The Use of Methylphenidate to Improve Executive Functioning in Pediatric Traumatic Brain Injury: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Searching
- Population: pediatric population (aged below 18 years of age) with a history of TBI.
- Intervention: MPH.
- Comparator: placebo or standard medical care.
- Primary outcome: attention.
- Secondary outcomes: other aspects of cognitive function, behaviour, and adverse events.
2.2. Search Terms
2.3. Inclusion and Exclusion Criteria
- An initial title and abstract screening performed by two independent reviewers.
- A full-text review.
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
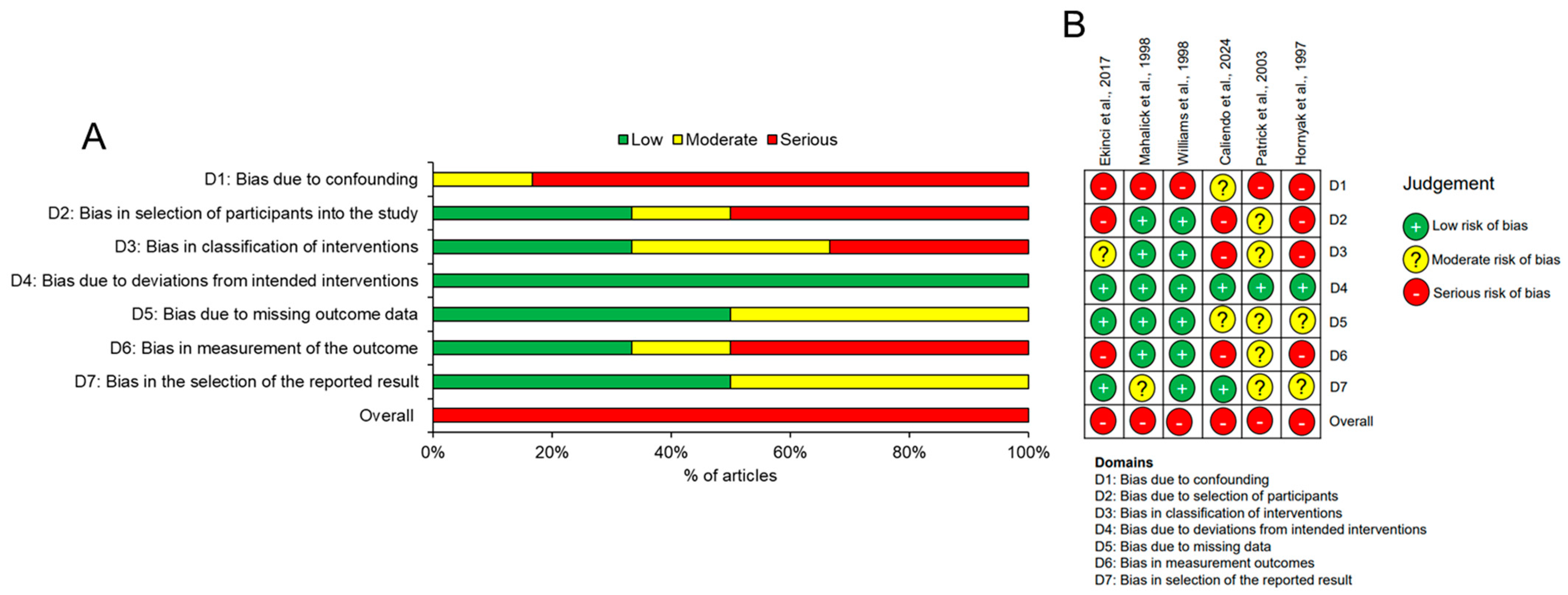
3.3. Risk-of-Bias Assessment
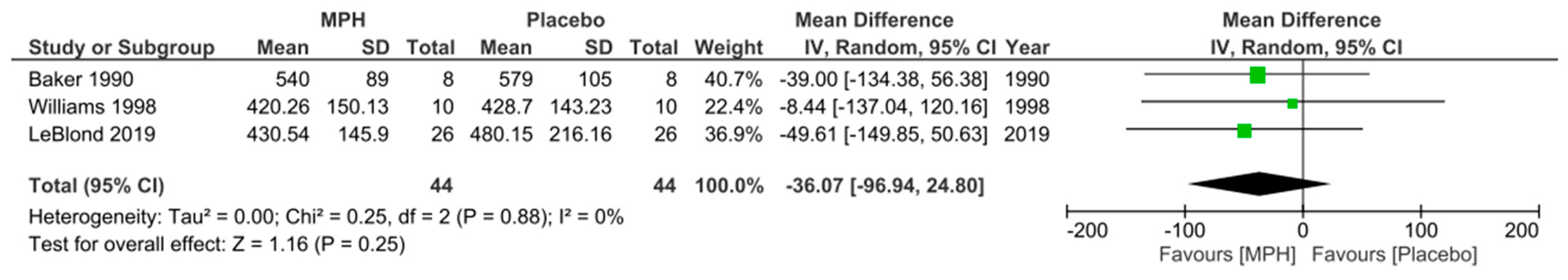
3.4. Primary Outcome
3.5. Secondary Outcomes
4. Discussion
Limitations of Individual Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forsyth, R.; Kirkham, F. Predicting outcome after childhood brain injury. Can. Med. Assoc. J. 2012, 184, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Bruns, N.; Trocchi, P.; Felderhoff-Müser, U.; Dohna-Schwake, C.; Stang, A. Hospitalization and morbidity rates after pediatric traumatic brain injury: A nation-wide population-based analysis. Front. Pediatr. 2021, 9, 747743. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.C.; Mummareddy, N.; Wellons, J.C.; Bonfield, C.M. Epidemiology of global pediatric traumatic brain injury: Qualitative review. World Neurosurg. 2016, 91, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I.; Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Stierwalt, J.A.G.; Murray, L.L. Attention impairment following traumatic brain injury. Semin. Speech Lang. 2002, 23, 129–138. [Google Scholar] [CrossRef]
- Babikian, T.; Asarnow, R. Neurocognitive outcomes and recovery after pediatric TBI: Meta-analytic review of the literature. Neuropsychology 2009, 23, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Narad, M.E.; Riemersma, J.; Wade, S.L.; Smith-Paine, J.; Morrison, P.; Taylor, H.G.; Yeates, K.O.; Kurowski, B.G. Impact of secondary ADHD on long-term outcomes after early childhood traumatic brain injury. J. Head Trauma Rehabil. 2020, 35, E271–E279. [Google Scholar] [CrossRef] [PubMed]
- Stojanovski, S.; Scratch, S.E.; Dunkley, B.T.; Schachar, R.; Wheeler, A.L. A systematic scoping review of new attention problems following traumatic brain injury in children. Front. Neurol. 2021, 12, 751736. [Google Scholar] [CrossRef] [PubMed]
- Narad, M.E.; Kennelly, M.; Zhang, N.; Wade, S.L.; Yeates, K.O.; Taylor, H.G.; Epstein, J.N.; Kurowski, B.G. Secondary attention-deficit/hyperactivity disorder in children and adolescents 5 to 10 years after traumatic brain injury. JAMA Pediatr. 2018, 172, 437–443. [Google Scholar] [CrossRef]
- Nazarova, V.A.; Sokolov, A.V.; Chubarev, V.N.; Tarasov, V.V.; Schiöth, H.B. Treatment of ADHD: Drugs, psychological therapies, devices, complementary and alternative methods as well as the trends in clinical trials. Front. Pharmacol. 2022, 13, 1066988. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.J.; Chien, Y.C.; Liu, C.T.; Wu, H.C.; Chang, C.Y.; Wu, M.Y. Effects of methylphenidate on cognitive function in adults with traumatic brain injury: A meta-analysis. Brain Sci. 2019, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Hagan, A.J.; Verity, S.J. The influence of methylphenidate on sustained attention in paediatric acquired brain injury: A meta-analytical review. Child Neuropsychol. 2022, 29, 710–741. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Searching for and selecting studies. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.4; Cochrane: London, UK, 2023. [Google Scholar]
- Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H. Optimal database combinations for literature searches in systematic reviews: A prospective exploratory study. Syst. Rev. 2017, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- British National Formulary. Available online: https://bnf.nice.org.uk/drugs/methylphenidate-hydrochloride/ (accessed on 29 July 2024).
- Covidence Systematic Review Software. Available online: https://www.covidence.org/ (accessed on 29 July 2024).
- The Oxford Levels of Evidence 2. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence/ (accessed on 29 July 2024).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Br. Med. J. 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. Br. Med. J. 2016, 355, i4919. [Google Scholar] [CrossRef]
- Review Manager (RevMan), Version 4.0. The Cochrane Collaboration. Available online: https://revman.cochrane.org (accessed on 10 August 2024).
- LeBlond, E.; Smith-Paine, J.; Riemersma, J.J.; Horn, P.S.; Wade, S.L.; Kurowski, B.G. Influence of methylphenidate on long-term neuropsychological and everyday executive functioning after traumatic brain injury in children with secondary attention problems. J. Int. Neuropsychol. Soc. 2019, 25, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Kurowski, B.G.; Epstein, J.N.; Pruitt, D.W.; Horn, P.S.; Altaye, M.; Wade, S.L. Benefits of methylphenidate for long-term attention problems after traumatic brain injury in childhood: A randomized, double-masked, placebo-controlled, dose-titration, crossover trial. J. Head Trauma Rehabil. 2019, 34, E1–E12. [Google Scholar] [CrossRef] [PubMed]
- Nikles, C.J.; McKinlay, L.; Mitchell, G.K.; Carmont, S.A.; Senior, H.E.; Waugh, M.C.; Epps, A.; Schluter, P.J.; Lloyd, O.T. Aggregated n-of-1 trials of central nervous system stimulants versus placebo for paediatric traumatic brain injury—A pilot study. Trials 2014, 15, 54. [Google Scholar] [CrossRef]
- Baker, B.K. The Effect of Methylphenidate on the Attending Behavior of Children with Closed Head Injuries. Ph.D. Thesis, The University of Utah, Salt Lake City, UT, USA, 1990. [Google Scholar]
- Clark, E.; Baker, B.K.; Gardner, M.K.; Pompa, J.L.; Tait, F.V. Effectiveness of stimulant drug treatment for attention problems: A look at head-injured children. Sch. Psychol. Int. 1990, 11, 227–234. [Google Scholar] [CrossRef]
- Ekinci, O.; Direk, M.; Gunes, S.; Teke, H.; Ekinci, N.; Yıldırım, F.; Okyuaz, C. Short-term efficacy and tolerability of methylphenidate in children with traumatic brain injury and attention problems. Brain Dev. 2017, 39, 327–336. [Google Scholar] [CrossRef]
- Mahalick, D.M.; Carmel, P.W.; Greenberg, J.P.; Molofsky, W.; Brown, J.A.; Heary, R.F.; Marks, D.; Zampella, E.; Hodosh, R.; von der Schmidt, E., 3rd. Psychopharmacologic treatment of acquired attention disorders in children with brain injury. Pediatr. Neurosurg. 1998, 29, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.E.; Ris, M.D.; Ayyangar, R.; Schefft, B.K.; Berch, D. Recovery in pediatric brain injury: Is psychostimulant medication beneficial? J. Head Trauma Rehabil. 1998, 13, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, E.; Lowder, R.; McLaughlin, M.J.; Watson, W.D.; Baum, K.T.; Blackwell, L.S.; Koterba, C.H.; Hoskinson, K.R.; Tlustos, S.J.; Shah, S.A.; et al. The use of methylphenidate during inpatient rehabilitation after pediatric traumatic brain injury: Population characteristics and prescribing patterns. J. Head Trauma Rehabil. 2024, 39, E122–E131. [Google Scholar] [CrossRef] [PubMed]
- Patrick, P.D.; Buck, M.L.; Conaway, M.R.; Blackman, J.A. The use of dopamine enhancing medications with children in low response states following brain injury. Brain Inj. 2003, 17, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Hornyak, J.E.; Nelson, V.S.; Hurvitz, E.A. The use of methylphenidate in paediatric traumatic brain injury. Pediatr. Rehabil. 1997, 1, 15–17. [Google Scholar] [CrossRef]
- Mena, J.H.; Sanchez, A.I.; Rubiano, A.M.; Peitzman, A.B.; Sperry, J.L.; Gutierrez, M.I.; Puyana, J.C. Effect of the modified Glasgow Coma Scale score criteria for mild traumatic brain injury on mortality prediction: Comparing classic and modified Glasgow Coma Scale score model scores of 13. J. Trauma Acute Care Surg. 2011, 71, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Roebuck, H.; Freigang, C.; Barry, J.G. Continuous Performance Tasks: Not just about sustaining attention. J. Speech Lang. Hear. Res. 2016, 59, 501–510. [Google Scholar] [CrossRef]
- Kumar, A.; Loane, D.J. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav. Immun. 2012, 26, 1191–1201. [Google Scholar] [CrossRef]
- Faraone, S.V. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci. Biobehav. Rev. 2018, 87, 255–270. [Google Scholar] [CrossRef]
- Peattie, A.R.D.; Manktelow, A.E.; Sahakian, B.J.; Menon, D.K.; Stamatakis, E.A. Methylphenidate ameliorates behavioural and neurobiological deficits in executive function for patients with chronic traumatic brain injury. J. Clin. Med. 2024, 13, 771. [Google Scholar] [CrossRef]
- Gualtieri, C.T.; Evans, R.W. Stimulant treatment for the neurobehavioural sequelae of traumatic brain injury. Brain Inj. 1988, 2, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.Y.; In, J. Considerations for crossover design in clinical study. Korean J. Anesthesiol. 2021, 74, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Hyun, S.E.; Oh, B.M. Rehabilitation for impaired attention in the acute and post-acute phase after traumatic brain injury: A narrative review. Korean J. Neurotrauma 2022, 19, 20–31. [Google Scholar] [CrossRef] [PubMed]


| Population | Intervention | Injury Type |
|---|---|---|
| p?ediatric * child * Child/ infan * Infant/ adolescen * Adolescent/ | Methylphenidate/ Concerta Ritalin Delmosart Equasym Medikinet | traumatic brain injury brain injury head injury head trauma concussion Brain injuries, traumatic/ Craniocerebral trauma/ |
| Inclusion Criteria | Exclusion Criteria | Justification |
|---|---|---|
| Including pediatric population aged 0–18 years | Only adult population | The focus of this review was to look at the efficacy in a pediatric population |
| History of traumatic brain injury | Other forms of brain injury | The focus of this review was the efficacy in a traumatic injury population |
| Studies in humans | Studies in animals | Scoping searches revealed that studies in humans were numerous enough to analyse and compare |
| Methylphenidate as intervention | Medications other than methylphenidate, including those in the same drug class | The focus of the review is to compare the efficacy of methylphenidate to placebo and standard care |
| Study designs meeting OCEBM levels one, two, or three, excluding reviews | Study designs meeting OCEBM level four or five and reviews | Low evidence levels would reduce the overall quality of the systematic review |
| English language translation available | No English language translation available | Time and resource constraints limited translation abilities |
| Full-text access available | Articles with abstract only, conference summaries, or no full-text available | Full-text was needed for a complete quality assessment and analysis of the data |
| No limitation on publication dates | Unpublished results | The field is novel, and previous systematic reviews did not focus on the same research question |
| Study | Study Origin | Type of Study | Number of Participants | Mean Age of Participants (Range) | Male:Female Ratio | Mean GCS (SD) | Dosing Regimen | Measurements Related to Primary and Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|
| LeBlond et al., 2019 [23] | USA | RCT | 26 | 11.25 (6–17) | 20:6 | 11.9 (4.2) | Four weeks of one of MPH or placebo, followed by four weeks of the other condition | BRIEF parent-report and self-report CPT D-KEFS VF WISCIV-PSI |
| Kurowski et al., 2019 [24] | USA | RCT | 26 (20 completed) | 11.5 (6–17) | 20:6 (15:5 completed) | 11.9 (4.2) | Four weeks of one of MPH or placebo, followed by four weeks of the other condition | VADPRS PSERS Vital signs |
| Nikles et al., 2014 [25] | Australia | RCT | 10 | 12.9 (6–16) | 6:4 | Moderate to severe | Three pairs of one week treatment periods (of placebo, MPH, and dexamphetamine) | Conners’ 3 rating scales (parent and teacher) BRIEF parent-report, teacher-report, and self-report ECBI |
| Baker et al., 1990 [26] | USA | RCT | 8 | 11 (7–15) | 5:3 | 11.4 (4.9) at hospital admission | Two weeks of one of MPH or placebo, followed by two weeks of the other condition | MFFT CPT Stroop test Conners’ abbreviated parent–teacher questionnaires Central-incidental method HRNB- seashore rhythm test, Trail making part A and B, progressive figures test ANSER PIC (short form) |
| Clark et al., 1990 [27] | USA | RCT | 8 | 11 (7–15) | 5:3 | Unknown (had to meet baseline test requirements) | Two weeks of one of MPH or placebo, followed by two weeks of the other condition | MFFT CPT Stroop test Seashore rhythm test Trail making part A and B Abbreviated parent–teacher questionnaires |
| Ekinci et al., 2017 [28] | Turkey | Prospective cohort | 20 | 12.7 (6–18) | 15:5 | 8.6 (2.7) | IR-MPH, increased to a dose of 10 mg twice daily for first week and 10 mg three times a day for second week | Turgay DSM-IV disruptive behavior disorders rating scale parent and teacher forms Conners’ 3 rating scale-revised (parent and teacher) CGI-S CGI-I Adverse effect scale |
| Mahalick et al., 1998 [29] | USA | Prospective controlled trial | 14 | 10.7 (5–14.5) | 11:3 | 8.1 (4.1) | 14 days of either MPH or placebo with a washout period of 12 h, then crossed over | Gordon diagnostic system (model III) The Woodcock–Johnson psychoeducational test battery- revised Ruff 2 and 7 cancellation test |
| Williams et al., 1998 [30] | USA | Prospective controlled study | 10 | 10.5 (8.3–16.7) | 9:1 | Unknown (had to meet baseline test requirements) | Two-week testing period, with four days of MPH or placebo and a three-day washout period before cross-over | Conners’ 3 rating scale (parent and teacher) SDMT CPT SMRTT SRT RANT Psychomotor skills- Purdue pegboard, finger tapping test, developmental test of VMI |
| Caliendo et al., 2024 [31] | USA | Retrospective review | 234 | 11.6 median (2 months–21 years) | 146:88 | Unknown | Had been given MPH | Demographic data MPH dosing patterns, adverse events Cognitive state (at admission, discharge, and other time points) |
| Patrick et al., 2003 [32] | USA | Retrospective review | 10 | 13.7 (8–19) | 7:3 | ‘Low response state’ for 30 days or more | Given a dopaminergic agonist (amantadine, pramipexole, bromocriptine, levodopa, or MPH) | WNSSP scores before and on medication |
| Hornyak et al., 1997 [33] | USA | Retrospective review | 10 | 10.9 (3–16) | 7:3 | 6.2 (range 5 to 9) | Had been given MPH | Ranchos Los Amigos level of cognitive functioning Subjective/qualitative comments on results |
| Study | Type of Score | Stimulant Group (Mean ± SD) | Placebo Group (Mean ± SD) |
|---|---|---|---|
| Nikles et al., 2014 [25] | Parent-rated | 10.9 ± 4.9 | 13.3 ± 5.4 |
| Teacher-rated | 6.5 ± 4.4 | 11.0 ± 5.2 | |
| Williams et al., 1998 * [30] | Parent-rated | 56.50 ± 14.71 | 56.90 ± 24.69 |
| Teacher-rated | 53.83 ± 13.78 | 61.29 ± 10.68 |
| Study | Type of Score | Stimulant Group (Mean ± SD) | Placebo Group (Mean ± SD) |
|---|---|---|---|
| LeBlond et al., 2019 [23] | Self-rated (GEC) | 41.10 ± 3.53 | 46.03 ± 3.53 |
| Parent-rated (BRI mean) | 58.49 ± 1.84 | 62.88 ± 1.84 | |
| Nikles et al., 2014 [25] | Parent-rated | 147.8 ± 29.8 | 152.3 ± 27.4 |
| Teacher-rated | 127.4 ± 24.1 | 143.2 ± 20.2 |
| Study | Stimulant Group (%) | Placebo Group (%) |
|---|---|---|
| Clark et al., 1990 [27] | 37.5 | 25.0 |
| Ekinci et al., 2017 [28] | 55 | - |
| Caliendo et al., 2024 [31] | 8.0 | - |
| Study | Type of Event | Stimulant Group (Mean ± SD) | Placebo Group (Mean ± SD) |
|---|---|---|---|
| Kurowski et al., 2019 [24] | Change in appetite | 0.3 ± 0.5 | 0.2 ± 0.4 |
| Extreme sadness | 0.0 ± 0.0 | 0.1 ± 0.2 | |
| Headache | 0.1 ± 0.3 | 0.2 ± 0.4 | |
| Irritability | 0.2 ± 0.4 | 0.3 ± 0.6 | |
| Listless | 0.1 ± 0.3 | 0.1 ± 0.3 | |
| Picking at | 0.3 ± 0.6 | 0.4 ± 0.6 | |
| Repetitive movements | 0.1 ± 0.2 | 0.1 ± 0.2 | |
| Sees/hears things | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Shaky | 0.1 ± 0.2 | 0.1 ± 0.4 | |
| Socially withdrawn | 0.0 ± 0.0 | 0.1 ± 0.2 | |
| Stomach-ache | 0.2 ± 0.5 | 0.0 ± 0.0 | |
| Suicidal/homicidal ideation | 0.0 ± 0.0 | 0.1 ± 0.2 | |
| Trouble sleeping | 0.2 ± 0.4 | 0.2 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitt-Francis, A.; Stevens, A.R.; Ahmed, Z.; Di Pietro, V. The Use of Methylphenidate to Improve Executive Functioning in Pediatric Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Trauma Care 2025, 5, 1. https://doi.org/10.3390/traumacare5010001
Pitt-Francis A, Stevens AR, Ahmed Z, Di Pietro V. The Use of Methylphenidate to Improve Executive Functioning in Pediatric Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Trauma Care. 2025; 5(1):1. https://doi.org/10.3390/traumacare5010001
Chicago/Turabian StylePitt-Francis, Anna, Andrew R. Stevens, Zubair Ahmed, and Valentina Di Pietro. 2025. "The Use of Methylphenidate to Improve Executive Functioning in Pediatric Traumatic Brain Injury: A Systematic Review and Meta-Analysis" Trauma Care 5, no. 1: 1. https://doi.org/10.3390/traumacare5010001
APA StylePitt-Francis, A., Stevens, A. R., Ahmed, Z., & Di Pietro, V. (2025). The Use of Methylphenidate to Improve Executive Functioning in Pediatric Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Trauma Care, 5(1), 1. https://doi.org/10.3390/traumacare5010001







