Immune Response to Extracellular Matrix Bioscaffolds: A Comprehensive Review
Abstract
1. Introduction
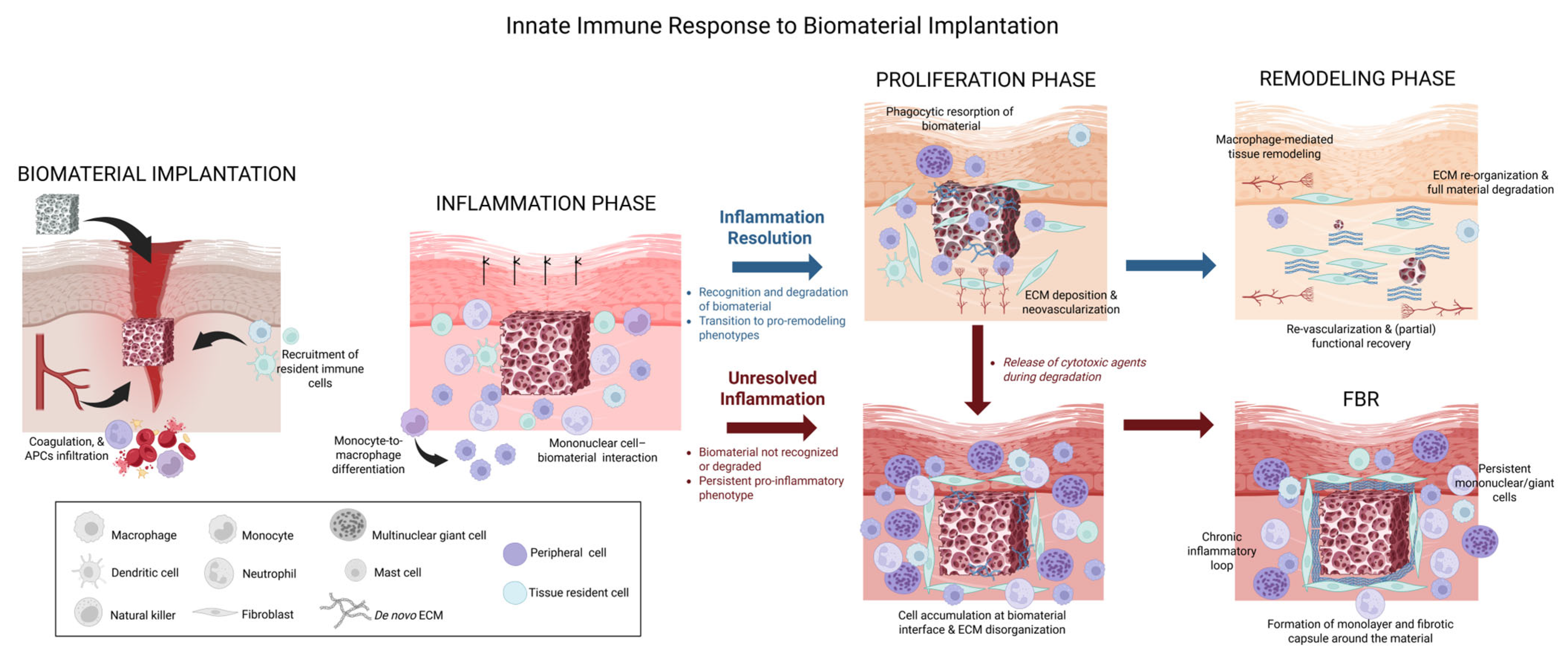
2. Overview of the Immune Response to Implanted Biomaterials
3. ECM Biomaterials
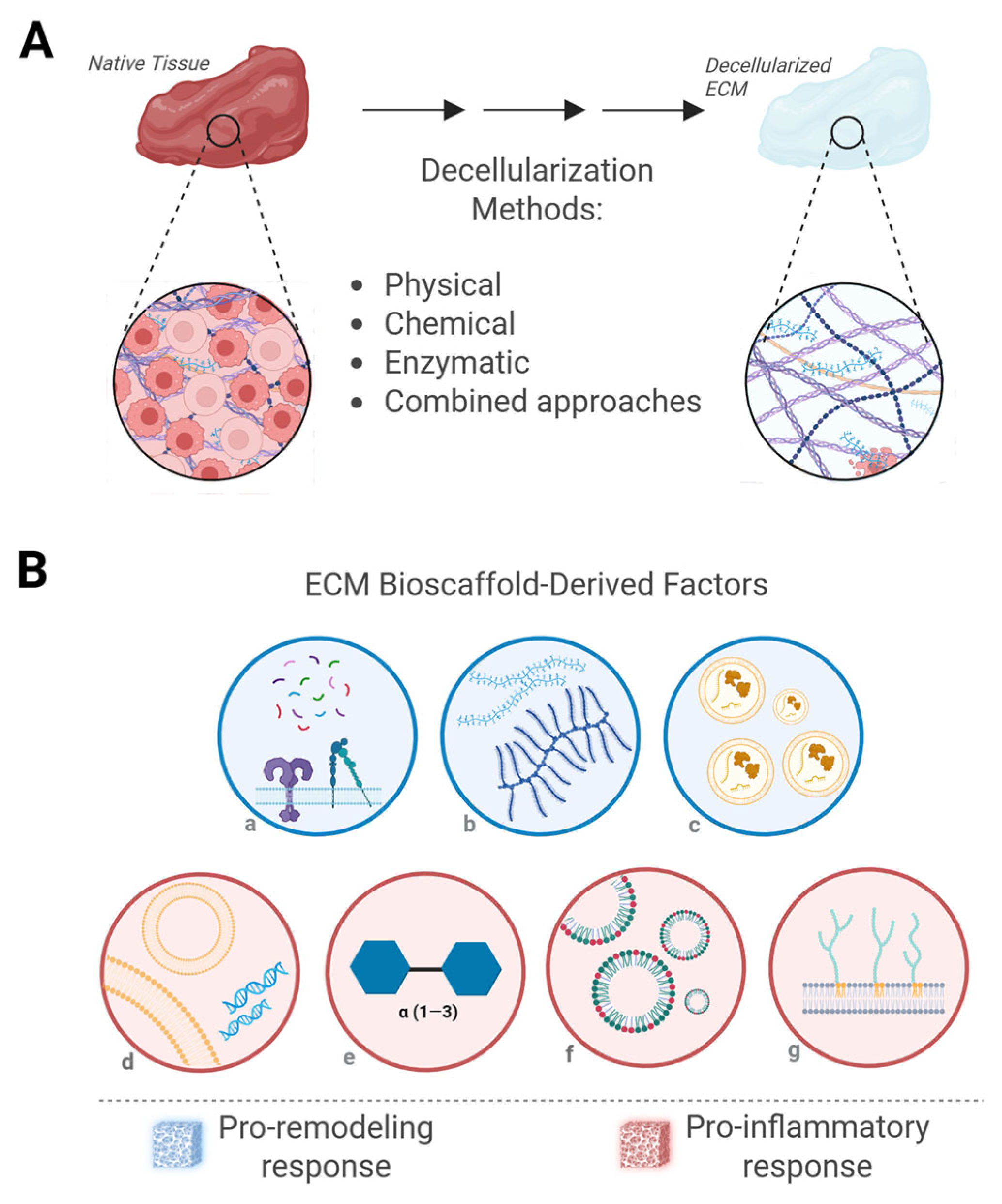
3.1. ECM Bioscaffolds
3.2. Purified ECM-Based Scaffolds
4. Factors Influencing Immune Response to ECM Bioscaffolds
4.1. Factors Promoting a Pro-Inflammatory Response
4.1.1. Residual DNA
4.1.2. Residuals from Processing Agents
4.1.3. ECM Carbohydrates
4.1.4. Endotoxins
4.2. Factors Promoting a Pro-Remodeling Response
4.2.1. Degradation Peptides and Recognition Motifs
4.2.2. ECM Carbohydrates: Friend or Fool?
4.2.3. Matrix-Bound Nanovesicles
4.3. Patient Factors Affecting the Immune Response
5. Implications in Clinical Translation
5.1. Implications in Manufacturing
5.2. Implications for Clinical Applications
5.3. Implications in Regulation as Medical Devices
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cramer, M.C.; Badylak, S.F. Extracellular Matrix-Based Biomaterials and Their Influence Upon Cell Behavior. Ann. Biomed. Eng. 2020, 48, 2132–2153. [Google Scholar] [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Kim, H.J.; Zheng, C.; Harkins, L.; Tao, W.; Deschenes, E. Foreign body response to synthetic polymer biomaterials and the role of adaptive immunity. Biomed. Mater. 2022, 17, 022007. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Dziki, J.L.; Giglio, R.M.; Sicari, B.M.; Wang, D.S.; Gandhi, R.M.; Londono, R.; Dearth, C.L.; Badylak, S.F. The Effect of Mechanical Loading Upon Extracellular Matrix Bioscaffold-Mediated Skeletal Muscle Remodeling. Tissue Eng. Part A 2018, 24, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Capella-Monsonís, H.; Kearns, S.; Kelly, J.; Zeugolis, D.I. Battling adhesions: From understanding to prevention. BMC Biomed. Eng. 2019, 1, 5. [Google Scholar] [CrossRef]
- Li, H.; Shan, W.; Zhao, X.; Sun, W. Neutrophils: Linking Inflammation to Thrombosis and Unlocking New Treatment Horizons. Int. J. Mol. Sci. 2025, 26, 1965. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Chung, L.; Maestas, D.R.; Housseau, F.; Elisseeff, J.H. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2017, 114, 184–192. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Cheng, Y.; Cao, X. Dendritic cell migration in inflammation and immunity. Cell. Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Alisjahbana, A.; Mohammad, I.; Gao, Y.; Evren, E.; Ringqvist, E.; Willinger, T. Human macrophages and innate lymphoid cells: Tissue-resident innate immunity in humanized mice. Biochem. Pharmacol. 2020, 174, 113672. [Google Scholar] [CrossRef] [PubMed]
- Behm, B.; Babilas, P.; Landthaler, M.; Schreml, S. Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M.; Loetscher, P. Chemokines in inflammation and immunity. Immunol. Today 2000, 21, 418–420. [Google Scholar] [CrossRef]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Lee, M.; Du, H.; Winer, D.A.; Clemente-Casares, X.; Tsai, S. Mechanosensing in macrophages and dendritic cells in steady-state and disease. Front. Cell Dev. Biol. 2022, 10, 1044729. [Google Scholar] [CrossRef]
- Capella-Monsonís, H.; Badylak, S.; Dewey, M. Extracellular matrix bioscaffolds: Structure-function. In Handbook of the Extracellular Matrix: Biologically-Derived Materials; Maia, F.R.A., Oliveira, J.M., Reis, R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–22. [Google Scholar]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS J. 2010, 12, 188–196. [Google Scholar] [CrossRef]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and tissue specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef]
- Coden, M.E.; Berdnikovs, S. Eosinophils in wound healing and epithelial remodeling: Is coagulation a missing link? J. Leukoc. Biol. 2020, 108, 93–103. [Google Scholar] [CrossRef]
- Vasanthan, V.; Hassanabad, A.F.; Belke, D.; Teng, G.; Isidoro, C.A.; Dutta, D.; Turnbull, J.; Deniset, J.F.; Fedak, P.W.M. Micronized Acellular Matrix Biomaterial Leverages Eosinophils for Postinfarct Cardiac Repair. JACC Basic. Transl. Sci. 2023, 8, 939–954. [Google Scholar] [CrossRef]
- Lokwani, R.; Fertil, D.; Hartigan, D.R.; Josyula, A.; Ngo, T.B.; Sadtler, K. Eosinophils Respond to Extracellular Matrix Treated Muscle Injuries but are Not Required for Macrophage Polarization. Adv. Healthc. Mater. 2025, 14, e2400134. [Google Scholar] [CrossRef]
- Tang, L.; Jennings, T.A.; Eaton, J.W. Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc. Natl. Acad. Sci. USA 1998, 95, 8841–8846. [Google Scholar] [CrossRef]
- Poto, R.; Loffredo, S.; Marone, G.; Di Salvatore, A.; de Paulis, A.; Schroeder, J.T.; Varricchi, G. Basophils beyond allergic and parasitic diseases. Front. Immunol. 2023, 14, 1190034. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, S.J.; Lee, J.H.; Knowles, J.C.; Lee, H.H.; Kim, H.W. Adaptive immunity of materials: Implications for tissue healing and regeneration. Bioact. Mater. 2024, 41, 499–522. [Google Scholar] [CrossRef] [PubMed]
- Kasravi, M.; Ahmadi, A.; Babajani, A.; Mazloomnejad, R.; Hatamnejad, M.R.; Shariatzadeh, S.; Bahrami, S.; Niknejad, H. Immunogenicity of decellularized extracellular matrix scaffolds: A bottleneck in tissue engineering and regenerative medicine. Biomater. Res. 2023, 27, 10. [Google Scholar] [CrossRef] [PubMed]
- Termeer, C.; Benedix, F.; Sleeman, J.; Fieber, C.; Voith, U.; Ahrens, T.; Miyake, K.; Freudenberg, M.; Galanos, C.; Simon, J.C. Oligosaccharides of Hyaluronan Activate Dendritic Cells via Toll-like Receptor 4. J. Exp. Med. 2002, 195, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Babelova, A.; Kiss, E.; Hausser, H.-J.; Baliova, M.; Krzyzankova, M.; Marsche, G.; Young, M.F.; Mihalik, D.; Götte, M.; et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J. Clin. Investig. 2005, 115, 2223–2233. [Google Scholar] [CrossRef]
- Sadtler, K.; Estrellas, K.; Allen, B.W.; Wolf, M.T.; Fan, H.; Tam, A.J.; Patel, C.H.; Luber, B.S.; Wang, H.; Wagner, K.R.; et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 2016, 352, 366–370. [Google Scholar] [CrossRef]
- Sadtler, K.; Sommerfeld, S.D.; Wolf, M.T.; Wang, X.; Majumdar, S.; Chung, L.; Kelkar, D.S.; Pandey, A.; Elisseeff, J.H. Proteomic composition and immunomodulatory properties of urinary bladder matrix scaffolds in homeostasis and injury. Semin. Immunol. 2017, 29, 14–23. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, X.; Wu, Y.; Xu, J.; Xiao, C.; Liu, Q.; Fang, L.; Liang, Y.; Zhou, J.; Wu, Y.; et al. Contribution of Tregs to the promotion of constructive remodeling after decellularized extracellular matrix material implantation. Mater. Today Bio 2024, 27, 101151. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Maestas, D.R.; Lebid, A.; Mageau, A.; Rosson, G.D.; Wu, X.; Wolf, M.T.; Tam, A.J.; Vanderzee, I.; Wang, X.; et al. Interleukin 17 and senescent cells regulate the foreign body response to synthetic material implants in mice and humans. Sci. Transl. Med. 2020, 12, eaax3799. [Google Scholar] [CrossRef] [PubMed]
- Allman, A.J.; McPherson, T.B.; Badylak, S.F.; Merrill, L.C.; Kallakury, B.; Sheehan, C.; Raeder, R.H.; Metzger, D.W. Xenogeneic extracellular matrix grafts elicit a Th2-restricted immune response. Transplantation 2001, 71, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.M.; Maestas, D.R.; Cherry, C.C.; Garcia, J.A.; Comeau, H.Y.; Davenport Huyer, L.; Kelly, S.H.; Peña, A.N.; Blosser, R.L.; Rosson, G.D.; et al. Biomaterials direct functional B cell response in a material-specific manner. Sci. Adv. 2021, 7, eabj5830. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Zielins, E.R.; Atashroo, D.A.; Maan, Z.N.; Duscher, D.; Walmsley, G.G.; Hu, M.; Senarath-Yapa, K.; McArdle, A.; Tevlin, R.; Wearda, T.; et al. Wound healing: An update. Regen. Med. 2014, 9, 817–830. [Google Scholar] [CrossRef]
- Rayahin, J.E.; Gemeinhart, R.A. Activation of macrophages in response to biomaterials. In Macrophages: Origin, Functions and Biointervention; Kloc, M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 317–351. [Google Scholar]
- Vogel, D.Y.; Glim, J.E.; Stavenuiter, A.W.; Breur, M.; Heijnen, P.; Amor, S.; Dijkstra, C.D.; Beelen, R.H. Human macrophage polarization in vitro: Maturation and activation methods compared. Immunobiology 2014, 219, 695–703. [Google Scholar] [CrossRef]
- Badylak, S.F.; Valentin, J.E.; Ravindra, A.K.; McCabe, G.P.; Stewart-Akers, A.M. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng. Part A 2008, 14, 1835–1842. [Google Scholar] [CrossRef]
- Martin, K.E.; García, A.J. Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies. Acta Biomater. 2021, 133, 4–16. [Google Scholar] [CrossRef]
- Klopfleisch, R. Macrophage reaction against biomaterials in the mouse model—Phenotypes, functions and markers. Acta Biomater. 2016, 43, 3–13. [Google Scholar] [CrossRef]
- Xiao, W.; Yang, Y.; Chu, C.; Rung, S.A.; Wang, Z.; Man, Y.; Lin, J.; Qu, Y. Macrophage response mediated by extracellular matrix: Recent progress. Biomed. Mater. 2023, 18, 012003. [Google Scholar] [CrossRef]
- Huleihel, L.; Dziki, J.L.; Bartolacci, J.G.; Rausch, T.; Scarritt, M.E.; Cramer, M.C.; Vorobyov, T.; LoPresti, S.T.; Swineheart, I.T.; White, L.J.; et al. Macrophage phenotype in response to ECM bioscaffolds. Semin. Immunol. 2017, 29, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Reing, J.E.; Brown, B.N.; Daly, K.A.; Freund, J.M.; Gilbert, T.W.; Hsiong, S.X.; Huber, A.; Kullas, K.E.; Tottey, S.; Wolf, M.T.; et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 2010, 31, 8626–8633. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef]
- Sridharan, R.; Cavanagh, B.; Cameron, A.R.; Kelly, D.J.; O’Brien, F.J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019, 89, 47–59. [Google Scholar] [CrossRef]
- Bendall, S.C.; Simonds, E.F.; Qiu, P.; Amir, E.-a.D.; Krutzik, P.O.; Finck, R.; Bruggner, R.V.; Melamed, R.; Trejo, A.; Ornatsky, O.I.; et al. Single-Cell Mass Cytometry of Differential Immune and Drug Responses Across a Human Hematopoietic Continuum. Science 2011, 332, 687–696. [Google Scholar] [CrossRef]
- Sommerfeld, S.D.; Cherry, C.; Schwab, R.M.; Chung, L.; Maestas, D.R.; Laffont, P.; Stein, J.E.; Tam, A.; Ganguly, S.; Housseau, F.; et al. Interleukin-36γ–producing macrophages drive IL-17–mediated fibrosis. Sci. Immunol. 2019, 4, eaax4783. [Google Scholar] [CrossRef]
- van der Maaten, L.; Hinton, G. Viualizing data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Van Gassen, S.; Callebaut, B.; Van Helden, M.J.; Lambrecht, B.N.; Demeester, P.; Dhaene, T.; Saeys, Y. FlowSOM: Using self-organizing maps for visualization and interpretation of cytometry data. Cytom. Part. A 2015, 87, 636–645. [Google Scholar] [CrossRef]
- Yang, Y.; Chu, C.; Liu, L.; Wang, C.; Hu, C.; Rung, S.; Man, Y.; Qu, Y. Tracing immune cells around biomaterials with spatial anchors during large-scale wound regeneration. Nat. Commun. 2023, 14, 5995. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-d.; Chu, C.-y.; Wang, C.-b.; Yang, Y.; Xu, Z.-y.; Qu, Y.-l.; Man, Y. Integrated-omics profiling unveils the disparities of host defense to ECM scaffolds during wound healing in aged individuals. Biomaterials 2024, 311, 122685. [Google Scholar] [CrossRef] [PubMed]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, H. Matrix metalloproteinases and cellular fibrinolytic activity. Biochemistry 2002, 67, 92–98. [Google Scholar] [CrossRef]
- Allman, A.J.; McPherson, T.B.; Merrill, L.C.; Badylak, S.F.; Metzger, D.W. The Th2-Restricted Immune Response to Xenogeneic Small Intestinal Submucosa Does Not Influence Systemic Protective Immunity to Viral and Bacterial Pathogens. Tissue Eng. 2002, 8, 53–62. [Google Scholar] [CrossRef]
- Wolf, M.T.; Ganguly, S.; Wang, T.L.; Anderson, C.W.; Sadtler, K.; Narain, R.; Cherry, C.; Parrillo, A.J.; Park, B.V.; Wang, G.; et al. A biologic scaffold-associated type 2 immune microenvironment inhibits tumor formation and synergizes with checkpoint immunotherapy. Sci. Transl. Med. 2019, 11, eaat7973. [Google Scholar] [CrossRef]
- Lehmann, N.; Christ, T.; Daugs, A.; Bloch, O.; Holinski, S. EDC-crosslinking of decellularized tissue—A promising approach? Tissue Eng. Part A 2017, 23, 13–14. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Leow-Dyke, S.F.; Rooney, P.; Kearney, J.N. Evaluation of copper and hydrogen peroxide treatments on the biology, biomechanics, and cytotoxicity of decellularized dermal allografts. Tissue Eng. Part C Methods 2016, 22, 290–300. [Google Scholar] [CrossRef]
- Pérez-Köhler, B.; Bayon, Y.; Bellón, J.M. Mesh infection and hernia repair: A review. Surg. Infect. 2015, 17, 124–137. [Google Scholar] [CrossRef]
- Capella-Monsonis, H.; Shridhar, A.; Chirravuri, B.; Figucia, M.; Learn, G.; Greenawalt, K.; Badylak, S.F. A Comparative Study of the Resorption and Immune Response for Two Starch-Based Hemostat Powders. J. Surg. Res. 2023, 282, 210–224. [Google Scholar] [CrossRef]
- Sicari, B.M.; Dziki, J.L.; Siu, B.F.; Medberry, C.J.; Dearth, C.L.; Badylak, S.F. The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials 2014, 35, 8605–8612. [Google Scholar] [CrossRef] [PubMed]
- Piatnitskaia, S.; Rafikova, G.; Bilyalov, A.; Chugunov, S.; Akhatov, I.; Pavlov, V.; Kzhyshkowska, J. Modelling of macrophage responses to biomaterials in vitro: State-of-the-art and the need for the improvement. Front. Immunol. 2024, 15, 1349461. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. A 2017, 105, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Sclamberg, S.G.; Tibone, J.E.; Itamura, J.M.; Kasraeian, S. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J. Shoulder Elb. Surg. 2004, 13, 538–541. [Google Scholar] [CrossRef]
- Bryant, D.; Holtby, R.; Willits, K.; Litchfield, R.; Drosdowech, D.; Spouge, A.; White, D.; Guyatt, G. A randomized clinical trial to compare the effectiveness of rotator cuff repair with or without augmentation using porcine small intestine submucosa for patients with moderate to large rotator cuff tears: A pilot study. J. Shoulder Elb. Surg. 2016, 25, 1623–1633. [Google Scholar] [CrossRef]
- Iannotti, J.P.; Codsi, M.J.; Kwon, Y.W.; Derwin, K.; Ciccone, J.; Brems, J.J. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J. Bone Jt. Surg. Am. Vol. 2006, 88, 1238–1244. [Google Scholar] [CrossRef]
- Deeken, C.R.; Abdo, M.S.; Frisella, M.M.; Matthews, B.D. Physicomechanical Evaluation of Polypropylene, Polyester, and Polytetrafluoroethylene Meshes for Inguinal Hernia Repair. J. Am. Coll. Surg. 2011, 212, 68–79. [Google Scholar] [CrossRef]
- Pott, P.P.; Schwarz, M.L.R.; Gundling, R.; Nowak, K.; Hohenberger, P.; Roessner, E.D. Mechanical Properties of Mesh Materials Used for Hernia Repair and Soft Tissue Augmentation. PLoS ONE 2012, 7, e46978. [Google Scholar] [CrossRef]
- Oelschlager, B.K.; Pellegrini, C.A.; Hunter, J.; Soper, N.; Brunt, M.; Sheppard, B.; Jobe, B.; Polissar, N.; Mitsumori, L.; Nelson, J.; et al. Biologic Prosthesis Reduces Recurrence After Laparoscopic Paraesophageal Hernia Repair: A Multicenter, Prospective, Randomized Trial. Ann. Surg. 2006, 244, 481–490. [Google Scholar] [CrossRef]
- Keane, T.J.; Londono, R.; Turner, N.J.; Badylak, S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012, 33, 1771–1781. [Google Scholar] [CrossRef]
- Brown, B.N.; Valentin, J.E.; Stewart-Akers, A.M.; McCabe, G.P.; Badylak, S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009, 30, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, L.; Bartolacci, J.G.; Dziki, J.L.; Vorobyov, T.; Arnold, B.; Scarritt, M.E.; Pineda Molina, C.; LoPresti, S.T.; Brown, B.N.; Naranjo, J.D.; et al. Matrix-bound nanovesicles recapitulate extracellular matrix effects on macrophage phenotype. Tissue Eng. Part A 2017, 23, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Crum, R.J.; Hall, K.; Molina, C.P.; Hussey, G.S.; Graham, E.; Li, H.; Badylak, S.F. Immunomodulatory matrix-bound nanovesicles mitigate acute and chronic pristane-induced rheumatoid arthritis. NPJ Regen. Med. 2022, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Capella-Monsonis, H.; Crum, R.J.; D’Angelo, W.; Hussey, G.S.; Badylak, S.F. Matrix-Bound Nanovesicles Promote Prohealing Immunomodulation Without Immunosuppression. Tissue Eng. Part A 2025. [Google Scholar] [CrossRef]
- Keane, T.J.; Badylak, S.F. The host response to allogeneic and xenogeneic biological scaffold materials. J. Tissue Eng. Regen. Med. 2015, 9, 504–511. [Google Scholar] [CrossRef]
- Reing, J.E.; Zhang, L.; Myers-Irvin, J.; Cordero, K.E.; Freytes, D.O.; Heber-Katz, E.; Bedelbaeva, K.; McIntosh, D.; Dewilde, A.; Braunhut, S.J.; et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng. Part A 2009, 15, 605–614. [Google Scholar] [CrossRef]
- Dziki, J.L.; Huleihel, L.; Scarritt, M.E.; Badylak, S.F. Extracellular Matrix Bioscaffolds as Immunomodulatory Biomaterials. Tissue Eng. Part A 2017, 23, 1152–1159. [Google Scholar] [CrossRef]
- Turner, J.B.; Corazzini, R.L.; Butler, T.J.; Garlick, D.S.; Rinker, B.D. Evaluating adhesion reduction efficacy of type I/III collagen membrane and collagen-GAG resorbable matrix in primary flexor tendon repair in a chicken model. Hand 2015, 10, 482–488. [Google Scholar] [CrossRef]
- Takanari, K.; Hong, Y.; Hashizume, R.; Huber, A.; Amoroso, N.J.; D’Amore, A.; Badylak, S.F.; Wagner, W.R. Abdominal wall reconstruction by a regionally distinct biocomposite of extracellular matrix digest and a biodegradable elastomer. J. Tissue Eng. Regen. Med. 2016, 10, 748–761. [Google Scholar] [CrossRef]
- Dziki, J.; Badylak, S.; Yabroudi, M.; Sicari, B.; Ambrosio, F.; Stearns, K.; Turner, N.; Wyse, A.; Boninger, M.L.; Brown, E.H.P.; et al. An acellular biologic scaffold treatment for volumetric muscle loss: Results of a 13-patient cohort study. npj Regen. Med. 2016, 1, 16008. [Google Scholar] [CrossRef] [PubMed]
- Dziki, J.L.; Badylak, S.F. Extracellular Matrix for Myocardial Repair. Adv. Exp. Med. Biol. 2018, 1098, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, J.M.; Hashizume, R.; Fujimoto, K.L.; Remlinger, N.T.; Pesyna, C.; Wagner, W.R.; Tobita, K.; Gilbert, T.W.; Badylak, S.F. Right ventricular outflow tract repair with a cardiac biologic scaffold. Cells Tissues Organs 2012, 195, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Kochupura, P.V.; Cohen, I.S.; Doronin, S.V.; Saltman, A.E.; Gilbert, T.W.; Kelly, D.J.; Ignotz, R.A.; Gaudette, G.R. The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium. Cell Transplant. 2006, 15 (Suppl. S1), S29–S40. [Google Scholar] [CrossRef]
- Sabetkish, S.; Sabetkish, N.; Kajbafzadeh, A.M. Regeneration of muscular wall of the bladder using a ureter matrix graft as a scaffold. Biotech. Histochem. 2022, 97, 207–214. [Google Scholar] [CrossRef]
- Badylak, S.F.; Kropp, B.; McPherson, T.; Liang, H.; Snyder, P.W. Small intestinal submucosa: A rapidly resorbed bioscaffold for augmentation cystoplasty in a dog model. Tissue Eng. 1998, 4, 379–387. [Google Scholar] [CrossRef]
- Kutten, J.C.; McGovern, D.; Hobson, C.M.; Luffy, S.A.; Nieponice, A.; Tobita, K.; Francis, R.J.; Reynolds, S.D.; Isenberg, J.S.; Gilbert, T.W. Decellularized tracheal extracellular matrix supports epithelial migration, differentiation, and function. Tissue Eng. Part A 2015, 21, 75–84. [Google Scholar] [CrossRef]
- Agrawal, V.; Tottey, S.; Johnson, S.A.; Freund, J.M.; Siu, B.F.; Badylak, S.F. Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng. Part A 2011, 17, 2435–2443. [Google Scholar] [CrossRef]
- Hoppo, T.; Badylak, S.F.; Jobe, B.A. A novel esophageal-preserving approach to treat high-grade dysplasia and superficial adenocarcinoma in the presence of chronic gastroesophageal reflux disease. World J. Surg. 2012, 36, 2390–2393. [Google Scholar] [CrossRef]
- Hynes, R.O.; Naba, A. Overview of the matrisome—An inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef]
- Shao, X.; Taha, I.N.; Clauser, K.R.; Gao, Y.; Naba, A. MatrisomeDB: The ECM-protein knowledge database. Nucleic Acids Res. 2019, 48, D1136–D1144. [Google Scholar] [CrossRef]
- Antisdel, J.L.; Janney, C.G.; Long, J.P.; Sindwani, R. Hemostatic agent microporous polysaccharide hemospheres (MPH) does not affect healing or intact sinus mucosa. Laryngoscope 2008, 118, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.A.; Gidwani, S.; Curtis, M.J. Early complications from the use of porcine dermal collagen implants (Permacol) as bridging constructs in the repair of massive rotator cuff tears. A report of 4 cases. Acta Orthop. Belg. 2007, 73, 432–436. [Google Scholar] [PubMed]
- Abdelfatah, M.M.; Rostambeigi, N.; Podgaetz, E.; Sarr, M.G. Long-term outcomes (>5-year follow-up) with porcine acellular dermal matrix (Permacol™) in incisional hernias at risk for infection. Hernia 2015, 19, 135–140. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Badylak, S.F. Host Response to Biomaterials: The Impact of Host Response on Biomaterial Selection; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Badylak, S.F. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: Factors that influence the host response. Ann. Biomed. Eng. 2014, 42, 1517–1527. [Google Scholar] [CrossRef]
- Marcal, H.; Ahmed, T.; Badylak, S.F.; Tottey, S.; Foster, L.J. A comprehensive protein expression profile of extracellular matrix biomaterial derived from porcine urinary bladder. Regen. Med. 2012, 7, 159–166. [Google Scholar] [CrossRef]
- Freytes, D.O.; Badylak, S.F.; Webster, T.J.; Geddes, L.A.; Rundell, A.E. Biaxial strength of multilaminated extracellular matrix scaffolds. Biomaterials 2004, 25, 2353–2361. [Google Scholar] [CrossRef]
- Badylak, S.F.; Lantz, G.C.; Coffey, A.; Geddes, L.A. Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg. Res. 1989, 47, 74–80. [Google Scholar] [CrossRef]
- Faulk, D.M.; Carruthers, C.A.; Warner, H.J.; Kramer, C.R.; Reing, J.E.; Zhang, L.; D’Amore, A.; Badylak, S.F. The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta Biomater. 2014, 10, 183–193. [Google Scholar] [CrossRef]
- Prasertsung, I.; Kanokpanont, S.; Bunaprasert, T.; Thanakit, V.; Damrongsakkul, S. Development of acellular dermis from porcine skin using periodic pressurized technique. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 85B, 210–219. [Google Scholar] [CrossRef]
- Agarwal, G.; Shumard, S.; McCrary, M.W.; Osborne, O.; Santiago, J.M.; Ausec, B.; Schmidt, C.E. Decellularized porcine peripheral nerve based injectable hydrogels as a Schwann cell carrier for injured spinal cord regeneration. J. Neural Eng. 2024, 21, 046002. [Google Scholar] [CrossRef]
- Chun, S.Y.; Ha, Y.S.; Yoon, B.H.; Lee, E.H.; Kim, B.M.; Gil, H.; Han, M.H.; Kwon, T.G.; Kim, B.S.; Lee, J.N. Optimal delipidation solvent to secure extracellular matrix from human perirenal adipose tissue. J. Biomed. Mater. Res. A 2022, 110, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Hosty, L.; Heatherington, T.; Quondamatteo, F.; Browne, S. Extracellular matrix-inspired biomaterials for wound healing. Mol. Biol. Rep. 2024, 51, 830. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Sklenářová, R.A.-O.X.; Akla, N.; Latorre, M.J.; Ulrichová, J.; Franková, J.A.-O. Collagen as a Biomaterial for Skin and Corneal Wound Healing. J. Funct. Biomater. 2022, 13, 249. [Google Scholar] [CrossRef]
- Gimeno, L.I.; Benito-Jardon, M.; Guerrero-Barbera, G.; Burday, N.; Costell, M. The Role of the Fibronectin Synergy Site for Skin Wound Healing. Cells 2022, 11, 2100. [Google Scholar] [CrossRef]
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef]
- Li, L.; Zheng, X.; Fan, D.; Yu, S.; Wu, D.; Fan, C.; Cui, W.; Ruan, H. Release of celecoxib from a bi-layer biomimetic tendon sheath to prevent tissue adhesion. Mater. Sci. Eng. C 2016, 61 (Suppl. C), 220–226. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, K.; Tan, J.; Zhou, R.; Chen, Y.; Chen, C.; Rong, Z.; Deng, M.; Yu, X.; Zhang, C.; et al. Laminin alpha 4 promotes bone regeneration by facilitating cell adhesion and vascularization. Acta Biomater. 2021, 126, 183–198. [Google Scholar] [CrossRef]
- Gonzalez de Torre, I.; Alonso, M.; Rodriguez-Cabello, J.C. Elastin-Based Materials: Promising Candidates for Cardiac Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 657. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M.; Bejenaru, L.E. Hyaluronic acid-based scaffolds for tissue engineering. Rom. J. Morphol. Embryol. 2018, 59, 71–76. [Google Scholar] [PubMed]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef] [PubMed]
- Pineda Molina, C.; Giglio, R.; Gandhi, R.M.; Sicari, B.M.; Londono, R.; Hussey, G.S.; Bartolacci, J.G.; Quijano Luque, L.M.; Cramer, M.C.; Dziki, J.L.; et al. Comparison of the host macrophage response to synthetic and biologic surgical meshes used for ventral hernia repair. J. Immunol. Regen. Med. 2019, 3, 13–25. [Google Scholar] [CrossRef]
- Chang, D.T.; Jones, J.A.; Meyerson, H.; Colton, E.; Kwon, I.K.; Matsuda, T.; Anderson, J.M. Lymphocyte/macrophage interactions: Biomaterial surface-dependent cytokine, chemokine, and matrix protein production. J. Biomed. Mater. Res. Part A 2008, 87A, 676–687. [Google Scholar] [CrossRef]
- Dutta, S.D.; An, J.M.; Hexiu, J.; Randhawa, A.; Ganguly, K.; Patil, T.V.; Thambi, T.; Kim, J.; Lee, Y.-k.; Lim, K.-T. 3D bioprinting of engineered exosomes secreted from M2-polarized macrophages through immunomodulatory biomaterial promotes in vivo wound healing and angiogenesis. Bioact. Mater. 2025, 45, 345–362. [Google Scholar] [CrossRef]
- Fink, J.; Fuhrmann, R.; Scharnweber, T.; Franke, R.P. Stimulation of monocytes and macrophages: Possible influence of surface roughness. Clin. Hemorheol. Microcirc. 2008, 39, 205–212. [Google Scholar] [CrossRef]
- Huo, Z.; Yang, W.; Harati, J.; Nene, A.; Borghi, F.; Piazzoni, C.; Milani, P.; Guo, S.; Galluzzi, M.; Boraschi, D. Biomechanics of Macrophages on Disordered Surface Nanotopography. ACS Appl. Mater. Interfaces 2024, 16, 27164–27176. [Google Scholar] [CrossRef]
- Yang, S.; Plotnikov, S.V. Mechanosensitive Regulation of Fibrosis. Cells 2021, 10, 994. [Google Scholar] [CrossRef]
- Xie, W.; Wei, X.; Kang, H.; Jiang, H.; Chu, Z.; Lin, Y.; Hou, Y.; Wei, Q. Static and Dynamic: Evolving Biomaterial Mechanical Properties to Control Cellular Mechanotransduction. Adv. Sci. 2023, 10, 2204594. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Ma, M.; Jiang, W.; Zhou, R. DAMPs and DAMP-sensing receptors in inflammation and diseases. Immunity 2024, 57, 752–771. [Google Scholar] [CrossRef]
- Bavaria, J.E.; Desai, N.D.; Cheung, A.; Petracek, M.R.; Groh, M.A.; Borger, M.A.; Schaff, H.V. The St Jude Medical Trifecta aortic pericardial valve: Results from a global, multicenter, prospective clinical study. J. Thorac. Cardiovasc. Surg. 2013, 147, 590–597. [Google Scholar] [CrossRef]
- Hussein, K.H.; Park, K.-M.; Kang, K.-S.; Woo, H.-M. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater. Sci. Eng. C 2016, 67, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Record Ritchie, R.D.; Salmon, S.L.; Hiles, M.C.; Metzger, D.W. Lack of immunogenicity of xenogeneic DNA from porcine biomaterials. Surg. Open Sci. 2022, 10, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Huang, Y.; Dai, J.; Zhao, M.; Wang, Y.; Turner, N.; Zhang, J. Endotoxin, not DNA, determines the host response and tissue regeneration behavior of acellular biologic scaffolds. Acta Biomater. 2025, 195, 157–168. [Google Scholar] [CrossRef]
- Zvarova, B.; Uhl, F.E.; Uriarte, J.J.; Borg, Z.D.; Coffey, A.L.; Bonenfant, N.R.; Weiss, D.J.; Wagner, D.E. Residual detergent detection method for nondestructive cytocompatibility evaluation of decellularized whole lung scaffolds. Tissue Eng. Part C Methods 2016, 22, 418–428. [Google Scholar] [CrossRef]
- Cebotari, S.; Tudorache, I.; Jaekel, T.; Hilfiker, A.; Dorfman, S.; Ternes, W.; Haverich, A.; Lichtenberg, A. Detergent decellularization of heart valves for tissue engineering: Toxicological effects of residual detergents on human endothelial cells. Artif. Organs 2010, 34, 206–210. [Google Scholar] [CrossRef]
- Ghorbani, F.; Ekhtiari, M.; Moeini Chaghervand, B.; Moradi, L.; Mohammadi, B.; Kajbafzadeh, A.M. Detection of the residual concentration of sodium dodecyl sulfate in the decellularized whole rabbit kidney extracellular matrix. Cell Tissue Bank. 2022, 23, 119–128. [Google Scholar] [CrossRef]
- Boekema, B.K.; Vlig, M.; Olde Damink, L.; Middelkoop, E.; Eummelen, L.; Buhren, A.V.; Ulrich, M.M. Effect of pore size and cross-linking of a novel collagen-elastin dermal substitute on wound healing. J. Mater. Sci. Mater. Med. 2014, 25, 423–433. [Google Scholar] [CrossRef]
- Cao, G.; Huang, Y.; Li, K.; Fan, Y.; Xie, H.; Li, X. Small intestinal submucosa: Superiority, limitations and solutions, and its potential to address bottlenecks in tissue repair. J. Mater. Chem. B 2019, 7, 5038–5055. [Google Scholar] [CrossRef] [PubMed]
- Delgado, L.M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng. Part B Rev. 2015, 21, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Stahl, E.C.; Bonvillain, R.W.; Skillen, C.D.; Burger, B.L.; Hara, H.; Lee, W.; Trygg, C.B.; Didier, P.J.; Grasperge, B.F.; Pashos, N.C.; et al. Evaluation of the host immune response to decellularized lung scaffolds derived from alpha-Gal knockout pigs in a non-human primate model. Biomaterials 2018, 187, 93–104. [Google Scholar] [CrossRef] [PubMed]
- McPherson, T.B.; Liang, H.; Record, R.D.; Badylak, S.F. Galalpha(1,3)Gal epitope in porcine small intestinal submucosa. Tissue Eng. 2000, 6, 233–239. [Google Scholar] [CrossRef]
- Raeder, R.H.; Badylak, S.F.; Sheehan, C.; Kallakury, B.; Metzger, D.W. Natural anti-galactose alpha1,3 galactose antibodies delay, but do not prevent the acceptance of extracellular matrix xenografts. Transpl. Immunol. 2002, 10, 15–24. [Google Scholar] [CrossRef]
- Cazzell, S.M.; Lange, D.L.; Dickerson, J.E., Jr.; Slade, H.B. The Management of Diabetic Foot Ulcers with Porcine Small Intestine Submucosa Tri-Layer Matrix: A Randomized Controlled Trial. Adv. Wound Care 2015, 4, 711–718. [Google Scholar] [CrossRef]
- Nherera, L.M.; Romanelli, M.; Trueman, P.; Dini, V. An Overview of Clinical and Health Economic Evidence Regarding Porcine Small Intestine Submucosa Extracellular Matrix in the Management of Chronic Wounds and Burns. Ostomy Wound Manag. 2017, 63, 38–47. [Google Scholar]
- Bejjani, G.K.; Zabramski, J.; Durasis Study, G. Safety and efficacy of the porcine small intestinal submucosa dural substitute: Results of a prospective multicenter study and literature review. J. Neurosurg. 2007, 106, 1028–1033. [Google Scholar] [CrossRef]
- Badylak, S.; Meurling, S.; Chen, M.; Spievack, A.; Simmons-Byrd, A. Resorbable bioscaffold for esophageal repair in a dog model. J. Pediatr. Surg. 2000, 35, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Kimble, A.; Hauschild, J.; McDonnell, G. Affinity and Inactivation of Bacterial Endotoxins for Medical Device Materials. Biomed. Instrum. Technol. 2023, 57, 153–162. [Google Scholar] [CrossRef]
- Alizzi, A.M.; Summers, P.; Boon, V.H.; Tantiongco, J.-P.; Thompson, T.; Leslie, B.J.; Williams, D.; Steele, M.; Bidstrup, B.P.; Diqer, A.-M.A. Reduction of post-surgical pericardial adhesions using a pig model. Heart Lung Circ. 2012, 21, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Marc, H.M.; Mees, U.; Hill, A.; Egbert, B.; Coker, G.; Estridge, T. Evaluation of a novel synthetic sealant for inhibition of cardiac adhesions and clinical experience in cardiac surgery procedures. Heart Surg. Forum. 2000, 4, 204–209. [Google Scholar]
- Daly, K.A.; Liu, S.; Agrawal, V.; Brown, B.N.; Huber, A.; Johnson, S.A.; Reing, J.; Sicari, B.; Wolf, M.; Zhang, X.; et al. The host response to endotoxin-contaminated dermal matrix. Tissue Eng. Part A 2012, 18, 1293–1303. [Google Scholar] [CrossRef]
- Ueki, N.; Someya, K.; Matsuo, Y.; Wakamatsu, K.; Mukai, H. Cryptides: Functional cryptic peptides hidden in protein structures. Biopolymers 2007, 88, 190–198. [Google Scholar] [CrossRef]
- Shakouri-Motlagh, A.; O’Connor, A.J.; Kalionis, B.; Heath, D.E. Improved ex vivo expansion of mesenchymal stem cells on solubilized acellular fetal membranes. J. Biomed. Mater. Res. Part A 2019, 107, 232–242. [Google Scholar] [CrossRef]
- Penolazzi, L.; Mazzitelli, S.; Vecchiatini, R.; Torreggiani, E.; Lambertini, E.; Johnson, S.; Badylak, S.F.; Piva, R.; Nastruzzi, C. Human mesenchymal stem cells seeded on extracellular matrix-scaffold: Viability and osteogenic potential. J. Cell. Physiol. 2012, 227, 857–866. [Google Scholar] [CrossRef]
- Capella-Monsonís, H.; De Pieri, A.; Peixoto, R.; Korntner, S.; Zeugolis, D.I. Extracellular matrix-based biomaterials as adipose-derived stem cell delivery vehicles in wound healing: A comparative study between a collagen scaffold and two xenografts. Stem Cell Res. Ther. 2020, 11, 510. [Google Scholar] [CrossRef]
- Capella-Monsonis, H.; Kelly, J.; Kearns, S.; Zeugolis, D.I. Decellularised porcine peritoneum as a tendon protector sheet. Biomed. Mater. 2019, 14, 044102. [Google Scholar] [CrossRef]
- Naranjo, J.D.; Saldin, L.T.; Sobieski, E.; Quijano, L.M.; Hill, R.C.; Chan, P.G.; Torres, C.; Dziki, J.L.; Cramer, M.C.; Lee, Y.C.; et al. Esophageal extracellular matrix hydrogel mitigates metaplastic change in a dog model of Barrett’s esophagus. Sci. Adv. 2020, 6, eaba4526. [Google Scholar] [CrossRef] [PubMed]
- Faulk, D.M.; Londono, R.; Wolf, M.T.; Ranallo, C.A.; Carruthers, C.A.; Wildemann, J.D.; Dearth, C.L.; Badylak, S.F. ECM hydrogel coating mitigates the chronic inflammatory response to polypropylene mesh. Biomaterials 2014, 35, 8585–8595. [Google Scholar] [CrossRef] [PubMed]
- de Castro Bras, L.E.; Frangogiannis, N.G. Extracellular matrix-derived peptides in tissue remodeling and fibrosis. Matrix Biol. 2020, 91–92, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.F.; Thomas, P.G. Towards integrating extracellular matrix and immunological pathways. Cytokine 2017, 98, 79–86. [Google Scholar] [CrossRef]
- Hamaia, S.; Farndale, R.W. Integrin recognition motifs in the human collagens. Adv. Exp. Med. Biol. 2014, 819, 127–142. [Google Scholar] [CrossRef]
- Solis-Cordova, J.; Edwards, J.H.; Fermor, H.L.; Riches, P.; Brockett, C.L.; Herbert, A. Characterisation of native and decellularised porcine tendon under tension and compression: A closer look at glycosaminoglycan contribution to tendon mechanics. J. Mech. Behav. Biomed. Mater. 2023, 139, 105671. [Google Scholar] [CrossRef]
- Zhang, F.; Zheng, L.; Cheng, S.; Peng, Y.; Fu, L.; Zhang, X.; Linhardt, R.J. Comparison of the interactions of different growth factors and glycosaminoglycans. Molecules 2019, 24, 3360. [Google Scholar] [CrossRef]
- Peloso, A.; Katari, R.; Tamburrini, R.; Duisit, J.; Orlando, G. Glycosaminoglycans as a measure of outcome of cell-on-scaffold seeding (decellularization) technology. Expert. Rev. Med. Devices 2016, 13, 1067–1068. [Google Scholar] [CrossRef]
- Huai, G.; Qi, P.; Yang, H.; Wang, Y. Characteristics of alpha-Gal epitope, anti-Gal antibody, alpha1,3 galactosyltransferase and its clinical exploitation (Review). Int. J. Mol. Med. 2016, 37, 11–20. [Google Scholar] [CrossRef]
- Huleihel, L.; Hussey, G.S.; Naranjo, J.D.; Zhang, L.; Dziki, J.L.; Turner, N.J.; Stolz, D.B.; Badylak, S.F. Matrix-bound nanovesicles within ECM bioscaffolds. Sci. Adv. 2016, 2, e1600502. [Google Scholar] [CrossRef]
- Liao, R.; Dewey, M.J.; Rong, J.; Johnson, S.A.; D’Angelo, W.A.; Hussey, G.S.; Badylak, S.F. Matrix-bound nanovesicles alleviate particulate-induced periprosthetic osteolysis. Sci. Adv. 2024, 10, eadn1852. [Google Scholar] [CrossRef]
- van der Merwe, Y.; Faust, A.E.; Sakalli, E.T.; Westrick, C.C.; Hussey, G.; Chan, K.C.; Conner, I.P.; Fu, V.L.N.; Badylak, S.F.; Steketee, M.B. Matrix-bound nanovesicles prevent ischemia-induced retinal ganglion cell axon degeneration and death and preserve visual function. Sci. Rep. 2019, 9, 3482. [Google Scholar] [CrossRef] [PubMed]
- Quijano, L.M.; Naranjo, J.D.; El-Mossier, S.O.; Turner, N.J.; Pineda Molina, C.; Bartolacci, J.; Zhang, L.; White, L.; Li, H.; Badylak, S.F. Matrix-Bound Nanovesicles: The effects of isolation method upon yield, purity, and function. Tissue Eng. Part C Methods 2020, 26, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Quijano, L.M.; Hussey, G.S.; Jiang, P.; Badylak, S.F. Matrix Bound Nanovesicles Have Tissue-Specific Characteristics That Suggest a Regulatory Role. Tissue Eng. Part A 2022, 28, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Crum, R.J.; Huckestien, B.R.; Dwyer, G.; Mathews, L.; Nascari, D.G.; Hussey, G.S.; Turnquist, H.R.; Alcorn, J.F.; Badylak, S.F. Mitigation of influenza-mediated inflammation by immunomodulatory matrix-bound nanovesicles. Sci. Adv. 2023, 9, eadf9016. [Google Scholar] [CrossRef]
- Brown, B.N.; Haschak, M.J.; Lopresti, S.T.; Stahl, E.C. Effects of age-related shifts in cellular function and local microenvironment upon the innate immune response to implants. Semin. Immunol. 2017, 29, 24–32. [Google Scholar] [CrossRef]
- Xu, J.; Nie, N.; Wu, B.; Li, Y.; Gong, L.; Yao, X.; Zou, X.; Ouyang, H. The personalized application of biomaterials based on age and sexuality specific immune responses. Biomaterials 2021, 278, 121177. [Google Scholar] [CrossRef]
- Kulkarni, M.M.; Popovic, B.; Nolfi, A.L.; Skillen, C.D.; Brown, B.N. Distinct impacts of aging on the immune responses to extracellular matrix-based versus synthetic biomaterials. Biomaterials 2025, 320, 123204. [Google Scholar] [CrossRef]
- Han, J.; Cherry, C.; Mejías, J.C.; Krishnan, K.; Ruta, A.; Maestas, D.R., Jr.; Peña, A.N.; Nguyen, H.H.; Nagaraj, S.; Yang, B.; et al. Age-associated Senescent—T Cell Signaling Promotes Type 3 Immunity that Inhibits the Biomaterial Regenerative Response. Adv. Mater. 2024, 36, 2310476. [Google Scholar] [CrossRef]
- Koons, G.L. Toward Sex-Specific Biomaterials Innovation: A Perspective. ACS Biomater. Sci. Eng. 2025. [Google Scholar] [CrossRef]
- Schussler, O.; Lila, N.; Perneger, T.; Mootoosamy, P.; Grau, J.; Francois, A.; Smadja, D.M.; Lecarpentier, Y.; Ruel, M.; Carpentier, A. Recipients with blood group A associated with longer survival rates in cardiac valvular bioprostheses. EBioMedicine 2019, 42, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Schussler, O.; Lila, N.; Grau, J.; Ruel, M.; Lecarpentier, Y.; Carpentier, A. Possible Link Between the ABO Blood Group of Bioprosthesis Recipients and Specific Types of Structural Degeneration. J. Am. Heart Assoc. 2020, 9, e015909. [Google Scholar] [CrossRef]
- Persson, M.; Edgren, G.; Dalén, M.; Glaser, N.; Olsson, M.L.; Franco-Cereceda, A.; Holzmann, M.J.; Sartipy, U. ABO blood type and risk of porcine bioprosthetic aortic valve degeneration: SWEDEHEART observational cohort study. BMJ Open 2019, 9, e029109. [Google Scholar] [CrossRef]
- Feingold, B.; Wearden, P.D.; Morell, V.O.; Galvis, D.; Galambos, C. Expression of A and B Blood Group Antigens on Cryopreserved Homografts. Ann. Thorac. Surg. 2009, 87, 211–214. [Google Scholar] [CrossRef]
- Baskett, R.J.F.; Nanton, M.A.; Warren, A.E.; Ross, D.B. Human leukocyte antigen-DR and ABO mismatch are associated with accelerated homograft valve failure in children: Implications for therapeutic interventions. J. Thorac. Cardiovasc. Surg. 2003, 126, 232–238. [Google Scholar] [CrossRef]
- Vogt, F.; Böll, B.M.; Boulesteix, A.-L.; Kilian, E.; Santarpino, G.; Reichart, B.; Schmitz, C. Homografts in aortic position: Does blood group incompatibility have an impact on patient outcomes? Interact. Cardiovasc. Thorac. Surg. 2013, 16, 619–624. [Google Scholar] [CrossRef]
- Izui, S.; Lambert, P.H.; Miescher, P.A. In vitro demonstration of a particular affinity of glomerular basement membrane and collagen for DNA. A possible basis for a local formation of DNA-anti-DNA complexes in systemic lupus erythematosus. J. Exp. Med. 1976, 144, 428–443. [Google Scholar] [CrossRef]
- Lim, H.-G.; Kim, G.B.; Jeong, S.; Kim, Y.J. Development of a next-generation tissue valve using a glutaraldehyde-fixed porcine aortic valve treated with decellularization, α-galactosidase, space filler, organic solvent and detoxification. Eur. J. Cardio-Thorac. Surg. 2015, 48, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Choi, S.-Y.; Sung, S.-C.; Lim, H.-G.; Park, S.-s.; Kim, S.-H.; Kim, Y.J. Changes of the structural and biomechanical properties of the bovine pericardium after the removal of α-Gal epitopes by decellularization and α-galactosidase treatment. Korean J. Thorac. Cardiovasc. Surg. 2012, 45, 380–389. [Google Scholar] [CrossRef]
- Gao, H.-W.; Li, S.-B.; Sun, W.Q.; Yun, Z.-M.; Zhang, X.; Song, J.-W.; Zhang, S.-K.; Leng, L.; Ji, S.-P.; Tan, Y.-X.; et al. Quantification of α-Gal antigen removal in the porcine dermal tissue by α-galactosidase. Tissue Eng. Part C Methods 2015, 21, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Oya, K.; Wahyudiono; Goto, M. Surfactant-Free Decellularization of Porcine Auricular Cartilage Using Liquefied Dimethyl Ether and DNase. Materials 2023, 16, 3172. [Google Scholar] [CrossRef]
- Capella-Monsonis, H.; Zeugolis, D.I. Decellularized xenografts in regenerative medicine: From processing to clinical application. Xenotransplantation 2021, 28, e12683. [Google Scholar] [CrossRef]
- Seddon, A.M.; Curnow, P.; Booth, P.J. Membrane proteins, lipids and detergents: Not just a soap opera. Biochim. Biophys. Acta (BBA)—Biomembr. 2004, 1666, 105–117. [Google Scholar] [CrossRef]
- Lim, L.S.; Riau, A.; Poh, R.; Tan, D.T.; Beuerman, R.W.; Mehta, J.S. Effect of dispase denudation on amniotic membrane. Mol. Vis. 2009, 15, 1962–1970. [Google Scholar] [PubMed]
- Duisit, J.; Amiel, H.; Wüthrich, T.; Taddeo, A.; Dedriche, A.; Destoop, V.; Pardoen, T.; Bouzin, C.; Joris, V.; Magee, D.; et al. Perfusion-decellularization of human ear grafts enables ECM-based scaffolds for auricular vascularized composite tissue engineering. Acta Biomater. 2018, 73, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Guyette, J.P.; Gilpin, S.E.; Charest, J.M.; Tapias, L.F.; Ren, X.; Ott, H.C. Perfusion decellularization of whole organs. Nat. Protoc. 2014, 9, 1451–1468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bao, J.; Wu, Q.; Zhou, Y.; Li, Y.; Wu, X.; Shi, Y.; Li, L.; Bu, H. Method for perfusion decellularization of porcine whole liver and kidney for use as a scaffold for clinical-scale bioengineering engrafts. Xenotransplantation 2015, 22, 48–61. [Google Scholar] [CrossRef]
- Capella-Monsonís, H.; Crum, R.J.; Hussey, G.S.; Badylak, S.F. Advances, challenges, and future directions in the clinical translation of ECM biomaterials for regenerative medicine applications. Adv. Drug Deliv. Rev. 2024, 211, 115347. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef]
- Ma, B.; Wang, X.; Wu, C.; Chang, J. Crosslinking strategies for preparation of extracellular matrix-derived cardiovascular scaffolds. Regen. Biomater. 2014, 1, 81–89. [Google Scholar] [CrossRef]
- McDade, J.K.; Brennan-Pierce, E.P.; Ariganello, M.B.; Labow, R.S.; Michael Lee, J. Interactions of U937 macrophage-like cells with decellularized pericardial matrix materials: Influence of crosslinking treatment. Acta Biomater. 2013, 9, 7191–7199. [Google Scholar] [CrossRef]
- Glynn, J.J.; Polsin, E.G.; Hinds, M.T. Crosslinking decreases the hemocompatibility of decellularized, porcine small intestinal submucosa. Acta Biomater. 2015, 14, 96–103. [Google Scholar] [CrossRef]
- Sepahi, M.; Hadadian, S.; Ahangari Cohan, R.; Norouzian, D. Lipopolysaccharide removal affinity matrices based on novel cationic amphiphilic peptides. Prep. Biochem. Biotechnol. 2021, 51, 386–394. [Google Scholar] [CrossRef]
- Crum, R.J.; Capella-Monsonís, H.; Chang, J.; Dewey, M.J.; Kolich, B.D.; Hall, K.T.; El-Mossier, S.O.; Nascari, D.G.; Hussey, G.S.; Badylak, S.F. Biocompatibility and biodistribution of matrix-bound nanovesicles in vitro and in vivo. Acta Biomater. 2023, 155, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Lohmander, F.; Lagergren, J.; Roy, P.G.; Johansson, H.; Brandberg, Y.; Eriksen, C.; Frisell, J. Implant based breast reconstruction with acellular dermal matrix: Safety data from an open-label, multicenter, randomized, controlled trial in the setting of breast cancer treatment. Ann. Surg. 2019, 269, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Schmidli, J.; Savolainen, H.; Heller, G.; Widmer, M.K.; Then-Schlagau, U.; Baumgartner, I.; Carrel, T.P. Bovine mesenteric vein graft (ProCol) in critical limb ischaemia with tissue loss and infection. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 251–253. [Google Scholar] [CrossRef]
- Owen, K.; Wilshaw, S.P.; Homer-Vanniasinkam, S.; Bojar, R.A.; Berry, H.; Ingham, E. Assessment of the antimicrobial activity of acellular vascular grafts. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, A.; Record, R.; Wu, C.C.; Tullius, B.; Badylak, S.; Ladisch, M. Antimicrobial activity associated with extracellular matrices. Tissue Eng. 2002, 8, 63–71. [Google Scholar] [CrossRef]
- Brennan, E.P.; Reing, J.; Chew, D.; Myers-Irvin, J.M.; Young, E.J.; Badylak, S.F. Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng. 2006, 12, 2949–2955. [Google Scholar] [CrossRef]
- Capella-Monsonis, H.; Tilbury, M.A.; Wall, J.G.; Zeugolis, D.I. Porcine mesothelium matrix as a biomaterial for wound healing applications. Mater. Today Bio 2020, 7, 100057. [Google Scholar] [CrossRef]
- Ferguson, D.P.; Lewington, M.R.; Smith, T.D.; Wong, I.H. Graft Utilization in the augmentation of large-to-massive rotator cuff repairs: A systematic review. Am. J. Sports Med. 2016, 44, 2984–2992. [Google Scholar] [CrossRef]
- Longo, U.G.; Lamberti, A.; Maffulli, N.; Denaro, V. Tendon augmentation grafts: A systematic review. Br. Med. Bull. 2010, 94, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Barber, F.A.; Herbert, M.A.; Coons, D.A. Tendon augmentation grafts: Biomechanical failure loads and failure patterns. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Golla, D.; Russo, C.C. Outcomes following placement of non–cross-linked porcine-derived acellular dermal matrix in complex ventral hernia repair. Int. Surg. 2014, 99, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.M.; Vargas, C.R.; Colakoglu, S.; Nguyen, J.T.; Lin, S.J.; Lee, B.T. Properties of meshes used in hernia repair: A comprehensive review of synthetic and biologic meshes. J. Reconstr. Microsurg. 2015, 31, 83–94. [Google Scholar] [CrossRef]
- Pavy, C.; Michielon, G.; Robertus, J.L.; Lacour-Gayet, F.; Ghez, O. Initial 2-year results of CardioCel® patch implantation in children. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 448–453. [Google Scholar] [CrossRef]
- Etnel, J.R.G.; Elmont, L.C.; Ertekin, E.; Mokhles, M.M.; Heuvelman, H.J.; Roos-Hesselink, J.W.; de Jong, P.L.; Helbing, W.A.; Bogers, A.J.J.C.; Takkenberg, J.J.M. Outcome after aortic valve replacement in children: A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2016, 151, 143–152.e143. [Google Scholar] [CrossRef]
- Huerta, S.; Varshney, A.; Patel, P.M.; Mayo, H.G.; Livingston, E.H. Biological mesh implants for abdominal hernia repair: US Food and Drug Administration approval process and systematic review of its efficacy. JAMA Surg. 2016, 151, 374–381. [Google Scholar] [CrossRef]
| Physical Properties of the ECM | Pro-Inflammatory Promotion | Pro-Remodeling Promotion | Limitations | Ref. |
|---|---|---|---|---|
| Surface charge and wettability | Anionic hydrophilic surfaces are related to inflammatory responses. | Cationic hydrophilic and hydrophobic surfaces are related to proremodeling responses and promote migration. | Few studies have been conducted in complex environments where the influence of the surface can be limited. | [119,120] |
| Surface topography | Higher roughness promotes migration and phagocytosis, but has no clear effect on phenotype. | Smoother surfaces can promote higher attachment of immune cells. | Very limited studies on synthetic materials that lack the biological component. | [121,122] |
| Stiffness | Higher stiffness (as compared to tissue in homeostasis) is usually related to inflammation. | Low stiffness can promote pro-remodeling but impair migration. | Few studies, mostly on 2D platforms. Complex interactions in 3D. | [48,123,124] |
| Component Present in the ECM | Risks | Removal Trade-Off |
|---|---|---|
| Glycosaminoglycans (GAGs, incl. hyaluronan/HA) | Low-MW HA fragments act as DAMPs recognized by PRRs. | Beneficial if preserved: hydration, growth-factor sequestration, support for recellularization; pro-remodeling cues. Avoid over-aggressive steps that fragment HA; prefer less-aggressive (non-ionic/zwitterionic) detergents with thorough rinsing. |
| Degradation peptides (matrikines/matricryptins) | Some peptides are sensed as DAMPs/chemotactic signals; overall bias toward pro-remodeling responses. | Beneficial if preserved: ECM behaves as a sustained peptide-release matrix. Avoid over-crosslinking, which reduces bioactive peptide release and promotes pro-inflammatory bias; avoid excessive fragmentation that increases the DAMP burden. |
| Integrin-recognition motifs (RGD, GxOGER, PHSRN) | NA | Beneficial if preserved: integrin-mediated adhesion and repair signaling. Minimize SDS exposure; avoid crosslinking that masks ligands. |
| Matrix-bound nanovesicles (MBV) | NA | Beneficial if preserved: potent local immunomodulators, replicate ECM pro-remodeling effects. Present across decellularization methods—resilient and tightly ECM-associated. |
| Collagen and basement-membrane (BM) architecture | Architectural damage elevates inflammatory bias and impairs endothelial behavior. | Beneficial if preserved: adhesion, epithelialization, angiogenesis; proper presentation of recognition motifs. Limit ionic detergents (SDS) that disrupt BM/collagen; use mechanical delamination; avoid over-crosslinking that reduces biodegradability and shifts immune tone. |
| Residual cellular membranes (processing residual) | Reservoir of antigens/DAMPs. | Emphasize complete membrane removal using physical (e.g., freeze–thaw, sonication) plus mild chemical steps. |
| α-gal epitope (largely membrane-associated carbohydrate) | Relevance remains under investigation; anti-Gal recognition may activate complement, recruit macrophages, and cause matrix degradation. | Reduce membrane reservoirs during decellularization. |
| Residual DNA (processing residual) | Quality control concern. | DNase with rinses to meet quality control without harsher steps that damage the ECM. |
| Residual detergents (processing residual) | Cytotoxic; hinders recellularization; damages BM/collagen. | Minimize ionic-detergent dose/time; favor non-ionic/zwitterionic where feasible; validate long rinses to sub-toxic levels. |
| Endotoxin (LPS) (processing residual) | Among the most potent inducers of innate activation, trace amounts can activate TLRs; FDA endotoxin limits apply. | Low-endotoxin sourcing and asepsis; validated depyrogenation; lot-release endotoxin testing; avoid over-sterilization that denatures ECM cues. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, D.J.; Hussey, G.; Capella-Monsonís, H. Immune Response to Extracellular Matrix Bioscaffolds: A Comprehensive Review. Biologics 2025, 5, 28. https://doi.org/10.3390/biologics5030028
Romero DJ, Hussey G, Capella-Monsonís H. Immune Response to Extracellular Matrix Bioscaffolds: A Comprehensive Review. Biologics. 2025; 5(3):28. https://doi.org/10.3390/biologics5030028
Chicago/Turabian StyleRomero, Daniela J., George Hussey, and Héctor Capella-Monsonís. 2025. "Immune Response to Extracellular Matrix Bioscaffolds: A Comprehensive Review" Biologics 5, no. 3: 28. https://doi.org/10.3390/biologics5030028
APA StyleRomero, D. J., Hussey, G., & Capella-Monsonís, H. (2025). Immune Response to Extracellular Matrix Bioscaffolds: A Comprehensive Review. Biologics, 5(3), 28. https://doi.org/10.3390/biologics5030028






