The Role of Prehospital REBOA for Hemorrhage Control in Civilian and Military Austere Settings: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Data Collection
2.3. Data Extraction and Synthesis
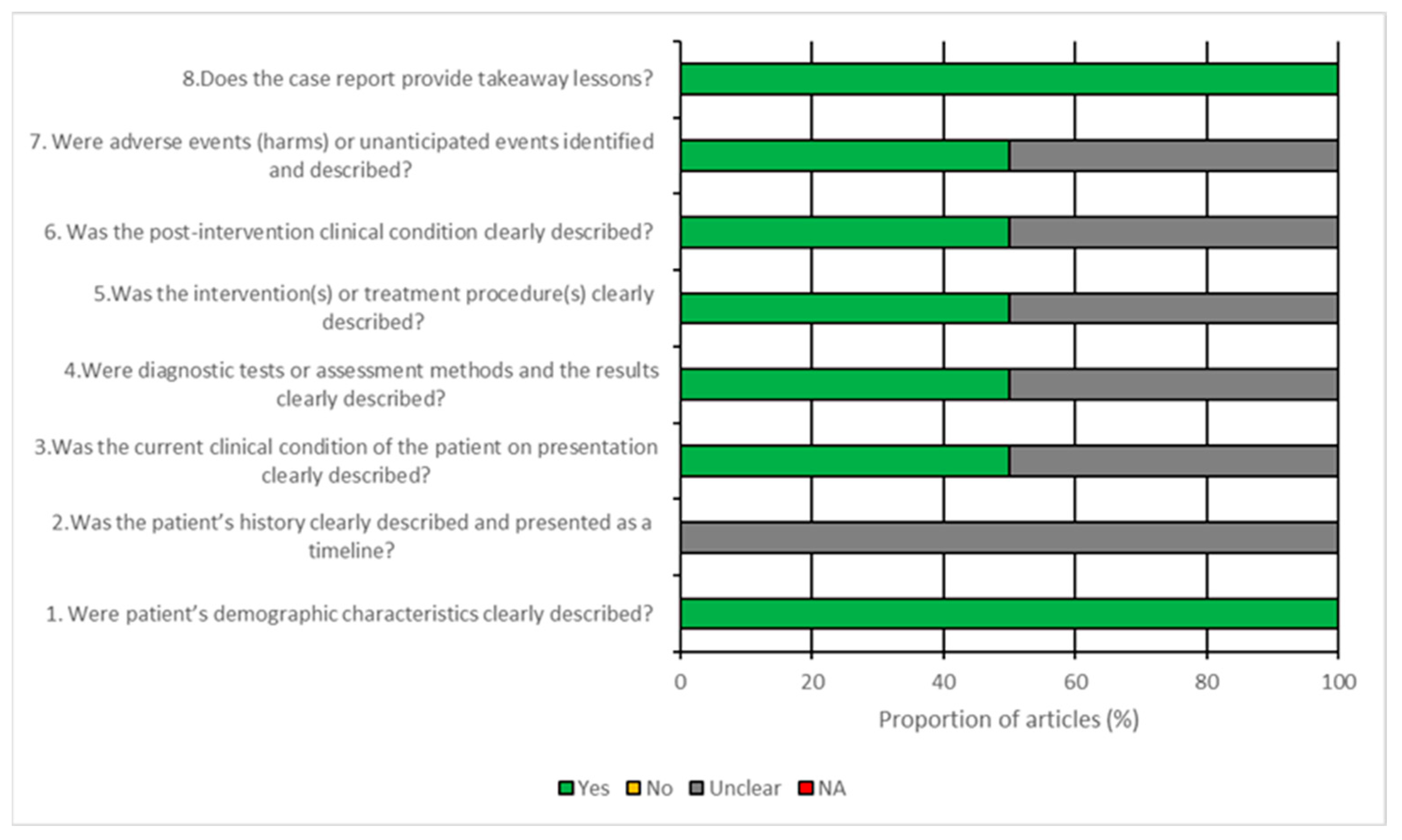
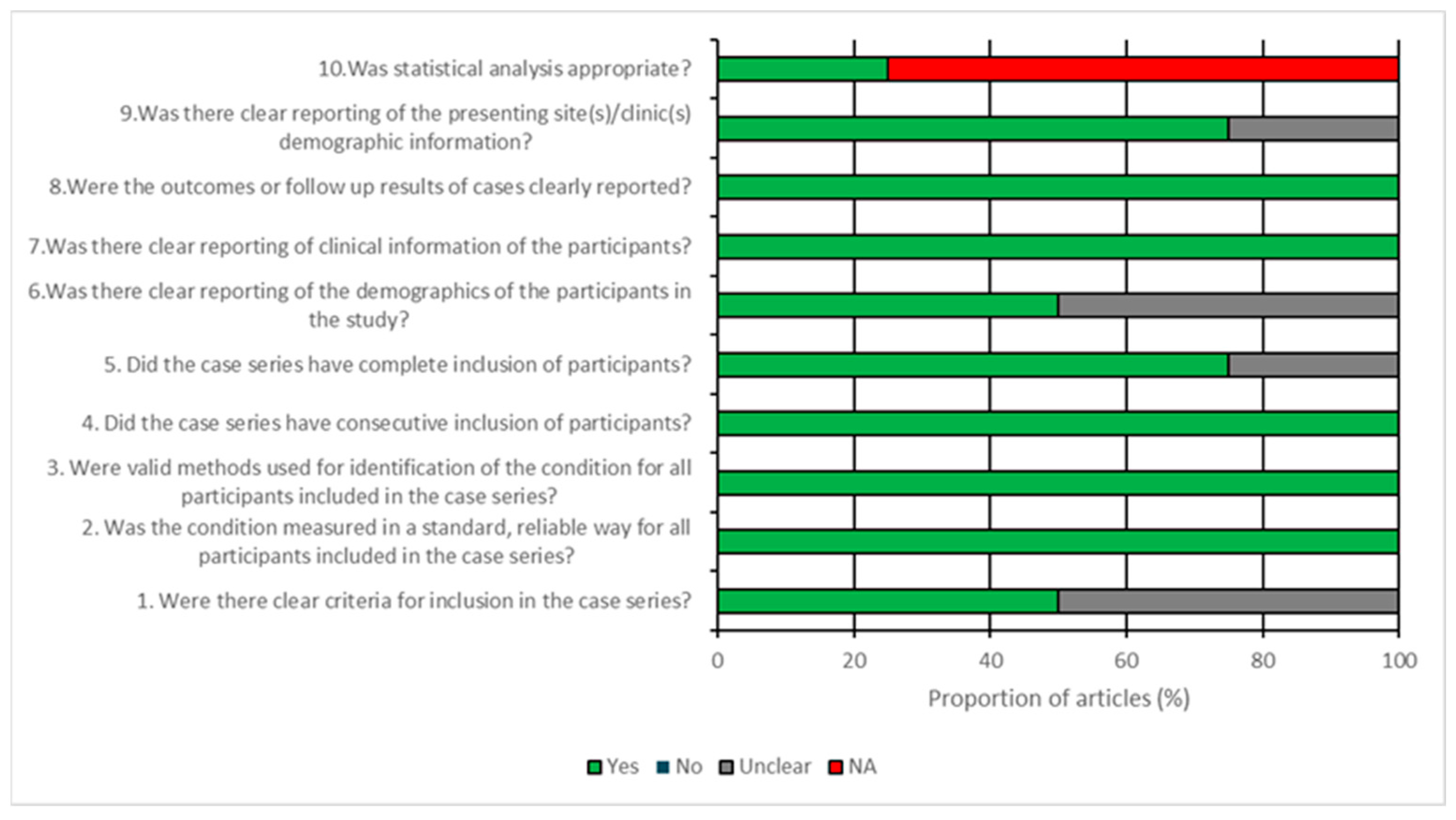
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
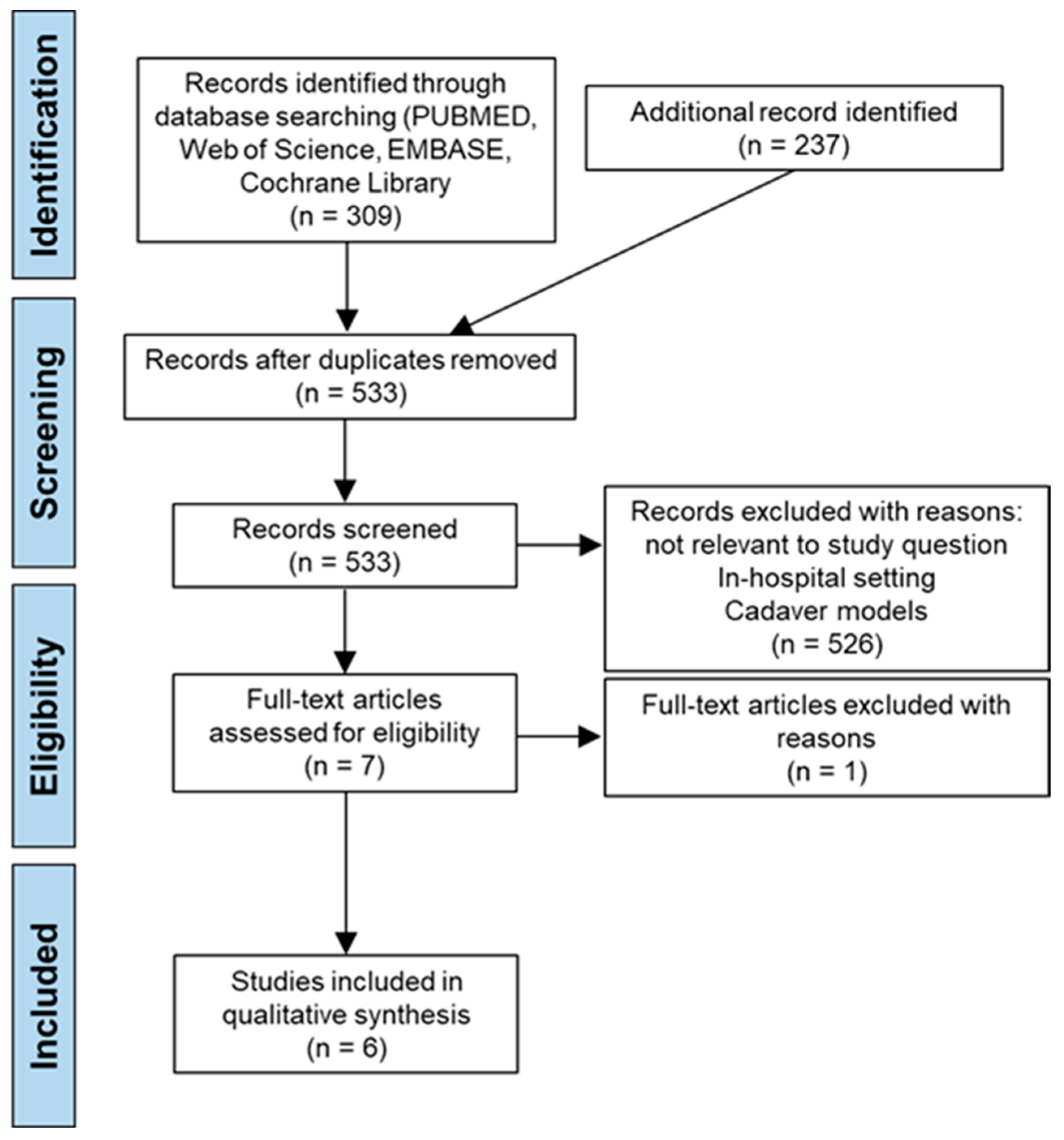
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
3.4. Patient Characteristics
3.5. Main Findings
3.6. Narrative Synthesis
4. Discussion
4.1. Complications
4.2. Surgical Cutdown versus Percutaneous Access
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kauvar, D.S.; Lefering, R.P.; Wade, C.E. Impact of Hemorrhage on Trauma Outcome: An Overview of Epidemiology, Clinical Presentations, and Therapeutic Considerations. J. Trauma Acute Care Surg. 2006, 60, S3–S11. [Google Scholar] [CrossRef] [Green Version]
- Alarhayem, A.Q.; Myers, J.G.; Dent, D.; Liao, L.; Muir, M.; Mueller, D.; Nicholson, S.; Cestero, R.; Johnson, M.C.; Stewart, R.; et al. Time is the enemy: Mortality in trauma patients with hemorrhage from torso injury occurs long before the “golden hour”. Am. J. Surg. 2016, 212, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.S.; Satahoo, S.S.; Butler, F.K.; Dermer, H.; Naranjo, D.; Julien, K.; Van Haren, R.M.; Namias, N.; Blackbourne, L.H.; Schulman, C.I.; et al. An analysis of prehospital deaths: Who can we save? J. Trauma Acute Care Surg. 2014, 77, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Eastridge, B.J.; Mabry, R.L.; Seguin, P.; Cantrell, J.; Tops, T.; Uribe, P.; Mallett, O.; Zubko, T.; Oetjen-Gerdes, L.; Rasmussen, T.E.; et al. Death on the battlefield (2001–2011): Implications for the future of combat casualty care. J. Trauma Acute Care Surg. 2012, 73, S431–S437. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.J.; Brenner, M.; Kozar, R.A.; Pasley, J.; Wade, C.E.; Baraniuk, M.S.; Scalea, T.; Holcomb, J.B. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J. Trauma Acute Care Surg. 2015, 79, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Manzano Nunez, R.; Naranjo, M.P.; Foianini, E.; Ferrada, P.; Rincon, E.; García-Perdomo, H.A.; Burbano, P.; Herrera, J.P.; García, A.F.; Ordoñez, C.A. A meta-analysis of resuscitative endovascular balloon occlusion of the aorta (REBOA) or open aortic cross-clamping by resuscitative thoracotomy in non-compressible torso hemorrhage patients. World J. Emerg. Surg. 2017, 12, 30. [Google Scholar] [CrossRef] [Green Version]
- Brenner, M.; Bulger, E.M.; Perina, D.G.; Henry, S.; Kang, C.S.; Rotondo, M.F.; Chang, M.C.; Weireter, L.J.; Coburn, M.; Winchell, R.J.; et al. Joint statement from the American College of Surgeons Committee on Trauma (ACS COT) and the American College of Emergency Physicians (ACEP) regarding the clinical use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). Trauma Surg. Acute Care Open. 2018, 3, e000154. [Google Scholar] [CrossRef] [Green Version]
- Hughes, C.W. Use of an intra-aortic balloon catheter tamponade for controlling intra-abdominal hemorrhage in man. Surgery 1954, 36, 65–68. [Google Scholar]
- Brenner, M.L.; Moore, L.J.; DuBose, J.J.; Tyson, G.H.; McNutt, M.K.; Albarado, R.P.; Holcomb, J.B.; Scalea, T.M.; Rasmussen, T.E. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J. Trauma Acute Care Surg. 2013, 75, 506–511. [Google Scholar] [CrossRef] [Green Version]
- Sato, R.; Kuriyama, A.; Takaesu, R.; Miyamae, N.; Iwanaga, W.; Tokuda, H.; Umemura, T. Resuscitative endovascular balloon occlusion of the aorta performed by emergency physicians for traumatic hemorrhagic shock: A case series from Japanese emergency rooms. J. Crit. Care 2018, 22, 103. [Google Scholar] [CrossRef] [Green Version]
- Kunitatsu, K.; Ueda, K.; Iwasaki, Y.; Yamazoe, S.; Yonemitsu, T.; Kawazoe, Y.; Kawashima, S.; Shibata, N.; Kato, S. Outcomes of abdominal trauma patients with hemorrhagic shock requiring emergency laparotomy: Efficacy of intra-aortic balloon occlusion. Acute Med. Surg. 2016, 3, 345–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borger van der Burg, B.L.S.; van Dongen, T.T.; Morrison, J.J.; Hedeman Joosten, P.P.A.; DuBose, J.J.; Hörer, T.M.; Hoencamp, R. A systematic review and meta-analysis of the use of resuscitative endovascular balloon occlusion of the aorta in the management of major exsanguination. Eur. J. Trauma Emerg. Surg. 2018, 44, 535–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joint Trauma System Clinical Practice Guideline (JTS CPG): Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for Hemorrhagic Shock (CPG ID: 38). San Antonio, TX: Joint Trauma System. 2014. Available online: http://www.usaisr.amedd.army.mil/cpgs/REBOA_%2006Jul2017CORRECTED.pdf (accessed on 24 August 2021).
- Stannard, A.; Morrison, J.J.; Scott, D.J.; Ivatury, R.A.; Ross, J.D.; Rasmussen, T.E. The epidemiology of noncompressible torso hemorrhage in the wars in Iraq and Afghanistan. J. Trauma Acute Care Surg. 2013, 74, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Eastridge, B.J.; Holcomb, J.B.; Shackelford, S. Outcomes of traumatic hemorrhagic shock and the epidemiology of preventable death from injury. Transfusion 2019, 59, 1423–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, Y.; Matsumoto, J.; Kondo, H.; Idoguchi, K.; Ishida, T.; Okada, Y.; Kon, Y.; Oka, K.; Ishida, K.; Toyoda, Y.; et al. Early arterial access for resuscitative endovascular balloon occlusion of the aorta is related to survival outcome in trauma. J. Trauma Acute Care Surg. 2018, 3, 507–511. [Google Scholar] [CrossRef]
- Inoue, J.; Shiraishi, A.; Yoshiyuki, A.; Haruta, K.; Matsui, H.; Otomo, Y. Resuscitative endovascular balloon occlusion of the aorta might be dangerous in patients with severe torso trauma: A propensity score analysis. J. Trauma Acute Care Surg. 2016, 80, 559–567. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: New York, NY, USA, 2021; Available online: www.training.cochrane.org/handbook (accessed on 8 June 2021).
- Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis. JBI Evid. Synth. 2020, 18, 2106–2107. [Google Scholar] [CrossRef]
- Sadek, S.; Lockey, D.J.; Lendrum, R.A.; Perkins, Z.; Price, J.; Davies, G.E. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in the pre-hospital setting: An additional resuscitation option for uncontrolled catastrophic haemorrhage. Resuscitation 2016, 107, 135–138. [Google Scholar] [CrossRef]
- Manley, J.D.; Mitchell, B.J.; DuBose, J.J.; Rasmussen, T.E. A Modern Case Series of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) in an Out-of-Hospital, Combat Casualty Care Setting. J. Spec. Oper. Med. 2017, 17, 1–8. [Google Scholar]
- Northern, D.M.; Manley, J.D.; Lyon, R.; Farber, D.; Mitchell, B.J.; Filak, K.J.; Lundy, J.; DuBose, J.J.; Rasmussen, T.E.; Holcomb, J.B. Recent advances in austere combat surgery: Use of aortic balloon occlusion as well as blood challenges by special operations medical forces in recent combat operations. J. Trauma Acute Care Surg. 2018, 85, S98–S103. [Google Scholar] [CrossRef]
- Lamhaut, L.; Qasim, Z.; Hutin, A.; Dagron, C.; Orsini, J.P.; Haegel, A.; Perkins, Z.; Pirracchio, R.; Carli, P. First description of successful use of zone 1 resuscitative endovascular balloon occlusion of the aorta in the prehospital setting. Resuscitation 2018, 133, e1–e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Schoutheete, J.C.; Fourneau, I.; Waroquier, F.; De Cupere, L.; O’Connor, M.; Van Cleynenbreugel, K.; Ceccaldi, J.C.; Nijs, S. Three cases of resuscitative endovascular balloon occlusion of the aorta (REBOA) in austere pre-hospital environment-technical and methodological aspects. World J. Emerg. Surg. 2018, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Lendrum, R.; Perkins, Z.; Chana, M.; Marsden, M.; Davenport, R.; Grier, G.; Sadek, S.; Davies, G. Pre-hospital Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for exsanguinating pelvic haemorrhage. Resuscitation 2019, 135, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Reva, V.A.; Petrov, A.N.; Samokhvalov, I.M. First Russian experience with endovascular balloon occlusion of the aorta in a zone of combat operations. Angiol. Vasc. Surg. 2020, 26, 61–75. [Google Scholar] [CrossRef]
- UK Ministry of Defence. Allied Joint Doctrine for Medical Support (AJP-4.10). 2015. Available online: https://www.gov.uk/government/publications/allied-joint-medical-support-doctrine-ajp-410 (accessed on 20 June 2021).
- Palmer, C. Major trauma and the injury severity score-where should we set the bar? Annu. Proc. Assoc. Adv. Automot. Med. 2007, 51, 13–29. [Google Scholar]
- Morrison, J.J.; Galgon, R.E.; Jansen, J.O.; Cannon, J.W.; Rasmussen, T.E.; Eliason, J.L. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J. Trauma Acute Care Surg. 2016, 80, 327–334. [Google Scholar] [CrossRef]
- Lansink, K.W.; Gunning, A.C.; Leenen, L.P. Cause of death and time of death distribution of trauma patients in a Level I trauma centre in the Netherlands. Eur. J. Trauma Emerg. Surg. 2013, 39, 375–383. [Google Scholar] [CrossRef]
- Butler, F.K.; Holcomb, J.B.; Schreiber, M.A.; Kotwal, R.S.; Jenkins, D.A.; Champion, H.R.; Bowling, F.; Cap, A.P.; Dubose, J.J.; Dorlac, W.C. Fluid resuscitation for hemorrhagic shock in tactical combat casualty care: TCCC Guide- lines Change 14-01- 2 June 2014. J. Spec. Oper Med. 2014, 14, 13–38. [Google Scholar]
- Brenner, M.; Teeter, W.; Hoehn, M.; Pasley, J.; Hu, P.; Yang, S.; Romagnoli, A.; Diaz, J.; Stein, D.; Scalea, T. Use of resuscitative endovascular balloon occlusion of the aorta for proximal aortic control in patients with severe hemorrhage and arrest. JAMA Surg. 2018, 153, 130–135. [Google Scholar] [CrossRef]
- Lockey, D.; Crewdson, K.; Davies, G. Traumatic cardiac arrest: Who are the survivors? Ann. Emerg. Med. 2006, 48, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Butler, F.K.; Holcomb, J.B.; Shackelford, S.; Barbabella, S.; Bailey, J.A.; Baker, J.B.; Cap, A.P.; Conklin, C.C.; Cunningham, C.W.; Davis, M.; et al. Advanced resuscitative care in tactical combat casualty care: TCCC guidelines change 18-01:14 October 2018. J. Spec. Oper. Med. 2018, 18, 37–55. [Google Scholar] [PubMed]
- Cotton, B.A.; Podbielski, J.; Camp, E.; Welch, T.; del Junco, D.; Bai, Y.; Hobbs, R.; Scroggins, J.; Hartwell, B.; Kozar, R.A.; et al. A randomized controlled pilot trial of modified whole blood versus component therapy in severely injured patients requiring large volume transfusions. Ann. Surg. 2013, 258, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Armand, R.; Hess, J.R. Treating coagulopathy in trauma patients. Transfus. Med. Rev. 2003, 17, 223–231. [Google Scholar] [CrossRef]
- Teeter, W.A.; Matsumoto, J.; Idoguchi, K.; Kon, Y.; Orita, T.; Funabiki, T.; Brenner, M.L.; Matsumura, Y. Smaller introducer sheaths for REBOA may be associated with fewer complications. J. Trauma Acute Care Surg. 2016, 81, 1039–1045. [Google Scholar] [CrossRef]
- Taylor, J.R., 3rd; Harvin, J.A.; Martin, C.; Holcomb, J.B.; Moore, L.J. Vascular complications from resuscitative endovascular balloon occlusion of the aorta: Life over limb? J. Trauma Acute Care Surg. 2017, 83, S120–S123. [Google Scholar] [CrossRef]
- Martinelli, T.; Thony, F.; Decléty, P.; Sengel, C.; Broux, C.; Tonetti, J.; Payen, J.F.; Ferretti, G. Intra-Aortic Balloon Occlusion to Salvage Patients With Life-Threatening Hemorrhagic Shocks From Pelvic Fractures. J. Trauma Acute Care Surg. 2010, 68, 942–948. [Google Scholar] [CrossRef] [Green Version]
- Tsurukiri, J.; Akamine, I.; Sato, T.; Sakurai, M.; Okumura, E.; Moriya, M.; Yamanaka, H.; Ohta, S. Resuscitative endovascular balloon occlusion of the aorta for uncontrolled haemorrahgic shock as an adjunct to haemostatic procedures in the acute care setting. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 13. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Matsumoto, J.; Kondo, H.; Idoguchi, K.; Ishida, T.; Kon, Y.; Tomita, K.; Ishida, K.; Hirose, T.; Umakoshi, K.; et al. Fewer REBOA complications with smaller devices and partial occlusion: Evidence from a multicentre registry in Japan. Emerg. Med. J. 2017, 34, 793–799. [Google Scholar] [CrossRef]
- Buck, D.B.; Karthaus, E.G.; Soden, P.A.; Ultee, K.H.; Van Herwaarden, J.A.; Moll, F.L.; Schermerhorn, M.L. Percutaneous versus femoral cutdown access for endovascular aneurysm repair. J. Vasc. Surg. 2015, 62, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Mayer, D.; Rancic, Z.; Wilhelm, M.; Genoni, M.; Veith, F.J.; Lachat, M. Improved hybrid technique for vascular access and closure. J. Endovasc. Ther. 2008, 15, 322–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitzer, S.G.; Wilbring, M.; Alexiou, K.; Stumpf, J.; Kappert, U.; Matschke, K. Surgical cut-down or percutaneous access-which is best for less vascular access complications in transfemoral TAVI? Catheter. Cardiovasc. Interv. 2016, 88, E52–E58. [Google Scholar] [CrossRef] [PubMed]
| Patient | Adults with traumatic haemorrhage |
| Intervention | Standard prehospital resuscitative interventions with REBOA |
| Comparison | Standard prehospital resuscitative interventions without REBOA |
| Outcome | Improved hemodynamic and reduced mortality |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Study | Design | Country | Setting | Inclusion and Exclusion Criteria | Intervention Protocol (Prehospital) | Outcomes Measures | Study Quality |
|---|---|---|---|---|---|---|---|
| Sadek et al., 2016 [21] | Case report | UK | Civilian |
|
|
| Low |
| Manley et al., 2017 [22] | Case series | FST operated by the US SOST | Military | Not specified |
|
| Moderate |
| Northern et al., 2018 [23] | Case series | FST operated by the US SOST | Military | Not specified |
|
| Moderate |
| Lamhaut et al., 2018 [24] | Case report | France | Civilian | Not specified |
|
| Low |
| de Schoutheete et al., 2018 [25] | Case series | FCCP operated by the Belgian SOST | Military | Based on the MIST acronym:
|
|
| Moderate |
| Lendrum et al., 2019 [26] | Case series | UK | Civilian |
|
|
| Moderate |
| Study | Sample Size | Mean Age (Years) | Gender | Specific Characteristics of Interest | Injury Severity | Shock/ Cardiac Arrest * | Mechanism of Injury/Injury Patterns | Initial SBP, Mean (Range) |
|---|---|---|---|---|---|---|---|---|
| Sadek et al. [21] | 1 | 32 | Male | N/A | ISS: 45 | Profound shock | Fell 15 m; pelvic haemorrhage | Not recordable |
| Manley et al. [22] | 4 | Not-mentioned | Male | Combat-related | Not mentioned | Shock | Significant NCTH penetrating injuries Gunshot wounds Diffuse fragmentation | 78 mmHg (70–90 mmHg) |
| Northern et al. [23] | 20 (19 successful AO, 1 failed) | 18–30 | Primarily male | Combatants | GCS: 7–15 | Shock | NCTH Gunshot wounds Explosion injuries | 71 mmHg (50–90 mmHg); |
| Lamhaut et al. [24] | 1 | 49 | Female | Diagnosed with advanced metastatic cancer in DCS | Not mentioned but patient had cardiac arrest at the scene; GCS should be 3 | Cardiac arrest | Fall from 30 feet Blunt trauma with abdominal torso haemorrhage | Cardiac arrest |
| de Schoutheete et al. [25] | 3 | 40 (25–54) | 2 Male, 1 female | No known peripheral vascular disease. | Mean ISS:36 (20–66) | 1 Shock, 2 Cardiac arrest | High-velocity penetrating trauma due to IEDs or gunshots | 2 patients: non-measurable 1 patient: 60 mmHg |
| Lendrum et al. [26] | 21 (19 trauma patients) | 22–79 | 10 female, 9 male | N/A | Median ISS 34, IQR: 27–43 | Profound shock | High-energy blunt trauma, pelvic haemorrhage due to fall, RTC | Median SBP: 57 mmHg (IQR: 40–68 mmHg) |
| Study | Zone of Balloon Deployment | Success Rate in Catheter Placement | Time of Occlusion (Mean) | Primary Outcomes (Change in BP) | Secondary Outcomes | ||
|---|---|---|---|---|---|---|---|
| Survival to the Next Higher Level of MTF | 30 day Mortality Rate | Complications | |||||
| Sadek et al. [21] | Zone 3 (n = 1) | 100% (Femoral arterial access- percutaneous) | >30 min no exact value | (Non-measurable -> 88/46 mmHg) | 100% | 0% | Not mentioned |
| Manley et al. [22] | Zone One (n = 3); Zone 3 (n = 1) | 100% (Femoral arterial access-3 percutaneous, 1 cutdown) | Zone One: 25 min Zone Three: 65 min | 51% (Mean SBP 78 mmHg ->118 mmHg) | 100% | N/A | No access-related site complication in open cut-down patients One patient had femoral sheath hematoma, exploration and arteriotomy repair done uneventfully One patient had distal migration of the balloon |
| Northern et al. [23] | Zone One (n = 17); Zone 3 (n = 3) * | 100% (Femoral arterial access 13 percutaneous, 6 cut down) | Zone One: 21 min * Zone Three: 9 min | 79%; (Mean SBP 71 mmHg ->127 mmHg) | 100% | N/A | No access-related site complication One patient had failed zone 3 REBOA with no pressure change, suspected balloon rupture due to overinflation, Shunting and ligation were done uneventfully to temporise the wound |
| Lamhaut et al. [24] | Zone One (n = 1) | 100% (Modified cutdown technique) | Zone One: 36 min | Asystole -> return of circulation | 100% | N/A—palliative care after diagnosis of advanced cancer in DCS | Not mentioned |
| de Schoutheete et al. [25] | Zone One (n = 3) | 100% (1 percutaneous, 2 cutdown) | Zone One: 31 min | Non-measurable & cardiac arrest & SBP 60 -> Mean SBP: 77 mmHg (70–90 mmHg) | 100% | N/A | Two patients developed thrombosis (One before surgical closure without clear cause, one after due to a technical error) |
| Lendrum et al. [26] | Zone Three (n = 13) | 68% (6/19 failed attempts in trauma patients due to inability to obtain arterial access resulting from poor US visualisation of CFA or failure to pass a guidewire) | Zone Three: 80 min median (IQR 75–115). | 100% (SBP 57 mmHg -> 114 mmHg (Median of differences 66, 95% CI: 25–74 mmHg; p < 0.001)) | 100% | 38% (Non-REBOA: 67%, p = 0.035) | 77% (10/13) patients developed distal arterial thrombus, requiring embolectomy or thrombectomy, (6/10 were directly related to a traumatic vascular injury) Lower limb amputation: REBOA group 31%, non REBOA 50%, p = 0.617) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, C.N.; Kadir, B.; Ahmed, Z. The Role of Prehospital REBOA for Hemorrhage Control in Civilian and Military Austere Settings: A Systematic Review. Trauma Care 2022, 2, 63-78. https://doi.org/10.3390/traumacare2010006
Chan CN, Kadir B, Ahmed Z. The Role of Prehospital REBOA for Hemorrhage Control in Civilian and Military Austere Settings: A Systematic Review. Trauma Care. 2022; 2(1):63-78. https://doi.org/10.3390/traumacare2010006
Chicago/Turabian StyleChan, Ching Nga, Bryar Kadir, and Zubair Ahmed. 2022. "The Role of Prehospital REBOA for Hemorrhage Control in Civilian and Military Austere Settings: A Systematic Review" Trauma Care 2, no. 1: 63-78. https://doi.org/10.3390/traumacare2010006
APA StyleChan, C. N., Kadir, B., & Ahmed, Z. (2022). The Role of Prehospital REBOA for Hemorrhage Control in Civilian and Military Austere Settings: A Systematic Review. Trauma Care, 2(1), 63-78. https://doi.org/10.3390/traumacare2010006