–62. [Google Scholar] [CrossRef]
- Dunbar, D.R.; Moonie, P.A.; Zeviani, M.; Holt, I.J. Complex I deficiency is associated with 3243G:C mitochondrial DNA in osteosarcoma cell cybrids. Hum. Mol. Genet. 1996, 5, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Trounce, I.; Wallace, D.C. Production of transmitochondrial mouse cell lines by cybrid rescue of rhodamine-6G pre-treated L-cells. Somat. Cell Mol. Genet. 1996, 22, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, A.; Kenyon, L.; Moraes, C.T. Human xenomitochondrial cybrids. Cellular models of mitochondrial complex I deficiency. J. Biol. Chem. 1998, 273, 14210–14217. [Google Scholar] [CrossRef]
- Pye, D.; Kyriakouli, D.S.; Taylor, G.A.; Johnson, R.; Elstner, M.; Meunier, B.; Chrzanowska-Lightowlers, Z.M.; Taylor, R.W.; Turnbull, D.M.; Lightowlers, R.N. Production of transmitochondrial cybrids containing naturally occurring pathogenic mtDNA variants. Nucleic Acids Res. 2006, 34, e95. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Hayashi, J. Generation of mtDNA-exchanged cybrids for determination of the effects of mtDNA mutations on tumor phenotypes. Methods Enzymol. 2009, 457, 335–346. [Google Scholar] [CrossRef]
- Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; Nakada, K.; Honma, Y.; Hayashi, J.I. ROS-Generating Mitochondrial DNA Mutations Can Regulate Tumor Cell Metastasis. Science 2008, 320, 661–664. [Google Scholar] [CrossRef]
- Hashizume, O.; Shimizu, A.; Yokota, M.; Sugiyama, A.; Nakada, K.; Miyoshi, H.; Itami, M.; Ohira, M.; Nagase, H.; Takenaga, K.; et al. Specific mitochondrial DNA mutation in mice regulates diabetes and lymphoma development. Proc. Natl. Acad. Sci. USA 2012, 109, 10528–10533. [Google Scholar] [CrossRef]
- Hashizume, O.; Yamanashi, H.; Taketo, M.M.; Nakada, K.; Hayashi, J. A Specific Nuclear DNA Background Is Required for High Frequency Lymphoma Development in Transmitochondrial Mice with G13997A mtDNA. PLoS ONE 2015, 10, e0118561. [Google Scholar] [CrossRef] [PubMed]
- Khozhukhar, N.; Spadafora, D.; Rodriguez, Y.; Alexeyev, M. Elimination of Mitochondrial DNA from Mammalian Cells. Curr. Protoc. Cell Biol. 2018, 78, 20.11.1–20.11.14. [Google Scholar] [CrossRef] [PubMed]
- Nass, M.M. Differential effects of ethidium bromide on mitochondrial and nuclear DNA synthesis in vivo in cultured mammalian cells. Exp. Cell Res. 1972, 72, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, A.; Attardi, G. Reversible tenfod reduction in mitochondria DNA content of human cells treated with ethidium bromide. Mol. Gen. Genet. 1978, 167, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, P.; de Muys, J.M.; Morais, R. An established avian fibroblast cell line without mitochondrial DNA. Somat. Cell Mol. Genet. 1986, 12, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Ito, S.; Takai, D.; Soejima, A.; Shisa, H.; LePecq, J.B.; Segal-Bendirdjian, E.; Kagawa, Y.; Hayashi, J.I. Isolation of mitochondrial DNA-less mouse cell lines and their application for trapping mouse synaptosomal mitochondrial DNA with deletion mutations. J. Biol. Chem. 1997, 272, 15510–15515. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Takai, D.; Hosaka, H.; Ito, S.; Shitara, H.; Isobe, K.; LePecq, J.B.; Segal-Bendirdjian, E.; Hayashi, J. Isolation and characterization of mitochondrial DNA-less lines from various mammalian cell lines by application of an anticancer drug, ditercalinium. Biochem. Biophys. Res. Commun. 1997, 239, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Okamaoto, M.; Ohsato, T.; Nakada, K.; Isobe, K.; Spelbrink, J.N.; Hayashi, J.; Hamasaki, N.; Kang, D. Ditercalinium chloride, a pro-anticancer drug, intimately associates with mammalian mitochondrial DNA and inhibits its replication. Curr. Genet. 2003, 43, 364–370. [Google Scholar] [CrossRef]
- Segal-Bendirdjian, E.; Coulaud, D.; Roques, B.P.; Le Pecq, J.B. Selective loss of mitochondrial DNA after treatment of cells with ditercalinium (NSC 335153), an antitumor bis-intercalating agent. Cancer Res. 1988, 48, 4982–4992. [Google Scholar]
- Shokolenko, I.N.; Wilson, G.L.; Alexeyev, M.F. Persistent damage induces mitochondrial DNA degradation. DNA Repair 2013, 12, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Spadafora, D.; Kozhukhar, N.; Chouljenko, V.N.; Kousoulas, K.G.; Alexeyev, M.F. Methods for Efficient Elimination of Mitochondrial DNA from Cultured Cells. PLoS ONE 2016, 11, e0154684. [Google Scholar] [CrossRef] [PubMed]
- Khozhukhar, N.; Spadafora, D.; Rodriguez, Y.A.R.; Fayzulin, R.; Alexeyev, M. Generation of Mammalian Cells Devoid of Mitochondrial DNA (rho(0) cells). Curr. Protoc. 2023, 3, e679. [Google Scholar] [CrossRef] [PubMed]
- Kukat, A.; Kukat, C.; Brocher, J.; Schafer, I.; Krohne, G.; Trounce, I.A.; Villani, G.; Seibel, P. Generation of rho0 cells utilizing a mitochondrially targeted restriction endonuclease and comparative analyses. Nucleic Acids Res. 2008, 36, e44. [Google Scholar] [CrossRef] [PubMed]
- Adkins, J.C.; Peters, D.H.; Faulds, D. Zalcitabine. An update of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in the management of HIV infection. Drugs 1997, 53, 1054–1080. [Google Scholar] [CrossRef] [PubMed]
- Walker, U.A.; Bauerle, J.; Laguno, M.; Murillas, J.; Mauss, S.; Schmutz, G.; Setzer, B.; Miquel, R.; Gatell, J.M.; Mallolas, J. Depletion of mitochondrial DNA in liver under antiretroviral therapy with didanosine, stavudine, or zalcitabine. Hepatology 2004, 39, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Walker, U.A.; Setzer, B.; Venhoff, N. Increased long-term mitochondrial toxicity in combinations of nucleoside analogue reverse-transcriptase inhibitors. AIDS 2002, 16, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Chariot, P.; Drogou, I.; de Lacroix-Szmania, I.; Eliezer-Vanerot, M.C.; Chazaud, B.; Lombes, A.; Schaeffer, A.; Zafrani, E.S. Zidovudine-induced mitochondrial disorder with massive liver steatosis, myopathy, lactic acidosis, and mitochondrial DNA depletion. J. Hepatol. 1999, 30, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Brivet, F.G.; Nion, I.; Megarbane, B.; Slama, A.; Brivet, M.; Rustin, P.; Munnich, A. Fatal lactic acidosis and liver steatosis associated with didanosine and stavudine treatment: A respiratory chain dysfunction? J. Hepatol. 2000, 32, 364–365. [Google Scholar] [CrossRef]
- Gerschenson, M.; Nguyen, V.T.; St Claire, M.C.; Harbaugh, S.W.; Harbaugh, J.W.; Proia, L.A.; Poirier, M.C. Chronic stavudine exposure induces hepatic mitochondrial toxicity in adult Erythrocebus patas monkeys. J. Hum. Virol. 2001, 4, 335–342. [Google Scholar]
- Gaou, I.; Malliti, M.; Guimont, M.C.; Letteron, P.; Demeilliers, C.; Peytavin, G.; Degott, C.; Pessayre, D.; Fromenty, B. Effect of stavudine on mitochondrial genome and fatty acid oxidation in lean and obese mice. J. Pharmacol. Exp. Ther. 2001, 297, 516–523. [Google Scholar] [PubMed]
- Kakuda, T.N. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin. Ther. 2000, 22, 685–708. [Google Scholar] [CrossRef] [PubMed]
- Sterky, F.H.; Lee, S.; Wibom, R.; Olson, L.; Larsson, N.G. Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 12937–12942. [Google Scholar] [CrossRef] [PubMed]
- Koi, M.; Morita, H.; Shimizu, M.; Oshimura, M. Construction of mouse A9 clones containing a single human chromosome (X/autosome translocation) via micro-cell fusion. Jpn. J. Cancer Res. 1989, 80, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Kozhukhar, N.; Fant, A.; Alexeyev, M.F. Quantification of mtDNA content in cultured cells by direct droplet digital PCR. Mitochondrion 2021, 61, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, J.; Tanaka, M.; Sato, W.; Ozawa, T.; Yonekawa, H.; Kagawa, Y.; Ohta, S. Effects of ethidium bromide treatment of mouse cells on expression and assembly of nuclear-coded subunits of complexes involved in the oxidative phosphorylation. Biochem. Biophys. Res. Commun. 1990, 167, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Fayzulin, R.Z.; Perez, M.; Kozhukhar, N.; Spadafora, D.; Wilson, G.L.; Alexeyev, M.F. A method for mutagenesis of mouse mtDNA and a resource of mouse mtDNA mutations for modeling human pathological conditions. Nucleic Acids Res. 2015, 43, e62. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Moreno, M.; Hermida-Gomez, T.; Gallardo, M.E.; Dalmao-Fernandez, A.; Rego-Perez, I.; Garesse, R.; Blanco, F.J. Generating Rho-0 Cells Using Mesenchymal Stem Cell Lines. PLoS ONE 2016, 11, e0164199. [Google Scholar] [CrossRef] [PubMed]
- Clayton, D.A.; Teplitz, R.L.; Nabholz, M.; Dovey, H.; Bodmer, W. Mitochondrial DNA of human-mouse cell hybrids. Nature 1971, 234, 560–562. [Google Scholar] [CrossRef]
- Attardi, B.; Attardi, G. Fate of mitochondrial DNA in human-mouse somatic cell hybrids (density gradient centrifugation-ethidium bromide-karyotype). Proc. Natl. Acad. Sci. USA 1972, 69, 129–133. [Google Scholar] [CrossRef]
- Kirsanov, K.I.; Lesovaya, E.A.; Yakubovskaya, M.G.; Belitsky, G.A. SYBR Gold and SYBR Green II are not mutagenic in the Ames test. Mutat. Res. 2010, 699, 1–4. [Google Scholar] [CrossRef]
- Singer, V.L.; Lawlor, T.E.; Yue, S. Comparison of SYBR Green I nucleic acid gel stain mutagenicity and ethidium bromide mutagenicity in the Salmonella/mammalian microsome reverse mutation assay (Ames test). Mutat. Res. 1999, 439, 37–47. [Google Scholar] [CrossRef]
- Singh, K.K.; Kulawiec, M.; Still, I.; Desouki, M.M.; Geradts, J.; Matsui, S. Inter-genomic cross talk between mitochondria and the nucleus plays an important role in tumorigenesis. Gene 2005, 354, 140–146. [Google Scholar] [CrossRef]
- Fan, H.; Penman, S. Mitochondrial RNA synthesis during mitosis. Science 1970, 168, 135–138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).