Enhancement of Biogas (Methane) Production from Cow Dung Using a Microbial Electrochemical Cell and Molecular Characterization of Isolated Methanogenic Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Collection of Cow Dung/Cattle Manure
2.3. Physiochemical Analysis of Substrate
2.4. Chemical Analysis of Substrate
2.5. Enrichment of the Collected Sample with Methanobacterium II Medium (MMII)
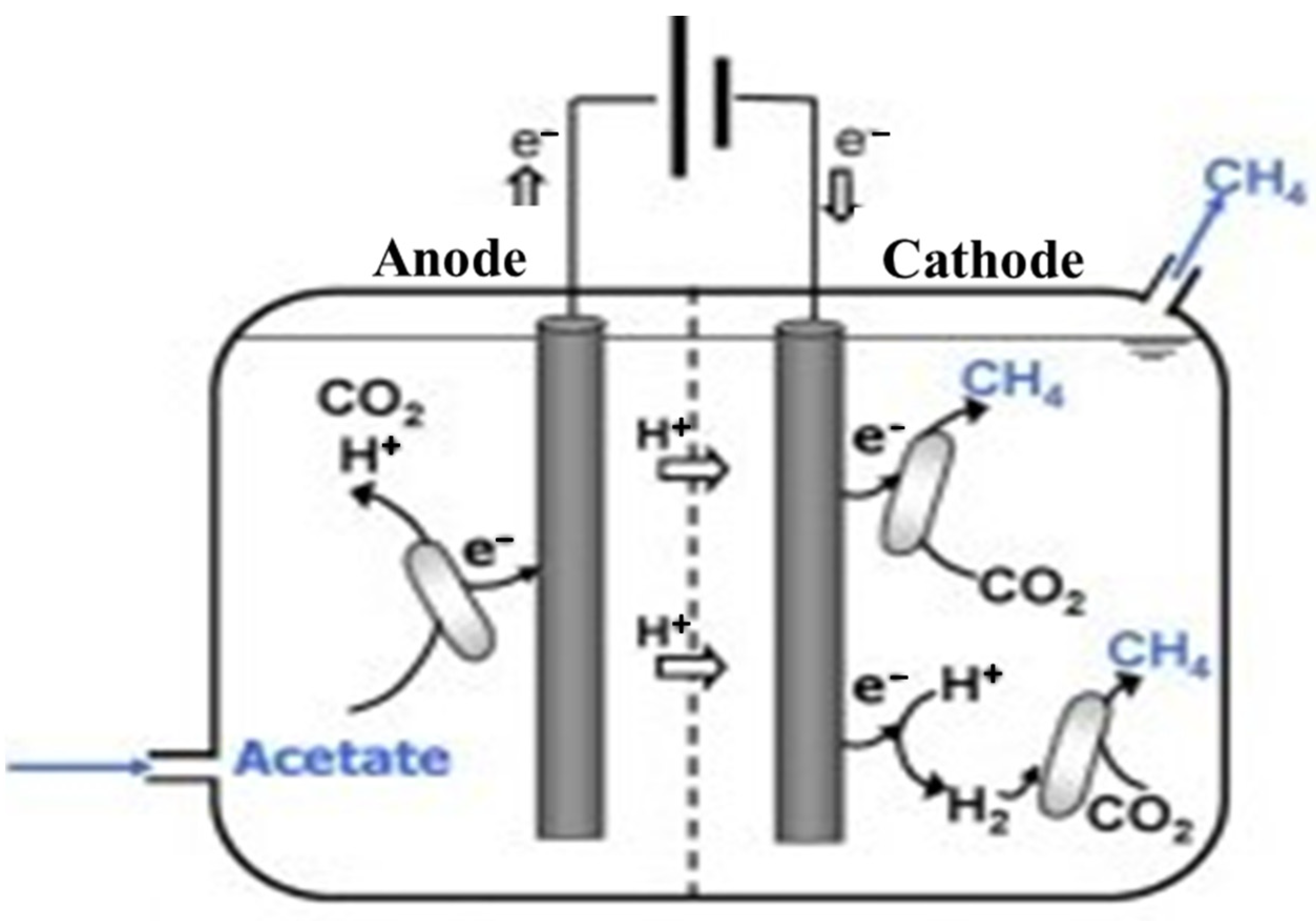
2.6. MEC Construction and Operation
2.7. Treatment of Graphite Electrodes
2.8. Cyclic Voltammetry
2.9. SEM Analysis of the Electrode
2.10. Isolation and Identification of Bacteria
2.11. Molecular Characterization of Isolates
2.12. Data Analysis
3. Results and Discussion
3.1. Determination of Environmental Parameters of the Collected Cow Dung
3.2. Optimization of Apparatus for Biogas Production
3.3. Operation of MFC
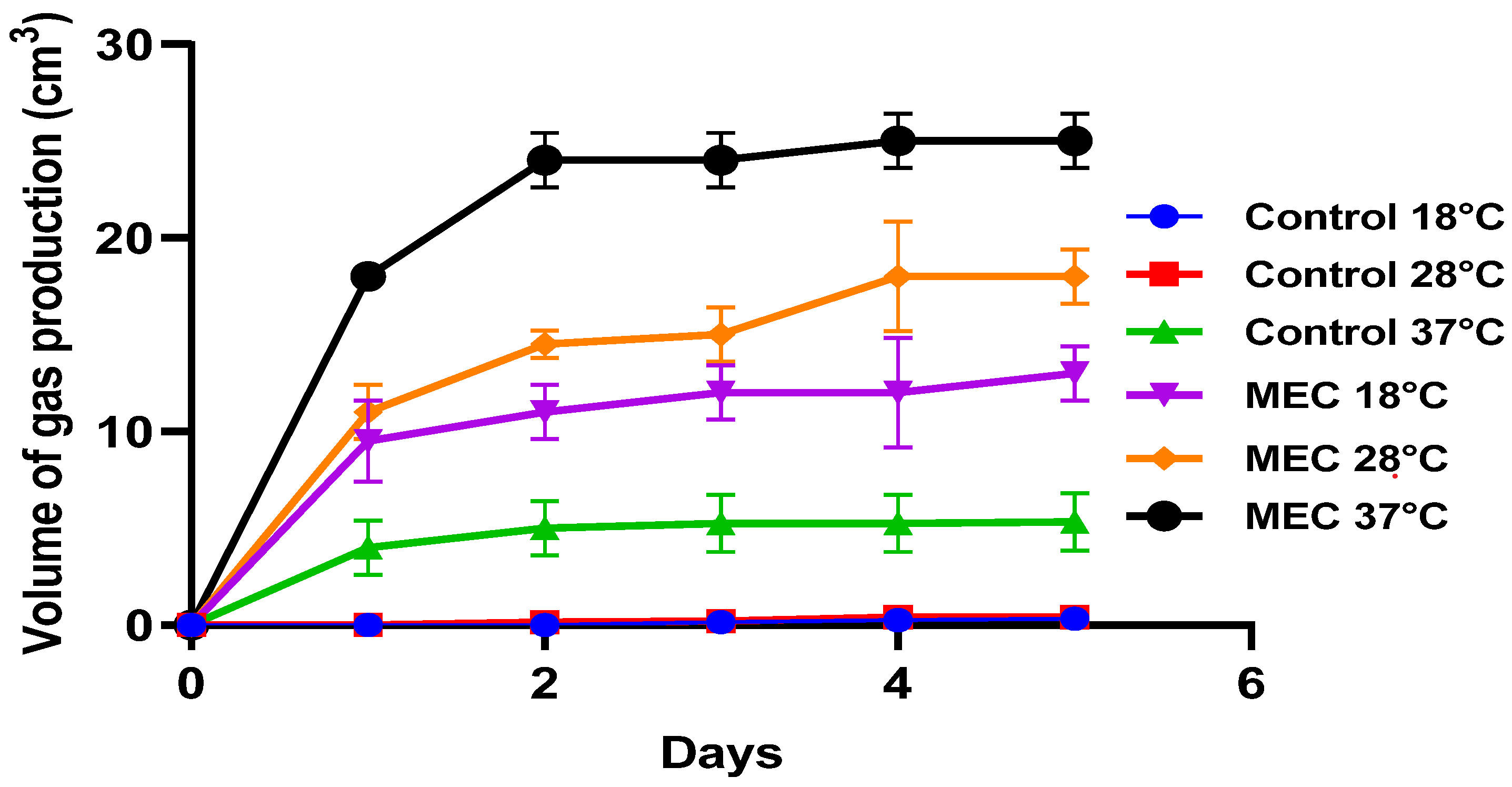
3.3.1. Temperature Dependent Production of Biogas
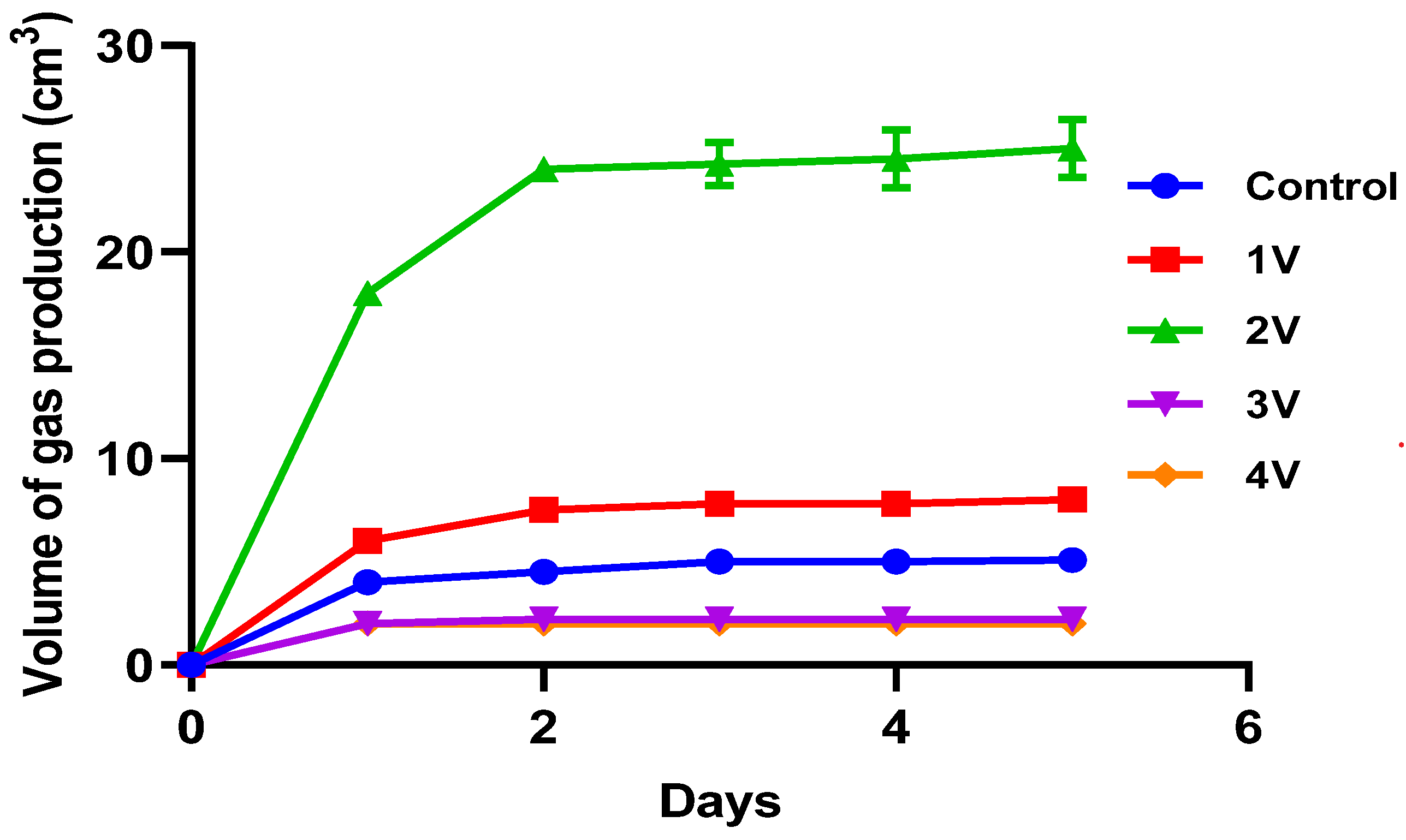
3.3.2. Voltage Optimization for Biogas Production
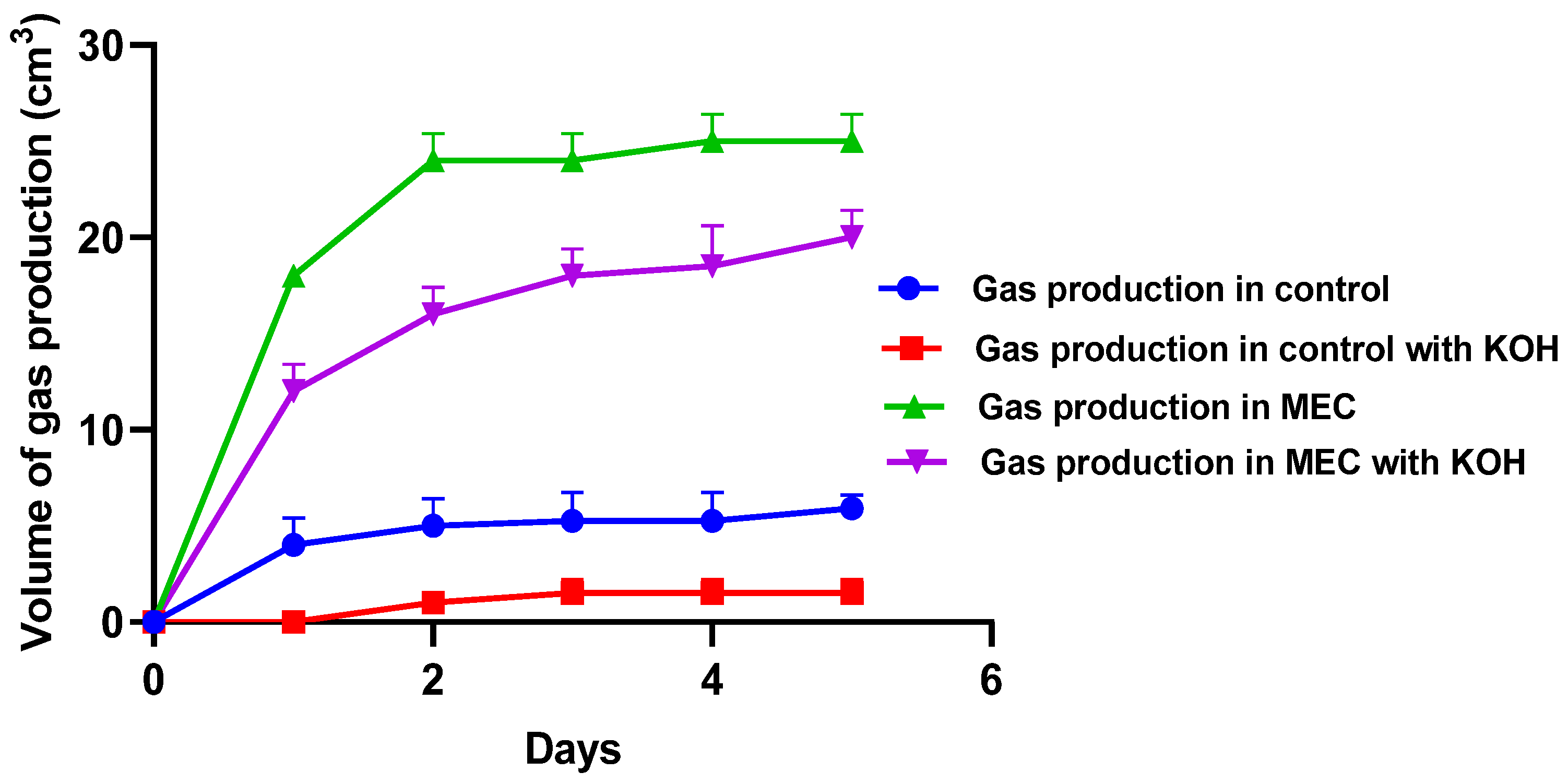
3.3.3. Biogas Collected in the Presence and Absence of KOH
3.3.4. Variation in Biogas Production with the Use of MWCNT Coated Electrodes
3.4. Removal of COD and Reducing Sugars
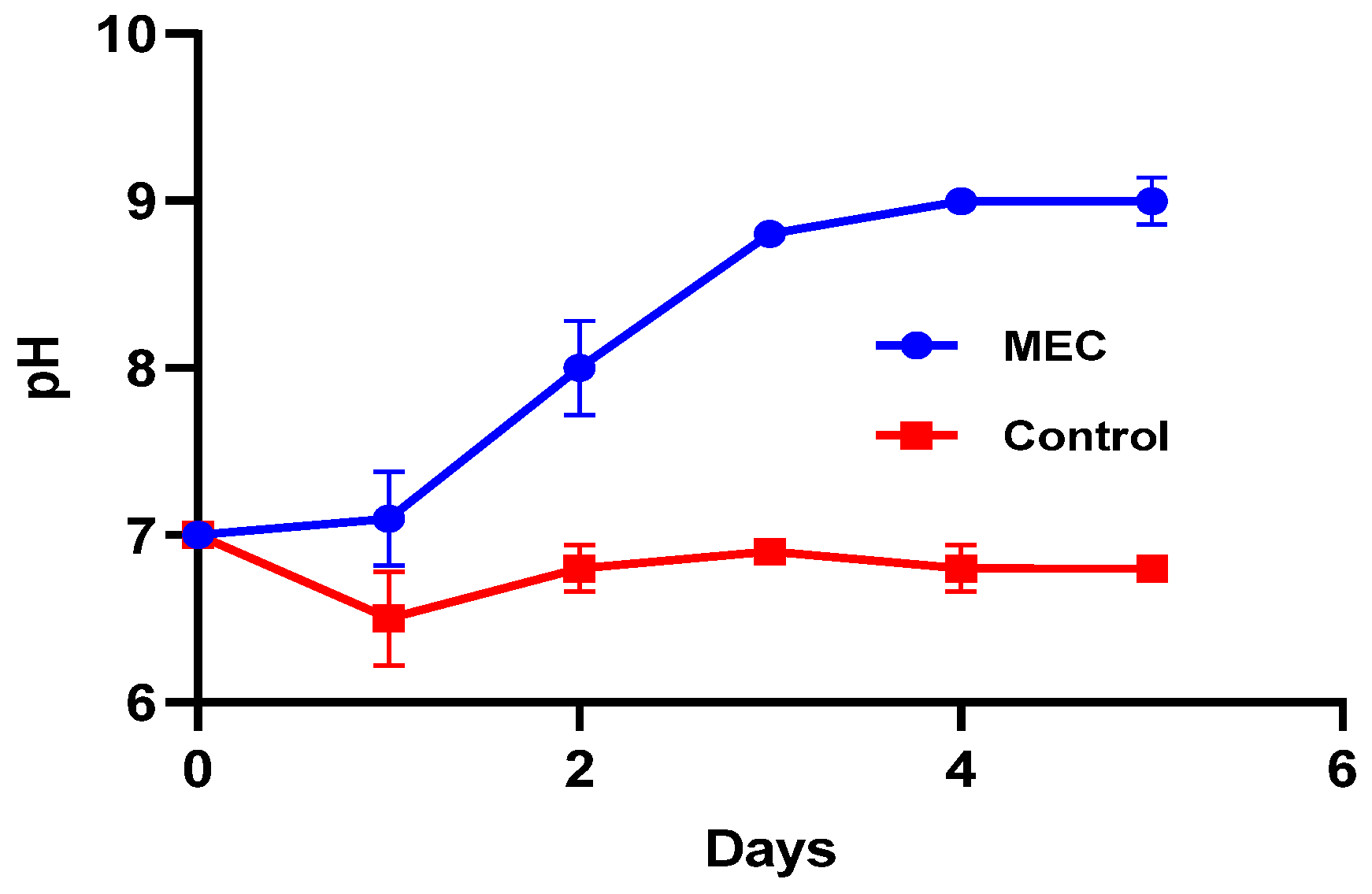
3.5. Change in the pH If the Anaerobic Reactor Contents
3.6. Scanning Electron Microscopy (SEM) Analysis of the Electrodes after Fermentation
3.7. Cyclic Voltammetry Measurements
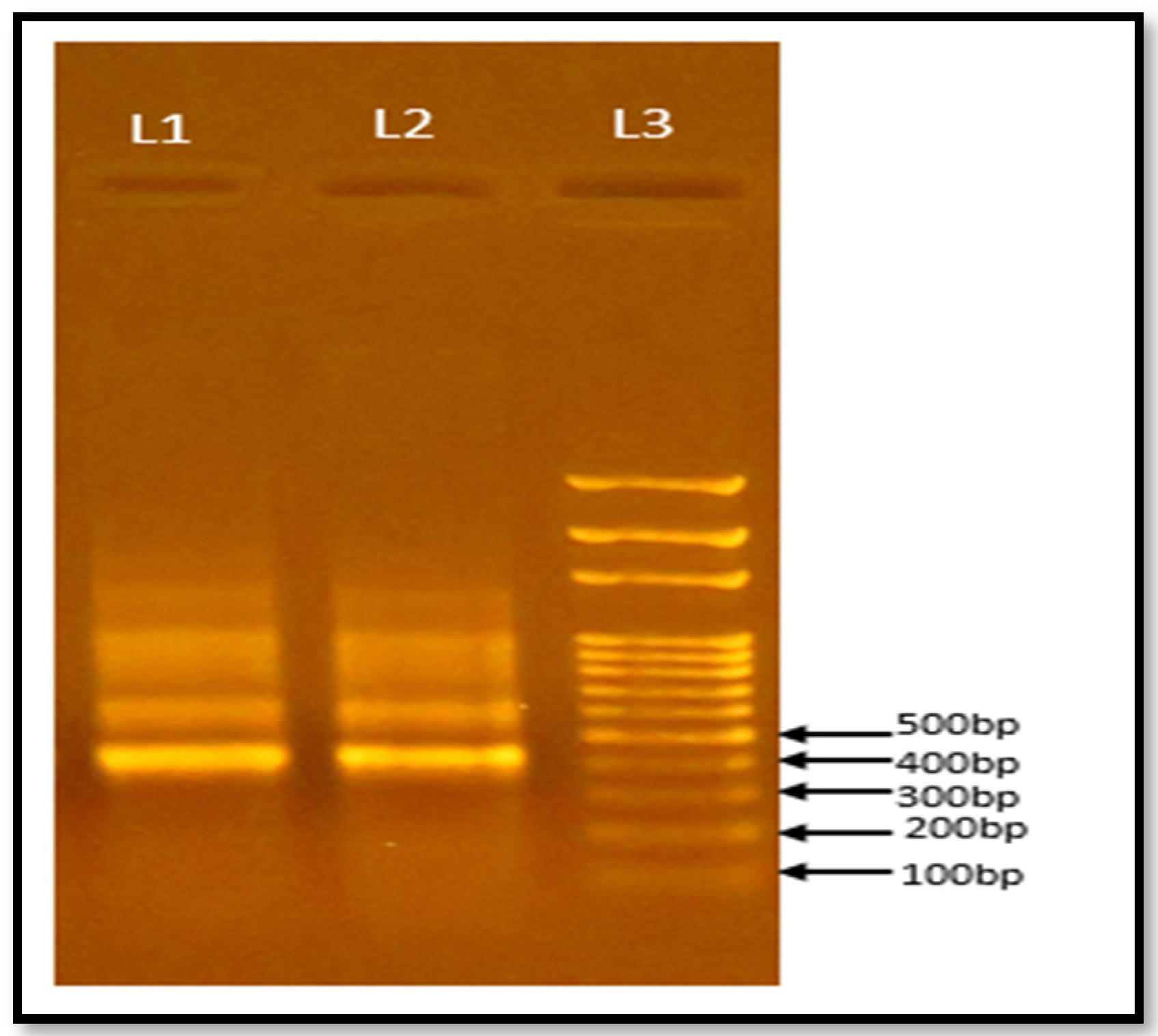
3.8. Characterization of Microbes Present in Methanogenic Inoculum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| S.N Components | Amount |
|---|---|
| 1 CaCl2 × 2 H2O | 0.10 g |
| 2 K2HPO4 | 0.30 g |
| 3 KH2PO4 | 0.30 g |
| 4 MgCl2 × 6 H2O | 0.20 g |
| 5 KCl 0.10 g NaCl | 0.60 g |
| 6 NH4 Cl | 1.00 g |
| 7 Trace element solution (i) | 10.00 mL |
| 8 Na-acetate | 0.50 g |
| 9 Na-resazurin solution (0.1% w/v) | 0.50 mL |
| 10 Vitamin solution (ii) | 10.00 mL |
| 11 Yeast extract | 1 g |
| 12 Na2S × 9 H2O | 0.50 g |
| 13 L-Cysteine-HCl × H2O | 0.50 g |
| 14 NaHCO3 | 4.00 g |
| 15 Distilled water | 1000.00 mL |
| (i)Trace element solution | |
| S.N Component | Amount |
| 1. Nitrilotriacetic acid | 1.50 g |
| 2. MgSO4 × 7 H2O | 3.00 g |
| 3. MnSO4 × H2O | 0.50 g |
| 4. NaCl | 1.00 g |
| 5. FeSO4 × 7 H2O | 0.10 g |
| 6. CoSO4 × 7 H2O | 0.18 g |
| 7. CaCl2 × 2 H2O | 0.10 g |
| 8. CuSO4 × 5 H2O | 0.01 g |
| 9. KAl(SO4)2 × 12 H2O | 0.02 g |
| 10. H3BO3 | 0.01 g |
| 11. Na2MoO4 × 2 H2O | 0.01 g |
| 12. NiCl2 × 6 H2O | 0.03 g |
| 13. Na2SeO3 × 5 H2O | 0.30 mg |
| 14. Na2WO4 × 2 H2O | 0.40 mg |
| (ii) Vitamin solution: | |
| S.N Components | Amount |
| 1. Biotin | 2.00 mg |
| 2. Folic acid | 2.00 mg |
| 3. Pyridoxine-HCl | 10.00 mg |
| 4. Thiamine-HCl × 2 H2O | 5.00 mg |
| 5. Riboflavin | 5.00 mg |
| 6. Nicotinic acid | 5.00 mg |
| 7. D-Ca-pantothenate | 5.00 mg |
| 8. Vitamin B12 | 0.10 mg |
| 9. p-Aminobenzoic acid | 5.00 mg |
| 10. Lipoic acid | 5.00 mg |
| 11. Distilled water | l L |
References
- Sampat, A.M.; Ruiz-Mercado, G.J.; Zavala, V.M. Economic and Environmental Analysis for Advancing Sustainable Management of Livestock Waste: A Wisconsin Case Study. ACS Sustain. Chem. Eng. 2018, 6, 6018–6031. [Google Scholar] [CrossRef] [PubMed]
- Dhungana, P.; Prajapati, B.; Maharjan, S.; Joshi, J. Current Trends in Lignocellulosic Bioethanol Production. Int. J. Appl. Sci. Biotechnol. 2022, 10, 1–11. [Google Scholar] [CrossRef]
- Demirbas, A.; Taylan, O.; Kaya, D. Biogas production from municipal sewage sludge (MSS). Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 3027–3033. [Google Scholar] [CrossRef]
- Qazi, W.A.; Abushammala, M.F.; Mohammed-Hasham, A.; Younes, M.K. Waste-to-Energy Technologies: A Literature Review. J. Solid Waste Technol. Manag. 2018, 44, 387–409. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sudharsana, T.; Jayamuthunagai, J.; Praveenkumar, R.; Chozhavendhan, S.; Iyyappan, J. RETRACTED: Biogas production–A review on composition, fuel properties, feed stock and principles of anaerobic digestion. Renew. Sustain. Energy Rev. 2018, 90, 570–582. [Google Scholar] [CrossRef]
- Faoagali, J. Meet the chief examiner microbiology 2010. Pathology 2010, 42, S51. [Google Scholar] [CrossRef]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Putri, D.A.; Saputro, R.R.; Budiyono, B. Biogas Production from Cow Manure. Int. J. Renew. Energy Dev. 2012, 1, 61–64. [Google Scholar] [CrossRef]
- Taherzadeh, M.; Bolton, K.; Wong, J.; Pandey, A. Sustainable Resource Recovery and Zero Waste Approaches; Elsevier: Amsterdam, The Netherlands, 2019; Available online: http://books.google.ie/books?id=mH2jDwAAQBAJ&pg=PA181&dq=978-1-910154-03-8&hl=&cd=1&source=gbs (accessed on 20 March 2024).
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct Biological Conversion of Electrical Current into Methane by Electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Factors Affecting the Effectiveness of Bioelectrochemical System Applications: Data Synthesis and Meta-Analysis. Batteries 2018, 4, 34. [Google Scholar] [CrossRef]
- AOAC, H.W. International A: Official Methods of Analysis of the AOAC International; The Association: Arlington County, VA, USA, 2000. [Google Scholar]
- Feldsine, P.T.; Abeyta, C.; Andrews, W.H. AOAC INTERNATIONAL Methods Committee Guidelines for Validation of Qualitative and Quantitative Food Microbiological Official Methods of Analysis. J. AOAC Int. 2002, 85, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Valo, A.; Carrère, H.; Delgenès, J.P. Thermal, chemical and thermo-chemical pre-treatment of waste activated sludge for anaerobic digestion. J. Chem. Technol. Biotechnol. 2004, 79, 1197–1203. [Google Scholar] [CrossRef]
- Olson, B.J.; Markwell, J. Assays for determination of protein concentration. Curr. Protoc. Pharmacol. 2007, 3, 3A. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, R.; Dhungana, P.; Sreerama, L.; Bhatt, P.; Prajapati, B.; Poudel, P.; Kandel, P.; Khadka, M.; Parajuli, A.; Joshi, J. The use of microbial fuel cell for efficient treatment of cauliflower waste and generation of electricity. Int. J. Sustain. Energy 2023, 42, 304–317. [Google Scholar] [CrossRef]
- Joshi, J.; Bhattarai, T.; Sreerama, L. Efficient Methods of Pretreatment for the Release of Reducing Sugars from Lignocellulosic Biomass Native to Nepal and Characterization of Pretreated Lignocellulosic Biomass Efficient Methods of Pretreatment for the Release of Reducing Sugars from Lignocel. Int. J. Adv. Biotechnol. Res. 2018, 9, 9–23. [Google Scholar]
- Houba, V.J.G.; Temminghoff, E.J.M. Behavior of phosphate in soil extracts using weak unbuffered extracting solutions. Commun. Soil Sci. Plant Anal. 1999, 30, 1367–1370. [Google Scholar] [CrossRef]
- Minilu Woldemariam, D.; Singh Chandravanshi, B. Concentration levels of essential and non-essential elements in selected Ethiopian wines. Bull. Chem. Soc. Ethiop. 2011, 25, 65852. [Google Scholar] [CrossRef]
- Joshi, J.; Dhungana, P.; Prajapati, B.; Maharjan, R.; Poudyal, P.; Yadav, M.; Mainali, M.; Yadav, A.P.; Bhattarai, T.; Sreerama, L. Enhancement of Ethanol Production in Electrochemical Cell by Saccharomyces cerevisiae (CDBT2) and Wickerhamomyces anomalus (CDBT7). Front. Energy Res. 2019, 7, 70. [Google Scholar] [CrossRef]
- Wang, Q.; Kuninobu, M.; Ogawa, H.I.; Kato, Y. Degradation of volatile fatty acids in highly efficient anaerobic digestion. Biomass Bioenergy 1999, 16, 407–416. [Google Scholar] [CrossRef]
- Molina, A.; González, J.; Laborda, E.; Wang, Y.; Compton, R.G. Catalytic mechanism in cyclic voltammetry at disc electrodes: An analytical solution. Phys. Chem. Chem. Phys. 2011, 13, 14694. [Google Scholar] [CrossRef] [PubMed]
- Stieglmeier, M.; Wirth, R.; Kminek, G.; Moissl-Eichinger, C. Cultivation of Anaerobic and Facultatively Anaerobic Bacteria from Spacecraft-Associated Clean Rooms. Appl. Environ. Microbiol. 2009, 75, 3484–3491. [Google Scholar] [CrossRef]
- Natarajan, V.P.; Zhang, X.; Morono, Y.; Inagaki, F.; Wang, F. A Modified SDS-Based DNA Extraction Method for High Quality Environmental DNA from Seafloor Environments. Front. Microbiol. 2016, 7, 986. [Google Scholar] [CrossRef]
- Tomlinson, M.S.; De Carlo, E.H. The need for high resolution time series data to characterize hawaiian streams1. Jawra J. Am. Water Resour. Assoc. 2003, 39, 113–123. [Google Scholar] [CrossRef]
- Hoekstra, N.; Bosker, T.; Lantinga, E. Effects of cattle dung from farms with different feeding strategies on germination and initial root growth of cress (Lepidium sativum L.). Agric. Ecosyst. Environ. 2002, 93, 189–196. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Chen, S.; Quan, X. Enhanced production of methane from waste activated sludge by the combination of high-solid anaerobic digestion and microbial electrolysis cell with iron–graphite electrode. Chem. Eng. J. 2015, 259, 787–794. [Google Scholar] [CrossRef]
- Cai, W.; Liu, W.; Zhang, Z.; Feng, K.; Ren, G.; Pu, C.; Li, J.; Deng, Y.; Wang, A. Electro-driven methanogenic microbial community diversity and variability in the electron abundant niche. Sci. Total Environ. 2019, 661, 178–186. [Google Scholar] [CrossRef]
- Tian, G.; Yang, B.; Dong, M.; Zhu, R.; Yin, F.; Zhao, X.; Wang, Y.; Xiao, W.; Wang, Q.; Zhang, W.; et al. The effect of temperature on the microbial communities of peak biogas production in batch biogas reactors. Renew. Energy 2018, 123, 15–25. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Dong, R.; Li, X. A large cathode surface area promotes electromethanogenesis at a proper external voltage in a single coaxial microbial electrolysis cell. Sci. Total Environ. 2023, 868, 161721. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Yang, Y.; Sun, G.; Wu, D. Impact of applied voltage on methane generation and microbial activities in an anaerobic microbial electrolysis cell (MEC). Chem. Eng. J. 2016, 283, 260–265. [Google Scholar] [CrossRef]
- Rastegar, Z.; Ghaemi, A. CO2 absorption into potassium hydroxide aqueous solution: Experimental and modeling. Heat Mass Transf. 2021, 58, 365–381. [Google Scholar] [CrossRef]
- Zhao, W.; Su, X.; Zhang, Y.; Xia, D.; Hou, S.; Zhou, Y.; Fu, H.; Wang, L.; Yin, X. Microbial electrolysis enhanced bioconversion of coal to methane compared with anaerobic digestion: Insights into differences in metabolic pathways. Energy Convers. Manag. 2022, 259, 115553. [Google Scholar] [CrossRef]
- Bajracharya, S.; ter Heijne, A.; Dominguez Benetton, X.; Vanbroekhoven, K.; Buisman, C.J.; Strik, D.P.; Pant, D. Carbon dioxide reduction by mixed and pure cultures in microbial electrosynthesis using an assembly of graphite felt and stainless steel as a cathode. Bioresour. Technol. 2015, 195, 14–24. [Google Scholar] [CrossRef]
- Merkoçi, A.; Pumera, M.; Llopis, X.; Pérez, B.; del Valle, M.; Alegret, S. New materials for electrochemical sensing VI: Carbon nanotubes. TrAC Trends Anal. Chem. 2005, 24, 826–838. [Google Scholar] [CrossRef]
- Salvador, A.F.; Martins, G.; Melle-Franco, M.; Serpa, R.; Stams, A.J.; Cavaleiro, A.J.; Pereira, M.A.; Alves, M.M. Carbon nanotubes accelerate methane production in pure cultures of methanogens and in a syntrophic coculture. Environ. Microbiol. 2017, 19, 2727–2739. [Google Scholar] [CrossRef]
- Liu, J.; Liu, F.; Yu, J.; Wang, Q.; Li, Z.; Liu, K.; Xu, C.; Yu, H.; Xiao, L. Proteomics reveal biomethane production process induced by carbon nanotube. Environ. Res. 2021, 200, 111417. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Rahim, R.A.; Abdullah, N.; Wright, A.D.G.; Shirai, Y.; Sakai, K.; Sulaiman, A.; Hassan, M.A. Importance of the methanogenic archaea populations in anaerobic wastewater treatments. Process Biochem. 2010, 45, 1214–1225. [Google Scholar] [CrossRef]
- Wagner, R.C.; Regan, J.M.; Oh, S.E.; Zuo, Y.; Logan, B.E. Hydrogen and methane production from swine wastewater using microbial electrolysis cells. Water Res. 2009, 43, 1480–1488. [Google Scholar] [CrossRef]
- Zhang, F.; Pant, D.; Logan, B.E. Long-term performance of activated carbon air cathodes with different diffusion layer porosities in microbial fuel cells. Biosens. Bioelectron. 2011, 30, 49–55. [Google Scholar] [CrossRef]
- Bo, T.; Zhu, X.; Zhang, L.; Tao, Y.; He, X.; Li, D.; Yan, Z. A new upgraded biogas production process: Coupling microbial electrolysis cell and anaerobic digestion in single-chamber, barrel-shape stainless steel reactor. Electrochem. Commun. 2014, 45, 67–70. [Google Scholar] [CrossRef]
- Bao-Kang, J.; Jian-Rong, Z.; Zu-Xun, Z. Theory and application of cyclic voltammetry for measurement of fast electrode kinetics at microdisk electrode. Chin. J. Chem. 2010, 14, 338–347. [Google Scholar] [CrossRef]
- Harnisch, F.; Freguia, S. A Basic Tutorial on Cyclic Voltammetry for the Investigation of Electroactive Microbial Biofilms. Chem. Asian J. 2012, 7, 466–475. [Google Scholar] [CrossRef]
- Begrem, S.; Jérôme, M.; Leroi, F.; Delbarre-Ladrat, C.; Grovel, O.; Passerini, D. Genomic diversity of Serratia proteamaculans and Serratia liquefaciens predominant in seafood products and spoilage potential analyses. Int. J. Food Microbiol. 2021, 354, 109326. [Google Scholar] [CrossRef]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- An, X.; Zong, Z.; Zhang, Q.; Li, Z.; Zhong, M.; Long, H.; Cai, C.; Tan, X. Novel thermo-alkali-stable cellulase-producing Serratia sp. AXJ-M cooperates with Arthrobacter sp. AXJ-M1 to improve degradation of cellulose in papermaking black liquor. J. Hazard. Mater. 2022, 421, 126811. [Google Scholar] [CrossRef]
- Sikora, A.; Detman, A.; Chojnacka, A.; Blaszczyk, M.K. Anaerobic Digestion: I. A Common Process Ensuring Energy Flow and the Circulation of Matter in Ecosystems. II. A Tool for the Production of Gaseous Biofuels. In Fermentation Processes; InTech: Rijeka, Croatia, 2017; Chapter 14; pp. 271–392. [Google Scholar] [CrossRef]





| Analytical Parameters of | Concentration |
|---|---|
| Total Soluble Protein | 116.25 ± 4.34 mg/L |
| COD | 438.75 ± 15.11 mg/L |
| Total phosphorus | 0.015 ± 0.007 mg/L |
| Reducing sugar | 0.515 ± 0.033 mg/L |
| Potassium | 0.015 ± 0.002 mg/L |
| Arsenic | 1.3 × 10−7 mg/L |
| pH | 7.35 ± 0.15 |
| TSS | 19.70 ± 1.5% |
| VSS | 10.50 ± 0.5% |
| Moisture content | 66.78 ± 2.5% |
| Sl. # | Type of Apparatus | Anaerobic State | Sampling Possibility | Volume Extension (Up to 1000 mL) | Application of Electrodes |
|---|---|---|---|---|---|
| 1 | Reagent Bottle | + | _ | _ | _ |
| 2 | Saline Bottle | + | _ | _ | _ |
| 3 | H-shaped two-chamber reactors | + | + | _ | + |
| 4 | Aspirator Bottle | + | + | + | + |
| S. N. | Temperature and Exptl Setup | Voltage Input | Electrode Used | Biogas Production, cm3 |
|---|---|---|---|---|
| 1 | 37 °C; Control | 0 V | N/A | 5 |
| 2 | 37 °C; MEC | 1 V | Graphite electrodes | 8 |
| 3 | 2 V | Graphite electrodes | 25 | |
| 4 | 3 V | Graphite electrodes | 2.2 | |
| 5 | 4 V | Graphite electrodes | 2 | |
| 6 | 28 °C; Control | 0 V | N/A | 0.45 |
| 7 | 28 °C; MEC | 2 V | Graphite electrodes | 19 |
| 8 | 18 °C; Control | 0 V | N/A | 0.2 |
| 9 | 18 °C; MEC | 2 V | Graphite electrodes | 13 |
| 10 | 18 °C; MEC | 2 V | MWCNT coated anode | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatt, P.; Poudyal, P.; Dhungana, P.; Prajapati, B.; Bajracharya, S.; Yadav, A.P.; Bhattarai, T.; Sreerama, L.; Joshi, J. Enhancement of Biogas (Methane) Production from Cow Dung Using a Microbial Electrochemical Cell and Molecular Characterization of Isolated Methanogenic Bacteria. Biomass 2024, 4, 455-471. https://doi.org/10.3390/biomass4020023
Bhatt P, Poudyal P, Dhungana P, Prajapati B, Bajracharya S, Yadav AP, Bhattarai T, Sreerama L, Joshi J. Enhancement of Biogas (Methane) Production from Cow Dung Using a Microbial Electrochemical Cell and Molecular Characterization of Isolated Methanogenic Bacteria. Biomass. 2024; 4(2):455-471. https://doi.org/10.3390/biomass4020023
Chicago/Turabian StyleBhatt, Puja, Pranita Poudyal, Pradip Dhungana, Bikram Prajapati, Suman Bajracharya, Amar Prasad Yadav, Tribikram Bhattarai, Lakshmaiah Sreerama, and Jarina Joshi. 2024. "Enhancement of Biogas (Methane) Production from Cow Dung Using a Microbial Electrochemical Cell and Molecular Characterization of Isolated Methanogenic Bacteria" Biomass 4, no. 2: 455-471. https://doi.org/10.3390/biomass4020023
APA StyleBhatt, P., Poudyal, P., Dhungana, P., Prajapati, B., Bajracharya, S., Yadav, A. P., Bhattarai, T., Sreerama, L., & Joshi, J. (2024). Enhancement of Biogas (Methane) Production from Cow Dung Using a Microbial Electrochemical Cell and Molecular Characterization of Isolated Methanogenic Bacteria. Biomass, 4(2), 455-471. https://doi.org/10.3390/biomass4020023






