1. Introduction
Animal and human nutrition is confronted with many challenges. The rising cost of feed ingredients is a major challenge confronting animal production [
1], while diseases remain the main challenge for animal production and human existence. Over the years, several attempts have been made to overcome these challenges, including the use of antibiotics, although the use of antibiotics in feed is no longer widely accepted owing to the potential risk of antibiotic resistance as well as the concerns of food safety arising from contamination or residual effects of chemicals and drugs in animal foods [
2,
3]. This has necessitated the shift in attention to probiotics to enhance growth performance in livestock, especially poultry, as well as to eliminate the risk of health hazards of antimicrobial drugs and vaccines [
4].
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [
5,
6]. They promote health and ensure the balance of bacterial composition in the digestive system, leading to the suppression of harmful pathogenic bacteria [
7,
8], with overall implications for animal health, welfare and performance. The documented benefits of probiotics include combating enteric pathogenic bacteria, improving growth performance, modulating the intestinal microflora, improving immune responses and improving feed conversion efficiency [
9,
10]. A high proportion of probiotic bacteria in the intestinal tract can form colonies that may suppress pathogenic bacteria, thereby protecting the intestinal tract from invasion by pathogenic bacteria [
11,
12]. The benefits probiotics offer have placed pressure on their demands for use in animal nutrition and as human supplements, for cosmetic and therapeutic purposes.
Among the lactic acid bacteria,
Lactobacillus has been proposed as the main candidate strain for probiotic potential [
13,
14]. A recent study reported that bacterial isolates belonging to
Lactobacillus plantarum and
Bacillus subtilis were the most promising probiotic agents in chicken nutrition among others tested [
15], although further studies to assess the safety and protective benefits of these probiotic candidates are recommended [
15].
Improved nutrient absorption and digestibility, enterotoxin neutralization, improved immune responses, reduced gastrointestinal colonization by foodborne pathogenic organisms (
Campylobacter,
Salmonella,
Clostridium and
Escherichia coli) and improved growth performance are some of the benefits of
Lactobacillus probiotic strains [
16,
17,
18]. However, to ensure the safety and sustainability of probiotics in nutrition and therapeutic applications, it is necessary to unravel most of the doubts associated with the constituents of the microorganism. In addition, many proteins from the
Lactobacillus acidophilus genome that may play important roles and assist in the biological understanding of the organism are uncharacterized. Uncharacterized proteins are proteins that are predicted to be expressed in an organism but to which no proper function has been assigned or known [
19]. Uncharacterized proteins have not been assigned any functions experimentally because they have not been studied or their functions are not yet known.
A lot remains unclear about the characteristics, safety profile and functional mechanisms of probiotics, owing in part to many of their proteins being uncharacterized or hypothetical. Understanding the proteins of the Lactobacillus acidophilus genome will shed more light on its functional mechanisms, safety profile and sustainable applications in animal and human nutrition and therapeutic and cosmetic products. Therefore, this study was carried out to stimulate interests in and lay foundations for in vivo functional characterization of these proteins in order to better understand their functional mechanisms, thereby ensuring their sustainable applications in animal and human nutrition and in cosmetic and therapeutic products. Investigating the selected uncharacterized proteins of a reference genome of Lactobacillus acidophilus may provide insights that may find applications in the animal nutrition, health, food production and nutraceutical industries.
4. Discussion
Previous authors have documented the importance of exploring hypothetical proteins in different organisms of interest [
31,
32,
33,
34,
35,
36,
37]. However, this is the first study in this area to focus on the structural and functional relationship, safety profile and IL-6-inducing capacity of uncharacterized proteins of probiotic
Lactobacillus acidophilus for sustainable applications in animal and human nutrition as well as for therapeutic and cosmetics purposes.
The importance of proteins as the ‘building blocks’ for the body cannot be over-emphasized. Proteins play significant roles in the structural formation and functions of organisms, performing key roles in the cell, including protecting the body from pathogens and carrying out chemical reactions. However, proteins’ discrete native structure influences its roles, exposes several channels, receptors and binding sites thereby controlling how they bind and interact with other molecules as well as how they form complexes used for regulatory and structural functions. A protein attains its functional native structure and reaches its final form (three-dimensional structure) by folding. Amino acid composition influences protein classification into different folding types, structural groups and functions. The physicochemical properties of a protein encoded in its amino acid determine its folding.
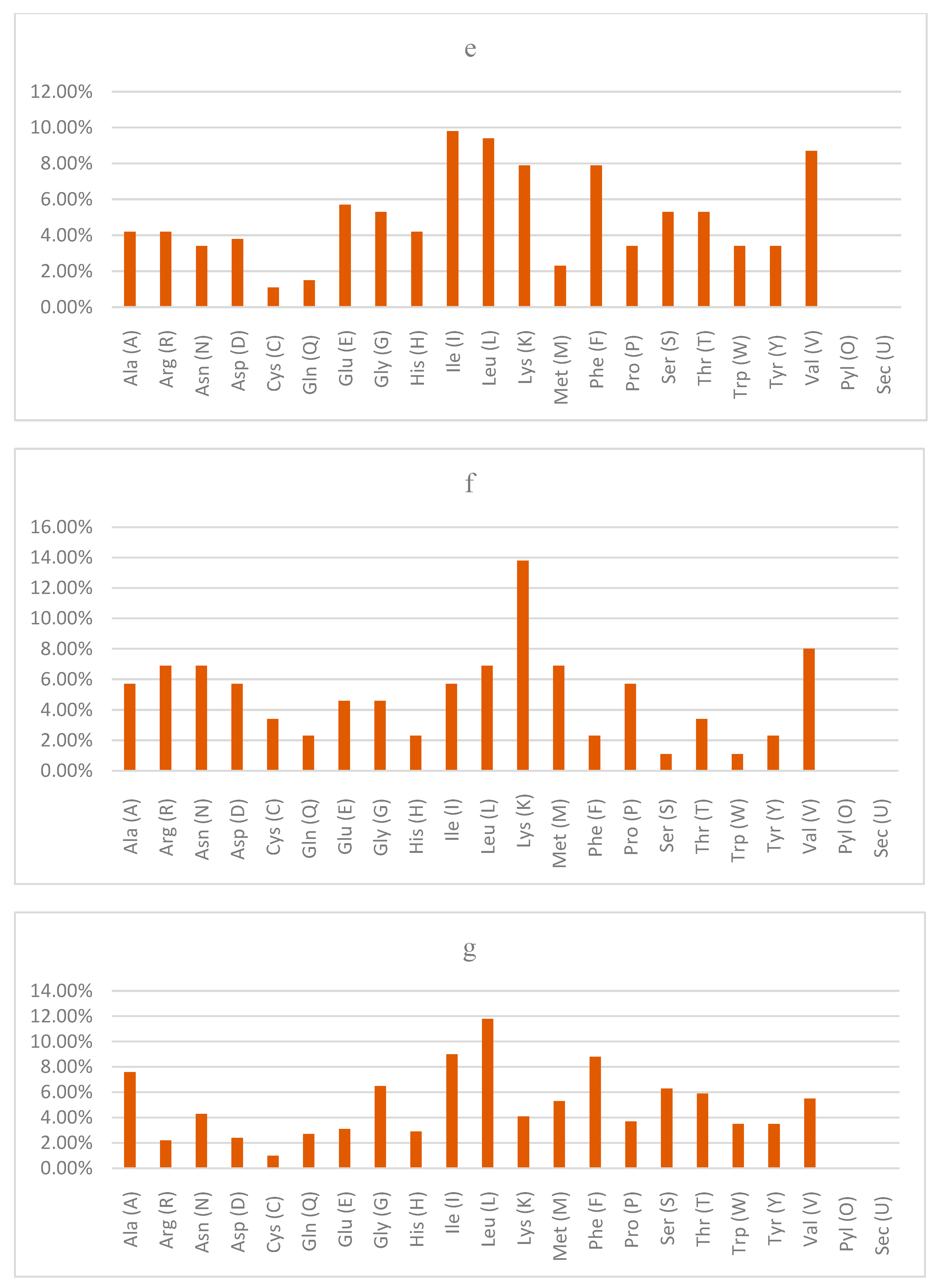
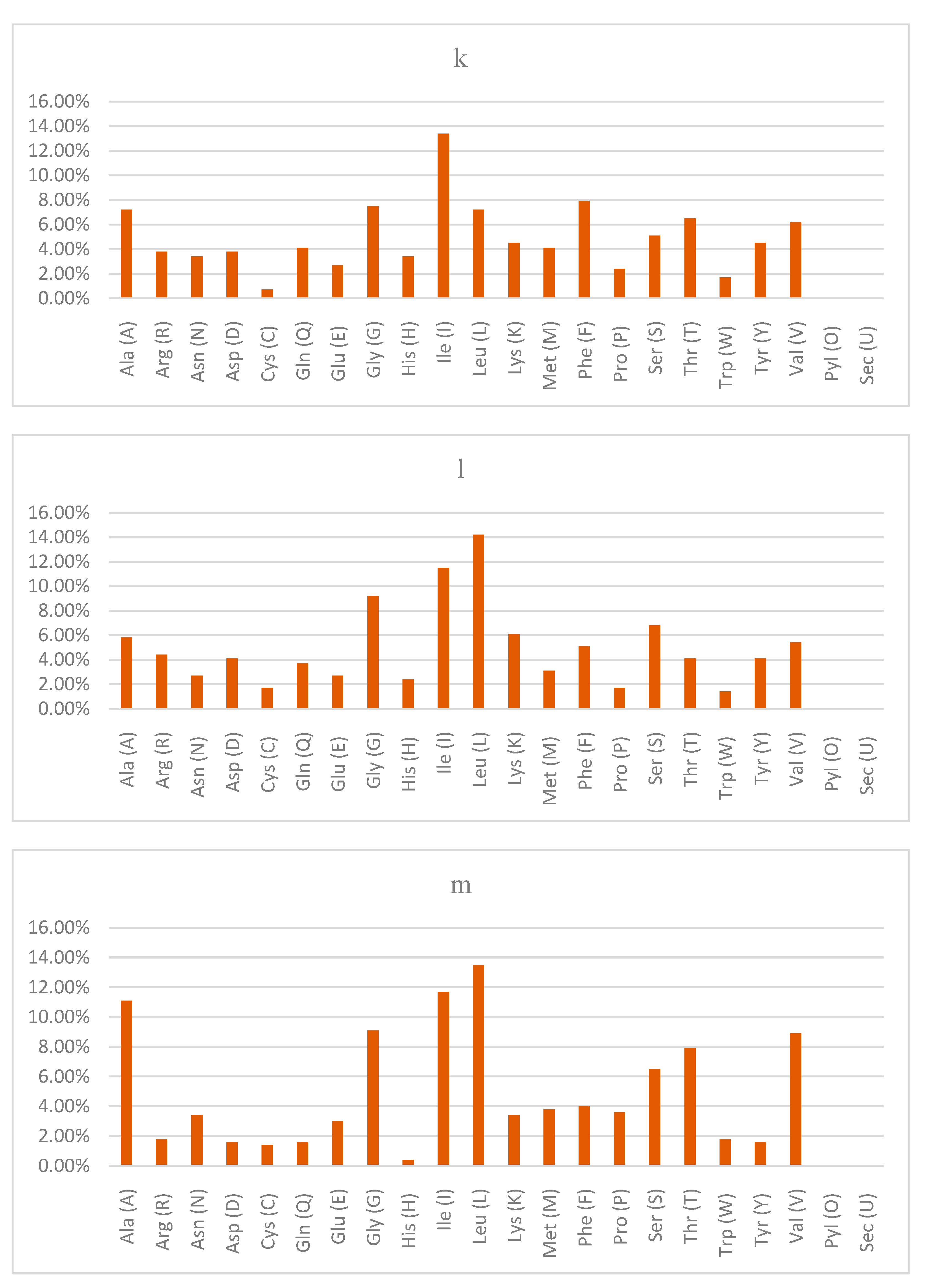
The physicochemical properties usually used in characterizing uncharacterized proteins include the molecular weight, theoretical isoelectric point (pI), amino acid composition, instability index, aliphatic index and grand average of hydropathicity (GRAVY) [
24]. In this study, the results of the physicochemical properties of the query proteins of the
Lactobacillus acidophilus reference genome revealed that LBA1446 had the highest pI. LBA0214 and LBA1788 had values less than 7.00 while the remaining query proteins had values greater than 7.00, indicating whether they are basic or acidic.
A protein has no net charge at the isoelectric point [
24]. At that point, the protein is not repelled or attracted by charged molecules. In a polar solvent, a charged molecule seems to be readily soluble. A protein with a pH above the pI is negatively charged and will not be attracted by a negatively charged surface, thereby decreasing its solubility. The pI plays an important role in protein purification, representing the pH during which solubility is minimal. The pI also talks about the basic or acidic nature of a protein. The pI of the amino acid and acidic protein will be lower and vice versa for a basic protein. Molecular weight is also useful in protein purification and separation.
The extinction coefficient has been defined as a measurement of how strongly a protein absorbs light at a given wavelength. Two values are usually produced by ProtParam for proteins measured in water at 280 nm. The first indicates that the computed value is based on the assumption that all cysteine residues appear as half cystines, that is, all pairs of Cys residues form cystines, while the second value is based on the assumption that no cysteine appears as half cystine, that is, the assumption that all Cys residues are reduced [
24,
35]. In this study, the extinction coefficient values are very high for LBA1510, LBA0214, LBA1446, LBA0037, LBA0995, yifk, LBA0899, LBA1209, LBA0972, LBA099513 and LBA0338 and high for LBA1705 and LBA1825, except for LBA1788. The high extinction coefficient may be an indication of the presence of a high concentration of Cys, Trp and Tyr in the query proteins of
Lactobacillus acidophilus, except for LBA1788, which does not contain Trp residues.
In this study, the half-life estimation was the same for all the query proteins. The estimated half-life is a prediction of the time it takes for half of the amount of protein in a cell to disappear after its synthesis in the cell, relying on the “N-end rule”, which relates the half-life of a protein to the identity of its N-terminal residue, in this case, for humans (in vitro), yeast (in vivo) and
Escherichia coli (in vivo) [
24].
An instability index less than 40 indicates that the protein is stable while a value above 40 indicates that the protein may be unstable [
24]. In the present study, all the query proteins had instability index values less than 40, which implies that they are stable, except for LBA0995, which may be regarded as unstable because it has an instability index greater than 40. The aliphatic index of a protein can be described as the relative volume occupied by aliphatic side chains, that is, alanine, isoleucine, leucine and valine, which may be regarded as a positive factor for the increase in thermostability of globular proteins [
24].
The importance of a protein thermostability cannot be over-emphasized. A thermostable protein is preferred for industrial applications and food processing. The ability to withstand extreme industrial and preservation conditions in food processing and storage is beneficial for probiotic bacteria. Hence, the ability of probiotic bacteria to withstand harsh environmental conditions, having its proteins being thermostable is an advantage to food, cosmetic and therapeutic industries benefiting from the health-promoting probiotics in the era of climate change. Proteins with high aliphatic indices are thermostable under a wide temperature range. In this study, the aliphatic indices are generally high for all the query proteins, although the values for LBA1825 and LBA0214 are not as high as the values obtained for the other query proteins. The high aliphatic index values obtained by the query proteins of the Lactobacillus acidophilus reference genome used in this study indicate that the proteins are stable over a wide temperature range.
The importance of the instability index is mainly in the storage of proteins in the correct solvent. Highly stable proteins are easy to store. For instance, an insulin monomer with an instability index of 43 is considered unstable and may macroscopically aggregate in aqueous solution during storage, which may consequently result in decline in biological activities.
The calculation of the grand average of hydropathy value for a peptide or protein is based on the sum of hydropathy values of all the amino acids, divided by the number of residues in the sequence [
24,
38]. In the present study, only LBA0214, LBA1825 and LBA1788 obtained negative GRAVY values, which is an indication of being hydrophilic, while the other query proteins had positive GRAVY values, which implies that they are hydrophobic [
39]. Another importance of GRAVY index is that it is used to determine whether a protein can be visualized on 2D gel. Proteins with GRAVY score greater than 0.4 are usually difficult to view on 2D gel because it lies outside the solubility range.
Proteins intended for use as food products or for use in consumer products are expected to be analyzed for their allergenic reaction potential before they are introduced into the market to determine and mitigate the risk of inducing an immediate type I (IgE-mediated) allergic response. Allergenicity or allergenic potential in this study is defined as the potential of a protein to cause or elicit immediate type (IgE-mediated) allergic reactions in humans [
40]. It is not certain whether probiotics fed to animals or offered for commercial sale are subjected to allergenic potential evaluations before they are introduced into the market. To ascertain the safety and sustainability of these probiotics, their safety profile including toxicity, allergenicity and antigenicity may be necessary, revealing the novelty of this study.
Bioinformatics tools such as AllerCatPro 2.0, VaxiJen ToxinPred [
26,
27,
28], for quick and cost-saving approaches, have been recommended. The findings from the present study revealed that none of the query proteins of the
Lactobacillus acidophilus reference genome studied were antigenic, allergenic or toxic for human or animal consumption.
The benefits of computational prediction of bacterial protein subcellular localization include the provision of rapid and cost-effective approaches for gaining insight into protein function, verifying experimental results, annotating newly sequenced bacterial genomes, detecting potential cell surface or secreted drug targets, and identifying microbial biomarkers. One of the most precise tools recommended for computational prediction of bacterial protein subcellular localization is PSORTb version 2.0 [
41,
42], which generates prediction results for five major locations for Gram-negative bacteria (cytoplasmic, inner membrane, periplasmic, outer membrane and extracellular) and four locations for Gram-positive bacteria (cytoplasmic, cytoplasmic membrane, cell wall and extracellular). However, the PSORTb version 3.0 used in this study is an updated version of 2.0 [
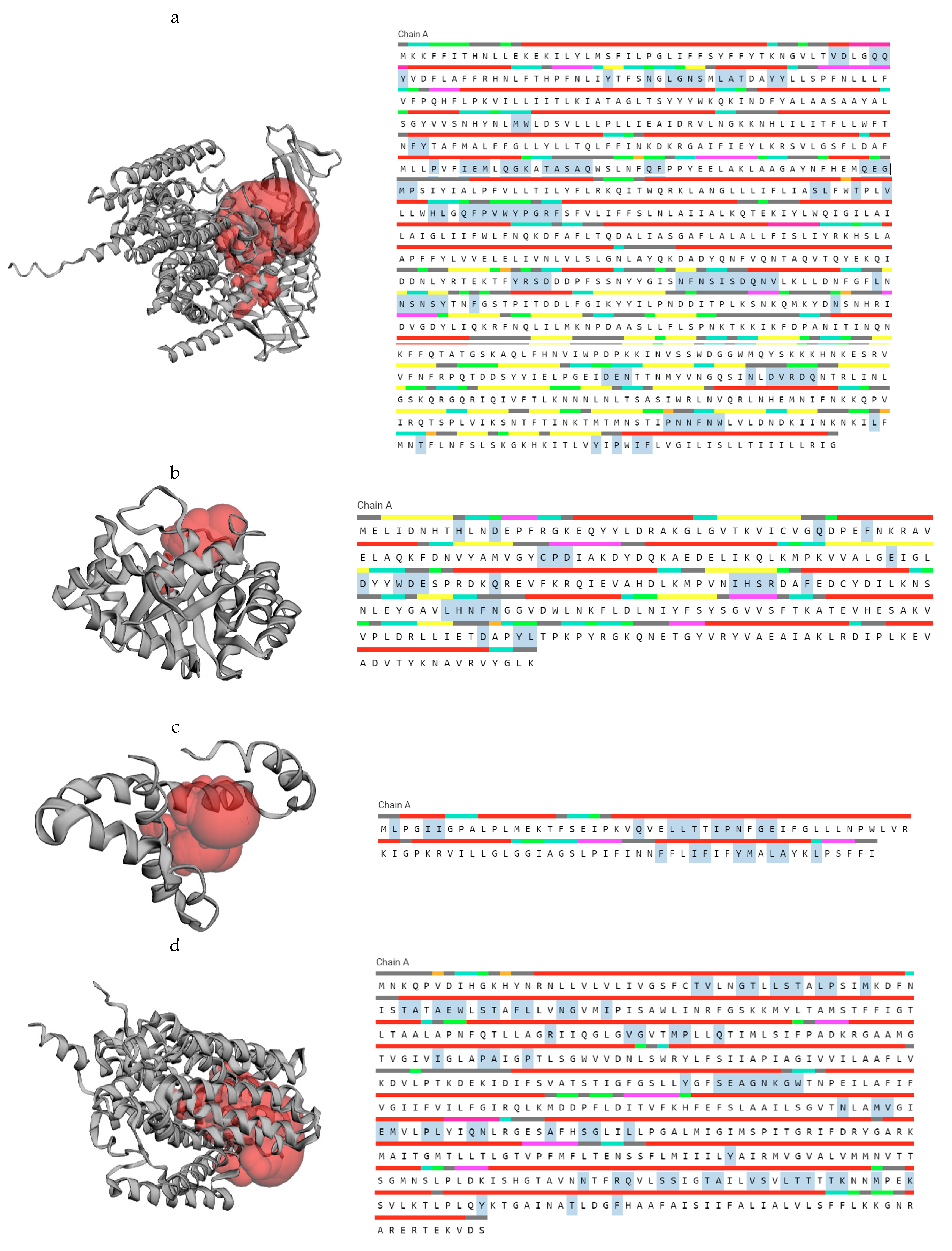
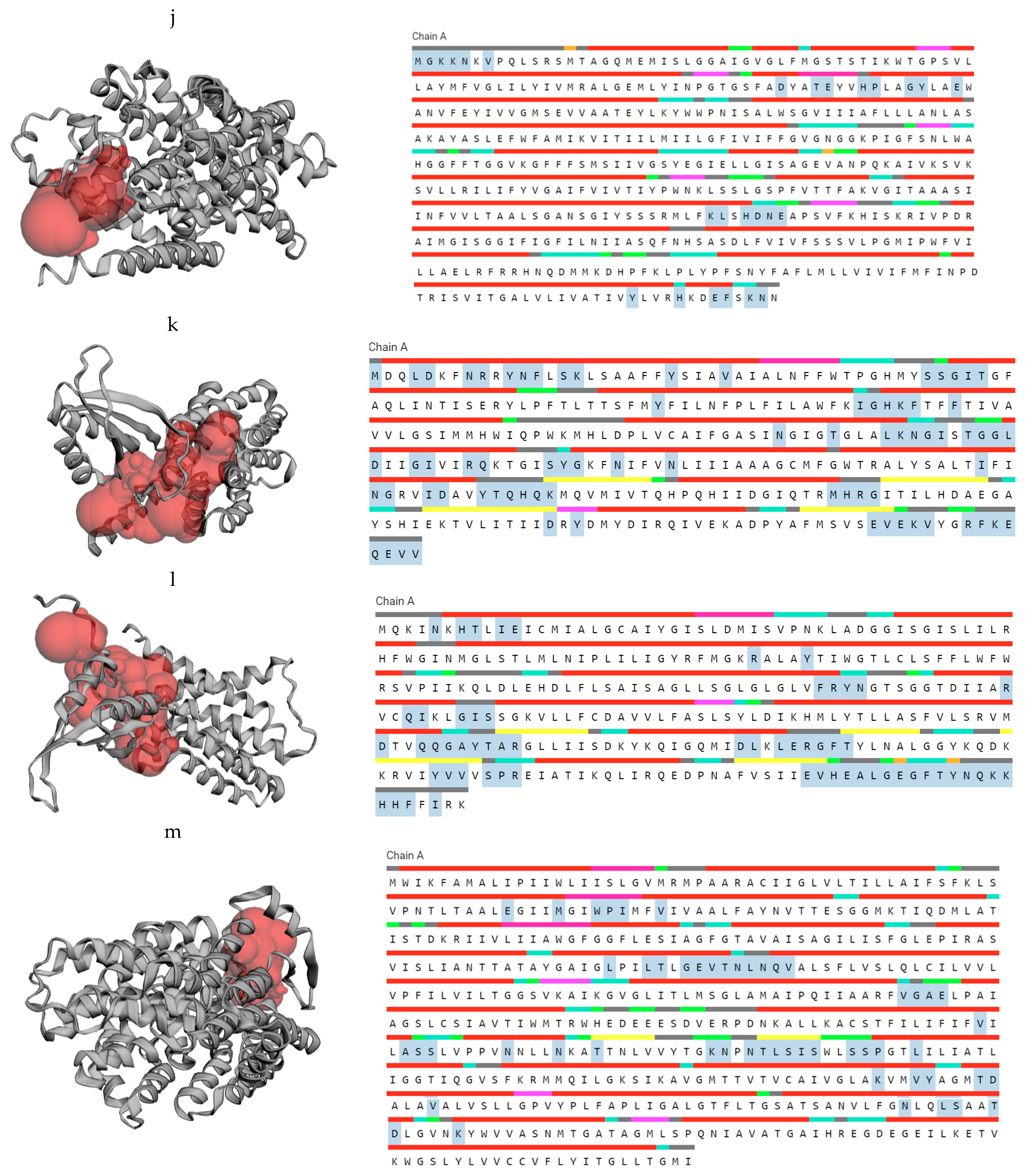
20] with more features. In this study, LBA1788 is cytoplasmic, LBA1510, LBA0214, LBA1705, LBA1446, LBA0037, LBA0995 and yifk-LBA0338 are found in the cytoplasmic membrane while the subcellular localization of LBA1825 is not known.
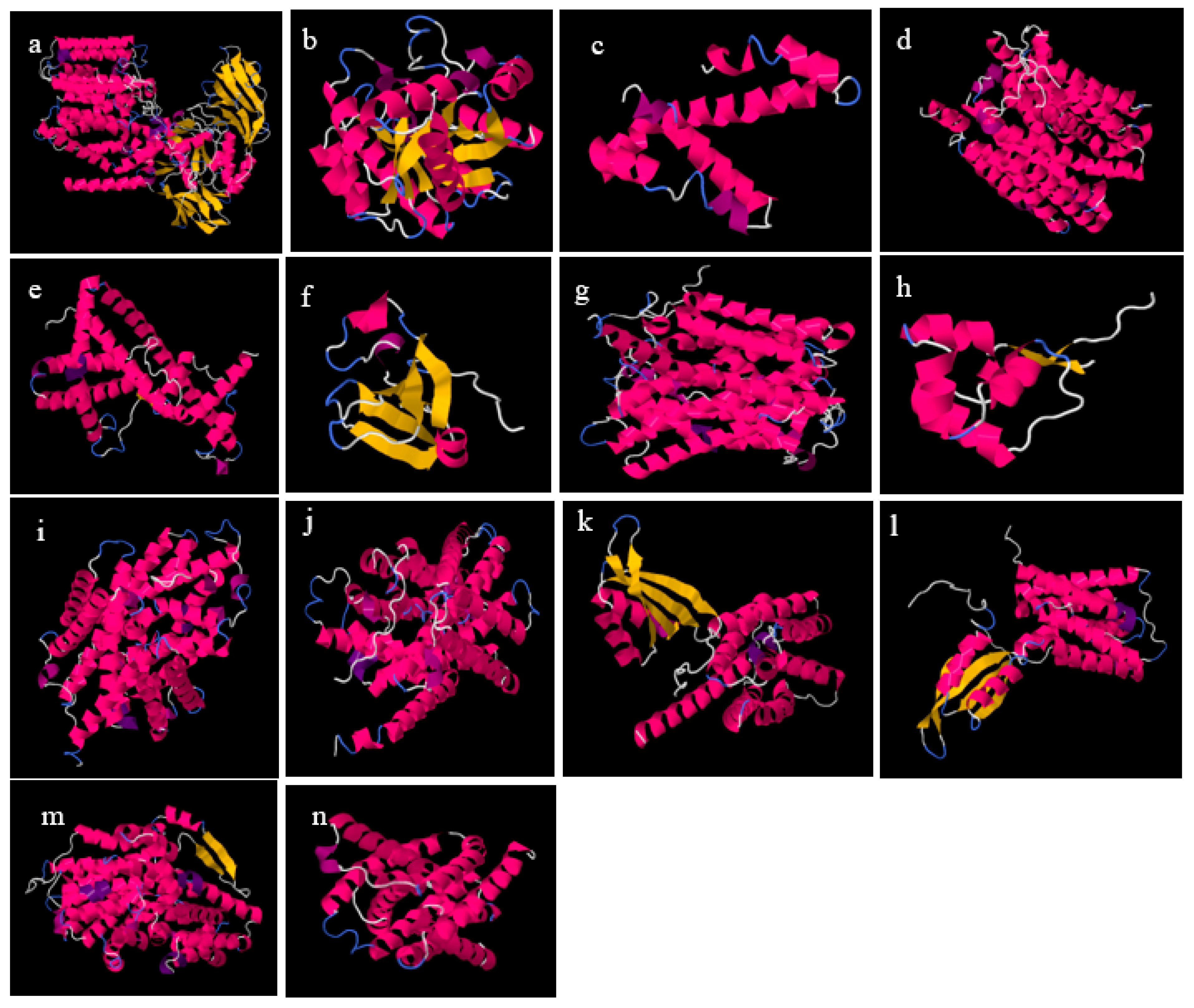
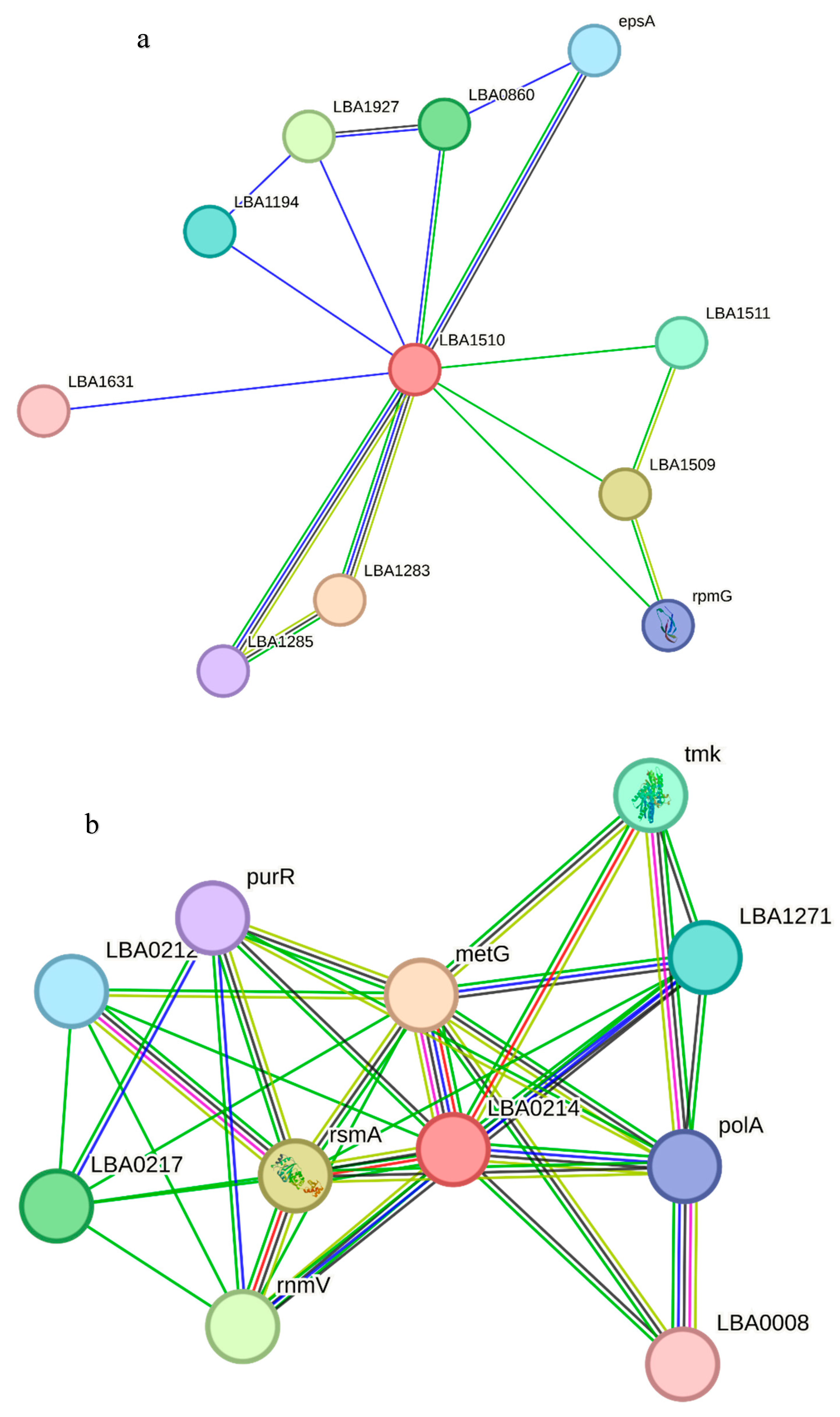
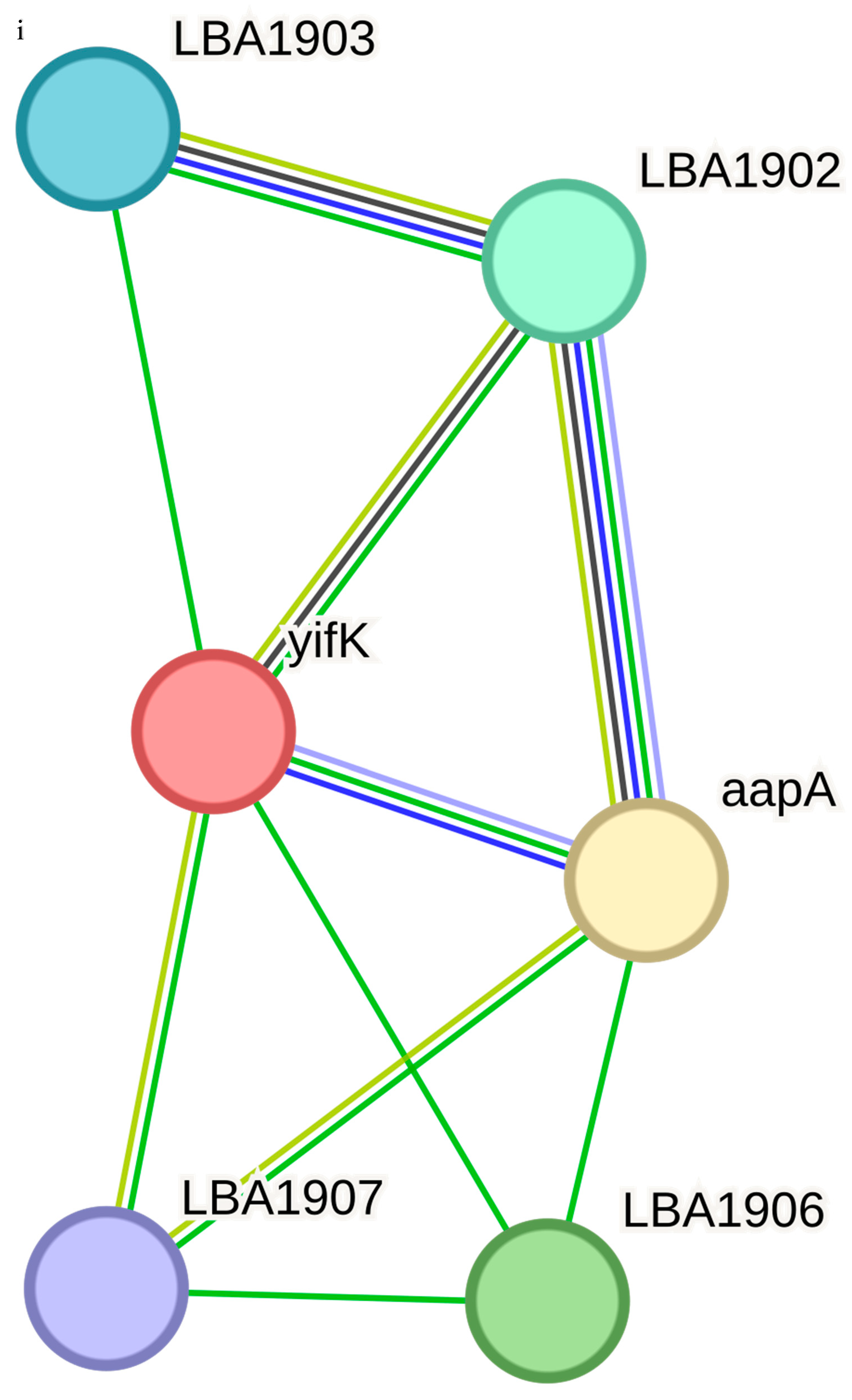
The functional associations of the query proteins of the
Lactobacillus acidophilus reference genome are presented in the Results section (
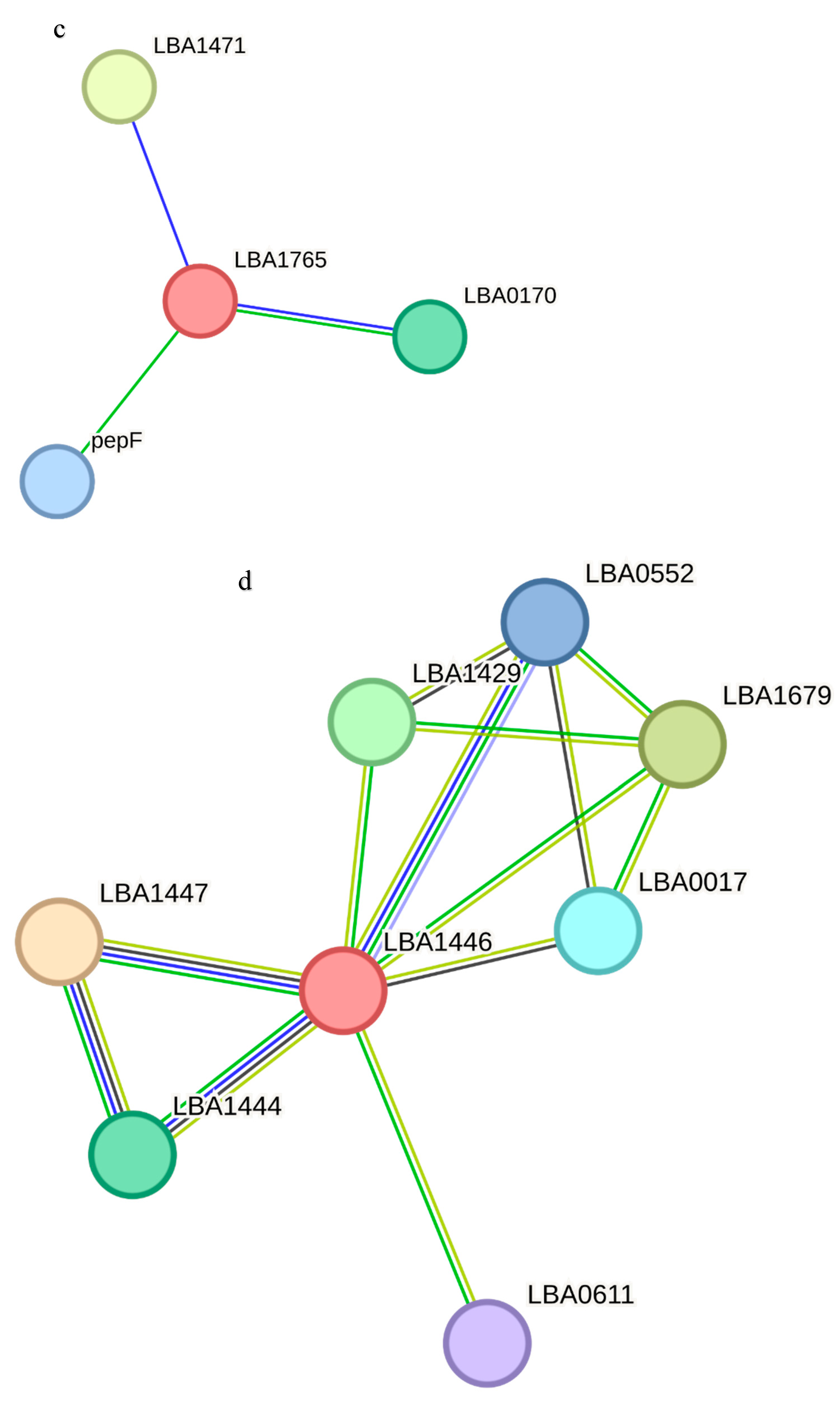
Figure 6). LBA1283 is a glycosil transferase, LBA1509 is a penicillin-binding protein and LBA1927 and LBA0860 are hypothetical proteins. metG is a met-rRNA synthetase that is not only required for the elongation of protein synthesis but also for the initiation of all mRNA translation. rsmA is a dimethyladenosine transferase, and mmV is a putative promase-like substance that is required for the correct processing of both the 5′ and 3′ ends of the 5S rRNA precursor. LBA0217 is a COG4466-uncharacterized protein conserved in bacteria while tmk is a thymidylate (dTMP) kinase; the phosphorylation of dTMP results in the formation of dTDP in both the de novo and salvage pathways of dTTP synthesis. LBA1471 is a putative multidrug efflux permease, LBA0170 is a putative 6-pyruvoyl-tetrahydropterin synthase and pepF is an oligopeptidase, endopeptidase F.
LBA1447 is a hypothetical protein, LBA1679 is an ABC transporter permease protein, LBA1429 is a putative transporter-membrane protein, LBA1444 is a transcriptional regulator family, MerR; LBA0017 is a putative general stress response and COG3237 is an uncharacterized protein conserved in bacteria belonging to the UPF0337 family. LBA0036 is a collagen-binding protein Cne precursor while pbpX-2 is a putative penicillin-binding protein. gldB is a glucose-inhibited division protein B that specifically methylates the N7 position of a guanine in 16S rRNA, ychF is a GTP-binding protein and parA, parB and parB-2 are chromosome-portioning proteins. Both parB and parB-2 are predicted transcriptional regulators of COG1475 and belong to the Par family.
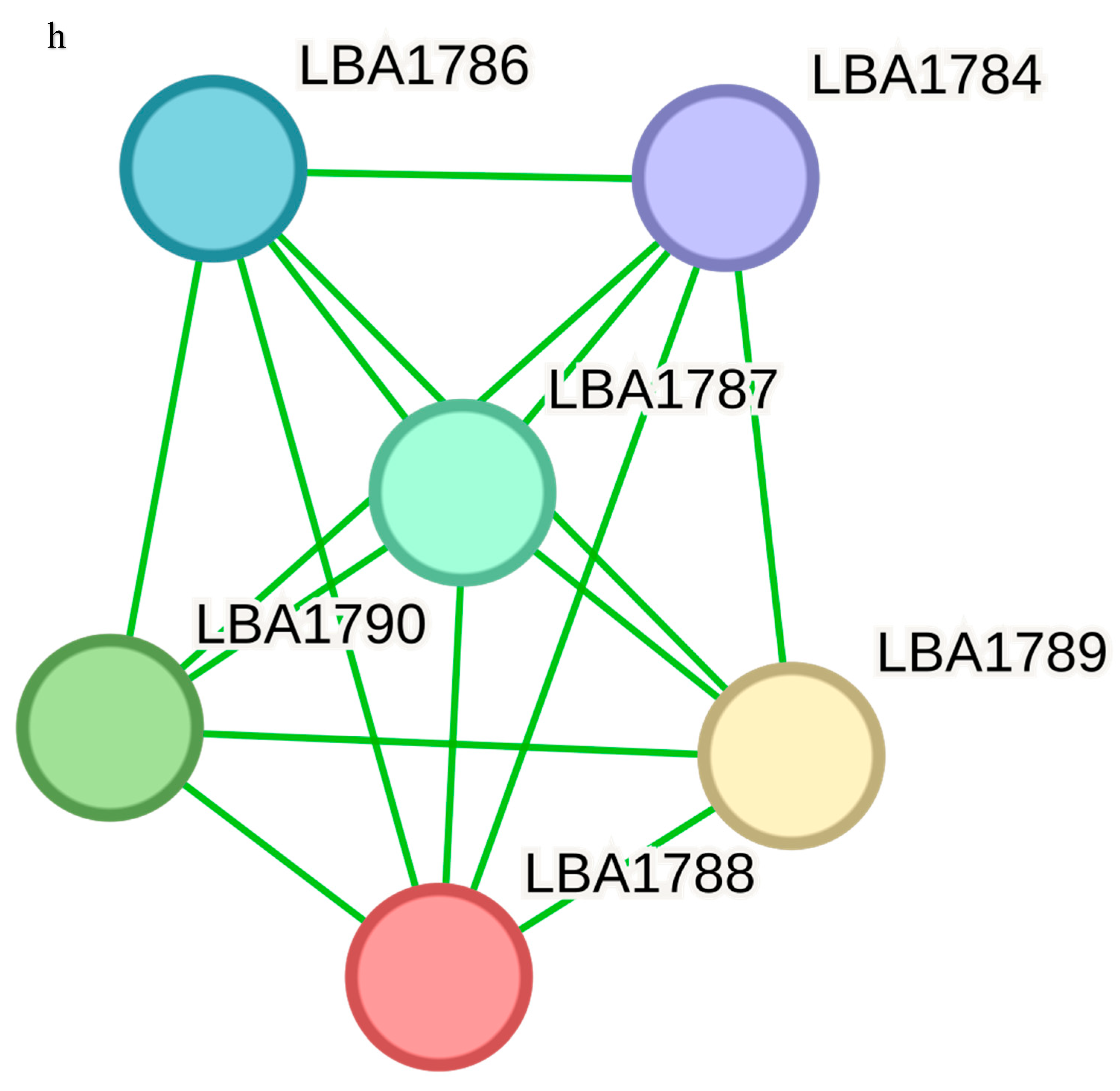
LBA0996 is a COG1982 arginine–lysine–ornithine decarboxylase, LBA0997 is an aluminum resistance protein, fabG is a 3-oxoacyl-(acyl-carrier protein) reductase and pepD is an aminoacyl-histidine dipeptidase and PepD is a carnosinase. LBA1789 and LBA1787 are hypothetical proteins while LBA1790 is a vacuolar sorting receptor protein homologueg of the PV72-cucur bit. The only functional associate of yifk, aapA is an amino acid permease. The functional associate of LBA0899, thyA is a thymidylate synthase, and dfrA is a dihydrofolate reductase, which is a key enzyme in folate metabolism.
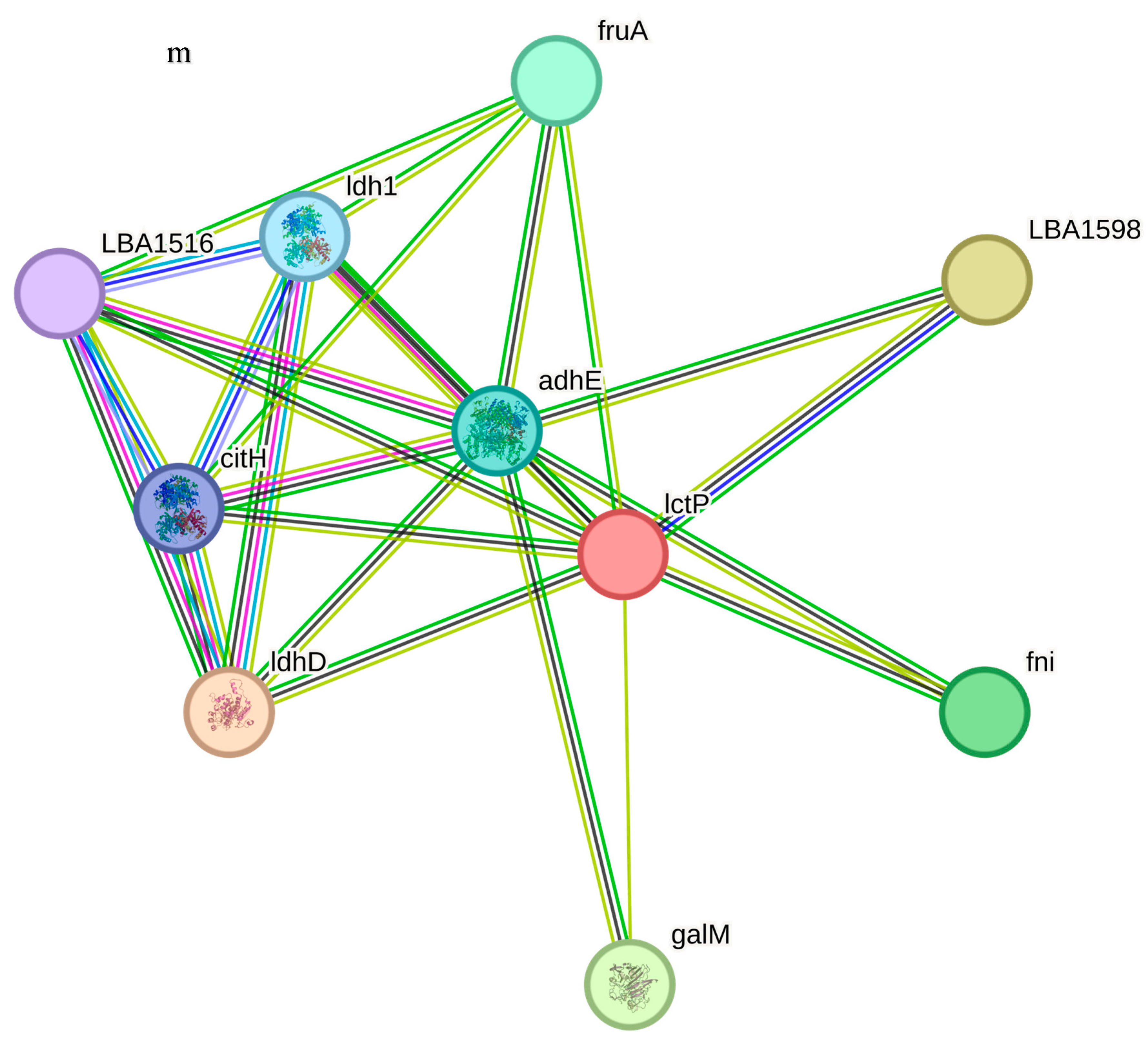
LBA0426 is a hypothetical protein, while cca is a tRNA nucleotidytransferase, polyA. LBA1598 is a glycolate oxidase, L-lactate dehydrogenase, cytochrome-type (lldD), ldhD is a D-lactate-dehydrogenase that belongs to the D-isomer specific 2-hydroxyacid dehydrogenase family, galM is a galactose-1-epimerase, mutarotase and fni are isopentenyl diphosphate isomerases, involved in the biosynthesis of isoprenoids. LBA0339, the functional associate of LBA0338, is a phosphoglycerate mutase, COG0406 fructose-2,6-bisphosphatase, which belongs to the phosphoglycerate mutase family. LBA0340 is a cation efflux protein that is a cation diffusion facilitator (CDF) transporter (TC 2.A. 4) Family. LBA0337 is a hypothetical protein while pheS is a phenylalanyl-tRNA synthetase, beta subunit, SyfB and COG0073 EMAP domain.
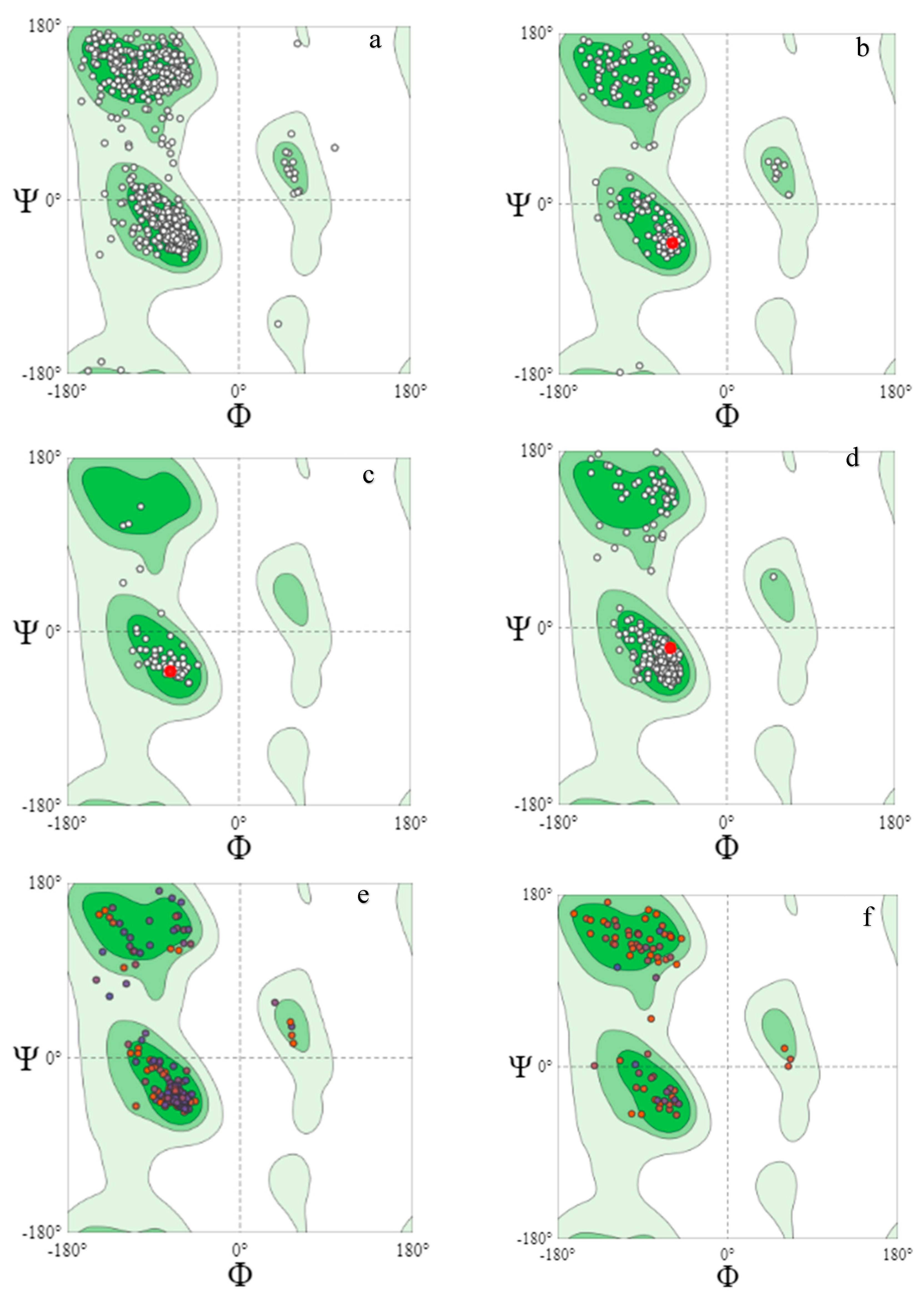
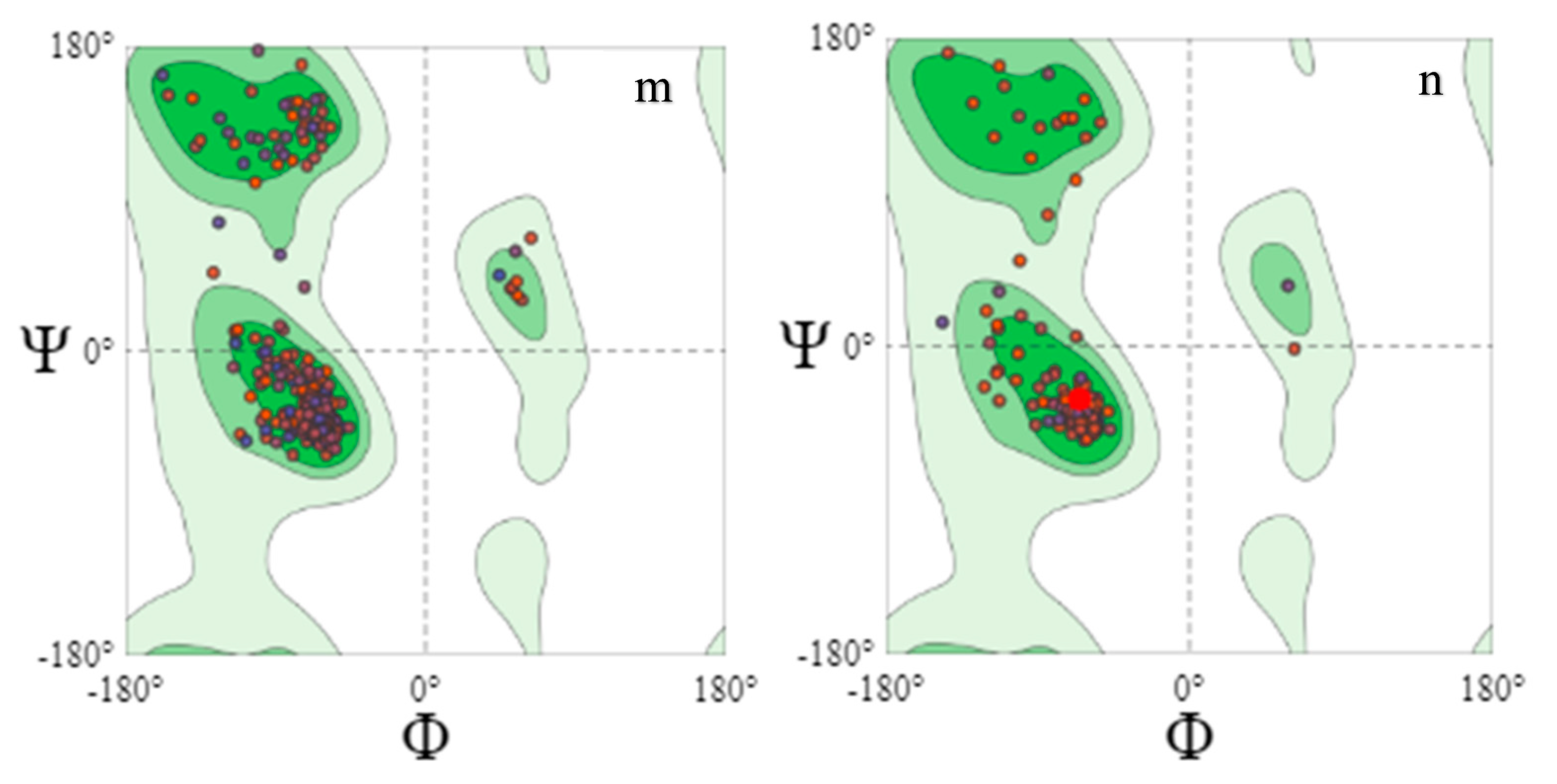
The Ramachandran plot, a graphical image of the dihedral angles φ (phi) and ψ (psi) in a protein’s backbone, indicates the combinations which are sterically permissible and favored for protein structure formation. The Ramachandran plots validated the structures obtained as the majority of the residues fell in the (B, A, L) areas. The favored Ramachandran areas (%) ranged between 92.31 and 99.59.
IL-6 is mainly produced by T lymphocytes, B lymphocytes, dendritic cells, microglia, fibroblasts, keratinocytes, mast cells, mesangial cells and vascular endothelial cells, binding to either soluble IL-6 receptors (sIL-6R) or the membrane-bound IL-6 receptors (mIL-6R) [
43]. It is a pleiotropic cytokine which plays several key roles in the body, such as influencing embryonic development, acute phase reactant pathways, B and T lymphocytes, synovial inflammation, blood–brain barrier permeability and hematopoiesis [
44]. It is known to play key functions in the normal, adaptive and innate autoimmunity. It is primarily expressed when tumor necrosis factor-alpha (TNFa) and interleukin 1 b (IL-1b) are activated. Prostaglandins, other cytokines, stress response, activated toll-like receptors (TLRs), and adipokines can also enhance its synthesis [
45]. When dysregulated, IL-6 can contribute to inflammatory cascades, indicating pathophysiology of many autoimmune conditions.
At the initial phase of inflammation, after IL-6 is produced, it moves to the liver (via the bloodstream), which is followed by the quick induction of an extensive range of acute phase proteins including serum amyloid A (SAA), C-reactive protein (CRP), a1-antichymotrypsin fibrinogen and haptoglobin [
46]. IL-6 also reduces albumin, transferrin and fibronectin production. A persistent high-level concentration of SAA may result in several chronic inflammatory conditions with a serious complication through amyloid A amyloidosis generation [
47], leading to deposition of amyloid fibril and progressive deterioration in organs [
48].
In addition, IL-6 regulates serum zinc and iron levels by regulating their transporters. It initiates the production of hepcidin that hinders the iron transporter ferroportin 1 action on the gut, thereby lowering the iron levels in the serum [
49], indicating the importance of IL-6–hepcidin axis in regulating hypoferremia and anemia associated with chronic inflammation. In the bone marrow, IL-6 promotes the maturation of megakaryocytes, thereby releasing platelets [
50]. Changes in acute phase protein levels and red blood cell and platelet counts can be used for determining the severity of inflammation in clinical laboratory examinations [
48].
The IL-6 axis has been successfully modulated (therapeutically), resulting in the approval of multiple therapeutic agents currently being investigated [
44]. IL-6 has been suggested to have potential applications in neuro-inflammatory conditions (not yet FDA approved) based on its ability to form complexes with other immunosuppressive medications (azathioprine and mycophenolate mofetil) [
44].
IL-6 has been reported to be produced by senescent cells and is involved in senescence-induced inflammation, age-dependent pathologies and cancer [
51]. It has been described as a crucial factor in inflammation, cancer and autoimmunity, performing its roles primarily via the IL-6–signal transducer, and acting as an activator of the transcription 3 (STAT3) pathway [
45,
52,
53]. In addition, the IL-6 amplifier (IL-6 Amp) is an amplification mechanism of IL-6, growth factors, chemokine and cytokine production via a synergic interaction between STAT3 and nuclear factor-kappa B (NF-κB), which also performs important functions in inflammatory conditions such as cancer, autoimmunity and cytokine storm syndromes [
54,
55,
56].
IL-6 inhibitors are now used clinically for conditions including rheumatoid arthritis (RA), Castleman’s disease and considered for treating COVID-19 [
57,
58] despite the involvement of many cytokines in inflammation-related diseases, based on the understanding that IL-6 is the main stimulator of STAT3 in inflammation. STA3 performs crucial functions in inflammation and oncogenesis together with NF-κB, expressing IL-6 as a target, indicating STAT3 and NF-κB as the basis of the IL-6 Amp. Injury, infection, obesity, stressors, senescence, smoking, pre-neoplastic mutation and cell death are factors which may activate the IL-6 Amp [
59]. The author further noted the importance of developing highly specific and effective small peptides against diseases with a requirement for high-resolution structural analysis of the ligand-receptor complex.
IL-6 is produced by various cell types such as epithelial, immune, liver, nerve, kidney and cartilage. It has been shown to play key regulatory functions in liver regeneration, cancer development, immunological regulation and inflammation [
60]. The dual roles of IL-6 have been established. IL-6 has a unique characteristic (dual signaling mechanism) referred to as classical signaling and trans-signaling. The pathway arises through the membrane-bound IL-6 receptor, while the trans-pathway is triggered through the soluble IL-6 receptor. IL-6 standard or trans-signaling ligand–receptor complex formation results in the stimulation of several intracellular signaling pathways [
60].
A recent study reported an increased IL-6 linked with long COVID-19 [
61]. The authors noted that IL6 levels in the serum were significantly raised in patients after COVID-19 infection in both long COVID-19 and the acute stage. Cytokine storms may cause the increase during the acute infection, but different dynamic changes in IL-6 and anti-bodies during the long COVID-19 phase may result in a decline. IL-6 was also significantly increased in patients with severe malaria compared with those in patients with non-severe malaria, demonstrating that IL-6 might be a candidate marker for severe malaria [
62]. The importance of IL-6 as a cytokine that facilitates a wide variety of biological functions upon binding to its receptor cannot be overstated. However, its excessive production and dysregulation of IL-6R signaling may result in several inflammatory diseases [
63].
Studies are on-going to provide potent and health-promoting alternatives to synthetic antibiotics in animal and human nutrition without any adverse effect to animals’ and human’s well-being [
64,
65,
66,
67,
68,
69,
70], underscoring the importance of the present study, which attempted to unravel the functional mechanisms of one of such alternatives. Different analyses were carried out to characterize and investigate the safety profiles of query proteins in the
L. acidophilus reference genome. The findings of the study revealed that none of the query proteins were allergenic or toxic, attesting to safety profile of LAB probiotics. All the query proteins are stable under a wide range of temperatures, indicating that they can withstand industrial processing and that climate change may not easily affect LAB probiotics. LBA0214, LBA1825 and LBA1788 are hydrophilic while the remaining ones are hydrophobic. Only LBA0995 has been determined to not be stable in nature, indicating that most of the proteins are stable, which is an advantage for their preservation and functionality. LBA1788 is cytoplasmic, while the rest are found in the cytoplasmic membrane. All the proteins produce peptides which induce IL-6 except LBA0037, LBA1825 and LBA1788, which are either non-immunogenic or non-IL-6-inducing. The immunogenic IL-6-inducing peptides are potential candidates for vaccine development and therapeutic purpose, further confirming the health-benefits of LAB probiotics in animal and human nutrition. The findings in this study are based on in silico analysis and a part of other preliminary studies that mainly aim at identifying and screening promising candidates for the next phases of the study focused on adopting an experimental approach (wet lab) to validate the claims in this preliminary study. Similar proposed studies are strongly recommended from other interested researchers. IL-6 is known to have dual roles: it contributes to host defense and mucosal immunity, but excessive or chronic induction is implicated in pathology. Therefore, any potential benefit of IL-6 induction must be context-dependent and carefully balanced against its pathogenic effects, highlighting the importance of downstream validation to assess physiological relevance.