Medial Sural Perforator “Nerve through Flap”: Anatomical Study and Clinical Application
Abstract
:1. Introduction
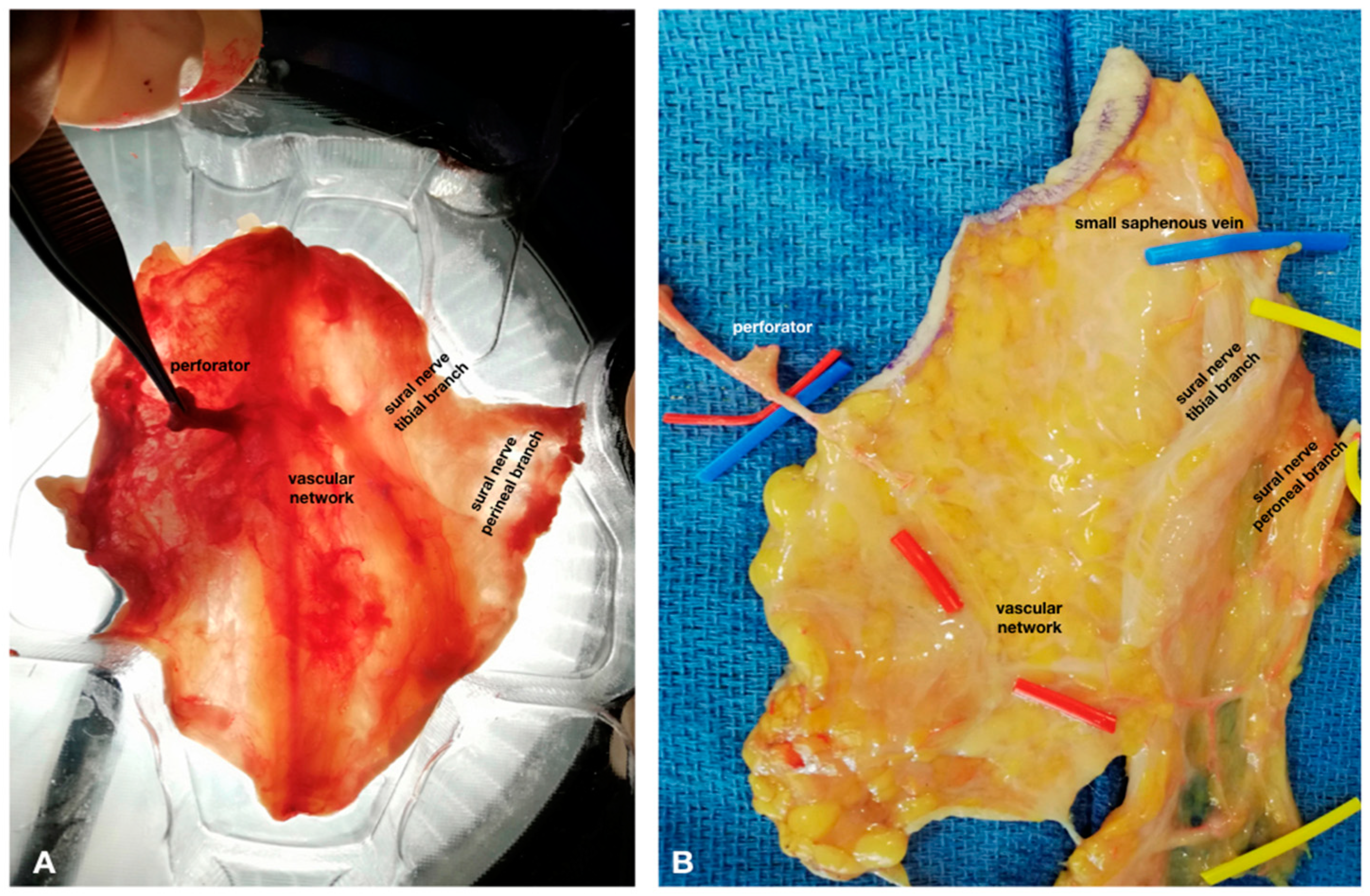
2. Materials and Methods
3. Results
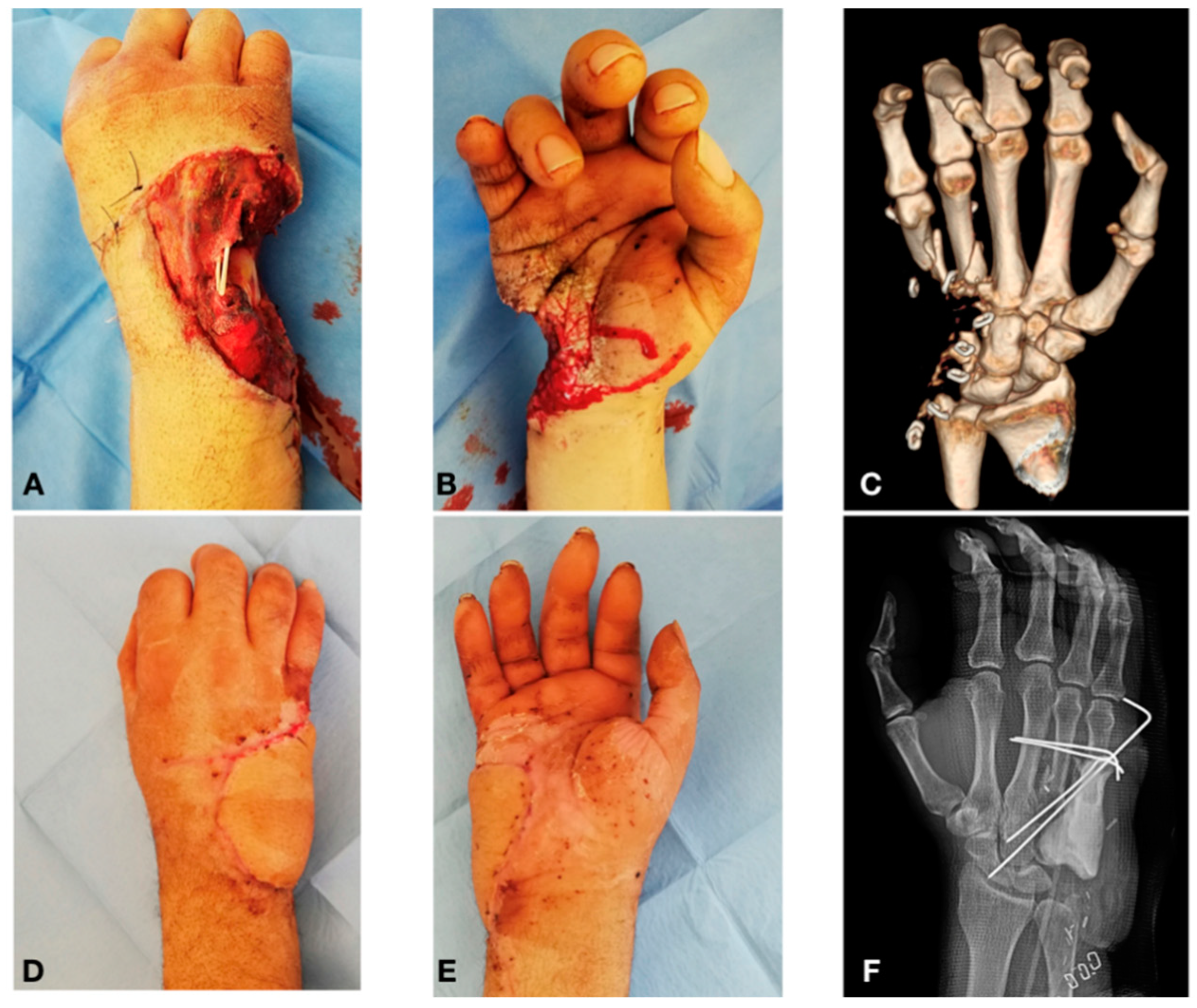
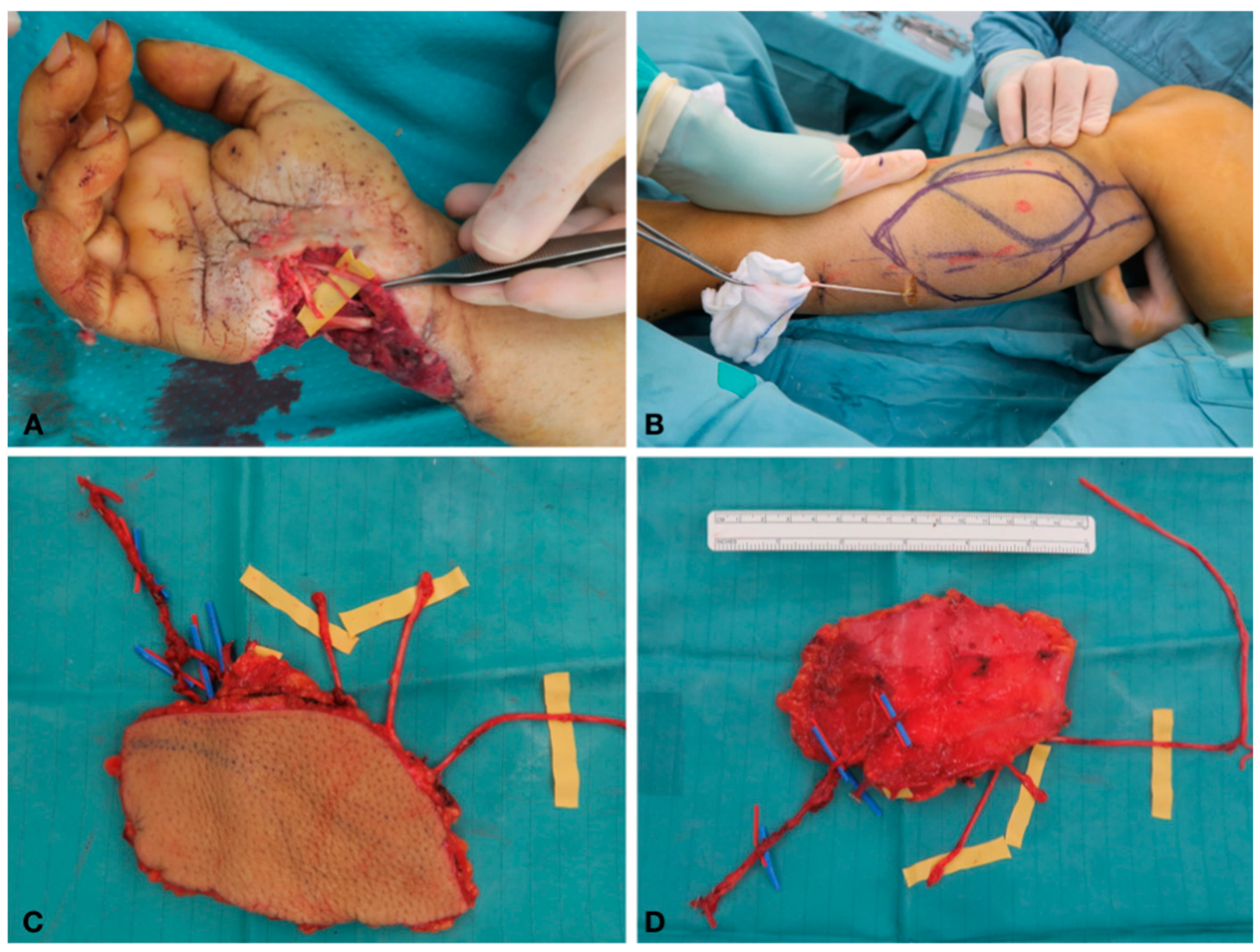
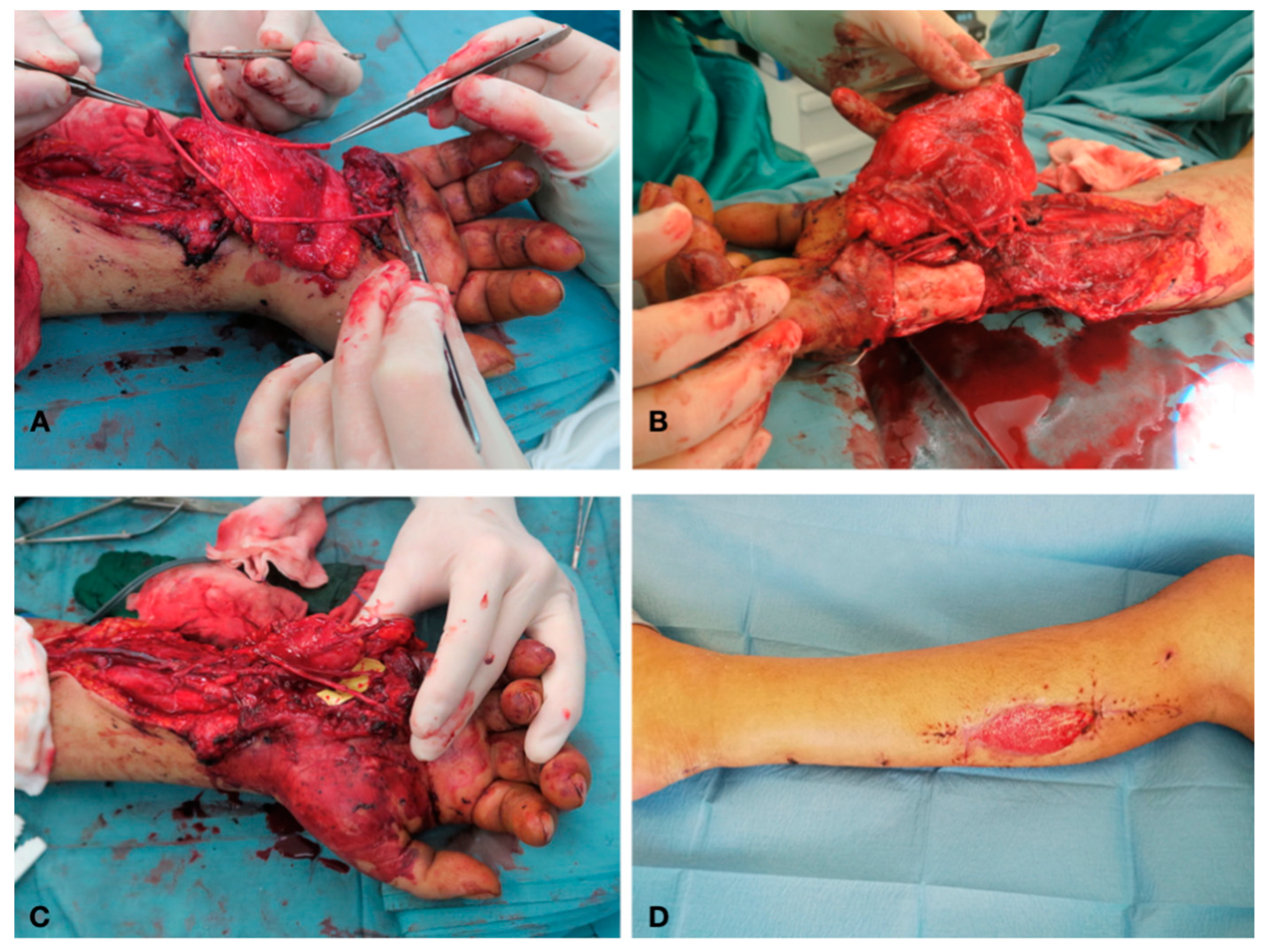
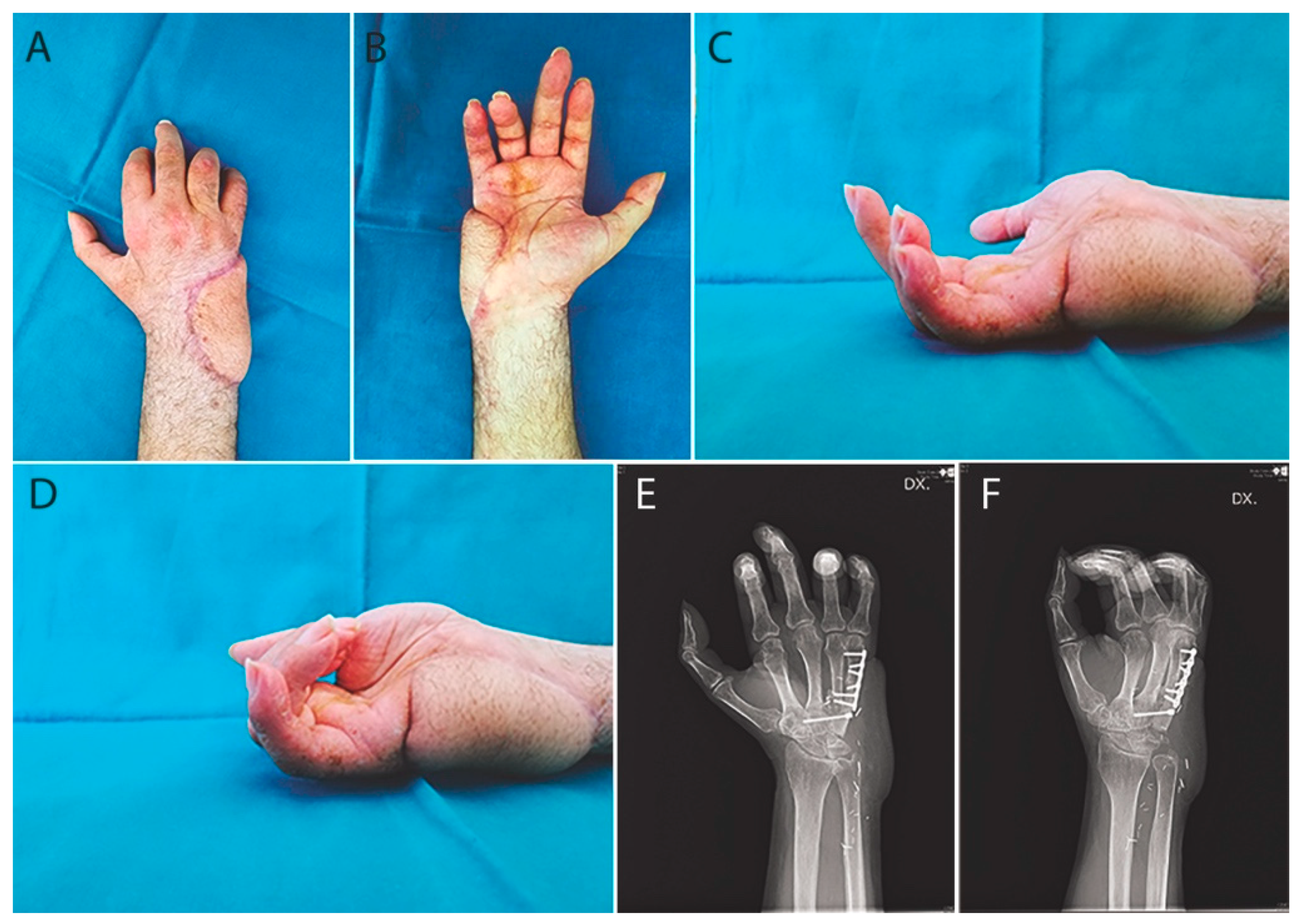
Case Report
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koshima, I.; Nanba, Y.; Tsutsui, T.; Takahashi, Y.; Kawai, A. Vascularized femoral nerve graft with anterolateral thigh true perforator flap for massive defects after cancer ablation in the upper arm. J. Reconstr. Microsurg. 2003, 19, 299–302. [Google Scholar]
- Iida, T.; Nakagawa, M.; Asano, T.; Fukushima, C.; Tachi, K. Free vascularized lateral femoral cutaneous nerve graft with anterolateral thigh flap for reconstruction of facial nerve defects. J. Reconstr. Microsurg. 2006, 22, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Narushima, M.; Kaji, N.; Yamamoto, T.; Yoshimatsu, H.; Hara, H.; Kikuchi, K.; Araki, J.; Yamashita, S.; Koshima, I. Superficial circumflex iliac artery pure skin perforator-based superthin flap for hand and finger reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.C.; Tsai, W.H.; Ting, P.S.; Hsueh, J.H.; Chen, L.W.; Lin, Y.S. Comparison between anterolateral thigh, radial forearm, and peroneal artery flap donor site thickness in Asian patients—A sonographic study. Microsurgery 2017, 37, 655–660. [Google Scholar] [CrossRef]
- Balan, J.R. Medial sural artery perforator free flap for the reconstruction of leg, foot and ankle defect: An excellent option. ANZ J. Surg. 2018, 88, E132–E136. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.Y.; Tien, H.Y.; Lin, Y.T.; Hsu, C.C.; Lin, C.H.; Chen, S.H. Prognosis of traumatic ulnar nerve injuries: A systematic review. Ann. Plast. Surg. 2019, 82, S45–S52. [Google Scholar] [CrossRef]
- Sunderland, S. The anatomy and physiology of nerve injury. Muscle Nerve 1990, 13, 771–784. [Google Scholar] [CrossRef]
- Hoke, A. A (heat) shock to the system promotes peripheral nerve regeneration. J. Clin. Investig. 2011, 121, 4231–4234. [Google Scholar] [CrossRef]
- Evriviades, D.; Jeffery, S.; Cubison, T.; Lawton, G.; Gill, M.; Mortiboy, D. Shaping the military wound: Issues surrounding the reconstruction of injured servicemen at the Royal Centre for Defence Medicine. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Moore, A.M.; Wagner, I.J.; Fox, I.K. Principles of nerve repair in complex wounds of the upper extremity. Semin. Plast. Surg. 2015, 29, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshima, I.; Harii, K. Experimental study of vascularized nerve grafts: Morphometric study of axonal regeneration of nerves transplanted into silicone tubes. Ann. Plast. Surg. 1985, 14, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, F.; Firrell, J.; Tsai, T.M.; Breidenbach, W.C. Functional results of vascularized versus nonvascularized nerve grafting. Plast. Reconstr. Surg. 1992, 89, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, G.; Shenaq, S.; Mirabi, B.; Spira, M. Nerve regeneration in a bony bed: Vascularized versus nonvascularized nerve grafts. Plast. Reconstr. Surg. 1993, 91, 1322–1331. [Google Scholar] [CrossRef]
- Mackinnon, S.E.; Kelly, L.; Hunter, D.A. Comparison of regeneration across a vascularized versus conventional nerve graft: Case report. Microsurgery 1988, 9, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Cherubino, M.; Pellegatta, I.; Crosio, A.; Valdatta, L.; Geuna, S.; Gornati, R.; Tos, P. Use of human fat grafting in the prevention of perineural adherence: Experimental study in athymic mouse. PLoS ONE 2017, 12, e0176393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campodonico, A.; Pangrazi, P.; De Francesco, F.; Riccio, M. Reconstruction of a long defect of the median nerve with a free nerve conduit flap. Arch. Plast. Surg. 2020, 47, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallock, G.G. Anatomic basis of the gastrocnemius perforator-based flap. Ann. Plast. Surg. 2001, 47, 517–522. [Google Scholar] [CrossRef]
- Cavadas, P.C.; Sanz-Giménez-Rico, J.R.; De la Càmara, G.A.; Navarro-Monzonìs, A.; Soler-Nomdedeu, S.; Martìnez-Soriano, F. The medial sural artery perforator free flap. Plast. Reconstr. Surg. 2001, 108, 1609–1617. [Google Scholar] [CrossRef]
- Wong, M.Z.; Wong, C.H.; Tan, B.K.; Chew, K.Y.; Tay, S.C. Surgical anatomy of the medial sural artery perforator flap. J. Reconstr. Microsurg. 2012, 28, 555–560. [Google Scholar] [CrossRef] [Green Version]
- Tapp, M.; Wenzinger, E.; Tarabishy, S.; Ricci, J.; Herrera, F.A. The epidemiology of upper extremity nerve injuries and associated cost in the US Emergency Departments. Ann. Plast. Surg. 2019, 83, 676–680. [Google Scholar] [CrossRef]
- Dy, C.J.; Mackinnon, S.E. Ulnar neuropathy: Evaluation and management. Curr. Rev. Musculoskelet. Med. 2016, 9, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Pfister, B.J.; Gordon, T.; Loverde, J.R.; Kochar, A.S.; Mackinnon, S.E.; Cullen, D.K. Biomedical engineering strategies for peripheral nerve repair: Surgical applications, state of the art, and future challenges. Crit. Rev. Biomed. Eng. 2011, 39, 81–124. [Google Scholar] [CrossRef]
- Griffin, M.F.; Malahias, M.; Hindocha, S.; Khan, W.S. Peripheral nerve injury: Principles for repair and regeneration. Open Orthop. J. 2014, 8, 199–203. [Google Scholar]
- Wang, M.L.; Rivlin, M.; Graham, J.G.; Beredjiklian, P.K. Peripheral nerve injury, scarring, and recovery. Connect. Tissue Res. 2019, 60, 3–9. [Google Scholar] [CrossRef]
- Yamamoto, R.; Motomiva, M.; Sakurai, K.; Sekiguchi, H.; Funakoshi, T.; Iwasaki, N. Application of free temporoparietal fascial flap for recurrent neural adhesion of superficial radial nerve—A case report. Microsurgery 2015, 35, 489–493. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugliese, P.; De Francesco, F.; Campodonico, A.; Pangrazi, P.P.; Antonini, A.; Riccio, M. Medial Sural Perforator “Nerve through Flap”: Anatomical Study and Clinical Application. Trauma Care 2021, 1, 15-22. https://doi.org/10.3390/traumacare1010002
Pugliese P, De Francesco F, Campodonico A, Pangrazi PP, Antonini A, Riccio M. Medial Sural Perforator “Nerve through Flap”: Anatomical Study and Clinical Application. Trauma Care. 2021; 1(1):15-22. https://doi.org/10.3390/traumacare1010002
Chicago/Turabian StylePugliese, Pierfrancesco, Francesco De Francesco, Andrea Campodonico, Pier Paolo Pangrazi, Andrea Antonini, and Michele Riccio. 2021. "Medial Sural Perforator “Nerve through Flap”: Anatomical Study and Clinical Application" Trauma Care 1, no. 1: 15-22. https://doi.org/10.3390/traumacare1010002
APA StylePugliese, P., De Francesco, F., Campodonico, A., Pangrazi, P. P., Antonini, A., & Riccio, M. (2021). Medial Sural Perforator “Nerve through Flap”: Anatomical Study and Clinical Application. Trauma Care, 1(1), 15-22. https://doi.org/10.3390/traumacare1010002





