Unleashing the Power of Biologics: Exploring the Governance and Regulation of Membrane-Based Virus Purification (MVP) Technologies
Abstract
1. Introduction
2. Regulatory Overview
2.1. Regulatory Scope
2.2. Feedback
3. Stakeholder Analysis
3.1. The Food and Drug Administration (FDA)
3.2. Healthcare Providers and Health Insurance Companies
3.3. Pharmaceutical Industry Stakeholders
3.4. Membrane Producers
3.5. Scientists and Researchers
3.6. Patients
3.7. The General Public
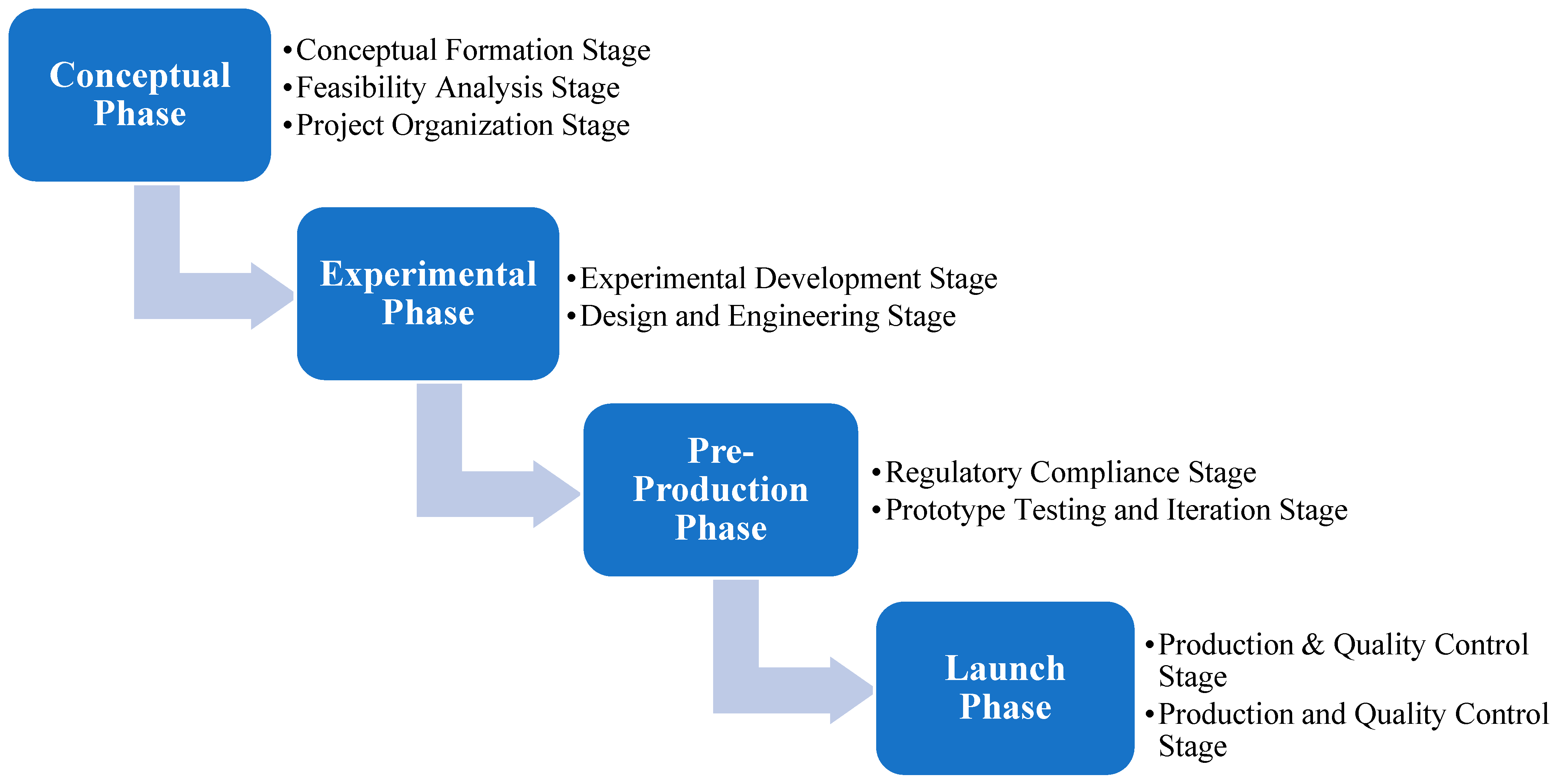
4. MVP Technology Development Process Model
4.1. The Conceptual Phase
4.2. The Experimental Phase
4.3. The Pre-Production Phase
4.4. The Launch Phase
5. Discussion
5.1. Future Research
5.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Precedence Research. Biologics Market Size to Surpass Around US$ 620.31 Bn by 2032. Available online: https://www.precedenceresearch.com/biologics-market (accessed on 15 June 2024).
- Makurvet, F.D. Biologics vs. small molecules: Drug costs and patient access. Med. Drug Discov. 2021, 9, 100075. [Google Scholar] [CrossRef]
- Gimenez, L.; Kawkabani, E.E.; Jacobs, P.; Malphettes, L. Overcoming the clarification challenges of high cell density culture. BMC Proc. 2015, 9, 35. [Google Scholar] [CrossRef]
- Gottschalk, U. Downstream processing of monoclonal antibodies: From high dilution to high purity. Biopharm Int. 2005, 18, 42. [Google Scholar]
- FDA. Guidance for Industry—Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications; FDA: Silver Spring, MD, USA, 2010. [Google Scholar]
- FDA. Q5A(R2) Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin; FDA: Silver Spring, MD, USA, 2024. [Google Scholar]
- Center for Drug Evaluation and Research. Q13 Continuous Manufacturing of Drug Substances and Drug Products. 2023. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q13-continuous-manufacturing-drug-substances-and-drug-products (accessed on 15 March 2024).
- Center for Biologics Evaluation and Research. About CBER; FDA: Silver Spring, MD, USA, 2024. [Google Scholar]
- Center for Biologics Evaluation and Research; Center for Drug Evaluation and Research. Q2(R1) Validation of Analytical Procedures: Text and Methodology Guidance for Industry. 2021. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2r1-validation-analytical-procedures-text-and-methodology-guidance-industry (accessed on 15 March 2024).
- FDA. Comment on Proposed Regulations and Submit Petitions. 2023. Available online: https://www.fda.gov/regulatory-information/dockets-management/comment-proposed-regulations-and-submit-petitions (accessed on 15 December 2024).
- Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research. Q5E Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process. 2020. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q5e-comparability-biotechnologicalbiological-products-subject-changes-their-manufacturing-process (accessed on 15 March 2024).
- Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research. Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients Guidance for Industry. 2020. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q7-good-manufacturing-practice-guidance-active-pharmaceutical-ingredients-guidance-industry (accessed on 15 March 2024).
- Yu, J.C.; Hlávka, J.P.; Joe, E.; Richmond, F.J.; Lakdawalla, D.N. Impact of non-binding FDA guidances on primary endpoint selection in Alzheimer’s disease trials. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12280. [Google Scholar] [CrossRef] [PubMed]
- Noah, L. Governance by the Backdoor: Administrative Law (Lessness) at the FDA. Neb. L. Rev. 2014, 93, 89. [Google Scholar]
- Noah, L.; Noah, B. Law, Medicine, and Medical Technology; Faculty Scholarship; Foundation Press: New York, NY, USA, 2002. [Google Scholar]
- Parrillo, N.R. Federal Agency Guidance and the Power to Bind: An Empirical Study of Agencies and Industries. Yale J. Regul. 2019, 36, 165. [Google Scholar]
- Hwang, T.J.; Avorn, J.; Kesselheim, A.S. Life Cycle of Medical Product Rules Issued by the US Food and Drug Administration. J. Health Politics Policy Law 2014, 39, 751–780. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.; Chattopadhyay, J.; Moffitt, S.; Nall, C. The complications of controlling agency time discretion: FDA review deadlines and postmarket drug safety. Am. J. Pol. Sci. 2012, 56, 98–114. [Google Scholar] [CrossRef]
- ICH Official Web Site: ICH. Available online: https://ich.org/ (accessed on 15 December 2024).
- FDA. Search for FDA Guidance Documents. 2023. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents (accessed on 15 March 2024).
- Gusmano, M.K. FDA Decisions and Public Deliberation:Challenges and Opportunities. Public Adm. Rev. 2013, 73, S115–S126. [Google Scholar] [CrossRef]
- Roth, D. A third seat at the table: An insider’s perspective on patient representatives. Hastings Cent. Rep. 2011, 41, 29–31. [Google Scholar] [CrossRef]
- Carpenter, D.; Zucker, E.J.; Avorn, J. Drug-review deadlines and safety problems. N. Engl. J. Med. 2008, 358, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Qato, D.M.; Alexander, G.C. Improving the Food and Drug Administration’s mandate to ensure postmarketing drug safety. JAMA 2011, 306, 1595–1596. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, A. How FDA Approves Drugs and Regulates Their Safety and Effectiveness; Congressional Research Service: Washington, DC, USA, 2018. [Google Scholar]
- Bendix, J. Value-based care gains ground Outcomes-based models are spreading, but fee-for-service still dominates payment landscape. Med. Econ. 2022, 99, 30–34. [Google Scholar]
- O’Sullivan, D.; Dooley, L. Applying Innovation; SAGE Publications: Thousand Oaks, CA, USA, 2008. [Google Scholar]
- Mattke, S.; Liu, H.; Orr, P. Medical Device Innovation in the Era of the Affordable Care Act: The End of Sexy. Rand Health Q. 2016, 6, 9. [Google Scholar] [PubMed]
- Edlin, R.; Hall, P.; Wallner, K.; McCabe, C. Sharing risk between payer and provider by leasing health technologies: An affordable and effective reimbursement strategy for innovative technologies? Value Health 2014, 7, 438–444. [Google Scholar] [CrossRef]
- Kocher, B. Incentives and information drive health care innovation: Lessons from Medicare Advantage. Better Healthcare Sooner. 2021. Available online: https://bobkocher.org/2021/01/04/incentives-and-information-drive-health-care-innovation-lessons-from-medicare-advantage/ (accessed on 15 September 2023).
- Clemens, J.; Gottlieb, J.D. Do Physicians’ Financial Incentives Affect Medical Treatment and Patient Health? Am. Econ. Rev. 2014, 104, 1320–1349. [Google Scholar] [CrossRef]
- Ex, P.; Vogt, V.; Busse, R.; Henschke, C. The reimbursement of new medical technologies in German inpatient care: What factors explain which hospitals receive innovation payments? Health Econ. Policy Law 2020, 15, 355–369. [Google Scholar] [CrossRef]
- Dowd, B.E.; Laugesen, M.J. Fee-for-service payment is not the (main) problem. Health Serv. Res. 2020, 55, 491. [Google Scholar] [CrossRef]
- Zilberman, D. The political economy of innovation and technological change. Environ. Dev. Econ. 2014, 19, 314–316. [Google Scholar] [CrossRef]
- Benson, T. Digital innovation evaluation: User perceptions of innovation readiness, digital confidence, innovation adoption, user experience and behaviour change. BMJ Health Care Inform. 2019, 26, e000018. [Google Scholar] [CrossRef]
- Hoed, M.W.; van den Backhaus, R.; Vries, E.; de Hamers, J.P.H.; Daniëls, R. Factors contributing to innovation readiness in health care organizations: A scoping review. BMC Health Serv. Res. 2022, 22, 997. [Google Scholar]
- Vaishnavi, V.; Suresh, M.; Dutta, P. Modelling the readiness factors for agility in healthcare organization: A TISM approach. Benchmarking Int. J. 2019, 26, 2372–2400. [Google Scholar] [CrossRef]
- Flessa, S.; Huebner, C. Innovations in Health Care—A Conceptual Framework. Int. J. Environ. Res. Public Health 2021, 18, 10026. [Google Scholar] [CrossRef]
- Moleirinho, M.G.; Silva, R.J.S.; Alves, P.M.; Carrondo, M.J.T.; Peixoto, C. Current challenges in biotherapeutic particles manufacturing. Expert Opin. Biol. Ther. 2020, 20, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Effio, C.L.; Hubbuch, J. Next generation vaccines and vectors: Designing downstream processes for recombinant protein-based virus-like particles. Biotechnol. J. 2015, 10, 715–727. [Google Scholar] [CrossRef]
- van der Loo, J.C.M.; Wright, J.F. Progress and challenges in viral vector manufacturing. Hum. Mol. Genet. 2016, 25, R42–R52. [Google Scholar] [CrossRef] [PubMed]
- Stille, S.; Steensma, E. Securing Today’s Biomanufacturing Supply Chain Using Transformative Supply | Cytiva. 2021. Available online: https://www.cytivalifesciences.com/en/us/solutions/bioprocessing/knowledge-center/Securing-Todays-Biomanufacturing-Supply-Chain-Using-Transformative-Supply (accessed on 15 January 2024).
- KMPG. The future of supply chain. The Future of Supply Chain. Available online: https://kpmg.com/us/en/articles/2023/future-supply-chain.html (accessed on 15 January 2024).
- Zhu, M.M.; Mollet, M.; Hubert, R.S.; Kyung, Y.S.; Zhang, G.G. Industrial Production of Therapeutic Proteins: Cell Lines, Cell Culture, and Purification. In Handbook of Industrial Chemistry and Biotechnology; Springer: Cham, Switzerland, 2017; pp. 1639–1669. [Google Scholar] [CrossRef]
- Shih, W.C. Global Supply Chains in a Post-Pandemic World. Harvard Business Review. 2020. Available online: https://hbr.org/2020/09/global-supply-chains-in-a-post-pandemic-world (accessed on 15 January 2024).
- Mulero, A. IRA Creates Unintended Complications for Biologics. BioSpace. 2023. Available online: https://www.biospace.com/ira-creates-unintended-complications-for-biologics (accessed on 15 January 2024).
- U.S. Department of the Treasury. How the Inflation Reduction Act’s Tax Incentives are Ensuring all Americans Benefit from the Growth of the Clean Energy Economy. 20 October 2023. Available online: https://home.treasury.gov/news/press-releases/jy1830 (accessed on 15 December 2023).
- Gaudet, D.; Méthot, J.; Déry, S.; Brisson, D.; Essiembre, C.; Tremblay, G.; Tremblay, K.; de Wal, J.; Twisk, J.; van den Bulk, N.; et al. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: An open-label trial. Gene Ther. 2013, 20, 361–369. [Google Scholar] [CrossRef]
- Keeler, A.; El-Mallah, M.; Flotte, T. Gene Therapy 2017: Progress and Future Directions. Clin. Transl. Sci. 2017, 10, 242–248. [Google Scholar] [CrossRef]
- Watanabe, N.; Yano, K.; Tsuyuki, K.; Okano, T.; Yamato, M. Re-examination of regulatory opinions in Europe: Possible contribution for the approval of the first gene therapy product Glybera. Mol. Ther. Methods Clin. Dev. 2015, 2, 14066. [Google Scholar] [CrossRef]
- Wright, J.F. Product-Related Impurities in Clinical-Grade Recombinant AAV Vectors: Characterization and Risk Assessment. Biomedicines 2014, 2, 80–97. [Google Scholar] [CrossRef]
- Allen, J.M.; Debelak, D.J.; Reynolds, T.C.; Miller, A.D. Identification and elimination of replication-competent adeno-associated virus (AAV) that can arise by nonhomologous recombination during AAV vector production. J. Virol. 1997, 71, 6816–6822. [Google Scholar] [CrossRef] [PubMed]
- Sommer, J.M.; Smith, P.H.; Parthasarathy, S.; Isaacs, J.; Vijay, S.; Kieran, J.; Powell, S.K.; McClelland, A.; Wright, J.F. Quantification of adeno-associated virus particles and empty capsids by optical density measurement. Mol. Ther. 2003, 7, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Barbier, L.; Simoens, S.; Vulto, A.G.; Huys, I. European Stakeholder Learnings Regarding Biosimilars: Part I-Improving Biosimilar Understanding and Adoption. BioDrugs 2020, 34, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Attridge, J. Innovation Models in the Biopharmaceutical Sector. Int. J. Innov. Manag. 2007, 11, 215–243. [Google Scholar] [CrossRef]
- Heled, Y. The Case for Disclosure of Biologics Manufacturing Information. J. Law Med. Ethics 2019, 47, 54–78. [Google Scholar] [CrossRef]
- Grabowski, H. Follow-on biologics: Data exclusivity and the balance between innovation and competition. Nat. Rev. Drug Discov. 2008, 7, 479–488. [Google Scholar] [CrossRef]
- Dobrowsky, T.; Gianni, D.; Pieracci, J.; Suh, J. AAV manufacturing for clinical use: Insights on current challenges from the upstream process perspective. Curr. Opin. Biomed. Eng. 2021, 20, 100353. [Google Scholar] [CrossRef]
- Cauchon, N.S.; Oghamian, S.; Hassanpour, S.; Abernathy, M. Innovation in Chemistry, Manufacturing, and Controls—A Regulatory Perspective from Industry. J. Pharm. Sci. 2019, 108, 2207–2237. [Google Scholar] [CrossRef]
- Felberbaum, R.S. The baculovirus expression vector system: A commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol. J. 2015, 10, 702–714. [Google Scholar] [CrossRef]
- Fleming, T.R.; Demets, D.L.; McShane, L.M. Discussion: The Role, Position, and Function of the FDA—The Past, Present, and Future. Biostatistics 2017, 18, 417–421. [Google Scholar] [CrossRef]
- Szabo, G. Clinical Trial Design for Alcoholic Hepatitis. Semin. Liver Dis. 2017, 37, 332–342. [Google Scholar] [CrossRef]
- Ho, M.; Saha, A.; McCleary, K.K.; Levitan, B.; Christopher, S.; Zandlo, K.; Braithwaite, R.S.; Hauber, A.B.; Medical Device Innovation Consortium’s Patient Centered Benefit-Risk Steering Committee. A Framework for Incorporating Patient Preferences Regarding Benefits and Risks into Regulatory Assessment of Medical Technologies. Value Health 2016, 19, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Tarver, M.E.; Neuland, C. Integrating Patient Perspectives into Medical Device Regulatory Decision Making to Advance Innovation in Kidney Disease. Clin. J. Am. Soc. Nephrol. 2021, 16, 636–638. [Google Scholar] [CrossRef]
- Johnson, F.R.; Zhou, M. Quantifying Patient Preferences for Regulatory Benefit–Risk Assessments. In Benefit-Risk Assessment Methods in Medical Product Development; Jiang, Q., He, W., Eds.; Chapman & Hall/CRC Biostatistics Series; Chapman and Hall/CRC, Taylor & Francis: Boca Raton, FL, USA, 2016; pp. 85–104. [Google Scholar] [CrossRef]
- Medical Device Innovation Consortium. Medical device innovation consortium (MDIC) patient centered benefit-risk project report: A framework for incorporating information on patient preferences regarding benefit and risk into regulatory assessments of new medical technology. Med. Dev. Innov. Consortium. 2015, 4, e34. [Google Scholar]
- Kamangar, F.; Isip, L.; Bhutani, T.; Dennis, M.; Heller, M.M.; Lee, E.S.; Nie, H.; Liao, W. How psoriasis patients perceive, obtain, and use biologic agents: Survey from an academic medical center. J. Dermatol. Treat. 2011, 24, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, Z.; Wang, X.; Yu, H.; Sun, J. Patients’ Perceptions of Biosimilars: A Systematic Review. BioDrugs 2023, 37, 829–841. [Google Scholar] [CrossRef]
- Gasteiger, C.; Scholz, U.; Petrie, K.J.; Dalbeth, N. A bio-what? Medical companions’ perceptions towards biosimilars and information needs in rheumatology. Rheumatol. Int. 2022, 42, 1993–2002. [Google Scholar] [CrossRef]
- Benz, H.L.; Lee, T.-H.J.; Tsai, J.-H.; Bridges, J.F.P.; Eggers, S.; Moncur, M.; Shaya, F.T.; Shoulson, I.; Spatz, E.S.; Wilson, L.; et al. Advancing the Use of Patient Preference Information as Scientific Evidence in Medical Product Evaluation: A Summary Report of the Patient Preference Workshop. Patient 2019, 12, 553–557. [Google Scholar] [CrossRef]
- Burstein, P. The Impact of Public Opinion on Public Policy: A Review and an Agenda. Political Res. Q. 2003, 56, 29–40. [Google Scholar] [CrossRef]
- Martinez, B.; Dailey, F.; Almario, C.V.; Keller, M.S.; Desai, M.; Dupuy, T.; Mosadeghi, S.; Whitman, C.; Lasch, K.; Ursos, L.; et al. Patient Understanding of the Risks and Benefits of Biologic Therapies in Inflammatory Bowel Disease: Insights from a Large-scale Analysis of Social Media Platforms. Inflamm. Bowel Dis. 2017, 23, 1057–1064. [Google Scholar] [CrossRef]
- Erikson, R.S. The Relationship between Public Opinion and State Policy: A New Look Based on Some Forgotten Data. Am. J. Political Sci. 1976, 20, 25–36. [Google Scholar] [CrossRef]
- Li, C.; Li, Y. Factors Influencing Public Risk Perception of Emerging Technologies: A Meta-Analysis. Sustainability 2023, 15, 3939. [Google Scholar] [CrossRef]
- Hu, Z.; Ding, S.; Li, S.; Chen, L.; Yang, S. Adoption Intention of Fintech Services for Bank Users: An Empirical Examination with an Extended Technology Acceptance Model. Symmetry 2019, 11, 340. [Google Scholar] [CrossRef]
- NW, 1615 L. St, Washington, S. 800 & Inquiries, D. 20036 U.-419-4300 | M.-857-8562 | F.-419-4372 | M. 2. Government, Regulation and the Social Safety Net. Pew Research Center—U.S. Politics & Policy. 2017. Available online: https://www.pewresearch.org/politics/2017/10/05/2-government-regulation-and-the-social-safety-net/ (accessed on 15 June 2024).
- Hoegberg, L. Risk perception, safety goals and regulatory decision-making. Reliab. Eng. Syst. Saf. 1998, 59, 135–139. [Google Scholar] [CrossRef]
- Hu, T.; Wang, S.; Luo, W.; Zhang, M.; Huang, X.; Yan, Y.; Liu, R.; Ly, K.; Kacker, V.; She, B.; et al. Revealing Public Opinion Towards COVID-19 Vaccines With Twitter Data in the United States: Spatiotemporal Perspective. J. Med. Internet Res. 2021, 23, e30854. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S. Exploring the Determinants of Perceived Risk of Middle East Respiratory Syndrome (MERS) in Korea. Int. J. Environ. Res. Public Health 2018, 15, 1168. [Google Scholar] [CrossRef]
- Tribble, S.J. Why the U.S. Remains the Most Expensive Market for ‘Biologic’ Drugs in the World; NPR: Washington, DC, USA, 2018. [Google Scholar]
- Dincer, O.C.; Fredriksson, P.G. Corruption and environmental regulatory policy in the United States: Does trust matter? Resour. Energy Econ. 2018, 54, 212–225. [Google Scholar] [CrossRef]
- Santoro, F.A.; Rothe, M.J.; Strober, B.E. Ethical considerations when prescribing biologics in dermatology. Clin. Dermatol. 2012, 30, 492–495. [Google Scholar] [CrossRef]
- Farina, V.; Reeves, J.T.; Senanayake, C.H.; Song, J.J. Asymmetric Synthesis of Active Pharmaceutical Ingredients. Chem. Rev. 2006, 106, 2734–2793. [Google Scholar] [CrossRef]
- Edgett, S. The Stage-Gate Model: An Overview. Stage-Gate International. 2022. Available online: https://www.stage-gate.com/blog/the-stage-gate-model-an-overview/ (accessed on 15 September 2024).
- Silverman, C. A Disorder of Affect: Love, Tragedy, Biomedicine, and Citizenship in American Autism Research, 1943–2003. Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 2004. Available online: https://www.proquest.com/openview/6675d743c744365aa2e7503542551611/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 15 December 2023).
- Mauro Caetano, C.S.A.; Amaral, D.; Guerrini, F. Open innovation and technology development process: The gap on partnership adoption from a case study perspective. Prod. Manag. Dev. 2011, 9, 111–120. [Google Scholar] [CrossRef][Green Version]
- Shimmin, C.; Wittmeier, K.; LaVoie, J.; Wicklund, E.; Sibley, K. Moving towards a more inclusive patient and public involvement in health research paradigm: The incorporation of a trauma-informed intersectional analysis. BMC Health Serv. Res. 2017, 17, 539. [Google Scholar] [CrossRef] [PubMed]
- Yuval-Davis, N. Nationalism, belonging, globalization and the ‘ethics of care’. Kvind. Køn Forsk. 2007, 2–3, 91–100. [Google Scholar] [CrossRef]
- Desouza, K.C.; Dombrowski, C.; Awazu, Y.; Baloh, P.; Papagari, S.; Jha, S.; Kim, J.Y. Crafting organizational innovation processes. Innovation 2009, 11, 6–33. [Google Scholar] [CrossRef]
| Regulation Title | Regulation Description | Year | Source |
|---|---|---|---|
| Guidance for Industry: Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications | Qualification of biological starters to test for adventitious agents is advised; validation of processes for inactivating adventitious agents using different model viruses is advised; ID-ing all potential contaminants is advised; current good manufacturing processes for “cell substrates” and “viral seeds” are advised; diploid cell strains; continuous cell lines; biological raw materials; ancillary reagents; serums, trypsin, amino acids, or biological reagents. | 2010 | https://www.fda.gov/media/78428/download (Accessed on 15 March 2024) |
| Guidance for Industry: Q2(R1) Validation of Analytical Procedures: Text and Methodology | This regulatory subdocument contains nonbinding recommendations for validating analytical procedures, largely in the context of the existing regulatory document “Guidance for Industry: Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications”. The scope of the regulations is contained within the topic of testing for the validity of various analytical procedures, categorized as testing for specificity, linearity, accuracy, range, precision, detection limit, quantitation limit, and robustness. | 2021 | https://www.fda.gov/media/152208/download (Accessed on 15 March 2024) |
| Q5A(R2) Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin Guidance for Industry | This guide describes the evaluation of the viral safety of biotechnology products, including viral clearance and testing. Project-wise, it refers to AAV, viral vectors writ large, and related components and materials. | 2024 | https://www.fda.gov/media/163115/download (Accessed on 15 March 2024) |
| Q13 Continuous Manufacturing of Drug Substances and Drug Products Guidance for Industry | This guidance applies to CM of drug substances and drug products for chemical entities and therapeutic proteins. It is applicable to CM for new products (e.g., new drugs, generic drugs, and biosimilars) and the conversion of batch manufacturing to CM for existing products. | 2023 | https://www.fda.gov/media/165775/download (Accessed on 15 March 2024) |
| Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients | The ICH guidance Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess. | 2018 | https://www.fda.gov/media/112426/download (Accessed on 15 March 2024) |
| Guidance for Industry: Q9 (R1) Quality Risk Management | This guideline provides principles and examples of tools for quality risk management that can be applied to different aspects of pharmaceutical quality. These aspects include development, manufacturing, distribution, and the inspection and submission/review processes throughout the lifecycle of drug substances, drug (medicinal) products, and biological and biotechnological products (including the use of raw materials, solvents, excipients, packaging and labeling materials in drug (medicinal) products, and biological and biotechnological products). | 2023 | https://www.fda.gov/media/167721/download (Accessed on 15 March 2024) |
| Guidance for Industry: Q5E Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process | This guidance is intended to assist manufacturers of biotechnological/biological products in the collection of relevant technical information that serves as evidence that the manufacturing process changes will not have an adverse impact on the quality, safety, and efficacy of the drug product. The document does not prescribe any particular analytical, nonclinical, or clinical strategy. The main emphasis of the document is on quality aspects. | 2005 | https://www.fda.gov/media/71489/download (Accessed on 15 March 2024) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galloway, B.; Stewart, P.A.; Gilmore, C.; Akakpo, V.; Borozdina, N.; Song, G.; Wickramasinghe, S.R.; Qian, X.; Arachchige, A.L.W.W.; Harcum, S.W. Unleashing the Power of Biologics: Exploring the Governance and Regulation of Membrane-Based Virus Purification (MVP) Technologies. Biologics 2025, 5, 9. https://doi.org/10.3390/biologics5020009
Galloway B, Stewart PA, Gilmore C, Akakpo V, Borozdina N, Song G, Wickramasinghe SR, Qian X, Arachchige ALWW, Harcum SW. Unleashing the Power of Biologics: Exploring the Governance and Regulation of Membrane-Based Virus Purification (MVP) Technologies. Biologics. 2025; 5(2):9. https://doi.org/10.3390/biologics5020009
Chicago/Turabian StyleGalloway, Ben, Patrick A. Stewart, Camille Gilmore, Victor Akakpo, Nataliia Borozdina, Geoboo Song, Sumith Ranil Wickramasinghe, Xianghong Qian, Asingsa Lakmini Weerasinghe Wickramasinghe Arachchige, and Sarah W. Harcum. 2025. "Unleashing the Power of Biologics: Exploring the Governance and Regulation of Membrane-Based Virus Purification (MVP) Technologies" Biologics 5, no. 2: 9. https://doi.org/10.3390/biologics5020009
APA StyleGalloway, B., Stewart, P. A., Gilmore, C., Akakpo, V., Borozdina, N., Song, G., Wickramasinghe, S. R., Qian, X., Arachchige, A. L. W. W., & Harcum, S. W. (2025). Unleashing the Power of Biologics: Exploring the Governance and Regulation of Membrane-Based Virus Purification (MVP) Technologies. Biologics, 5(2), 9. https://doi.org/10.3390/biologics5020009









