Cognitive Predictors of Posttraumatic Stress in Children 6 Months after Paediatric Intensive Care Unit Admission
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Setting
2.2. Measures
2.2.1. Premorbid and Trauma Characteristics
2.2.2. PTSS and PTSD
2.2.3. Cognitive Variables
Peri-Trauma Affect
Peri-Trauma Cognitive Processing
Acute Trauma Memory
2.3. Procedure
2.4. Data Analysis
3. Results
3.1. Demographics
3.2. Predictors of PTSS at 6 Months
3.3. Predictors of PTSD-AA
3.4. Mediation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Australian and New Zealand Paediatric Intensive Care Registry. ANZICS Centre for Outcome and Resource Evaluation 2020 Report Melbourne, Australia. 2020. Available online: https://www.anzics.com.au/australian-and-new-zealand-paediatric-intensive-care-registry-anzpicr/ (accessed on 22 March 2023).
- Dow, B.L.; Kenardy, J.; Le Brocque, R.; Long, D. Brief Report: The utility of the Children’s Revised Impact of Event Scale in screening for Posttraumatic Stress Disorder in children following admission to Intensive Care. J. Trauma. Stress 2012, 25, 602–605. [Google Scholar] [CrossRef]
- Landolt, M.A.; Buehlmann, C.; Maag, T.; Schiestl, C. Brief Report: Quality of life is impaired in pediatric burn survivors with posttraumatic stress disorder. J. Pediatr. Psychol. 2009, 34, 14–21. [Google Scholar] [CrossRef]
- Seng, J.S.; Graham-Bermann, S.A.; Clark, C.R.; McCarthy, A.M.; Ronis, D.L. Posttraumatic stress disorder and physical comorbidity among female children and adolescents: Results from service-use data. Pediatrics 2005, 116, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Zatzick, D.F.; Jurkovich, G.J.; Fan, M.Y.; Grossman, D.; Russo, J.; Katon, W.; Rivara, F.P. Association between posttraumatic stress and depressive symptoms and functional outcomes in adolescents followed up longitudinally after injury hospitalization. Arch. Pediatr. Adolesc. Med. 2008, 162, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Shears, D.; Nadel, S.; Gledhill, J.; Garralda, M.E. Short-term psychiatric adjustment of children and their parents following meningoccal disease. Pediatr. Crit. Care Med. 2005, 6, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Colville, G.; Kerry, S.; Pierce, C. Children’s factual and delusional memories of Intensive Care. Am. J. Respir. Crit. Care Med. 2008, 177, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Bronner, M.B.; Knoester, H.; Bos, A.P.; Last, B.F.; Grootenhuis, M.A. Follow-up after paediatric intensive care treatment: Parental posttraumatic stress. Acta Paediatr. 2008, 97, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Rees, G.; Gledhill, J.; Garralda, M.E.; Nadel, S. Psychiatric outcome following paediatric intensive care unit (PICU) admission: A cohort study. Intensive Care Med. 2004, 30, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Dow, B.; Kenardy, J.; Long, D.; Le Brocque, R. Children’s post-traumatic stress and the role of memory following admission to intensive care: A review. Clin. Psychol. 2012, 16, 1–14. [Google Scholar] [CrossRef]
- Rennick, J.E.; Johnston, C.C.; Dougherty, G.; Platt, R.; Ritchie, J.A. Children’s psychological responses after critical illness and exposure to invasive technology. J. Dev. Behav. Pediatr. JDBP 2002, 23, 133–144. [Google Scholar] [CrossRef]
- Ehlers, A.; Clark, D.M. A cognitive model of posttraumatic stress disorder. Behav. Res. Ther. 2000, 38, 319–345. [Google Scholar] [CrossRef]
- Marsac, M.L.; Kassam-Adams, N.; Delahanty, D.L.; Widaman, K.; Barakat, L.P. Posttraumatic stress following acute medical trauma in children: A proposed model of bio-psycho-social processes during the peri-trauma period. Clin. Child Fam. Psychol. Rev. 2014, 17, 399–411. [Google Scholar] [CrossRef]
- Meiser-Stedman, R. Towards a cognitive-behavioral model of PTSD in children and adolescents. Clin. Child Fam. Psychol. Rev. 2002, 5, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Kassam-Adams, N.; Winston, F. Predicting Child PTSD: The relationship between Acute Stress Disorder and PTSD in injured children. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Meiser-Stedman, R.; Dalgleish, T.; Smith, P.; Yule, W.; Glucksman, E. Diagnostic, demographic, memory quality, and cognitive variables associated with acute stress disorder in children and adolescents. J. Abnorm. Psychol. 2007, 116, 65–79. [Google Scholar] [CrossRef]
- McKinnon, A.C.; Nixon, R.D.V.; Brewer, N. The influence of data-driven processing on perceptions of memory quality and intrusive symptoms in children following traumatic events. Behav. Res. Ther. 2008, 46, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, A.; Mayou, R.A.; Bryant, B. Cognitive predictors of posttraumatic stress disorder in children: Results of a prospective longitudinal study. Behav. Res. Ther. 2003, 41, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, A.; Brewer, N.; Cameron, K.; Nixon, R.D.V. The relationship between processing style, trauma memory processes, and the development of posttraumatic stress symptoms in children and adolescents. J. Behav. Ther. Exp. Psychiatry 2017, 57, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Dow, B.L.; Kenardy, J.A.; Long, D.A.; Le Brocque, R.M. Cognitive/Affective Factors are Associated with Children’s Acute Posttraumatic Stress Following Pediatric Intensive Care. Psychol. Trauma Theory Res. Pract. Policy 2019, 11, 55–63. [Google Scholar] [CrossRef]
- Smith, P.; Perrin, S.; Dyregrov, A.; Yule, W. Principal components analysis of the Impact of Event Scale with children in war. Personal. Individ. Differ. 2003, 34, 315–322. [Google Scholar] [CrossRef]
- Giannopoulou, I.; Smith, P.; Ecker, C.; Strouthos, M.; Dikaiakou, A.; Yule, W. Factor structure of the Children’s Revised Impact of Event Scale (CRIES) with children exposed to earthquake. Personal. Individ. Differ. 2006, 40, 1027–1037. [Google Scholar] [CrossRef]
- Saigh, P.A.; Yasik, A.E.; Oberfield, R.A.; Green, B.L.; Halamandaris, P.V.; Rubenstein, H.; Nester, J.; Resko, J.; Hetz, B.; McHugh, M. The Children’s PTSD Inventory: Development and reliability. J. Trauma. Stress 2000, 13, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Yasik, A.E.; Saigh, P.A.; Oberfield, R.A.; Green, B.; Halamandaris, P.; McHugh, M. The validity of the Children’s PTSD Inventory. J. Trauma. Stress 2001, 14, 81–94. [Google Scholar] [CrossRef]
- Scheeringa, M.S.; Wright, M.J.; Hunt, J.P.; Zeanah, C.H. Factors affecting the diagnosis and prediction of PTSD symptomatology in children and adolescents. Am. J. Psychiatry 2006, 163, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Dow, B.L.; Kenardy, J.A.; Le Brocque, R.M.; Long, D.A. The Diagnosis of Posttraumatic Stress Disorder in School-Aged Children and Adolescents Following Pediatric Intensive Care Unit Admission. J. Child Adolesc. Psychopharmacol. 2013, 23, 614–619. [Google Scholar] [CrossRef]
- Jones, C.; Humphris, G.; Griffiths, R.D. Preliminary validation of the ICUM Tool: A tool for assessing memory of the intensive care experience. Clin. Intensive Care 2000, 11, 252–255. [Google Scholar]
- Meiser-Stedman, R.; Smith, P.; Yule, W.; Dalgleish, T. The Trauma Memory Quality Questionnaire: Preliminary development and validation of a measure of trauma memory characteristics for children and adolescents. Memory 2007, 15, 271–279. [Google Scholar] [CrossRef]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; The Guilford Press: New York, NY, USA, 2013. [Google Scholar]
- Pai, A.; Heining, M. Ketamine. Continuing Education in Anaesthesia. Crit. Care Pain 2007, 7, 59–63. [Google Scholar]
- Le Brocque, R.M.; Hendrikz, J.; Kenardy, J.A. The course of posttraumatic stress in children: Examination of recovery trajectories following traumatic injury. J. Pediatr. Psychol. 2010, 35, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Trickey, D.; Siddaway, A.; Meiser-Stedman, R.; Serpell, L.; Field, A. A meta-analysis of risk factors for post-traumatic stress disorder in children and adolescents. Clin. Psychol. Rev. 2012, 32, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Böhringer, A.; Wolf, O.T. Stress disrupts context-dependent memory. Learn. Mem. 2009, 16, 110–113. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | n (%) | M (SD) |
|---|---|---|
| Age, years | 10.78 (2.65) | |
| Gender, male | 32 (58%) | |
| Family of origin, both biological parents a | 38 (69%) | |
| Participating parent’s highest level of education a | ||
| Did not complete high school | 6 (11%) | |
| Completed high school | 7 (13%) | |
| College certificate | 16 (29%) | |
| University degree | 19 (35%) | |
| Prior trauma exposure a (# of traumas) | 1.39 (1.42) | |
| Premorbid behavioural problems a | 5 (11%) | |
| Length of stay in PICU > 48 h | 12 (22%) | |
| PIM2 Risk of Mortality | 1.81 (2.55) | |
| Mechanically ventilated | 16 (29%) | |
| Reason for admission | ||
| Post-operative care | 22 (40%) | |
| Traumatic Injury | 13 (24%) | |
| Respiratory | 6 (11%) | |
| Other | 14 (25%) | |
| Admission status, elective | 21 (38%) | |
| Number of invasive procedures | 4.98 (4.98) | |
| Received therapeutic agents | ||
| Midazolam | 13 (24%) | |
| Morphine | 28 (51%) | |
| Propofol | 9 (16%) | |
| Ketamine | 13 (24%) | |
| Other patient death during admission | 5 (9%) | |
| Exposed to distressing event in PICU a | 3 (6%) | |
| Acute PTSS (>30 = elevated) | 19.87 (18.00) | |
| Peri-trauma affect | ||
| Peri-trauma fear | 21 (38%) | |
| Peri-trauma panic | 19 (35%) | |
| Peri-trauma sadness | 21 (38%) | |
| Cognitive variables | ||
| Confusion | 27 (49%) | |
| Delusional experiences | 28 (51%) | |
| Total PICU recall | 1.91 (1.09) | |
| Sensory memory quality | 23.75 (5.58) |
| Variables | PTSS at 6 Months | PTDS-AA Positive |
|---|---|---|
| Premorbid factors | ||
| Age | 0.02 | −0.05 |
| Gender | 0.08 | 0.11 |
| Prior trauma exposure a | 0.15 | −0.04 |
| Premorbid behavioural problems a | 0.23 | 0.09 |
| Trauma characteristics | ||
| Disease-related | ||
| Length of stay in PICU > 48 h | 0.02 | 0.05 |
| PIM2 Risk of death | 0.05 | 0.06 |
| Admission for traumatic injury | 0.01 | 0.02 |
| Treatment-related | ||
| Number of invasive procedures | 0.12 | 0.16 |
| Mechanically ventilated | 0.12 | 0.03 |
| Intubated | 0.08 | 0.01 |
| Therapeutic agents | ||
| Midazolam | 0.12 | 0.12 |
| Morphine | 0.22 | 0.23 |
| Propofol | 0.06 | 0.04 |
| Ketamine | 0.33 * | 0.21 |
| Environment-related | ||
| Other patient death during admission | 0.01 | 0.08 |
| Exposed to distressing event in PICU a | 0.03 | 0.05 |
| Peri-trauma affect | ||
| Peri-trauma fear | 0.28 * | 0.24 |
| Peri-trauma panic | 0.47 *** | 0.38 ** |
| Peri-trauma sadness | 0.27 * | 0.24 |
| Cognitive variables | ||
| Peri-trauma cognitive processing | ||
| Confusion | 0.45 ** | 0.33 * |
| Delusional experiences | 0.47 *** | 0.38 ** |
| Acute trauma memory | ||
| Total PICU recall | −0.13 | 0.02 |
| Sensory memory quality | 0.42 ** | 0.38 ** |
| Variables | Acute PTSS | Peri-Trauma Fear | Peri-Trauma Panic | Peri-Trauma Sadness | Confusion | Peri-Trauma Delusions | Acute Sensory Memory Quality |
|---|---|---|---|---|---|---|---|
| Ketamine | −0.062 | −0.085 | 0.046 | −0.173 | 0.139 | 0.094 | −0.052 |
| Acute PTSS | - | 0.195 | 0.368 ** | 0.213 | 0.356 ** | 0.299 * | 0.437 *** |
| Peri-trauma fear | - | - | 0.452 *** | 0.461 *** | 0.501 *** | 0.260 | 0.077 |
| Peri-trauma panic | - | - | - | 0.531 *** | 0.204 | 0.412 ** | 0.359 ** |
| Peri-trauma sadness | - | - | - | - | 0.201 | 0.485 *** | 0.314 * |
| Confusion | - | - | - | - | - | 0.345 * | 0.157 |
| Peri-trauma delusions | - | - | - | - | - | - | 0.240 |
| B | SE B | β | R2 | ∆R2 | |
|---|---|---|---|---|---|
| Step 1 | 0.21 *** | 0.21 *** | |||
| Acute PTSS | 0.13 | 0.04 | 0.46 *** | ||
| Step 2 | 0.34 *** | 0.13 ** | |||
| Acute PTSS | 0.14 | 0.03 | 0.48 *** | ||
| Ketamine | 3.04 | 0.95 | 0.36 ** | ||
| Step 3 | 0.55 *** | 0.21 *** | |||
| Acute PTSS | 0.07 | 0.03 | 0.23 * | ||
| Ketamine | 2.81 | 0.83 | 0.33 ** | ||
| Peri-trauma affect | 0.41 | 0.36 | 0.14 | ||
| Peri-trauma cognitive processing | 1.39 | 0.49 | 0.33 ** | ||
| Acute sensory memory quality | 0.16 | 0.07 | 0.24 * |
| Independent Variables | B | SE B | OR | 95% CI for OR | χ2 STEP | χ2 MODEL | Correctly Classified | Cox & Snell R2 | Negelkerke R2 |
|---|---|---|---|---|---|---|---|---|---|
| Step 1 | 5.30 * | 5.30 * | 71% | 0.10 | 0.10 | ||||
| Acute PTSS | 0.06 | 0.03 | 1.06 * | 1.01–1.11 | |||||
| Step 2 | 3.22 (*) | 8.53 * | 78% | 0.14 | 0.14 | ||||
| Acute PTSS | 0.06 | 0.03 | 1.07 * | 1.01–1.13 | |||||
| Ketamine | 1.30 | 0.73 | 3.65 (*) | 0.88–15.23 | |||||
| Step 3 | 10.31 * | 18.84 ** | 80% | 0.29 | 0.29 | ||||
| Acute PTSS | 0.02 | 0.04 | 1.02 | 0.95–1.10 | |||||
| Ketamine | 1.32 | 0.86 | 3.74 | 0.69–20.33 | |||||
| Peri-trauma affect | 0.26 | 0.35 | 1.30 | 0.65–2.58 | |||||
| Peri-trauma cognitive processing | 0.83 | 0.51 | 2.30 | 0.84–6.29 | |||||
| Acute sensory memory quality | 0.13 | 0.08 | 1.14 | 0.97–1.32 |
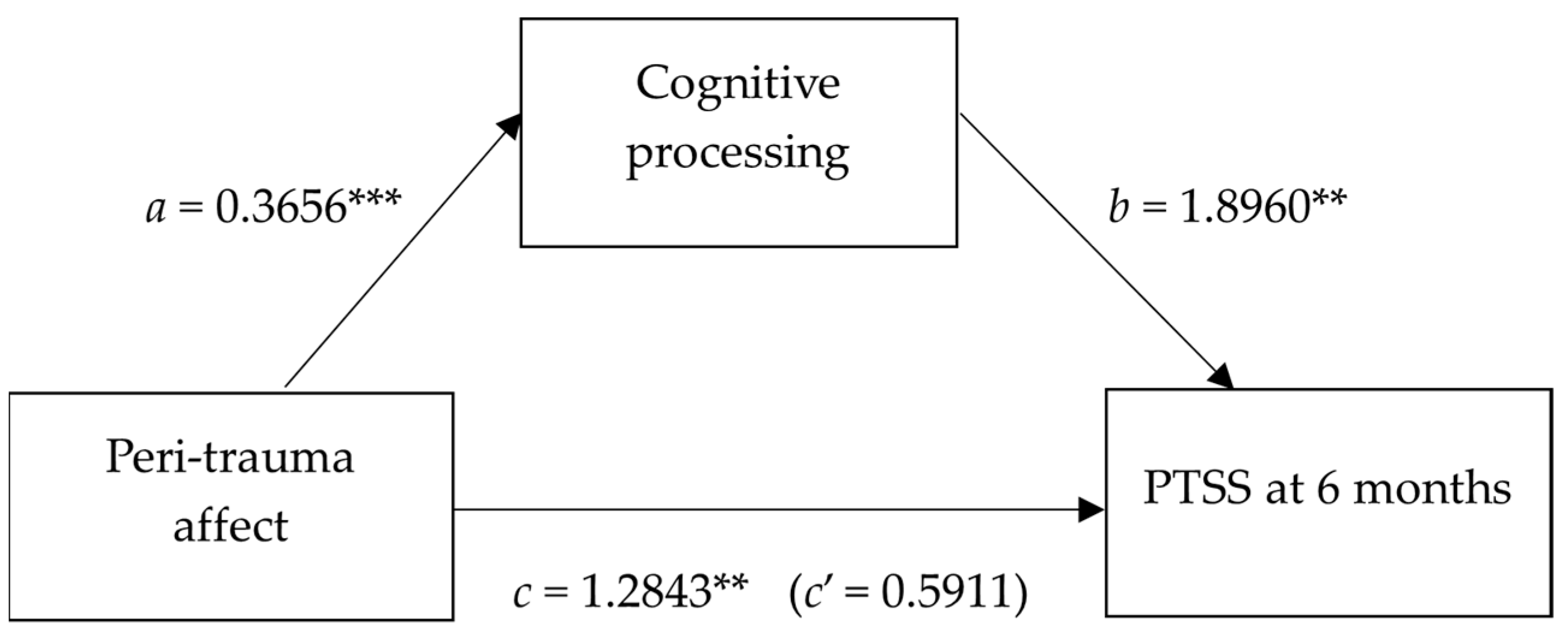
| Antecedent | Consequent | |||||||
|---|---|---|---|---|---|---|---|---|
| Cognitive Processing | PTSS at 6 Months | |||||||
| Coeff. | SE | p | Coeff. | SE | p | |||
| Peri-trauma affect | a | 0.3656 | 0.0844 | 0.0001 | c’ | 0.5911 | 0.4070 | 0.152 |
| Cognitive processing | - | - | - | b | 1.8960 | 0.5690 | 0.002 | |
| Constant | iM | 0.5036 | 0.1362 | <0.001 | iY | 2.4935 | 0.6327 | <0.001 |
| R2 = 0.2613 | R2 = 0.3210 | |||||||
| F (1, 53) = 18.74, p < 0.001 | F (2, 52) = 12.29, p < 0.001 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dow, B.L.; Kenardy, J.A.; Le Brocque, R.M.; Long, D.A. Cognitive Predictors of Posttraumatic Stress in Children 6 Months after Paediatric Intensive Care Unit Admission. Trauma Care 2023, 3, 82-92. https://doi.org/10.3390/traumacare3020009
Dow BL, Kenardy JA, Le Brocque RM, Long DA. Cognitive Predictors of Posttraumatic Stress in Children 6 Months after Paediatric Intensive Care Unit Admission. Trauma Care. 2023; 3(2):82-92. https://doi.org/10.3390/traumacare3020009
Chicago/Turabian StyleDow, Belinda L., Justin A. Kenardy, Robyne M. Le Brocque, and Debbie A. Long. 2023. "Cognitive Predictors of Posttraumatic Stress in Children 6 Months after Paediatric Intensive Care Unit Admission" Trauma Care 3, no. 2: 82-92. https://doi.org/10.3390/traumacare3020009
APA StyleDow, B. L., Kenardy, J. A., Le Brocque, R. M., & Long, D. A. (2023). Cognitive Predictors of Posttraumatic Stress in Children 6 Months after Paediatric Intensive Care Unit Admission. Trauma Care, 3(2), 82-92. https://doi.org/10.3390/traumacare3020009







