Fibrotic Chronic Eosinophilic Pneumonia Treated with an Anti-IL-5 Monoclonal Antibody: A Case Report
Abstract
1. Introduction
2. Case Presentation
2.1. Patient History
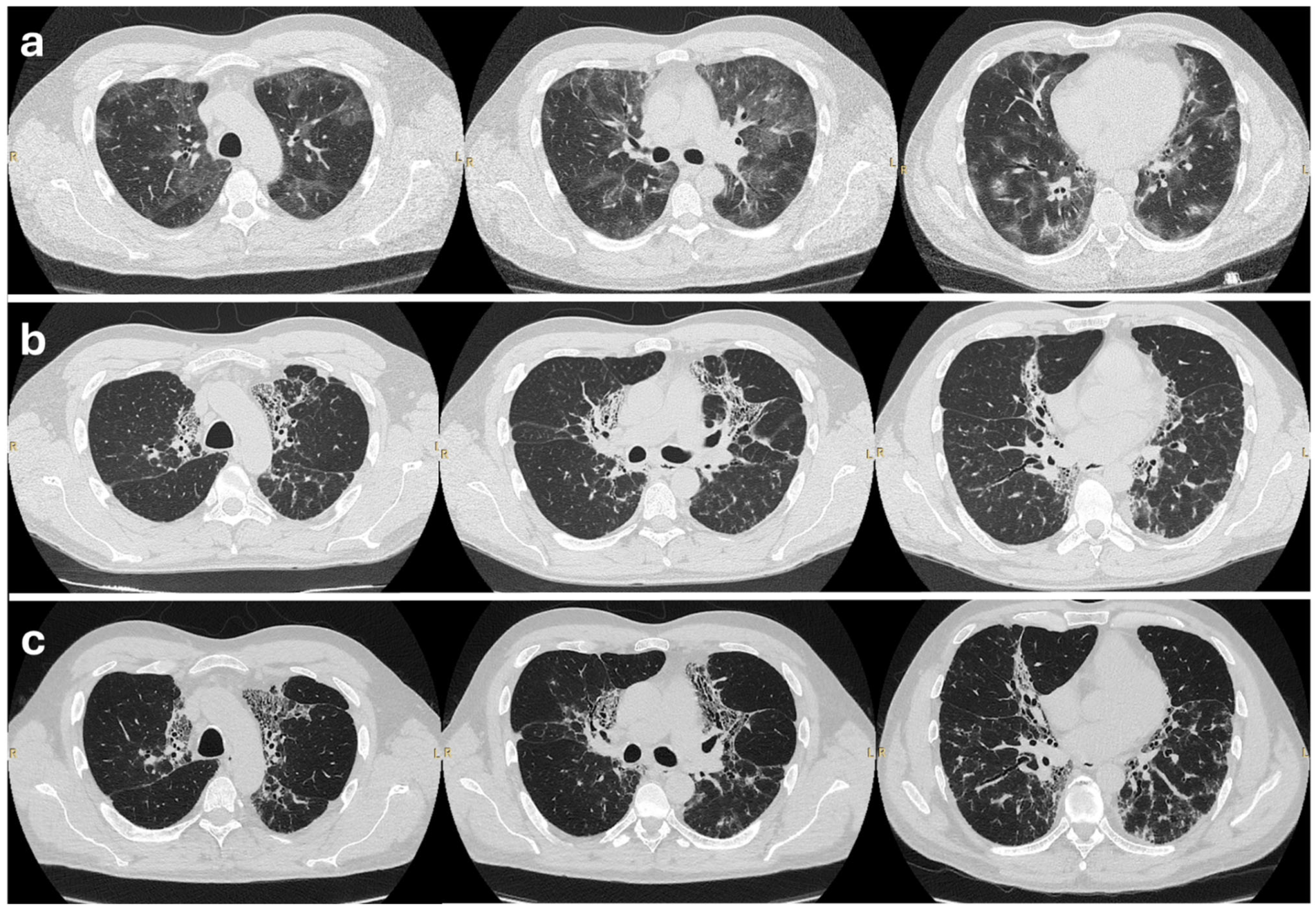
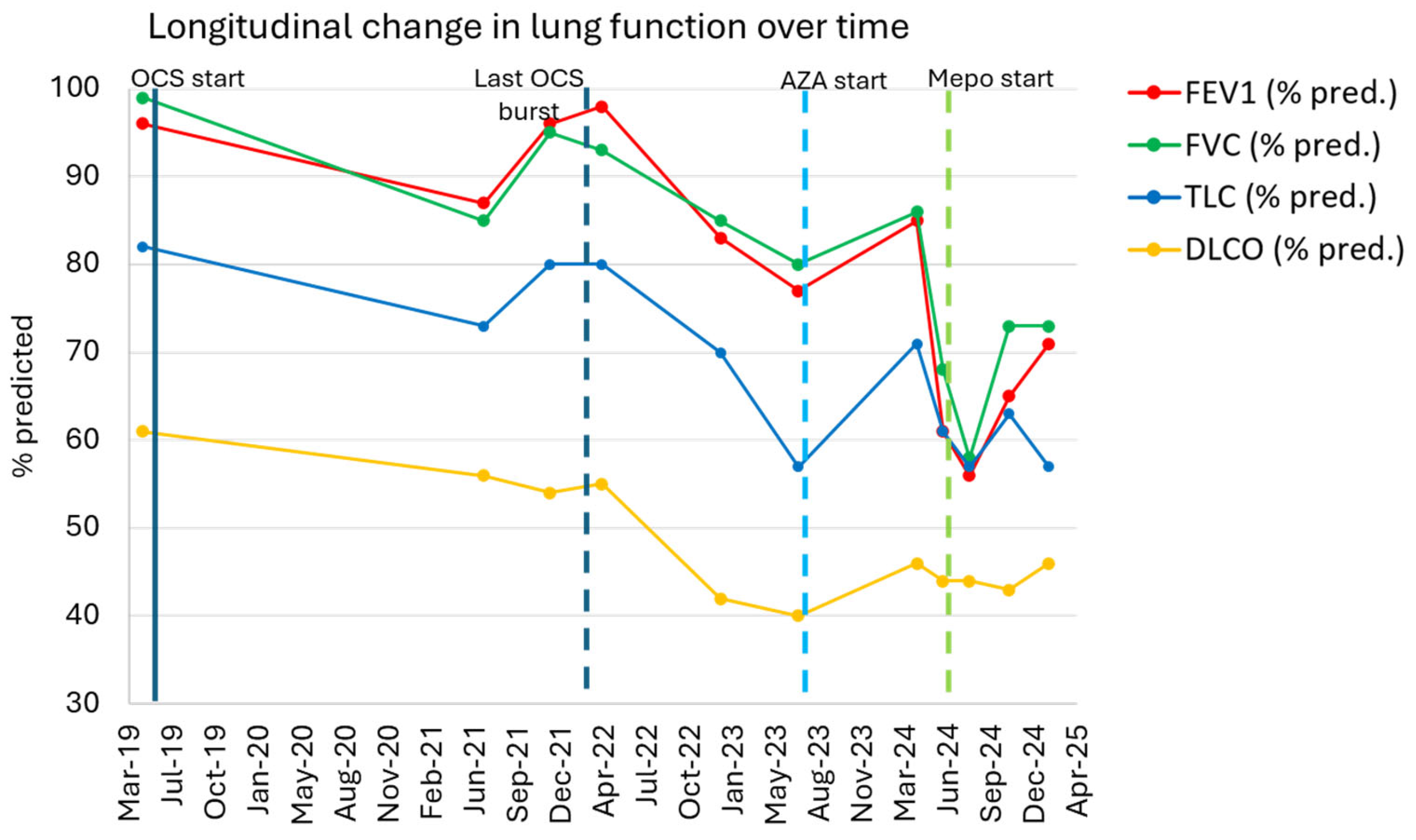
2.2. Initial Treatment and Outcome
2.3. Further Management
2.4. Outcome on Mepolizumab
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANAs | Antinuclear Antibodies |
| ANCAs | Antineutrophil Cytoplasmic Antibodies |
| AZA | Azathioprine |
| BAL | Bronchoalveolar Lavage |
| CEP | Chronic Eosinophilic Pneumonia |
| DLCO | Diffusing Capacity of the Lung for Carbon Monoxide |
| ENA | Extractable Nuclear Antigens |
| FeNO | Fractional exhaled Nitric Oxide |
| FEV1 | Forced Expiratory Volume in 1 Second |
| FVC | Forced Vital Capacity |
| HRCT | High-Resolution Computed Tomography |
| ICSs | Inhaled Corticosteroids |
| IgE | Immunoglobulin E |
| IL-5 | Interleukin-5 |
| IL-5Rα | Interleukin-5 Receptor Alpha |
| ILD | Interstitial Lung Disease |
| mMRC | Modified Medical Research Council Dyspnea Scale |
| PFTs | Pulmonary Function Tests |
| PPF | Progressive Pulmonary Fibrosis |
| RAST | RadioAllergoSorbent Test |
| SpO2 | Peripheral Oxygen Saturation |
| TLC | Total Lung Capacity |
| TPMT | Thiopurine Methyltransferase |
References
- Marchand, E.; Cordier, J.-F. Idiopathic Chronic Eosinophilic Pneumonia. Orphanet J. Rare Dis. 2006, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Crowe, M.; Robinson, D.; Sagar, M.; Chen, L.; Ghamande, S. Chronic Eosinophilic Pneumonia: Clinical Perspectives. Ther. Clin. Risk Manag. 2019, 15, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Volmer, T.; Effenberger, T.; Trautner, C.; Buhl, R. Consequences of Long-Term Oral Corticosteroid Therapy and Its Side-Effects in Severe Asthma in Adults: A Focused Review of the Impact Data in the Literature. Eur. Respir. J. 2018, 52, 1800703. [Google Scholar] [CrossRef] [PubMed]
- Murillo, A.D.; Castrillon, A.I.; Serrano, C.D.; Fernandez-Trujillo, L. Monoclonal Antibodies in Idiopathic Chronic Eosinophilic Pneumonia: A Scoping Review. BMC Pulm. Med. 2024, 24, 74. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Nucala 100 mg solution for injection–Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_en.pdf (accessed on 16 May 2024).
- Brenard, E.; Pilette, C.; Dahlqvist, C.; Colinet, B.; Schleich, F.; Roufosse, F.; Froidure, A. Real-Life Study of Mepolizumab in Idiopathic Chronic Eosinophilic Pneumonia. Lung 2020, 198, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Takahashi, K.; Kurihara, Y.; Sadamatsu, H.; Kuwahara, Y.; Kimura, S.; Sueoka-Aragane, N. Anti-IL-5 Agents for the Treatment of Idiopathic Chronic Eosinophilic Pneumonia: A Case Series. J. Asthma Allergy 2022, 15, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Shijubo, N.; Koba, H.; Mori, Y.; Satoh, M.; Morikawa, T.; Abe, S. Chronic Eosinophilic Pneumonia Progressing to Lung Fibrosis. Eur. Respir. J. 1994, 7, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Baqir, M.; Peikert, T.; Johnson, T.; Tandon, Y.; Yi, E.; Schroeder, D.; Ryu, J. Idiopathic Chronic Eosinophilic Pneumonia Evolving to Pulmonary Fibrosis: A Retrospective Analysis: Eosinophilic Pneumonia and Fibrosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2022, 39, e2022020. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Baratella, E.; Borghesi, A.; Calandriello, L.; Cortese, G.; Della Casa, G.; Giraudo, C.; Grassedonio, E.; Larici, A.R.; Palmucci, S.; Romei, C.; et al. Quantification of Progressive Pulmonary Fibrosis by Visual Scoring of HRCT Images: Recommendations from Italian Chest Radiology Experts. Radiol. Med. 2025, 130, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-E.; Lee, W.-J.; Son, E.-J.; Kim, T.-H.; Jeon, D.-S.; Kim, Y.-S. The Experience of Reslizumab Treatment for Frequent Relapsing Chronic Eosinophilic Pneumonia Accompanying Asthma: A Case Series. Eur. Respir. J. 2023, 62, PA4101. [Google Scholar] [CrossRef]
- Davoine, F.; Lacy, P. Eosinophil Cytokines, Chemokines, and Growth Factors: Emerging Roles in Immunity. Front. Immunol. 2014, 5, 570. [Google Scholar] [CrossRef] [PubMed]
- Rochester, C.L.; Ackerman, S.J.; Zheng, T.; Elias, J.A. Eosinophil-Fibroblast Interactions. Granule Major Basic Protein Interacts with IL-1 and Transforming Growth Factor-Beta in the Stimulation of Lung Fibroblast IL-6-Type Cytokine Production. J. Immunol. 1996, 156, 4449–4456. [Google Scholar] [CrossRef] [PubMed]
- Levi-Schaffer, F.; Garbuzenko, E.; Rubin, A.; Reich, R.; Pickholz, D.; Gillery, P.; Emonard, H.; Nagler, A.; Maquart, F.A.X. Human Eosinophils Regulate Human Lung- and Skin-Derived Fibroblast Properties in Vitro: A Role for Transforming Growth Factor β (TGF-β). Proc. Natl. Acad. Sci. USA 1999, 96, 9660–9665. [Google Scholar] [CrossRef] [PubMed]
- Zagai, U.; Dadfar, E.; Lundahl, J.; Venge, P.; Sköld, C.M. Eosinophil Cationic Protein Stimulates TGF-Β1 Release by Human Lung Fibroblasts In Vitro. Inflammation 2007, 30, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Bois, R.M.; du Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenzato, U.; Previtero, D.; Castelli, G.; Ruzzini, E.; Cocconcelli, E.; Tinè, M.; Padoan, R.; Balestro, E.; Baraldo, S.; Spagnolo, P. Fibrotic Chronic Eosinophilic Pneumonia Treated with an Anti-IL-5 Monoclonal Antibody: A Case Report. Biologics 2025, 5, 23. https://doi.org/10.3390/biologics5030023
Semenzato U, Previtero D, Castelli G, Ruzzini E, Cocconcelli E, Tinè M, Padoan R, Balestro E, Baraldo S, Spagnolo P. Fibrotic Chronic Eosinophilic Pneumonia Treated with an Anti-IL-5 Monoclonal Antibody: A Case Report. Biologics. 2025; 5(3):23. https://doi.org/10.3390/biologics5030023
Chicago/Turabian StyleSemenzato, Umberto, Daniele Previtero, Gioele Castelli, Eleonora Ruzzini, Elisabetta Cocconcelli, Mariaenrica Tinè, Roberto Padoan, Elisabetta Balestro, Simonetta Baraldo, and Paolo Spagnolo. 2025. "Fibrotic Chronic Eosinophilic Pneumonia Treated with an Anti-IL-5 Monoclonal Antibody: A Case Report" Biologics 5, no. 3: 23. https://doi.org/10.3390/biologics5030023
APA StyleSemenzato, U., Previtero, D., Castelli, G., Ruzzini, E., Cocconcelli, E., Tinè, M., Padoan, R., Balestro, E., Baraldo, S., & Spagnolo, P. (2025). Fibrotic Chronic Eosinophilic Pneumonia Treated with an Anti-IL-5 Monoclonal Antibody: A Case Report. Biologics, 5(3), 23. https://doi.org/10.3390/biologics5030023





