Modeling of an Innovative Nitrogen-Free Cryotherapy Device
Abstract
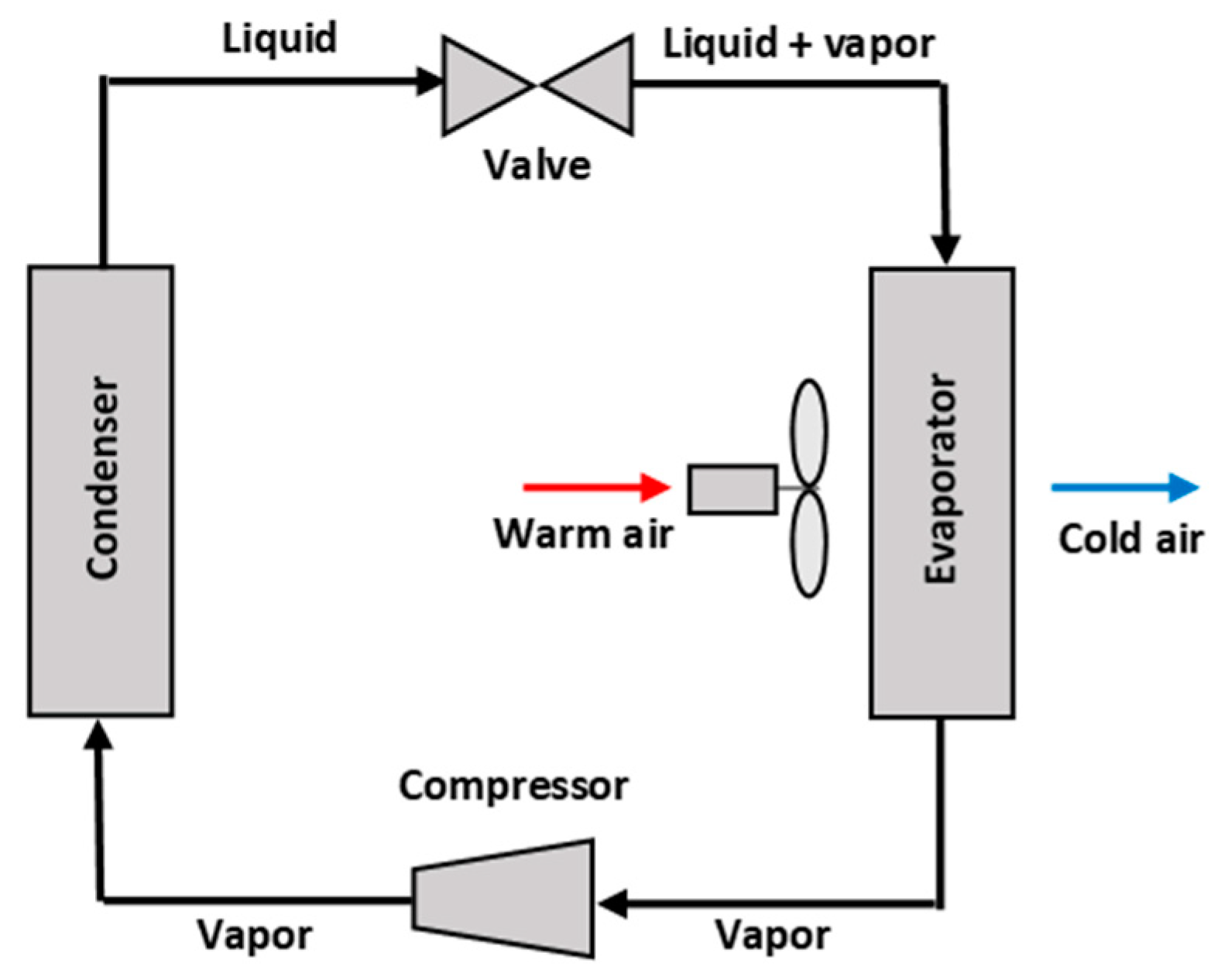
:1. Introduction
2. Materials and Methods
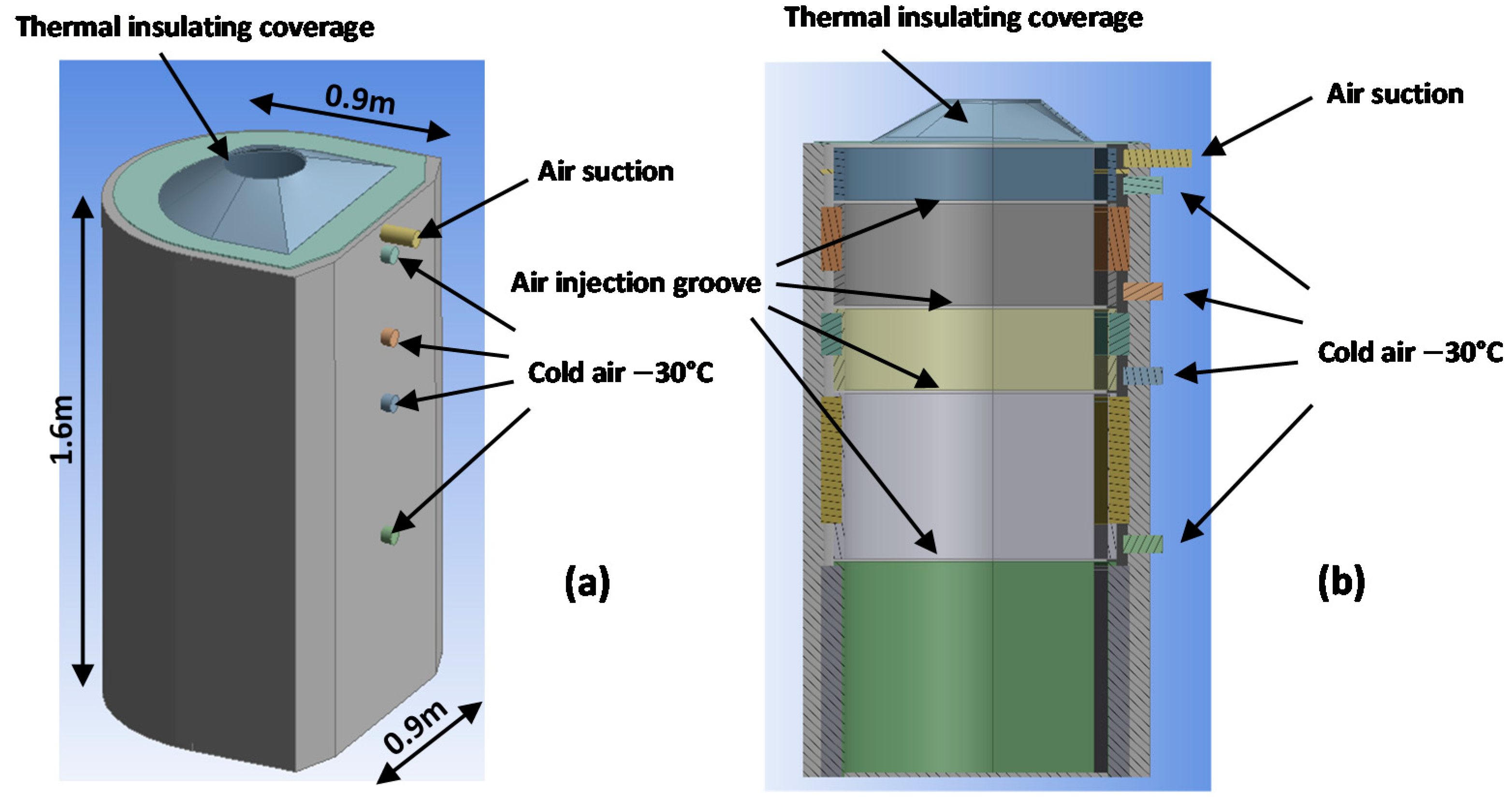
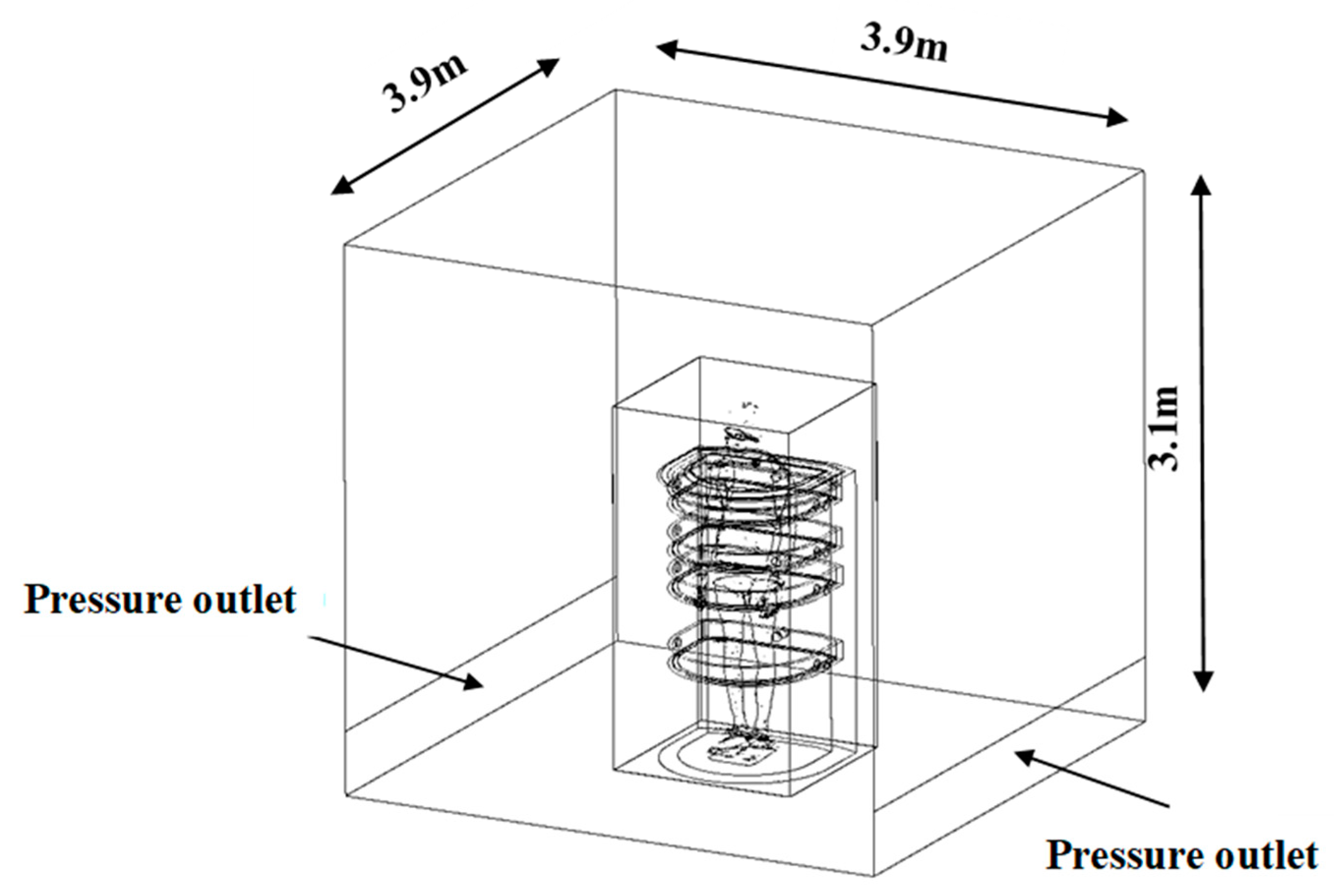
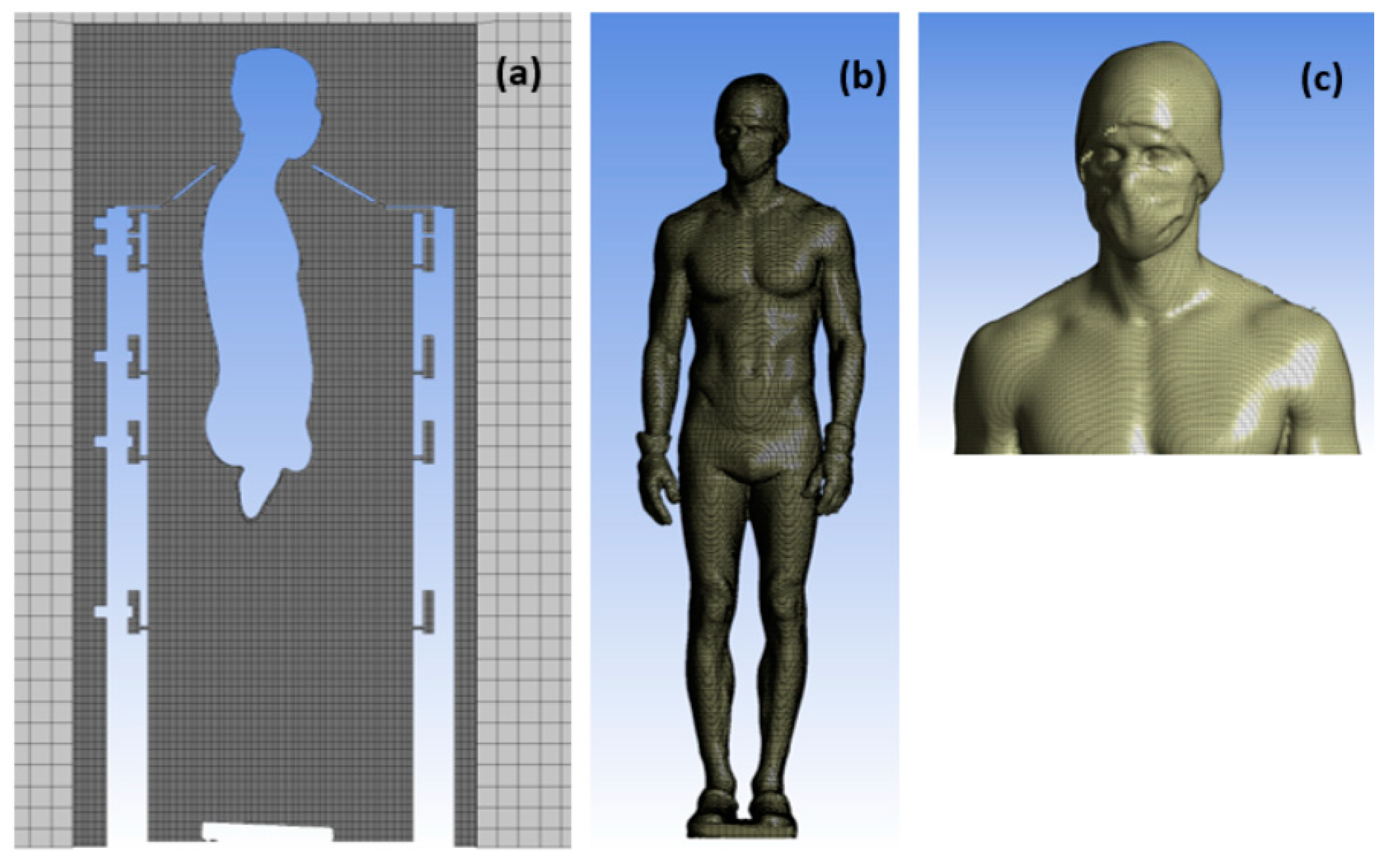
2.1. Geometry of the PBC Cabin
2.2. Mesh
2.3. Boundary Conditions
2.4. Numerical Setup
3. Results
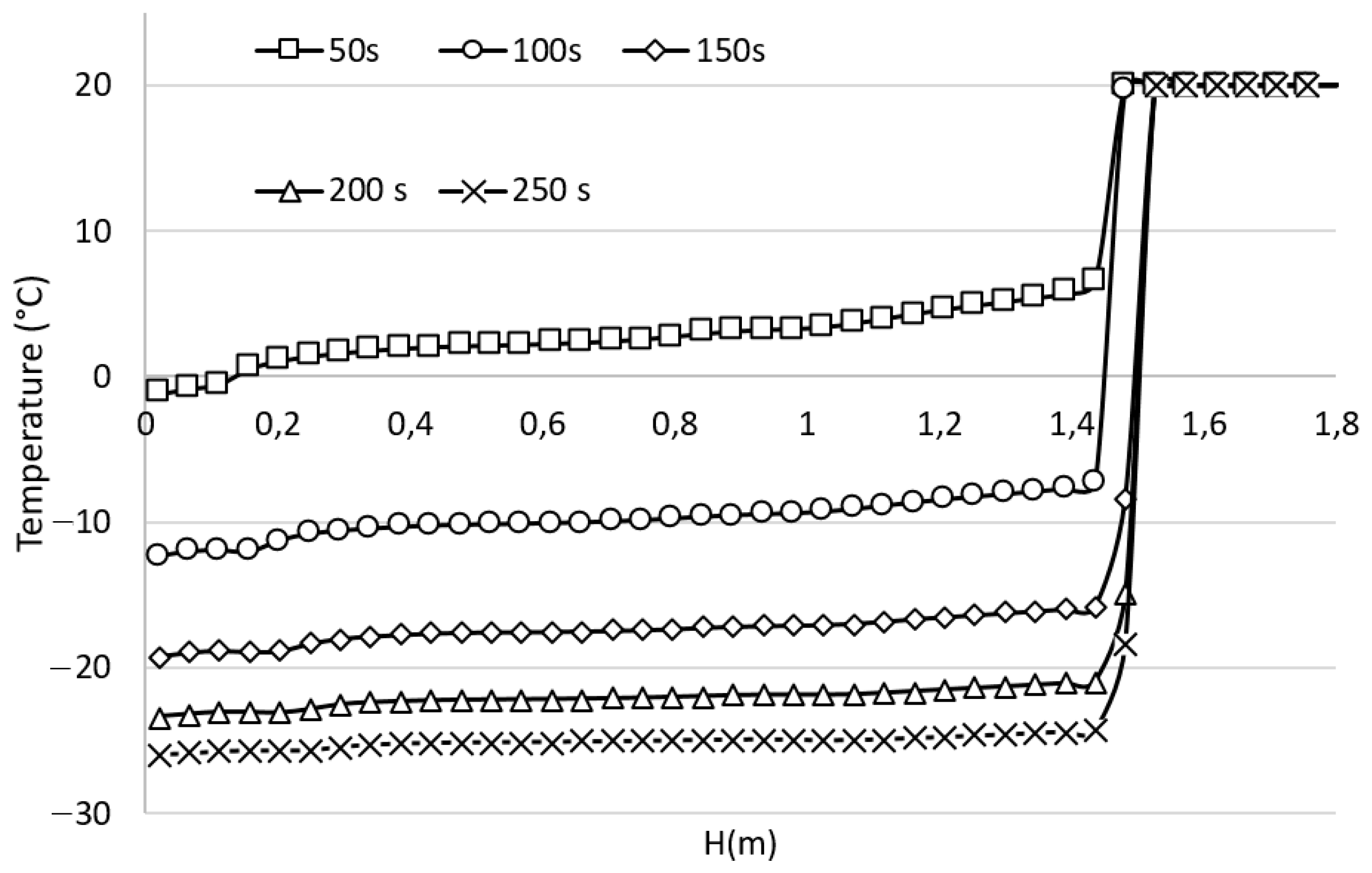
3.1. Time Evolution of the Temperature Inside the Cabin, Pre-Cooling Phase
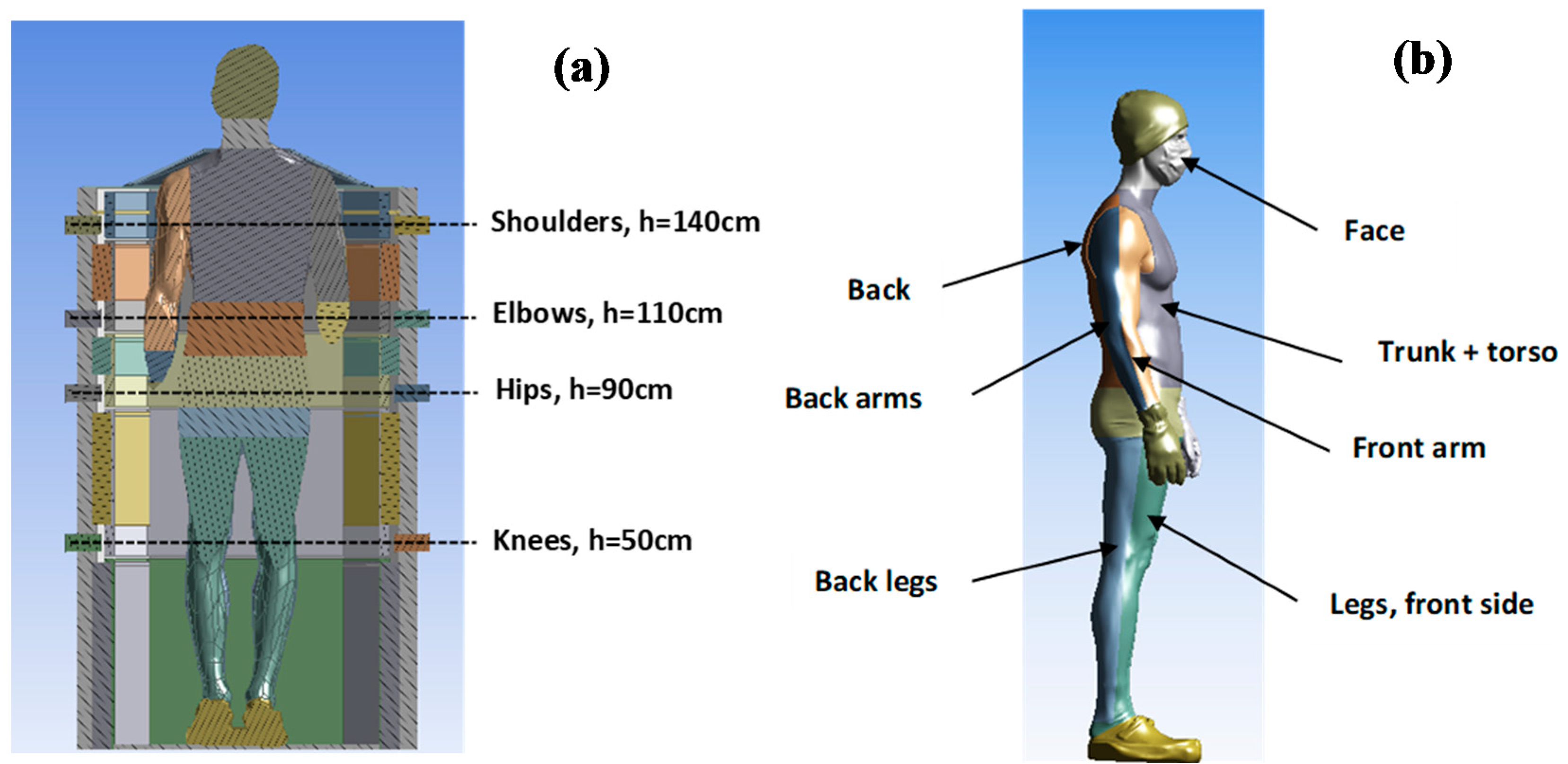
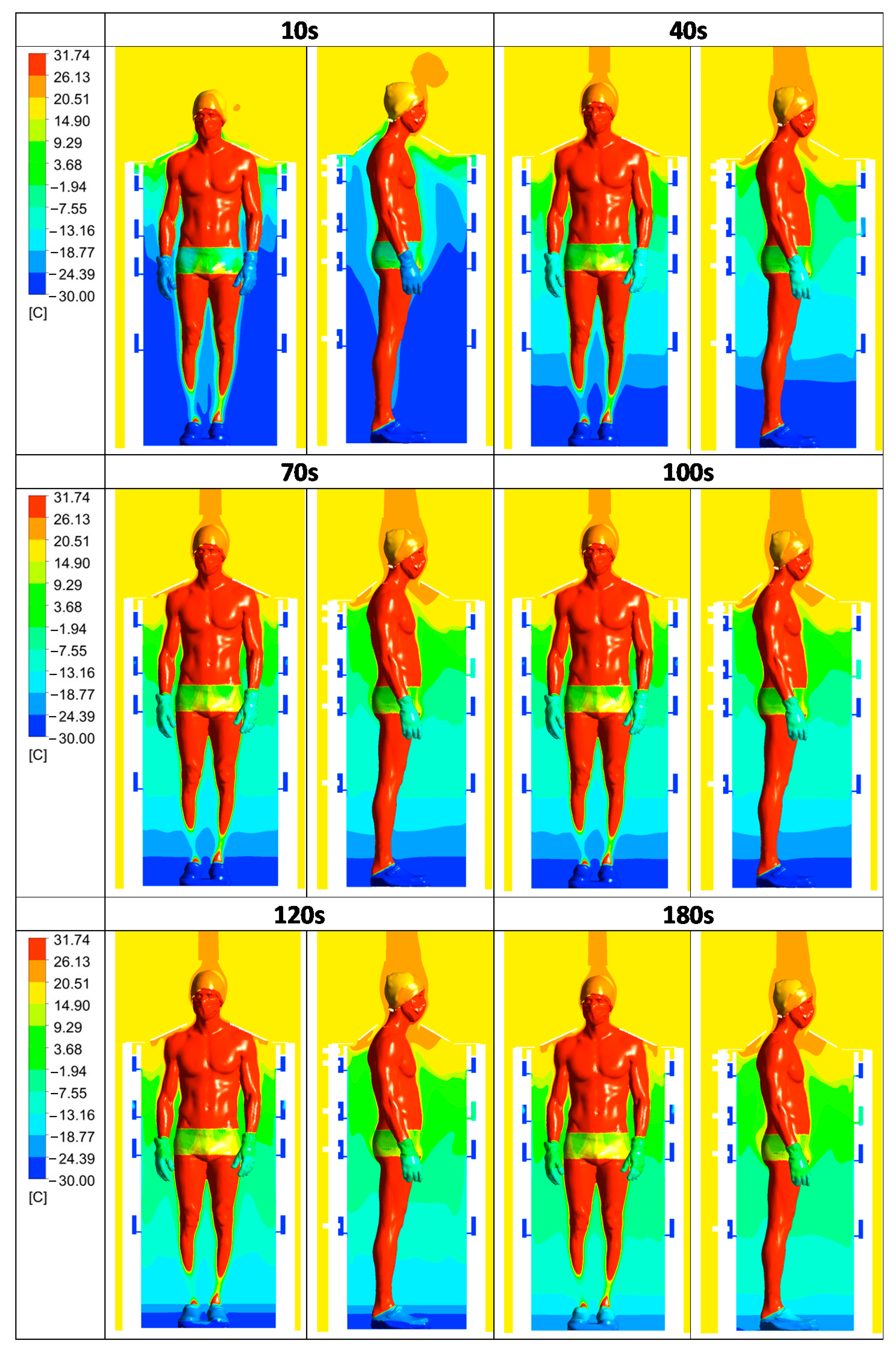
3.2. Convective Heat Transfer between the Human Body and Its Environment
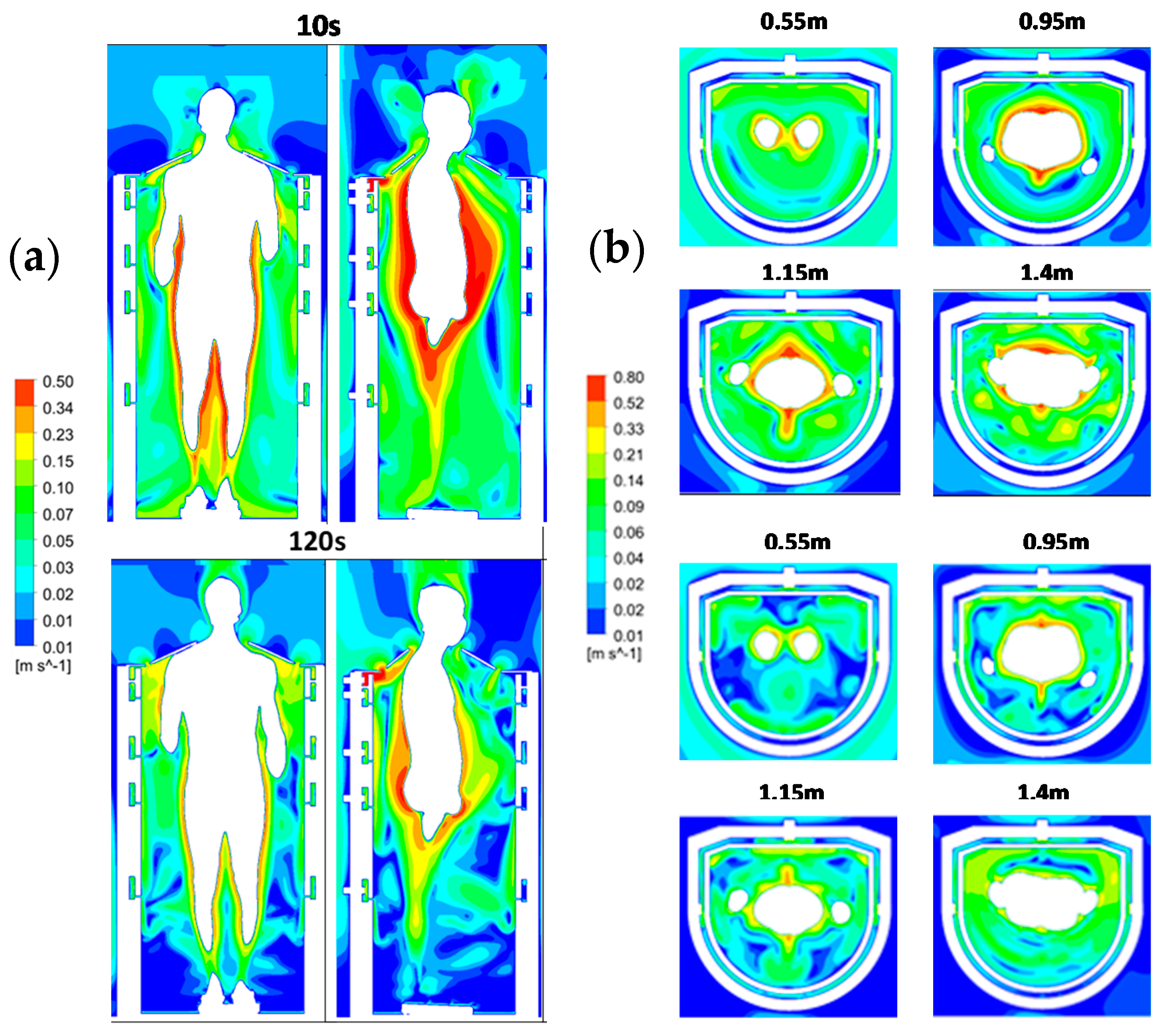
3.3. Time Evolution of the Air Velocity Inside the Cabin during a Cryotherapy Session
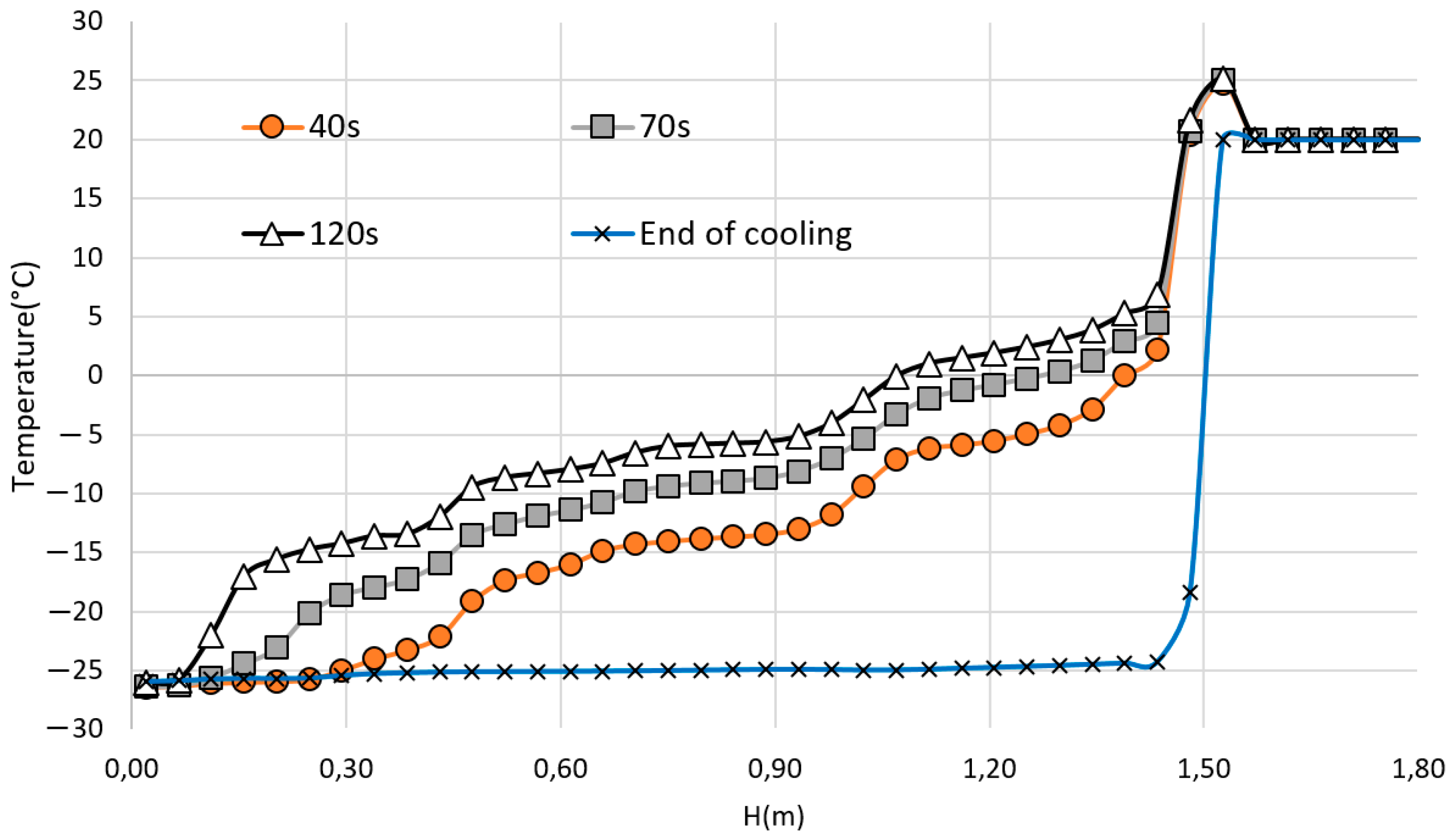
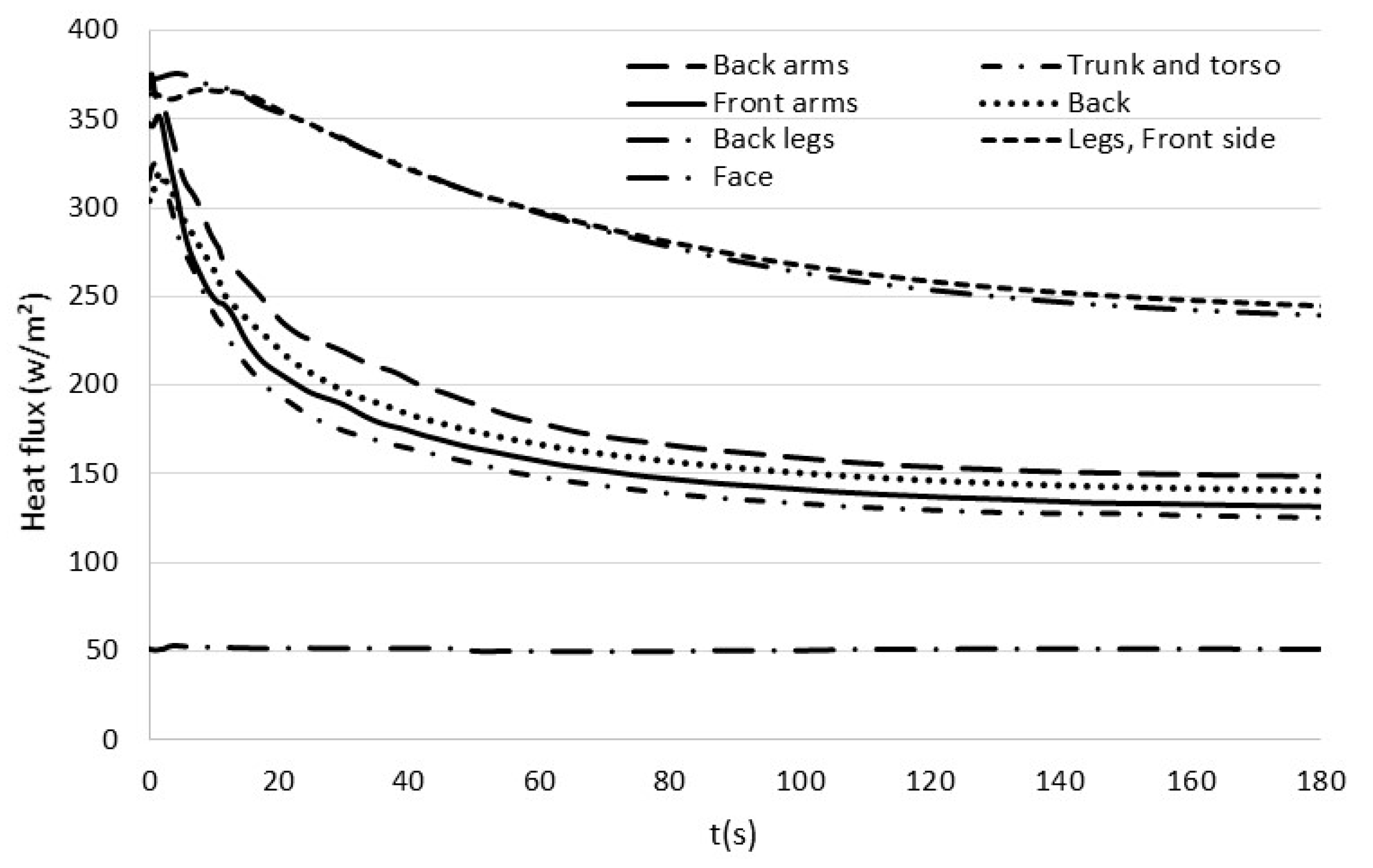
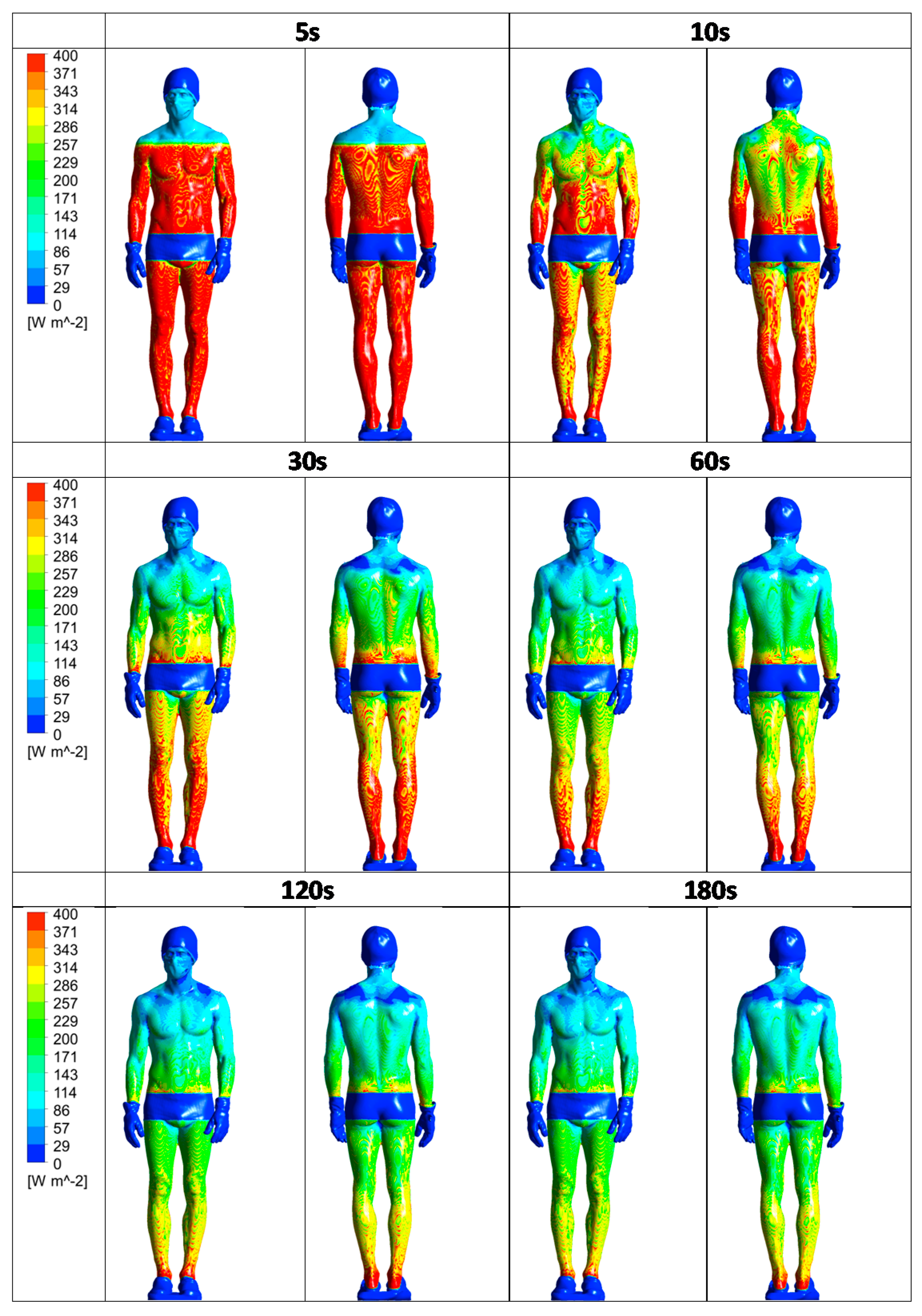
3.4. Time Evolution of the Heat Flux on the Body Surface
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polidori, G.; Taiar, R.; Legrand, F.; Beaumont, F.; Murer, S.; Bogard, F.; Boyer, F.C. Infrared thermography for assessing skin temperature differences between Partial Body Cryotherapy and Whole Body Cryotherapy devices at −140 °C. Infrared Phys. Technol. 2018, 93, 158–161. [Google Scholar] [CrossRef]
- Hausswirth, C.; Schaal, K.; Le Meur, Y.; Bieuzen, F.; Filliard, J.-R.; Volondat, M.; Louis, J. Parasympathetic activity and blood catecholamine responses following a single partial-body cryostimulation and a whole-body cryostimulation. PLoS ONE 2013, 8, e72658. [Google Scholar] [CrossRef] [Green Version]
- Banfi, G.; Melegati, G.; Barassi, A.; Dogliotti, G.; Melzi d’Eril, G.; Dugué, B.; Corsi, M.M. Effects of whole-body cryotherapy on serum mediators of inflammation and serum muscle enzymes in athletes. J. Therm. Biol. 2009, 34, 55–59. [Google Scholar] [CrossRef]
- Straburzyńska-Lupa, A.; Kasprzak, M.P.; Romanowski, M.W.; Kwaśniewska, A.; Romanowski, W.; Iskra, M.; Rutkowski, R. The Effect of Whole-Body Cryotherapy at Different Temperatures on Proinflammatory Cytokines, Oxidative Stress Parameters, and Disease Activity in Patients with Ankylosing Spondylitis. Oxid. Med. Cell. Longev. 2018, 2018, 2157496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Algafly, A.A.; George, K.P. The effect of cryotherapy on nerve conduction velocity, pain threshold and pain tolerance. Br. J. Sports Med. 2007, 41, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Rymaszewska, J.; Ramsey, D.; Chładzińska-Kiejna, S. Whole-body cryotherapy as adjunct treatment of depressive and anxiety disorders. Arch. Immunol. Ther. Exp. 2008, 56, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Vitenet, M.; Tubez, F.; Marreiro, A.; Polidori, G.; Taiar, R.; Legrand, F.; Boyer, F.C. Effect of whole body cryotherapy interventions on health-related quality of life in fibromyalgia patients: A randomized controlled trial. Complement. Ther. Med. 2018, 36, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Bouzigon, R.; Grappe, F.; Ravier, G.; Dugue, B. Whole- and partial-body cryostimulation/cryotherapy: Current technologies and practical applications. J. Therm. Biol. 2016, 61, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Huizenga, C.; Zhang, H.; Arens, E.; Wang, D. Skin and core temperature response to partial-and whole-body heating and cooling. J. Therm. Biol. 2004, 29, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Stocks, J.M.; Taylor, N.A.S.; Tipton, M.J.; Greenleaf, J.E. Human physiological responses to cold exposure. Aviat. Space Environ. Med. 2004, 75, 444–457. [Google Scholar]
- Castellani, J.W.; Young, A.J. Human physiological responses to cold exposure: Acute responses and acclimatization to prolonged exposure. Auton. Neurosci. Thermoregul. 2016, 196, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Cholewka, A.; Stanek, A.; Sieroń, A.; Drzazga, Z. Thermography study of skin response due to whole-body cryotherapy. Skin Res. Technol. 2012, 18, 180–187. [Google Scholar] [CrossRef]
- Fonda, B.; De Nardi, M.; Sarabon, N. Effects of whole-body cryotherapy duration on thermal and cardio-vascular response. J. Therm. Biol. 2014, 42, 52–55. [Google Scholar] [CrossRef]
- Costello, J.T.; McInerney, C.D.; Bleakley, C.M.; Selfe, J.; Donnelly, A.E. The use of thermal imaging in assessing skin temperature following cryotherapy: A review. J. Therm. Biol. 2012, 37, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Bleakley, C.; Hopkins, J. Is it possible to achieve optimal levels of tissue cooling in cryotherapy? Phys. Ther. Rev. 2010, 15, 344–350. [Google Scholar] [CrossRef]
- Polidori, G.; Elfahem, R.; Abbes, B.; Bogard, F.; Legrand, F.; Bouchet, B.; Beaumont, F. Preliminary study on the effect of sex on skin cooling response during whole body cryostimulation (−110 °C): Modeling and prediction of exposure durations. Cryobiology 2020, 97, 12–19. [Google Scholar] [CrossRef]
- Osczevski, R.; Bluestein, M. The new wind chill equivalent temperature chart. Bull. Am. Meteorol. Soc. 2005, 86, 1453–1458. [Google Scholar] [CrossRef] [Green Version]
- Bouzigon, R.; Arfaoui, A.; Grappe, F.; Ravier, G.; Jarlot, B.; Dugue, B. Validation of a new whole-body cryotherapy chamber based on forced convection. J. Therm. Biol. 2017, 65, 138–144. [Google Scholar] [CrossRef]
- Chorowski, M.; Piotrowska, A.; Sieron, A.; Stanek, A. Safety Aspects of Cryochamber Operation. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2013; Volume 1573. [Google Scholar] [CrossRef]
- Agnieszka, P. Safety aspects of whole-body cryochamber and cryosauna operation. IOP Conf. Ser. Mater. Sci. Eng. 2017, 278, 012125. [Google Scholar] [CrossRef]
- Dugue, B.; Bernard, J.; Bouzigon, R.; Nardi, M.; Wafa, D.; Junior, J.; Guilpart, J.; Lombardi, G.; Miller, E.; Tiemessen, I. Note D’information-Institut International du Froid-Cryothérapie ou Cryostimulation à Corps Entier; International Institute of Refrigeration: Paris, France, 2020. [Google Scholar]
- Bernard, J.P.; Dallais, A. Safe and Efficient Use of Liquid Nitrogen in Cryotherapy Applications. In Proceedings of the 3rd IIR Conference on Cold Applications in Life Sciences-Cryotherapy and Cryopreservation, Saint Petersbourg, Russia, 12–14 September 2018. [Google Scholar]
- Greenwald, E.; Christman, M.; Penn, L.; Brinster, N.; Liebman, T.N. Cold panniculitis: Adverse cutaneous effect of whole-body cryotherapy. JAAD Case Rep. 2018, 4, 344–345. [Google Scholar] [CrossRef] [Green Version]
- Gużda, A.; Szmolke, N. Compressors in Heat Pumps. Mach. Dyn. Res. 2015, 39, 71–83. [Google Scholar]
- Baranov, A.; Pakhomov, O.; Fedorov, A.; Ivanov, V.; Zaitsev, A.; Polyakov, R. Technique and Technology of Whole-Body Cryotherapy (WBC). Low-Temp. Technol. 2019, 30, 1–25. [Google Scholar] [CrossRef] [Green Version]
- De Nardi, M.; Silvani, S.; Facheris, C.; Pagnoncelli, M.; Bisio, A.; Faelli, E.; La Torre, A.; Ruggeri, P.; Codella, R. Effectiveness and safety of a thermal insulating coverage on the top of the cryo-cabin during a partial-body cryostimulation. J. Therm. Biol. 2021, 97, 102901. [Google Scholar] [CrossRef]
- Savic, M.; Fonda, B.; Sarabon, N. Actual temperature during and thermal response after whole-body cryotherapy in cryo-cabin. J. Therm. Biol. 2013, 38, 186–191. [Google Scholar] [CrossRef]
- Schlichting, H.; Gersten, K. Boundary-Layer Theory; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Liu, Y.; Liu, Z.; Luo, J. Numerical Investigation of the Unsteady Thermal Plume around Human Body in Closed Space. Procedia Eng. 2015, 131, 1919–1926. [Google Scholar] [CrossRef] [Green Version]
| Mass Flow Rate | Temperature | |
|---|---|---|
| Air injection nozzles | 0.0013 kg/s | −30 °C |
| Suction nozzle | 0.016 kg/s | |
| Pression | ||
| Pressure outlet room | 1 ATM | 20 °C |
| Surface heat flux | ||
| Cabin walls + room | = 0 W/m² | |
| Caps + gloves + clogs | = 0 W/m² | |
| Température | ||
| Back legs | 31.16 °C | |
| Arms on the back side | 31.98 °C | |
| Trunk + torso | 31.53 °C | |
| Back | 29.74 °C | |
| Arms on the front side | 31.66 °C | |
| Legs on the front side | 30.55 °C | |
| Face | 31.53 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaumont, F.; Bogard, F.; Hakim, H.; Murer, S.; Bouchet, B.; Polidori, G. Modeling of an Innovative Nitrogen-Free Cryotherapy Device. Dynamics 2021, 1, 204-216. https://doi.org/10.3390/dynamics1020013
Beaumont F, Bogard F, Hakim H, Murer S, Bouchet B, Polidori G. Modeling of an Innovative Nitrogen-Free Cryotherapy Device. Dynamics. 2021; 1(2):204-216. https://doi.org/10.3390/dynamics1020013
Chicago/Turabian StyleBeaumont, Fabien, Fabien Bogard, Hassen Hakim, Sébastien Murer, Bastien Bouchet, and Guillaume Polidori. 2021. "Modeling of an Innovative Nitrogen-Free Cryotherapy Device" Dynamics 1, no. 2: 204-216. https://doi.org/10.3390/dynamics1020013
APA StyleBeaumont, F., Bogard, F., Hakim, H., Murer, S., Bouchet, B., & Polidori, G. (2021). Modeling of an Innovative Nitrogen-Free Cryotherapy Device. Dynamics, 1(2), 204-216. https://doi.org/10.3390/dynamics1020013







