Functional Hydrogels in Bone Tissue Engineering: From Material Design to Translational Applications
Abstract
1. Introduction
2. Hydrogel’s Properties
2.1. Classification and Design
2.2. Properties of Hydrogel
2.2.1. Physical Properties
2.2.2. Chemical Properties
2.2.3. Mechanical Properties
2.3. Bioactive Delivery
3. Preclinical and Clinical Applications
4. Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECM | Bone extracellular matrix |
| MSCs | Mesenchymal stem cells |
| PEG | Polyethylene glycol |
| PLA | Polylactic acid |
| PCL | Polycaprolactone |
| PVA | Polyvinyl alcohol |
| PPF | Polypropylene fumarate |
| OGP | Osteogenic growth peptide |
| GelMA | Gelatin methacrylate |
| MMP | Matrix metalloproteinase |
| BMP-2 | Bone morphogenetic protein-2 |
| VEGF | Vascular endothelial growth factor |
| PDGF | Platelet-derived growth factor |
| TGF-β | Transforming growth factor beta |
| PRP | Platelet-rich plasma |
| OSA | Oxidised alginate |
| Dex-TA | Dextran-tyramine |
| bFGF | Basic fibroblast growth factor |
| mPGA | Methacrylated poly(γ-glutamic acid) |
| PLGA | Poly(lactic-co-glycolic acid) |
| NGF | Nerve growth factor |
| DEXGEL | Dextrin-based injectable hydrogel |
References
- Vanderkarr, M.F.; Ruppenkamp, J.W.; Vanderkarr, M.; Holy, C.E.; Blauth, M. Risk factors and healthcare costs associated with long bone fracture non-union: A retrospective US claims database analysis. J. Orthop. Surg. Res. 2023, 18, 745. [Google Scholar] [CrossRef]
- Maisenbacher, T.C.; Rollmann, M.F.; Menger, M.M.; Braun, N.R.; Braun, B.J.; Herath, S.C.; Stuby, F.; Nuessler, A.K.; Histing, T.; Reumann, M.K. Direct and indirect costs of long bone fracture nonunions of the lower limb: The economic burden on the German healthcare system. Bone Jt. Res. 2025, 14, 338–347. [Google Scholar] [CrossRef]
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Thomas, J.D.; Kehoe, J.L. Bone nonunion. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Bowers, K.M.; Anderson, D.E. Delayed union and nonunion: Current concepts, prevention, and correction: A review. Bioengineering 2024, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Zura, R.; Xiong, Z.; Einhorn, T.; Watson, J.T.; Ostrum, R.F.; Prayson, M.J.; Della Rocca, G.J.; Mehta, S.; McKinley, T.; Wang, Z. Epidemiology of fracture nonunion in 18 human bones. JAMA Surg. 2016, 151, e162775. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Bi, Q.; Zhao, C.; Chen, J.-Y.; Cai, M.-H.; Chen, X.-Y. Recent advances in biomaterials for the treatment of bone defects. Organogenesis 2020, 16, 113–125. [Google Scholar] [CrossRef]
- De Pace, R.; Molinari, S.; Mazzoni, E.; Perale, G. Bone regeneration: A review of current treatment strategies. J. Clin. Med. 2025, 14, 1838. [Google Scholar] [CrossRef]
- Santoro, A.; Voto, A.; Fortino, L.; Guida, R.; Laudisio, C.; Cillo, M.; D’Ursi, A.M. Bone defect treatment in regenerative medicine: Exploring natural and synthetic bone substitutes. Int. J. Mol. Sci. 2025, 26, 3085. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Sun, H.; Xu, J.; Wang, Y.; Shen, S.; Xu, X.; Zhang, L.; Jiang, Q. Bone microenvironment regulative hydrogels with ROS scavenging and prolonged oxygen-generating for enhancing bone repair. Bioact. Mater. 2023, 24, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic and Bone tissue engineering graft substitutes: What is the future? Injury 2021, 52, S72–S77. [Google Scholar] [CrossRef]
- Dos Santos, R.L.; Ahmed, A.; Hunn, B.E.; Addison, A.E.; Marques, D.W.; Bruce, K.A.; Martin, J.R. Oxidation-responsive, settable bone substitute composites for regenerating critically-sized bone defects. Biomater. Sci. 2025, 13, 1975–1992. [Google Scholar] [CrossRef]
- Liu, H.; Chen, H.; Han, Q.; Sun, B.; Liu, Y.; Zhang, A.; Fan, D.; Xia, P.; Wang, J. Recent advancement in vascularized tissue-engineered bone based on materials design and modification. Mater. Today Bio 2023, 23, 100858. [Google Scholar] [CrossRef] [PubMed]
- Madhavarapu, S.; Lakshmikanthan, A.; Cipriano, J.; Mai, L.; Frazier, B.; Cook-Chennault, K.; Kanna, A.J.; Franco, F.; Freeman, J.W. 3D-printed polymer scaffolds for vascularized bone regeneration using mineral and extracellular matrix deposition. Regen. Eng. Transl. Med. 2025, 11, 512–523. [Google Scholar] [CrossRef]
- Ghasabpour, T.; Sharifianjazi, F.; Bazli, L.; Tebidze, N.; Sorkhabi, M. Bioactive glasses, ceramics, glass-ceramics and composites: State-of-the-art review and future challenges. J. Compos. Compd. 2025, 7, 1–16. [Google Scholar] [CrossRef]
- Niazvand, F.; Hosseinzadeh, J.; Zahed, P.; Azizabadi, N. Effect of SrO incorporation on the morphology of sol-gel derived 58S bioactive glass. J. Compos. Compd. 2025, 7, 1–5. [Google Scholar]
- Kubiak-Mihkelsoo, Z.; Kostrzębska, A.; Błaszczyszyn, A.; Pitułaj, A.; Dominiak, M.; Gedrange, T.; Nawrot-Hadzik, I.; Matys, J.; Hadzik, J. Ionic doping of hydroxyapatite for bone regeneration: Advances in structure and properties over two decades—A narrative review. Appl. Sci. 2025, 15, 1108. [Google Scholar] [CrossRef]
- Zheng, Y.; Ke, Z.; Hu, G.; Tong, S. Hydrogel promotes bone regeneration through various mechanisms: A review. Biomed. Eng./Biomed. Tech. 2025, 70, 103–114. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, X.; Zhou, X.; Tan, W.; Luo, H.; Lei, S.; Yang, Y. GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: Therapeutic strategies and recent advances. Biomater. Res. 2023, 27, 86. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, Z.; Zheng, Z.; Chen, H.; Chen, Y. Nanomaterial-integrated injectable hydrogels for craniofacial bone reconstruction. J. Nanobiotechnol. 2024, 22, 525. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, J.; Hu, C.; Wang, Y.; He, C. Advances in hydrogel systems for bone regeneration: Trends, innovations, and prospects. J. Mater. Chem. B 2025, 13, 14869–14908. [Google Scholar] [CrossRef] [PubMed]
- Gough, J.E.; Saiani, A.; Miller, A.F. Peptide hydrogels: Mimicking the extracellular matrix. Bioinspired Biomim. Nanobiomater. 2012, 1, 4–12. [Google Scholar] [CrossRef]
- Cota Quintero, J.L.; Ramos-Payán, R.; Romero-Quintana, J.G.; Ayala-Ham, A.; Bermúdez, M.; Aguilar-Medina, E.M. Hydrogel-Based Scaffolds: Advancing Bone Regeneration Through Tissue Engineering. Gels 2025, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Fedorovich, N.E.; Alblas, J.; de Wijn, J.R.; Hennink, W.E.; Verbout, A.J.; Dhert, W.J.A. Hydrogels as extracellular matrices for skeletal tissue engineering: State-of-the-art and novel application in organ printing. Tissue Eng. 2007, 13, 1905–1925. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shin, H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 339–359. [Google Scholar] [CrossRef]
- Jurczak, P.; Lach, S. Hydrogels as Scaffolds in Bone-Related Tissue Engineering and Regeneration. Macromol. Biosci. 2023, 23, 2300152. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Maisani, M.; Pezzoli, D.; Chassande, O.; Mantovani, D. Cellularizing hydrogel-based scaffolds to repair bone tissue: How to create a physiologically relevant micro-environment? J. Tissue Eng. 2017, 8, 2041731417712073. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Liu, K.; Gao, F. Hydrogel scaffolds in bone regeneration: Their promising roles in angiogenesis. Front. Pharmacol. 2023, 14, 1050954. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Jia, Z.; Jiao, K.; Liu, C.; Deng, Z.; Bai, Y.; Wei, X.; Zhou, X. Bioprinted hydrogels in bone regeneration: A bibliometric analysis. Front. Pharmacol. 2025, 16, 1532629. [Google Scholar] [CrossRef]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C., Jr.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Bhatia, S. Natural polymers vs synthetic polymer. In Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Springer: Berlin/Heidelberg, Germany, 2016; pp. 95–118. [Google Scholar]
- Yang, J.; Sun, X.; Zhang, Y.; Chen, Y. The application of natural polymer–based hydrogels in tissue engineering. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–307. [Google Scholar]
- Hu, T.; Fang, J.; Shen, Y.; Li, M.; Wang, B.; Xu, Z.; Hu, W. Advances of naturally derived biomedical polymers in tissue engineering. Front. Chem. 2024, 12, 1469183. [Google Scholar] [CrossRef]
- Morwood, A.J.; El-Karim, I.A.; Clarke, S.A.; Lundy, F.T. The role of extracellular matrix (ECM) adhesion motifs in functionalised hydrogels. Molecules 2023, 28, 4616. [Google Scholar] [CrossRef] [PubMed]
- Radwan-Pragłowska, J.; Piątkowski, M.; Deineka, V.; Janus, Ł.; Korniienko, V.; Husak, E.; Holubnycha, V.; Liubchak, I.; Zhurba, V.; Sierakowska, A. Chitosan-based bioactive hemostatic agents with antibacterial properties—Synthesis and characterization. Molecules 2019, 24, 2629. [Google Scholar] [CrossRef]
- Ren, Z.; Li, M.; Wang, F.; Qiao, J.; Kaya, M.G.A.; Tang, K. Antibacterial chitosan-based composite sponge with synergistic hemostatic effect for massive haemorrhage. Int. J. Biol. Macromol. 2023, 252, 126344. [Google Scholar] [CrossRef]
- Neuman, M.G.; Nanau, R.M.; Oruña-Sanchez, L.; Coto, G. Hyaluronic acid and wound healing. J. Pharm. Pharm. Sci. 2015, 18, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Price, R.D.; Myers, S.; Leigh, I.M.; Navsaria, H.A. The role of hyaluronic acid in wound healing: Assessment of clinical evidence. Am. J. Clin. Dermatol. 2005, 6, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, A.; Liu, S.; Lv, M.; Chen, S. Research progress in decellularized extracellular matrix-derived hydrogels. Regen. Ther. 2021, 18, 88–96. [Google Scholar] [CrossRef]
- Yao, Q.; Zheng, Y.-W.; Lan, Q.-H.; Kou, L.; Xu, H.-L.; Zhao, Y.-Z. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mater. Sci. Eng. C 2019, 104, 109942. [Google Scholar] [CrossRef]
- Kim, Y.S.; Majid, M.; Melchiorri, A.J.; Mikos, A.G. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 2019, 4, 83–95. [Google Scholar] [CrossRef]
- Gaihre, B.; Liu, X.; Lee Miller, A.; Yaszemski, M.; Lu, L. Poly (caprolactone fumarate) and oligo [poly (ethylene glycol) fumarate]: Two decades of exploration in biomedical applications. Polym. Rev. 2021, 61, 319–356. [Google Scholar] [CrossRef]
- Krishna, L.; Jayabalan, M. Synthesis and characterization of biodegradable poly (ethylene glycol) and poly (caprolactone diol) end capped poly (propylene fumarate) cross linked amphiphilic hydrogel as tissue engineering scaffold material. J. Mater. Sci. Mater. Med. 2009, 20, 115–122. [Google Scholar] [CrossRef]
- Neves, S.C.; Pereira, R.F.; Araújo, M.; Barrias, C.C. Bioengineered peptide-functionalized hydrogels for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Elsevier: Amsterdam, The Netherlands, 2018; pp. 101–125. [Google Scholar]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural polymer-based hydrogels with enhanced mechanical performances: Preparation, structure, and property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Saik, J.E.; Gould, D.J.; Dickinson, M.E.; West, J.L. Immobilization of cell-adhesive laminin peptides in degradable PEGDA hydrogels influences endothelial cell tubulogenesis. BioRes. Open Access 2013, 2, 241–249. [Google Scholar] [CrossRef]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and synthetic polymers for bone scaffolds optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Furko, M.; Balázsi, K.; Balázsi, C. Calcium phosphate loaded biopolymer composites—A comprehensive review on the most recent progress and promising trends. Coatings 2023, 13, 360. [Google Scholar] [CrossRef]
- Kucko, S.K.; Raeman, S.M.; Keenan, T.J. Current advances in hydroxyapatite-and β-tricalcium phosphate-based composites for biomedical applications: A review. Biomed. Mater. Devices 2023, 1, 49–65. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, Y.; Kuang, R.; Liu, H.; Sun, D.; Mao, T.; Jiang, K.; Yang, X.; Watanabe, N.; Mayo, K.H. Injectable hydrogel-loaded nano-hydroxyapatite that improves bone regeneration and alveolar ridge promotion. Mater. Sci. Eng. C 2020, 116, 111158. [Google Scholar] [CrossRef]
- Fu, M.; Li, J.; Liu, M.; Yang, C.; Wang, Q.; Wang, H.; Chen, B.; Fu, Q.; Sun, G. Sericin/nano-hydroxyapatite hydrogels based on graphene oxide for effective bone regeneration via immunomodulation and osteoinduction. Int. J. Nanomed. 2023, 18, 1875–1895. [Google Scholar] [CrossRef]
- Chen, R.; Chen, H.-B.; Xue, P.-P.; Yang, W.-G.; Luo, L.-Z.; Tong, M.-Q.; Zhong, B.; Xu, H.-L.; Zhao, Y.-Z.; Yuan, J.-D. HA/MgO nanocrystal-based hybrid hydrogel with high mechanical strength and osteoinductive potential for bone reconstruction in diabetic rats. J. Mater. Chem. B 2021, 9, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, T.; Mesgar, A.S.; Mohammadi, Z. Bioactive glasses: A promising therapeutic ion release strategy for enhancing wound healing. ACS Biomater. Sci. Eng. 2020, 6, 5399–5430. [Google Scholar] [CrossRef]
- Van Rijt, S.; De Groot, K.; Leeuwenburgh, S.C.G. Calcium phosphate and silicate-based nanoparticles: History and emerging trends. Tissue Eng. Part A 2022, 28, 461–477. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive glasses and glass-ceramics for healthcare applications in bone regeneration and tissue engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef]
- Taye, M.B. Biomedical applications of ion-doped bioactive glass: A review. Appl. Nanosci. 2022, 12, 3797–3812. [Google Scholar] [CrossRef]
- Qin, S.; Niu, Y.; Zhang, Y.; Wang, W.; Zhou, J.; Bai, Y.; Ma, G. Metal ion-containing hydrogels: Synthesis, properties, and applications in bone tissue engineering. Biomacromolecules 2024, 25, 3217–3248. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Weng, J.; Yu, F.; Zhang, W.; Li, G.; Qin, H.; Tan, Z.; Zeng, H. Insights into the role of magnesium ions in affecting osteogenic differentiation of mesenchymal stem cells. Biol. Trace Elem. Res. 2021, 199, 559–567. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, J.; Zhang, X.; Liang, G.; Xu, T.; Niu, W. Three-dimensional printed Mg-doped β-TCP bone tissue engineering scaffolds: Effects of magnesium ion concentration on osteogenesis and angiogenesis in vitro. Tissue Eng. Regen. Med. 2019, 16, 415–429. [Google Scholar] [CrossRef]
- Fisher, S.A.; Baker, A.E.G.; Shoichet, M.S. Designing peptide and protein modified hydrogels: Selecting the optimal conjugation strategy. J. Am. Chem. Soc. 2017, 139, 7416–7427. [Google Scholar] [CrossRef]
- Mondal, S.; Das, S.; Nandi, A.K. A review on recent advances in polymer and peptide hydrogels. Soft Matter 2020, 16, 1404–1454. [Google Scholar] [CrossRef]
- Giordano, S.; Gallo, E.; Diaferia, C.; Rosa, E.; Carrese, B.; Borbone, N.; Scognamiglio, P.L.; Franzese, M.; Oliviero, G.; Accardo, A. Multicomponent peptide-based hydrogels containing chemical functional groups as innovative platforms for biotechnological applications. Gels 2023, 9, 903. [Google Scholar] [CrossRef]
- Gallo, E.; Diaferia, C.; Giordano, S.; Rosa, E.; Carrese, B.; Piccialli, G.; Borbone, N.; Morelli, G.; Oliviero, G.; Accardo, A. Ultrashort cationic peptide Fmoc-FFK as hydrogel building block for potential biomedical applications. Gels 2023, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Ligorio, C.; Mata, A. Synthetic extracellular matrices with function-encoding peptides. Nat. Rev. Bioeng. 2023, 1, 518–536. [Google Scholar] [CrossRef]
- Unal, A.Z.; West, J.L. Synthetic ECM: Bioactive synthetic hydrogels for 3D tissue engineering. Bioconjug. Chem. 2020, 31, 2253–2271. [Google Scholar] [CrossRef] [PubMed]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef]
- Burdick, J.A.; Anseth, K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 2002, 23, 4315–4323. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, Y.; Wang, M.; Huang, Y.; Xu, H.; Su, Y.; Zhao, Y.; Shang, Y. Supramolecular hydrogel based on an osteogenic growth peptide promotes bone defect repair. ACS Omega 2022, 7, 11395–11404. [Google Scholar] [CrossRef]
- Luo, P.; Fang, J.; Yang, D.; Yu, L.; Chen, H.; Jiang, C.; Guo, R.; Zhu, T.; Tang, S. OP3-4 peptide sustained-release hydrogel inhibits osteoclast formation and promotes vascularization to promote bone regeneration in a rat femoral defect model. Bioeng. Transl. Med. 2023, 8, e10414. [Google Scholar]
- Qiao, Y.; Liu, X.; Zhou, X.; Zhang, H.; Zhang, W.; Xiao, W.; Pan, G.; Cui, W.; Santos, H.A.; Shi, Q. Gelatin templated polypeptide co-cross-linked hydrogel for bone regeneration. Adv. Healthc. Mater. 2020, 9, 1901239. [Google Scholar] [CrossRef]
- Benoit, D.S.W.; Collins, S.D.; Anseth, K.S. Multifunctional hydrogels that promote osteogenic human mesenchymal stem cell differentiation through stimulation and sequestering of bone morphogenic protein 2. Adv. Funct. Mater. 2007, 17, 2085–2093. [Google Scholar] [CrossRef]
- Reddi, A.H. Morphogenesis and tissue engineering of bone and cartilage: Inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000, 6, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Tharakan, S.; Raja, I.; Pietraru, A.; Sarecha, E.; Gresita, A.; Petcu, E.; Ilyas, A.; Hadjiargyrou, M. The use of hydrogels for the treatment of bone osteosarcoma via localized drug-delivery and tissue regeneration: A narrative review. Gels 2023, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.; Li, C.; Weir, M.D.; Wang, P.; Reynolds, M.A.; Zhao, L.; Xu, H.H.K. Injectable calcium phosphate with hydrogel fibers encapsulating induced pluripotent, dental pulp and bone marrow stem cells for bone repair. Mater. Sci. Eng. C 2016, 69, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Wang, N.; You, X.-G.; Zhang, A.-D.; Chen, X.-G.; Liu, Y. Collagen-based biocomposites inspired by bone hierarchical structures for advanced bone regeneration: Ongoing research and perspectives. Biomater. Sci. 2022, 10, 318–353. [Google Scholar] [CrossRef]
- Agrawal, V.; Sinha, M. A review on carrier systems for bone morphogenetic protein-2. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 904–925. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhao, L.; Zhang, J.; Luo, H. Constructions and properties of physically cross-linked hydrogels based on natural polymers. Polym. Rev. 2023, 63, 574–612. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B: Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef]
- Tordi, P.; Ridi, F.; Samorì, P.; Bonini, M. Cation-alginate complexes and their hydrogels: A powerful toolkit for the development of next-generation sustainable functional materials. Adv. Funct. Mater. 2025, 35, 2416390. [Google Scholar]
- Van Damme, L.; Blondeel, P.; Van Vlierberghe, S. Injectable biomaterials as minimal invasive strategy towards soft tissue regeneration—An overview. J. Phys. Mater. 2021, 4, 022001. [Google Scholar] [CrossRef]
- Young, S.A.; Riahinezhad, H.; Amsden, B.G. In situ-forming, mechanically resilient hydrogels for cell delivery. J. Mater. Chem. B 2019, 7, 5742–5761. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.L.; Woodrow, K.A. Medical applications of porous biomaterials: Features of porosity and tissue-specific implications for biocompatibility. Adv. Healthc. Mater. 2022, 11, 2102087. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in porous scaffold design for bone and cartilage tissue engineering and regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, B.; Yang, C.; Wu, J.; Zhao, Q. Macroporous hydrogels prepared by ice templating: Developments and challenges. Chin. J. Chem. 2023, 41, 3082–3096. [Google Scholar] [CrossRef]
- De France, K.J.; Xu, F.; Hoare, T. Structured macroporous hydrogels: Progress, challenges, and opportunities. Adv. Healthc. Mater. 2018, 7, 1700927. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Braschler, T.M.; Renaud, P. Advances in the design of macroporous polymer scaffolds for potential applications in dentistry. J. Periodontal Implant Sci. 2013, 43, 251. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R.S.R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Bejenaru, L.E.; Bejenaru, C.; Blendea, A.; Mogoşanu, G.D.; Biţă, A.; Boia, E.R. Advancements in Hydrogels: A Comprehensive Review of Natural and Synthetic Innovations for Biomedical Applications. Polymers 2025, 17, 2026. [Google Scholar] [CrossRef] [PubMed]
- Carriero, V.C.; Di Muzio, L.; Petralito, S.; Casadei, M.A.; Paolicelli, P. Cryogel scaffolds for tissue-engineering: Advances and challenges for effective bone and cartilage regeneration. Gels 2023, 9, 979. [Google Scholar] [CrossRef]
- Mukasheva, F.; Moazzam, M.; Yernaimanova, B.; Shehzad, A.; Zhanbassynova, A.; Berillo, D.; Akilbekova, D. Design and characterization of 3D printed pore gradient hydrogel scaffold for bone tissue engineering. Bioprinting 2024, 39, e00341. [Google Scholar] [CrossRef]
- Castanheira, E.J.; Maia, J.R.; Monteiro, L.P.G.; Sobreiro-Almeida, R.; Wittig, N.K.; Birkedal, H.; Rodrigues, J.M.M.; Mano, J.F. 3D-printed injectable nanocomposite cryogel scaffolds for bone tissue regeneration. Mater. Today Nano 2024, 28, 100519. [Google Scholar] [CrossRef]
- Yang, D. Recent advances in hydrogels. Chem. Mater. 2022, 34, 1987–1989. [Google Scholar] [CrossRef]
- Arkenberg, M.R.; Nguyen, H.D.; Lin, C.-C. Recent advances in bio-orthogonal and dynamic crosslinking of biomimetic hydrogels. J. Mater. Chem. B 2020, 8, 7835–7855. [Google Scholar] [CrossRef]
- Bednarek, C.; Schepers, U.; Thomas, F.; Bräse, S. Bioconjugation in materials science. Adv. Funct. Mater. 2024, 34, 2303613. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, K.; Mitragotri, S. Covalently Crosslinked hydrogels via step-growth reactions: Crosslinking chemistries, polymers, and clinical impact. Adv. Mater. 2021, 33, 2006362. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, F.; Liu, Y. A review of the mechanism, properties, and applications of hydrogels prepared by enzymatic cross-linking. J. Agric. Food Chem. 2023, 71, 10238–10249. [Google Scholar] [CrossRef]
- Teixeira, L.S.M.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef]
- Yao, H.; Wang, J.; Mi, S. Photo processing for biomedical hydrogels design and functionality: A review. Polymers 2017, 10, 11. [Google Scholar] [CrossRef]
- Dikovsky, D.; Bianco-Peled, H.; Seliktar, D. Proteolytically Degradable Photo-Polymerized Hydrogels Made From PEG–Fibrinogen Adducts. Adv. Eng. Mater. 2010, 12, B200–B209. [Google Scholar] [CrossRef]
- Sun, J.; Chen, C.; Zhang, B.; Yao, C.; Zhang, Y. Advances in 3D-printed scaffold technologies for bone defect repair: Materials, biomechanics, and clinical prospects. BioMed. Eng. OnLine 2025, 24, 51. [Google Scholar] [CrossRef] [PubMed]
- Houchin, M.L.; Topp, E.M. Chemical degradation of peptides and proteins in PLGA: A review of reactions and mechanisms. J. Pharm. Sci. 2008, 97, 2395–2404. [Google Scholar] [CrossRef]
- Martins, C.; Sousa, F.; Araújo, F.; Sarmento, B. Functionalizing PLGA and PLGA derivatives for drug delivery and tissue regeneration applications. Adv. Healthc. Mater. 2018, 7, 1701035. [Google Scholar] [CrossRef]
- Yildirimer, L.; Seifalian, A.M. Three-dimensional biomaterial degradation—Material choice, design and extrinsic factor considerations. Biotechnol. Adv. 2014, 32, 984–999. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Zhang, W. Control of scaffold degradation in tissue engineering: A review. Tissue Eng. Part B Rev. 2014, 20, 492–502. [Google Scholar] [CrossRef]
- Lee, K.Y.; Bouhadir, K.H.; Mooney, D.J. Controlled degradation of hydrogels using multi-functional cross-linking molecules. Biomaterials 2004, 25, 2461–2466. [Google Scholar] [CrossRef]
- Konieczynska, M.D.; Grinstaff, M.W. On-demand dissolution of chemically cross-linked hydrogels. Acc. Chem. Res. 2017, 50, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sheng, S.; Tan, K.; Wang, S.; Wu, X.; Yang, J.; Hu, Y.; Cao, L.; Xu, K.; Zhou, F. Injectable hydrogels for bone regeneration with tunable degradability via peptide chirality modification. Mater. Horiz. 2024, 11, 4367–4377. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, Y.; Wang, Y.; Zhou, T.; Ma, B.; Zhou, P. A multifunctional nanocomposite hydrogel with controllable release behavior enhances bone regeneration. Regen. Biomater. 2023, 10, rbad046. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Luo, H.; Chen, J.; Chen, G.; Yu, X.; Ye, Z. BMP-2 releasing mineral-coated microparticle-integrated hydrogel system for enhanced bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1217335. [Google Scholar] [CrossRef] [PubMed]
- El-Rashidy, A.A.; El Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.S.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Effect of polymeric matrix stiffness on osteogenic differentiation of mesenchymal stem/progenitor cells: Concise review. Polymers 2021, 13, 2950. [Google Scholar] [CrossRef]
- Yu, X.; Qin, Z.; Wu, H.; Lv, H.; Yang, X. Tuning hydrogel mechanics by kinetically dependent cross-linking. Macromolecules 2019, 52, 1249–1256. [Google Scholar] [CrossRef]
- Kong, H.J.; Wong, E.; Mooney, D.J. Independent control of rigidity and toughness of polymeric hydrogels. Macromolecules 2003, 36, 4582–4588. [Google Scholar] [CrossRef]
- Lin, P.; Ma, S.; Wang, X.; Zhou, F. Molecularly engineered dual-crosslinked hydrogel with ultrahigh mechanical strength, toughness, and good self-recovery. Adv. Mater. 2015, 27, 2054–2059. [Google Scholar] [CrossRef]
- Panteli, P.A.; Patrickios, C.S. Multiply interpenetrating polymer networks: Preparation, mechanical properties, and applications. Gels 2019, 5, 36. [Google Scholar] [CrossRef]
- Abd El-Aziz, A.M.; Abd El-Fattah, A.; El-Maghraby, A.; Ghareeb, D.A.; Kandil, S. Viscoelasticity, mechanical properties, and in vitro bioactivity of gelatin/borosilicate bioactive glass nanocomposite hydrogels as potential scaffolds for bone regeneration. Polymers 2021, 13, 2014. [Google Scholar] [CrossRef]
- Fisher, J.P.; Dean, D.; Mikos, A.G. Photocrosslinking characteristics and mechanical properties of diethyl fumarate/poly (propylene fumarate) biomaterials. Biomaterials 2002, 23, 4333–4343. [Google Scholar] [CrossRef]
- Tao, D.; Wang, H.; Chang, S.; Cheng, J.; Da, N.; Zhang, L.; Yang, J.; Wang, W.; Xu, F.; Li, B. Matrix Viscoelasticity Orchestrates Osteogenesis via Mechanotransduction Mediated Metabolic Switch in Macrophages. Adv. Healthc. Mater. 2025, 14, 2405097. [Google Scholar] [CrossRef] [PubMed]
- Bernero, M.; Zauchner, D.; Müller, R.; Qin, X.-H. Interpenetrating network hydrogels for studying the role of matrix viscoelasticity in 3D osteocyte morphogenesis. Biomater. Sci. 2024, 12, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; Zhou, Y.-N.; Yu, X.-G.; Fu, Z.-Y.; Zhao, C.-C.; Hu, Y.; Lin, K.-L.; Xu, Y.-J. A xonotlite nanofiber bioactive 3D-printed hydrogel scaffold based on osteo-/angiogenesis and osteoimmune microenvironment remodeling accelerates vascularized bone regeneration. J. Nanobiotechnol. 2024, 22, 59. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, T.; Peng, L.; Sun, Q.; Wei, Y.; Han, B. Advancements in hydrogel-based drug sustained release systems for bone tissue engineering. Front. Pharmacol. 2020, 11, 622. [Google Scholar] [CrossRef]
- Du, Z.; Cao, G.; Li, K.; Zhang, R.; Li, X. Nanocomposites for the delivery of bioactive molecules in tissue repair: Vital structural features, application mechanisms, updated progress and future perspectives. J. Mater. Chem. B 2020, 8, 10271–10289. [Google Scholar] [CrossRef]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef]
- Shan, B.H.; Wu, F.G. Hydrogel-based growth factor delivery platforms: Strategies and recent advances. Adv. Mater. 2024, 36, 2210707. [Google Scholar] [CrossRef]
- Tayalia, P.; Mooney, D.J. Controlled growth factor delivery for tissue engineering. Adv. Mater. 2009, 21, 3269–3285. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Alsberg, E. Bioactive factor delivery strategies from engineered polymer hydrogels for therapeutic medicine. Prog. Polym. Sci. 2014, 39, 1235–1265. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Ji, H.; Qian, Y.; Wang, Q.; Liu, X.; Zhao, W.; Zhao, C. Heparin-based and heparin-inspired hydrogels: Size-effect, gelation and biomedical applications. J. Mater. Chem. B 2019, 7, 1186–1208. [Google Scholar] [CrossRef] [PubMed]
- Safi, S.Z.; Fazil, S.; Saeed, L.; Shah, H.; Arshad, M.; Alobaid, H.M.; Rehman, F.; Sharif, F.; Selvaraj, C.; Orakzai, A.H. Chitosan-and heparin-based advanced hydrogels: Their chemistry, structure and biomedical applications. Chem. Pap. 2024, 78, 9287–9309. [Google Scholar] [CrossRef]
- Qi, J.; Wu, H.; Liu, G. Novel strategies for spatiotemporal and controlled BMP-2 delivery in bone tissue engineering. Cell Transplant. 2024, 33, 09636897241276733. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Kim, K. Bone morphogenetic protein-2 associated multiple growth factor delivery for bone tissue regeneration. J. Pharm. Investig. 2018, 48, 187–197. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Li, B.; Chen, L. Controlled dual delivery of low doses of BMP-2 and VEGF in a silk fibroin–nanohydroxyapatite scaffold for vascularized bone regeneration. J. Mater. Chem. B 2017, 5, 6963–6972. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.-L.; Deng, Z.-P.; Wang, Z.-L.; Song, F.; Zhu, L.-L. Emerging Delivery Strategies of Platelet-Rich Plasma with Hydrogels for Wound Healing. Adv. Polym. Technol. 2022, 2022, 5446291. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Law, J.X.; Ng, S.-F. Delivery systems for platelet derived growth factors in wound healing: A review of recent developments and global patent landscape. J. Drug Deliv. Sci. Technol. 2022, 71, 103270. [Google Scholar] [CrossRef]
- Wang, J.-F.; Jan, J.-S.; Hu, J.-J. Heparin-Based Growth Factor Delivery Platforms: A Review. Pharmaceutics 2025, 17, 1145. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Pedrares, N.; Fuentes-Boquete, I.; Díaz-Prado, S.; Rey-Rico, A. Hydrogel-based localized nonviral gene delivery in regenerative medicine approaches—An overview. Pharmaceutics 2020, 12, 752. [Google Scholar] [CrossRef]
- Jahangiri, S.; Rahimnejad, M.; Nasrollahi Boroujeni, N.; Ahmadi, Z.; Motamed Fath, P.; Ahmadi, S.; Safarkhani, M.; Rabiee, N. Viral and non-viral gene therapy using 3D (bio) printing. J. Gene Med. 2022, 24, e3458. [Google Scholar] [CrossRef]
- Hosseinkhani, H.; Domb, A.J.; Sharifzadeh, G.; Nahum, V. Gene therapy for regenerative medicine. Pharmaceutics 2023, 15, 856. [Google Scholar] [CrossRef]
- Santoro, A.; Ferrara, Y.V.; De Angelis, A. Therapeutic strategies in cystinosis: A focus on cysteamine and beyond. Exp. Mol. Pathol. 2025, 144, 104995. [Google Scholar] [CrossRef]
- Giordano, S.; Terracciano, M.; Gallo, E.; Diaferia, C.; Falanga, A.P.; Accardo, A.; Franzese, M.; Salvatore, M.; Piccialli, G.; Borbone, N. Investigating the Interactions of Peptide Nucleic Acids with Multicomponent Peptide Hydrogels for the Advancement of Healthcare Technologies. Gels 2025, 11, 367. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, K.-H.; Kim, S.; Lee, Y.-M.; Seol, Y.-J. BMP-2 gene delivery-based bone regeneration in dentistry. Pharmaceutics 2019, 11, 393. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Y.; Luo, J.; Liu, X.; Yang, Q.; Shi, X.; Wang, Y. Black phosphorus nanosheets-enabled DNA hydrogel integrating 3D-printed scaffold for promoting vascularized bone regeneration. Bioact. Mater. 2023, 21, 97–109. [Google Scholar] [CrossRef]
- Shi, R.; Zhan, H.; Jiang, S.; Lin, K.; Yuan, C. DNA-Based Hydrogels for Musculoskeletal Reconstruction: Harnessing Dynamic Programmability and Multimodal Therapeutic Integration. Adv. Sci. 2025, 12, e11099. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Zhou, L.; Luo, Z.; Liu, G.; Hang, Z.; Li, H.; Li, F.; Wen, Y. Emerging delivery systems for enabling precision nucleic acid therapeutics. ACS Nano 2025, 19, 4039–4083. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Role of biological scaffolds, hydro gels and stem cells in tissue regeneration therapy. Adv. Tissue Eng. Regen. Med. Open Access 2017, 2, 121. [Google Scholar] [CrossRef]
- Kostadinova, M.; Raykovska, M.; Simeonov, R.; Lolov, S.; Mourdjeva, M. Recent Advances in Bone Tissue Engineering: Enhancing the Potential of Mesenchymal Stem Cells for Regenerative Therapies. Curr. Issues Mol. Biol. 2025, 47, 287. [Google Scholar] [CrossRef]
- Shoji, S.; Uchida, K.; Saito, W.; Sekiguchi, H.; Inoue, G.; Miyagi, M.; Kuroda, A.; Takaso, M. Acceleration of Bone Healing by In Situ-Forming Dextran-Tyramine Conjugates Containing Basic Fibroblast Growth Factor in Mice. Cureus 2020, 12, e10085. [Google Scholar] [CrossRef]
- Hulsart-Billström, G.; Piskounova, S.; Gedda, L.; Andersson, B.-M.; Bergman, K.; Hilborn, J.; Larsson, S.; Bowden, T. Morphological differences in BMP-2-induced ectopic bone between solid and crushed hyaluronan hydrogel templates. J. Mater. Sci. Mater. Med. 2013, 24, 1201–1209. [Google Scholar] [CrossRef]
- Chen, J.; Yan, X.; Nie, L.; Zhou, S.; Ji, P.; Zhang, H. Injectable hydrogel microsphere orchestrates immune regulation and bone regeneration via sustained release of calcitriol. Mater. Today Bio 2025, 32, 101687. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Yang, Q.; Chen, A.; Dong, S.; Liao, L.; Zhang, C. 3D Printed Microsphere-Hydrogel Scaffold Facilitates Restoration of Reinnervation in Bone Regeneration through Programmable Release of NGF/BMP-2 Mimetic Peptides. Adv. Healthc. Mater. 2025, 14, 2501594. [Google Scholar] [CrossRef] [PubMed]
- Ingavle, G.C.; Gionet-Gonzales, M.; Vorwald, C.E.; Bohannon, L.K.; Clark, K.; Galuppo, L.D.; Leach, J.K. Injectable mineralized microsphere-loaded composite hydrogels for bone repair in a sheep bone defect model. Biomaterials 2019, 197, 119–128. [Google Scholar] [CrossRef]
- Machado, A.; Pereira, I.; Costa, F.; Brandão, A.; Pereira, J.E.; Maurício, A.C.; Santos, J.D.; Amaro, I.; Falacho, R.; Coelho, R. Randomized clinical study of injectable dextrin-based hydrogel as a carrier of a synthetic bone substitute. Clin. Oral Investig. 2023, 27, 979–994. [Google Scholar] [CrossRef]
- Meenakshi, S.S.; Sankari, M. Effectiveness of chitosan nanohydrogel as a bone regenerative material in intrabony defects in patients with chronic periodontitis: A randomized clinical trial. J. Adv. Oral Res. 2021, 12, 222–228. [Google Scholar] [CrossRef]
- Neovius, E.; Lemberger, M.; Skogh, A.C.D.; Hilborn, J.; Engstrand, T. Alveolar bone healing accompanied by severe swelling in cleft children treated with bone morphogenetic protein-2 delivered by hydrogel. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 37–42. [Google Scholar] [CrossRef]
- Vallmajo-Martin, Q.; Millan, C.; Müller, R.; Weber, F.E.; Ehrbar, M.; Ghayor, C. Enhanced bone regeneration in rat calvarial defects through BMP2 release from engineered poly (ethylene glycol) hydrogels. Sci. Rep. 2024, 14, 4916. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Gan, D.; Li, Z.; Shen, J.; Tang, P.; Luo, S.; Li, P.; Lu, X.; Zheng, W. The characteristics of mussel-inspired nHA/OSA injectable hydrogel and repaired bone defect in rabbit. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1814–1825. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, A.V.; Kuznetsova, V.S.; Bukharova, T.B.; Grigoriev, T.E.; Zagoskin, Y.D.; Nedorubova, I.A.; Babichenko, I.I.; Chvalun, S.N.; Goldstein, D.V.; Kulakov, A.A. Influence of the degree of deacetylation of chitosan and BMP-2 concentration on biocompatibility and osteogenic properties of BMP-2/PLA granule-loaded chitosan/β-glycerophosphate hydrogels. Molecules 2021, 26, 261. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.U.; Srinivasan, S.S.; Kumbar, S.G.; Moss, I.L. Hydrogel-based strategies for intervertebral disc regeneration: Advances, challenges and clinical prospects. Gels 2024, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, J.; Gao, L.; Zhang, W. Hydrogels in Alveolar Bone Regeneration. ACS Biomater. Sci. Eng. 2024, 10, 7337–7351. [Google Scholar] [CrossRef]
- Shen, X.; Zhu, D.; Hu, H.; Su, L.; Wu, G.; Forouzanfar, T.; Yang, G.; Jiang, Z. Bone powder-laden hydrogel scaffolds in bone tissue engineering. Biomater. Sci. 2026, 14, 31–55. [Google Scholar] [CrossRef]
- Bonetti, L.; Scalet, G. 4D fabrication of shape-changing systems for tissue engineering: State of the art and perspectives. Prog. Addit. Manuf. 2025, 10, 1913–1943. [Google Scholar] [CrossRef]
- Omidian, H.; Mfoafo, K. Three-dimensional printing strategies for enhanced hydrogel applications. Gels 2024, 10, 220. [Google Scholar] [CrossRef]
- Lima, C.S.A.d.; Balogh, T.S.; Varca, J.P.R.O.; Varca, G.H.C.; Lugão, A.B.; A. Camacho-Cruz, L.; Bucio, E.; Kadlubowski, S.S. An updated review of macro, micro, and nanostructured hydrogels for biomedical and pharmaceutical applications. Pharmaceutics 2020, 12, 970. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Bae, J.; Zhou, X.; Guo, Y.; Yu, G. Nanostructured functional hydrogels as an emerging platform for advanced energy technologies. Adv. Mater. 2018, 30, 1801796. [Google Scholar] [CrossRef]
- Günay, M.; Meral, T. Biomedical Applications with Multiscale Structures Produced by Additive Manufacturing. J. Mol. Eng. Mater. 2025, 13, 2430004. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Innovative Micro-and Nano-Architectures in Biomedical Engineering for Therapeutic and Diagnostic Applications. Micromachines 2025, 16, 419. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Saiding, Q.; Cai, X.; Xiao, Y.; Wang, P.; Cai, Z.; Gong, X.; Gong, W.; Zhang, X.; Cui, W. Intelligent vascularized 3D/4D/5D/6D-printed tissue scaffolds. Nano-Micro Lett. 2023, 15, 239. [Google Scholar] [CrossRef]
- Lu, C.; Jin, A.; Liu, H.; Gao, C.; Sun, W.; Zhang, Y.; Dai, Q.; Liu, Y. Advancing tissue engineering through vascularized cell spheroids: Building blocks of the future. Biomater. Sci. 2025, 13, 1901–1922. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Lu, D.C.; Tumialán, L.M.; Chou, D. Multilevel anterior cervical discectomy and fusion with and without rhBMP-2: A comparison of dysphagia rates and outcomes in 150 patients. J. Neurosurg. Spine 2013, 18, 43–49. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone morphogenetic protein-2 in development and bone homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Bordukalo-Nikšić, T.; Kufner, V.; Vukičević, S. The role of BMPs in the regulation of osteoclasts resorption and bone remodeling: From experimental models to clinical applications. Front. Immunol. 2022, 13, 869422. [Google Scholar] [CrossRef]
- Omidian, H.; Akhzarmehr, A.; Dey Chowdhury, S. Hydrogel composites for multifunctional biomedical applications. J. Compos. Sci. 2024, 8, 154. [Google Scholar] [CrossRef]
- Virdi, C.; Lu, Z.; Zreiqat, H.; No, Y.J. Theta-Gel-Reinforced Hydrogel Composites for Potential Tensile Load-Bearing Soft Tissue Repair Applications. J. Funct. Biomater. 2023, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Cui, Z.; Xu, D.; Liu, C.; Zhou, C. Chitin nanocrystal-reinforced chitin/collagen composite hydrogels for annulus fibrosus repair after discectomy. Mater. Today Bio 2025, 31, 101537. [Google Scholar] [CrossRef] [PubMed]
- Emon, N.U.; Zhang, L.; Osborne, S.D.; Lanoue, M.A.; Huang, Y.; Tian, Z.R. Novel nanomaterials for developing bone scaffolds and tissue regeneration. Nanomaterials 2025, 15, 1198. [Google Scholar] [CrossRef]
- Babo, P.S.; Pires, R.L.; Santos, L.; Franco, A.; Rodrigues, F.; Leonor, I.; Reis, R.L.; Gomes, M.E. Platelet lysate-loaded photocrosslinkable hyaluronic acid hydrogels for periodontal endogenous regenerative technology. ACS Biomater. Sci. Eng. 2017, 3, 1359–1369. [Google Scholar] [CrossRef]
- Jooybar, E.; Abdekhodaie, M.J.; Alvi, M.; Mousavi, A.; Karperien, M.; Dijkstra, P.J. An injectable platelet lysate-hyaluronic acid hydrogel supports cellular activities and induces chondrogenesis of encapsulated mesenchymal stem cells. Acta Biomater. 2019, 83, 233–244. [Google Scholar] [CrossRef]
- Flegeau, K.; Gauthier, O.; Rethore, G.; Autrusseau, F.; Schaefer, A.; Lesoeur, J.; Veziers, J.; Brésin, A.; Gautier, H.; Weiss, P. Injectable silanized hyaluronic acid hydrogel/biphasic calcium phosphate granule composites with improved handling and biodegradability promote bone regeneration in rabbits. Biomater. Sci. 2021, 9, 5640–5651. [Google Scholar] [CrossRef]
- Machado, A.; Pereira, I.; Pereira, J.E.; Maltez, L.; Brandão, A.; Alvites, R.; Sousa, A.C.; Branquinho, M.; Caseiro, A.R.; Pedrosa, S.S. Dextrin hydrogel loaded with a macroporous Bonelike® scaffold and dental pulp stem cells for critical-sized defect repair. Materialia 2023, 30, 101859. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-healing injectable hydrogels for tissue regeneration. Chem. Rev. 2022, 123, 834–873. [Google Scholar] [CrossRef]
- Erezuma, I.; Lukin, I.; Desimone, M.; Zhang, Y.S.; Dolatshahi-Pirouz, A.; Orive, G. Progress in self-healing hydrogels and their applications in bone tissue engineering. Biomater. Adv. 2023, 146, 213274. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, N.; Li, X.; Liu, Z.; Li, K.; Jiao, T.; Liu, F. Recent progress of 3D printed responsive scaffolds for bone repair: A review. Mater. Today Bio 2025, 35, 102351. [Google Scholar] [CrossRef]
- Shreya, L.; Suma, U.S. Recent Advances in Oral In-situ Gel Drug Delivery System: A Polymeric Approach. Drug Dev. Ind. Pharm. 2025, 51, 1639–1649. [Google Scholar]
- Su, N.; Villicana, C.; Yang, F. Immunomodulatory strategies for bone regeneration: A review from the perspective of disease types. Biomaterials 2022, 286, 121604. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, Z.; Yilmaz-Aykut, D.; Tourk, F.M.; Bassous, N.; Barroso-Zuppa, M.; Shawl, A.I.; Ashraf, S.S.; Avci, H.; Hassan, S. Immunomodulating hydrogels as stealth platform for drug delivery applications. Pharmaceutics 2022, 14, 2244. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Xiong, Y.; Zha, K.; Cao, F.; Zhou, W.; Abbaszadeh, S.; Ouyang, L.; Liao, Y.; Hu, W.; Dai, G. Immune homeostasis modulation by hydrogel-guided delivery systems: A tool for accelerated bone regeneration. Biomater. Sci. 2023, 11, 6035–6059. [Google Scholar] [CrossRef]
- Zhang, B.; Su, Y.; Zhou, J.; Zheng, Y.; Zhu, D. Toward a better regeneration through implant-mediated immunomodulation: Harnessing the immune responses. Adv. Sci. 2021, 8, 2100446. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; He, Y.; Li, Q.; Yang, K.; Sun, L.; Xu, H.; Wang, R. Intelligent design and medical applications of antimicrobial hydrogels. Colloid Interface Sci. Commun. 2023, 53, 100696. [Google Scholar] [CrossRef]
- Yao, H.; Wu, M.; Lin, L.; Wu, Z.; Bae, M.; Park, S.; Wang, S.; Zhang, W.; Gao, J.; Wang, D. Design strategies for adhesive hydrogels with natural antibacterial agents as wound dressings: Status and trends. Mater. Today Bio 2022, 16, 100429. [Google Scholar] [CrossRef]
- Li, Z.; Ren, K.; Chen, J.; Zhuang, Y.; Dong, S.; Wang, J.; Liu, H.; Ding, J. Bioactive hydrogel formulations for regeneration of pathological bone defects. J. Control. Release 2025, 380, 686–714. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Zhang, X.; Wu, T.; Huang, K.; Wang, B.; Liao, J. Self-healing hydrogels for bone defect repair. RSC Adv. 2023, 13, 16773–16788. [Google Scholar] [CrossRef]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M. Bioactive materials for bone regeneration: Biomolecules and delivery systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef]
- Kang, H.-J.; Park, S.-S.; Tripathi, G.; Lee, B.-T. Injectable demineralized bone matrix particles and their hydrogel bone grafts loaded with β-tricalcium phosphate powder and granules: A comparative study. Mater. Today Bio 2022, 16, 100422. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Lee, C.-S. Nanoclay-composite hydrogels for bone tissue engineering. Gels 2024, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Alizadeh, P.; Kadumudi, F.B.; Orive, G.; Gaharwar, A.K.; Castilho, M.; Golafshan, N.; Dolatshahi-Pirouz, A. Multi-leveled nanosilicate implants can facilitate near-perfect bone healing. ACS Appl. Mater. Interfaces 2023, 15, 21476–21495. [Google Scholar] [CrossRef]
- Wen, Z.; Shi, X.; Li, X.; Liu, W.; Liu, Y.; Zhang, R.; Yu, Y.; Su, J. Mesoporous TiO2 coatings regulate ZnO nanoparticle loading and Zn2+ release on titanium dental implants for sustained osteogenic and antibacterial activity. ACS Appl. Mater. Interfaces 2023, 15, 15235–15249. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]
- Protsak, I.S.; Morozov, Y.M. Fundamentals and advances in stimuli-responsive hydrogels and their applications: A review. Gels 2025, 11, 30. [Google Scholar] [CrossRef]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart hydrogels–synthetic stimuli-responsive antitumor drug release systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Zhou, Q.; Zhou, F.; Zhang, Q.; Su, J. Smart hydrogels for bone reconstruction via modulating the microenvironment. Research 2023, 6, 0089. [Google Scholar] [CrossRef]
- Mofrad, Y.M.; Asiaei, S.; Shaygani, H.; Cheraghi, F.; Amirsadat, S.; Soltani, M.; Derarandash, F.D.N.; Shams, M.; Zare, S.; Shamloo, A. Advances in Smart Hydrogels for Nerve Repair: A Review Focusing on Criteria and Applications. J. Sci. Adv. Mater. Devices 2025, 10, 100996. [Google Scholar] [CrossRef]
- Li, M.; Fan, Y.; Ran, M.; Chen, H.; Han, J.; Zhai, J.; Wang, Z.; Ning, C.; Shi, Z.; Yu, P. Hydrogel coatings of implants for pathological bone repair. Adv. Healthc. Mater. 2024, 13, 2401296. [Google Scholar] [CrossRef] [PubMed]
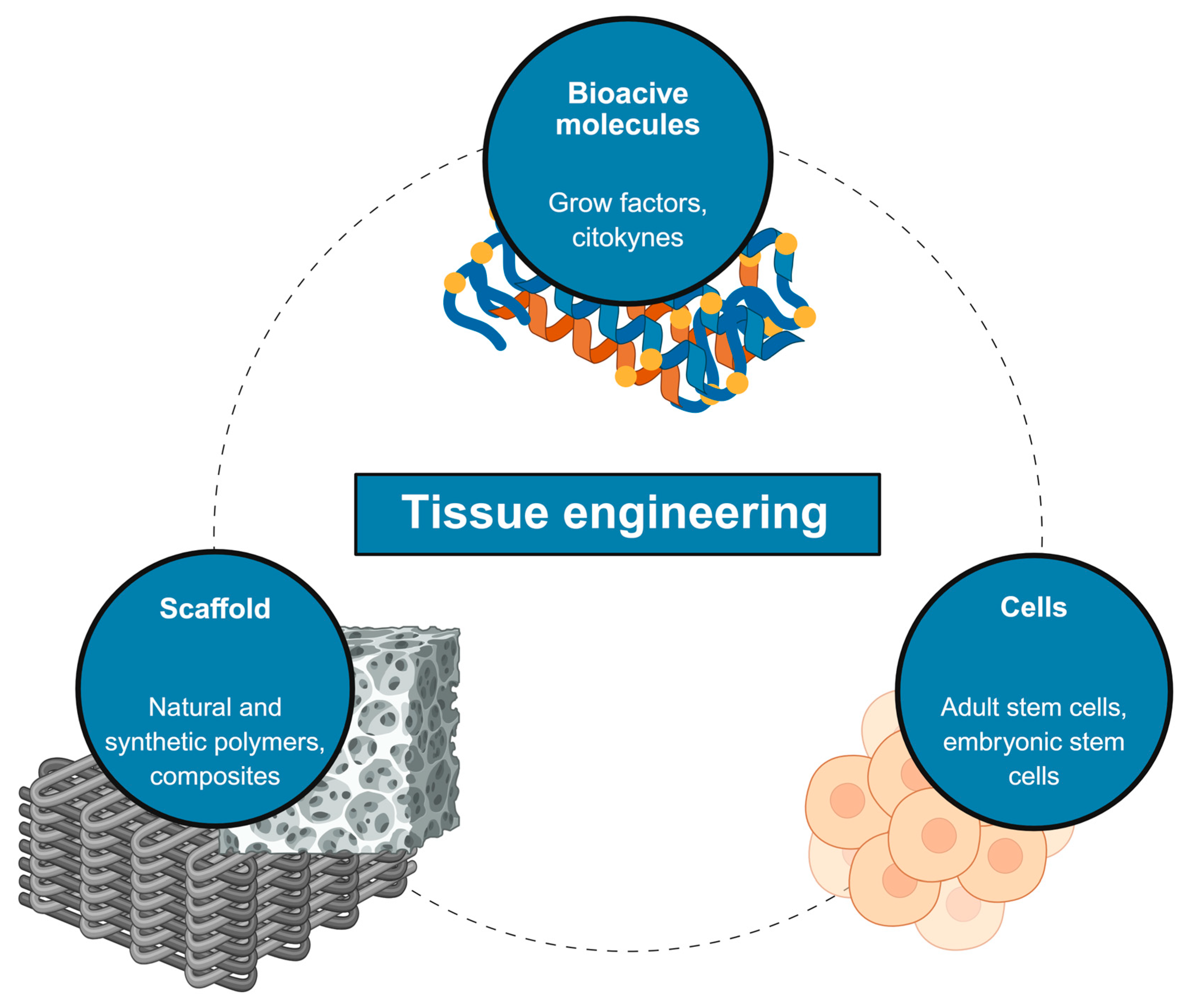
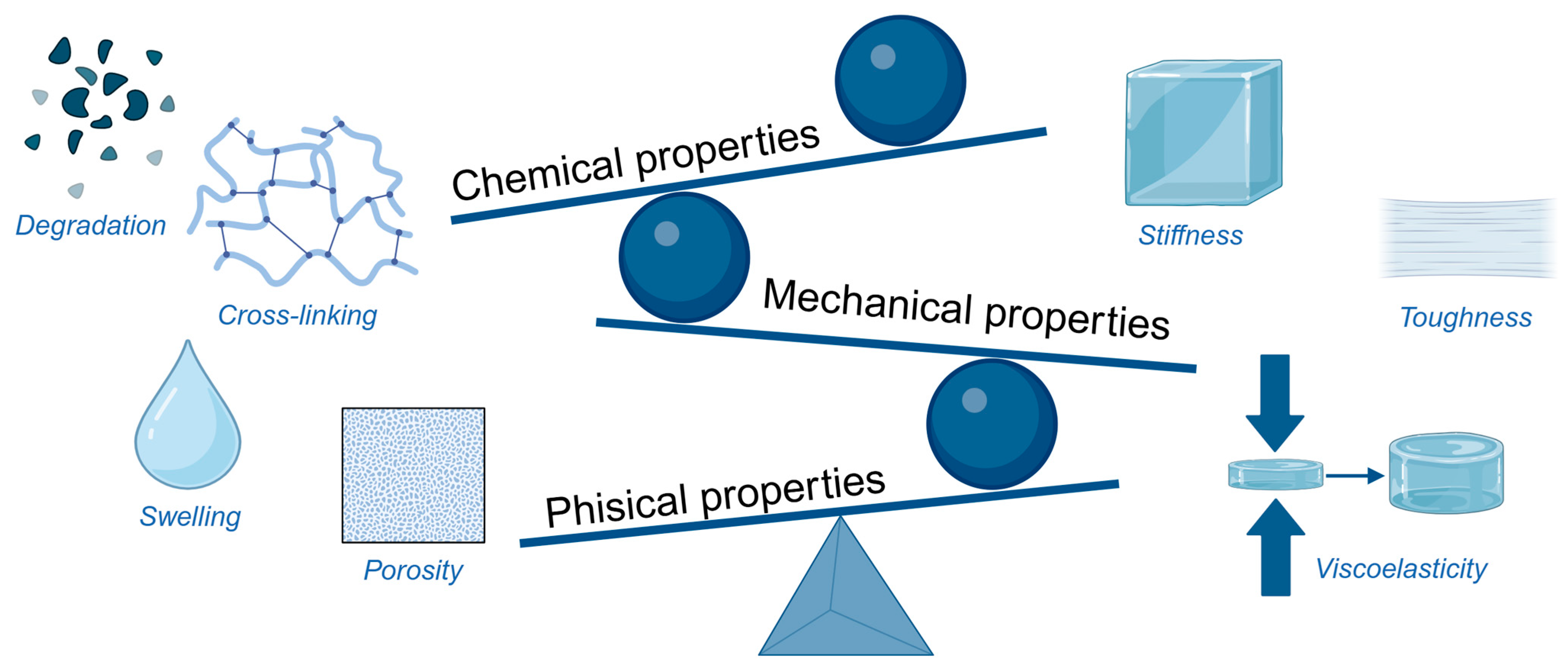
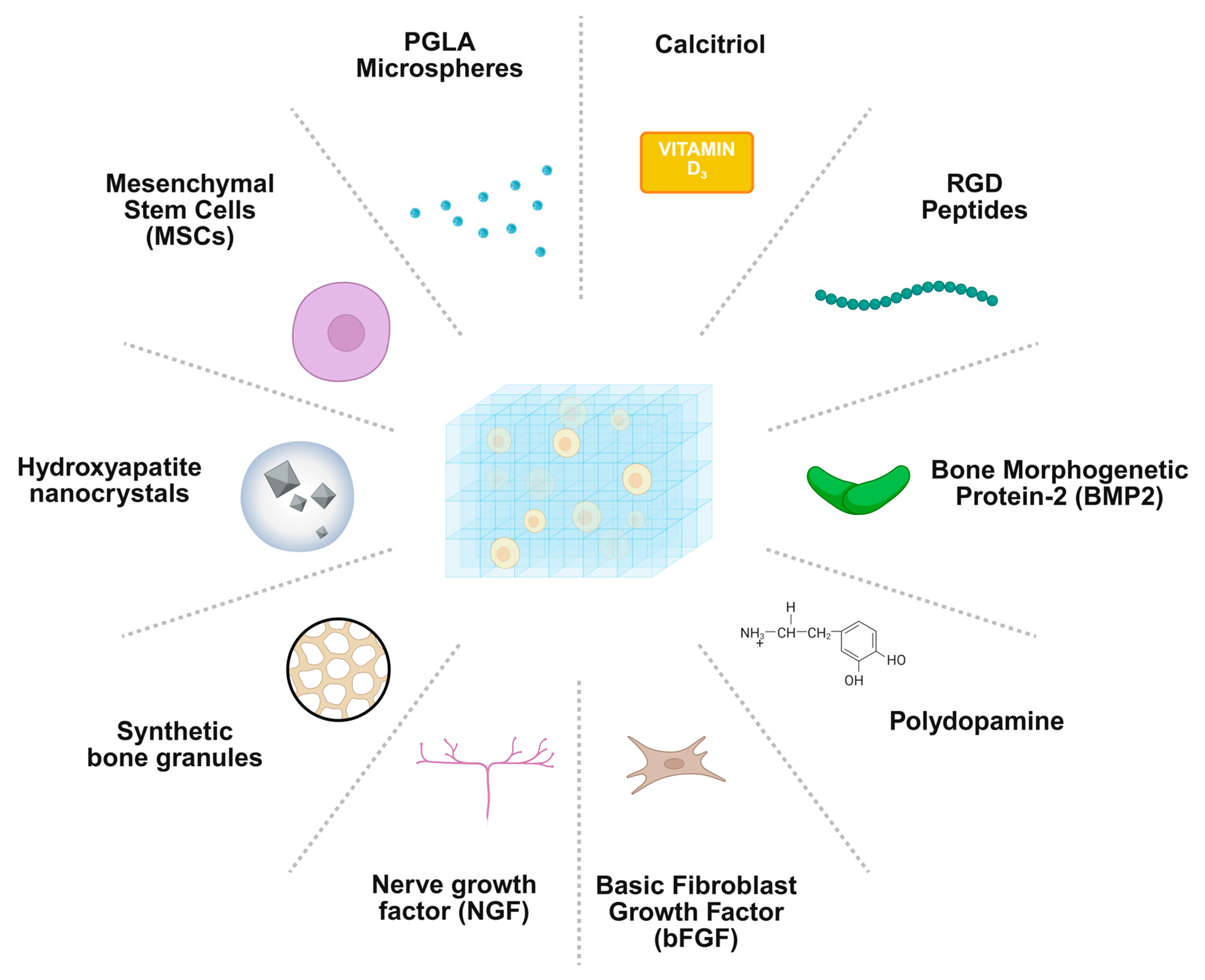
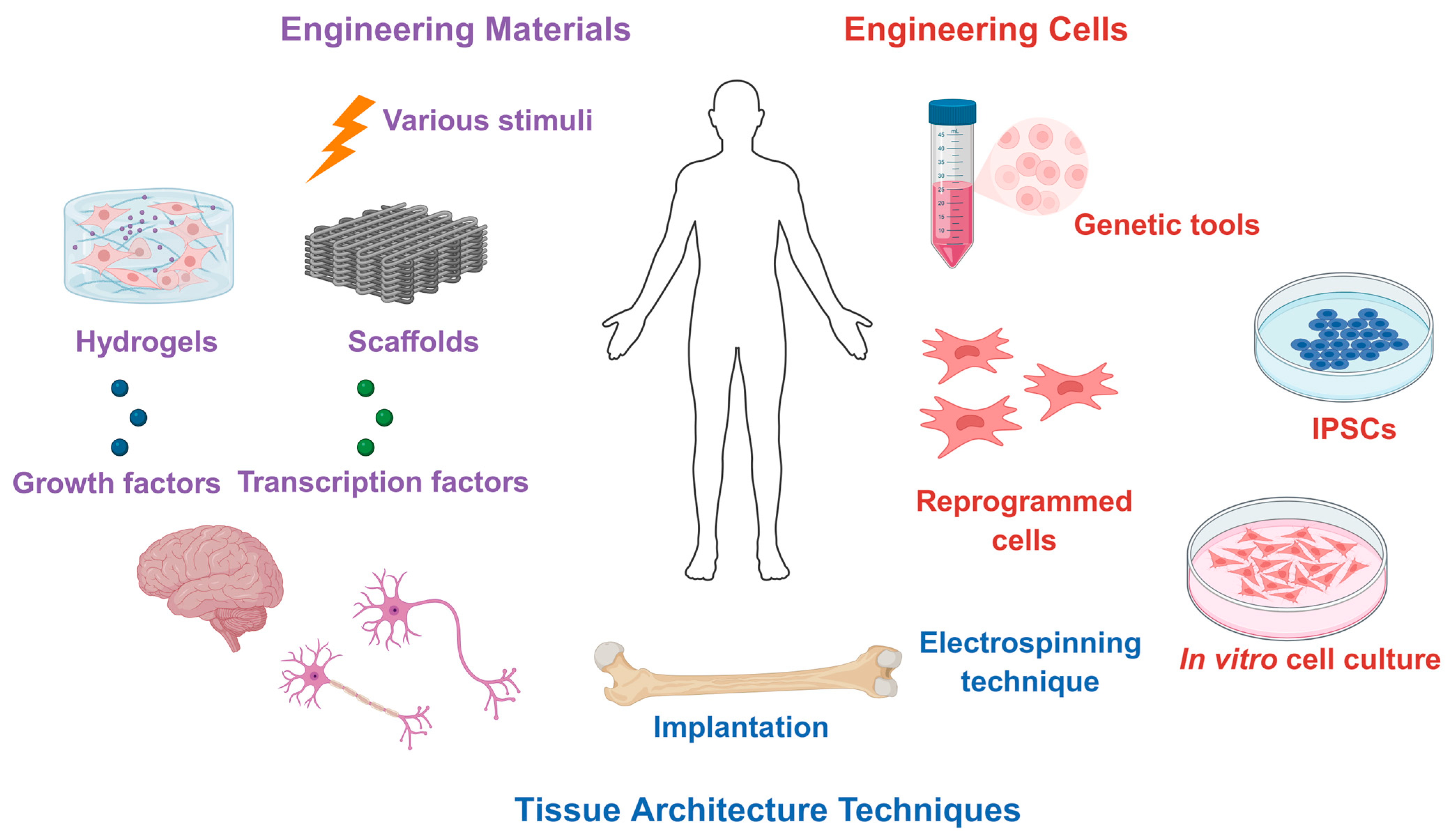

| Type | Examples | Advantages | Limitations | Applications in Regeneration |
|---|---|---|---|---|
| Natural | Collagen, Gelatin, Hyaluronic acid, Alginate, Chitosan | Biocompatibility, bioactivity, cell-adhesive sites, enzymatic degradation | Low mechanical stability, batch-to-batch variability | Osteoconduction, support for cells and vessels, natural release of signals |
| Synthetic | PEG, PVA, PLA/PCL, PPF | Tunable chemical and mechanical properties, reproducibility, large-scale production | Bioinert, require biofunctionalization | Suitable for applications requiring higher robustness |
| Bioactive Molecules | Hydrogel-Mediated Features | Primary Regenerative Role |
|---|---|---|
| Growth factors (BMPs, VEGF, PDGF, TGF-β) | Preserve bioactivity, enable localised and sustained release, allow temporal and spatial control | Drive osteogenesis and angiogenesis by mimicking natural bone signalling cascades |
| Recombinant proteins | Encapsulation via electrostatic interactions, covalent tethering, or microspheres; strategies to reduce burst release (e.g., heparin-based systems) | Maintain osteoinductive activity while minimising rapid clearance and off-target effects |
| Platelet-rich plasma (PRP)/platelet-derived growth factors | Harness endogenous regenerative cues when incorporated within hydrogels | Support local tissue regeneration and signalling |
| Gene therapy (plasmid DNA, RNA, viral vectors) | Enable sustained local protein production, modulate immune response, support osteogenesis and angiogenesis | Fine-tune the regenerative microenvironment and prolong therapeutic effects |
| Living cells (MSCs, pre-osteoblasts) | Hydrogels protect transplanted cells, ensure retention and survival; contribute directly via differentiation and indirectly via paracrine signalling | Enhance bone formation through combined cellular and paracrine mechanisms |
| Hydrogel Type | Model Used | Results |
|---|---|---|
| In situ-forming dextran–tyramine (Dex-TA) hydrogel loaded with bFGF | Mouse femoral fracture model | Direct injection of bFGF-loaded Dex-TA hydrogel at the fracture site accelerated bone healing. This method resulted in increased callus formation, greater bone density observed on radiographs, and enhanced bone strength compared to treatment with saline or hydrogel lacking bFGF [159]. |
| Chemically cross-linked hyaluronan hydrogel used either as solid plugs or crushed fragments, all loaded with BMP-2 | Rat ectopic bone formation model (subcutaneous/muscle implantation) | Both hydrogels induced ectopic bone formation. Solid hydrogels generated a more organised cortical-like shell with a marrow-like centre, while crushed hydrogels resulted in more dispersed, trabecular bone. These findings indicate that the macroarchitecture of the hydrogel significantly influences BMP-2–induced bone morphology [160]. |
| Injectable hydrogel microsphere system (hydrogel microspheres loaded with calcitriol) designed for sustained release | Rat inflammatory bone-defect model | The calcitriol-releasing microsphere hydrogel scavenged ROS, shifted macrophages from an M1 to an M2 phenotype, reduced inflammation, and significantly enhanced new bone formation compared with free calcitriol or blank controls, showing coupled immunomodulation and osteogenesis [161]. |
| 3D-printed GelMA/mPGA hydrogel scaffold containing PLGA microspheres for sequential NGF-mimetic (fast) and BMP-2-mimetic (sustained) release | Rat critical-size calvarial defect | The dual-peptide microsphere–hydrogel scaffold enhanced neurite outgrowth, Schwann cell migration, and mesenchymal stem cell (MSC) osteogenesis in vitro. In vivo, this scaffold resulted in increased bone volume, improved trabecular organisation, and robust re-innervation compared to single-factor or unloaded scaffolds. This suggests that coordinated neural and bone signalling significantly improves bone regeneration [162]. |
| Composite alginate/hyaluronate hydrogel loaded with mineralised polymeric microspheres and autologous MSCs | Sheep iliac-crest segmental defect (critical-size) | After 12 weeks, composite hydrogels containing mesenchymal stem cells (MSCs) demonstrated significantly greater new bone formation and vascularization than acellular or microsphere-only controls. These hydrogels also exhibited more complete defect bridging and a higher bone volume fraction [163]. |
| Injectable dextrin-based hydrogel (DEXGEL Bone) used as a carrier for glass-reinforced hydroxyapatite synthetic bone substitute (Bonelike®) | Human randomised clinical trial—alveolar ridge preservation after tooth extraction | The hydrogel-reinforced bone substitute exhibited improved handling characteristics, superior defect filling, and favourable primary stability of implants while maintaining ridge dimensions. The study confirmed both the safety and clinical performance of DEXGEL Bone as an injectable carrier [164]. |
| Injectable chitosan nanohydrogel used as a periodontal bone-regenerative material | Human randomised clinical trial—intrabony periodontal defects in chronic periodontitis | Treatment with chitosan nanohydrogel resulted in significantly greater reductions in probing depth, increased clinical attachment gain, and enhanced radiographic bone fill compared to the control treatment, demonstrating improved periodontal bone regeneration [165]. |
| Hyaluronan-based hydrogel carrier delivering recombinant human BMP-2 | Children with cleft lip/palate—secondary alveolar bone reconstruction | BMP-2-hydrogel treatment resulted in alveolar bone healing adequate for tooth eruption and orthodontic applications; however, it was often associated with severe postoperative swelling, with some cases necessitating intensive care. This finding underscores a trade-off between effective bone regeneration and the risk of soft-tissue complications [166]. |
| Functional Feature | Description | Example Materials |
|---|---|---|
| Self-healing | Self-repair for irregular defects, reducing inflammation | Chitin, oxidised hyaluronic acid |
| Bone-powder-laden hydrogels | Improve repair efficiency and preclinical osteoinduction | Polymers and xenogeneic bone powder |
| Injectable nanocomposites | Support angiogenesis and controlled growth factor release | Gelatin/PEG and BMP-2 |
| Functionalized biomaterials | High porosity and stiffness for cell adhesion and colonisation | Chitosan-based hydrogels, graphene-containing composites |
| Antimicrobial hydrogels | Local infection control and biofilm prevention, integrating antibacterial and regenerative functions | GelMA/CS/PEG hydrogels loaded with antibiotics, Ag+/Zn2+ ions, or antimicrobial peptides |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Petraglia, F.M.; Giordano, S.; Santoro, A. Functional Hydrogels in Bone Tissue Engineering: From Material Design to Translational Applications. Biologics 2026, 6, 2. https://doi.org/10.3390/biologics6010002
Petraglia FM, Giordano S, Santoro A. Functional Hydrogels in Bone Tissue Engineering: From Material Design to Translational Applications. Biologics. 2026; 6(1):2. https://doi.org/10.3390/biologics6010002
Chicago/Turabian StylePetraglia, Francesco Maria, Sabrina Giordano, and Angelo Santoro. 2026. "Functional Hydrogels in Bone Tissue Engineering: From Material Design to Translational Applications" Biologics 6, no. 1: 2. https://doi.org/10.3390/biologics6010002
APA StylePetraglia, F. M., Giordano, S., & Santoro, A. (2026). Functional Hydrogels in Bone Tissue Engineering: From Material Design to Translational Applications. Biologics, 6(1), 2. https://doi.org/10.3390/biologics6010002






