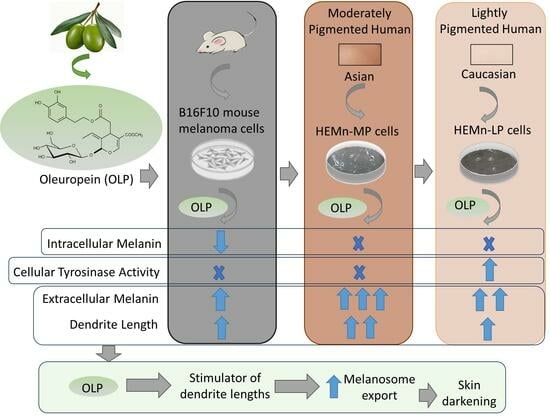
Oleuropein Is a Stimulator of Melanocyte Dendricity: Potential for Treatment of Hypopigmentation
Abstract
1. Introduction
2. Materials and Methods
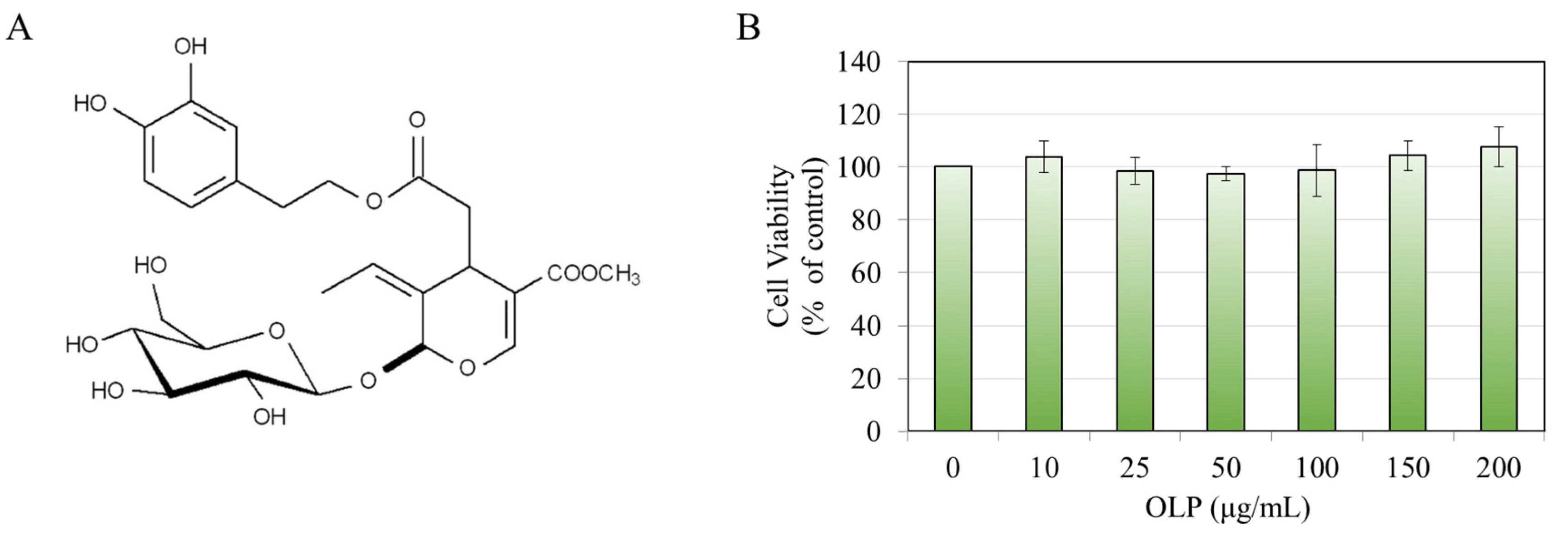
2.1. Materials
2.2. Cell Culture
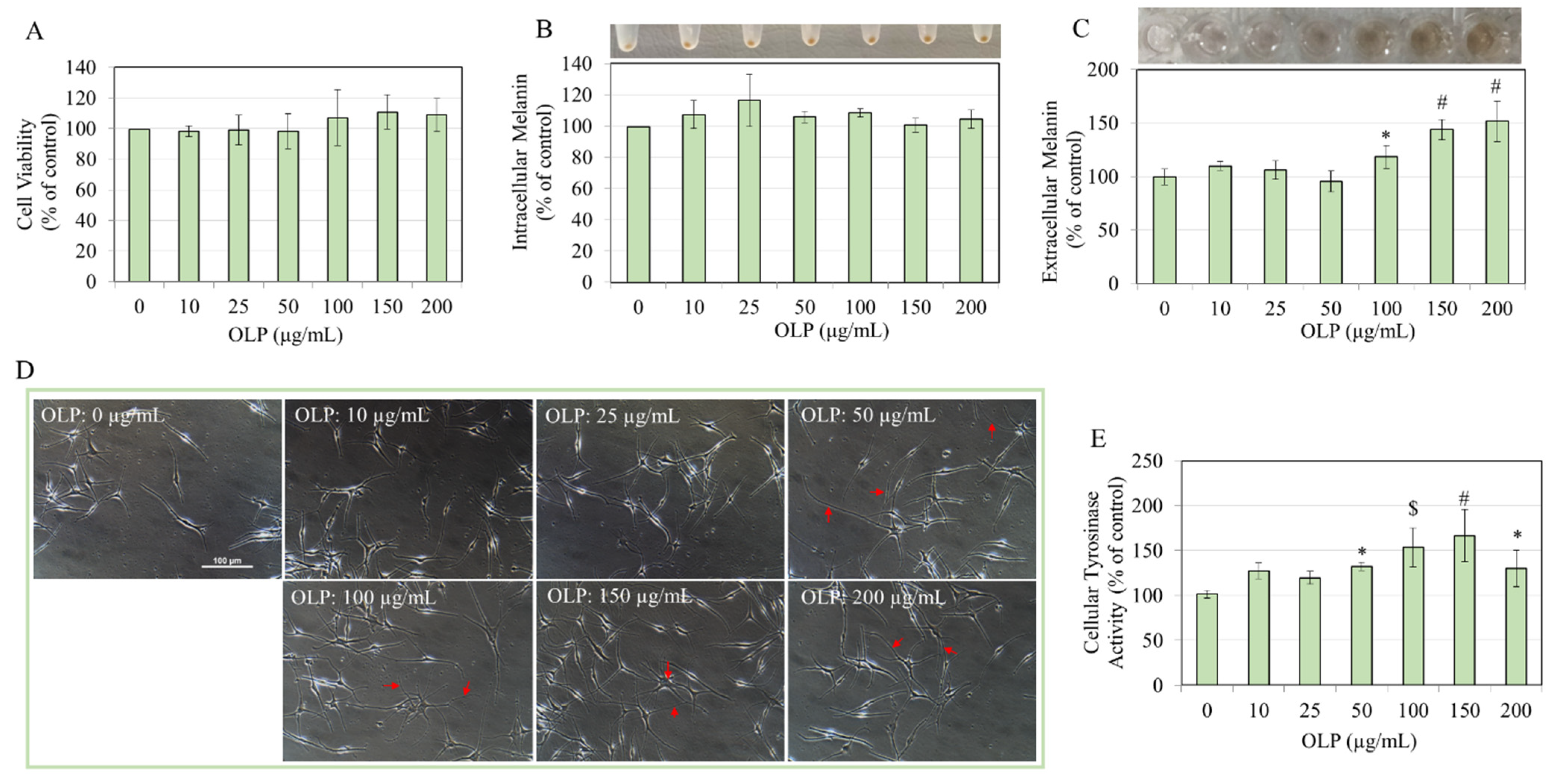
2.3. MTS Cytotoxicity Assay
2.4. Extracellular and Intracellular Melanin Assay
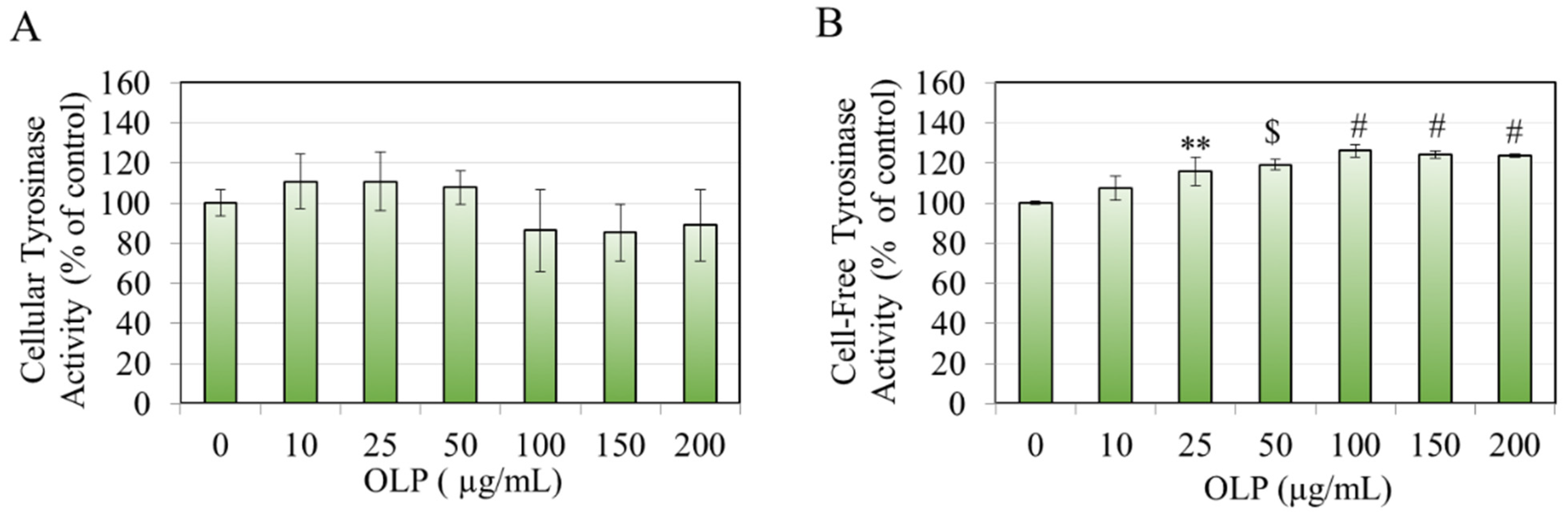
2.5. Cellular Tyrosinase Activity
2.6. Cell-Free Tyrosinase Activity
2.7. Quantitation of Dendricity in Human Melanocytes
2.8. Statistical Analysis
3. Results
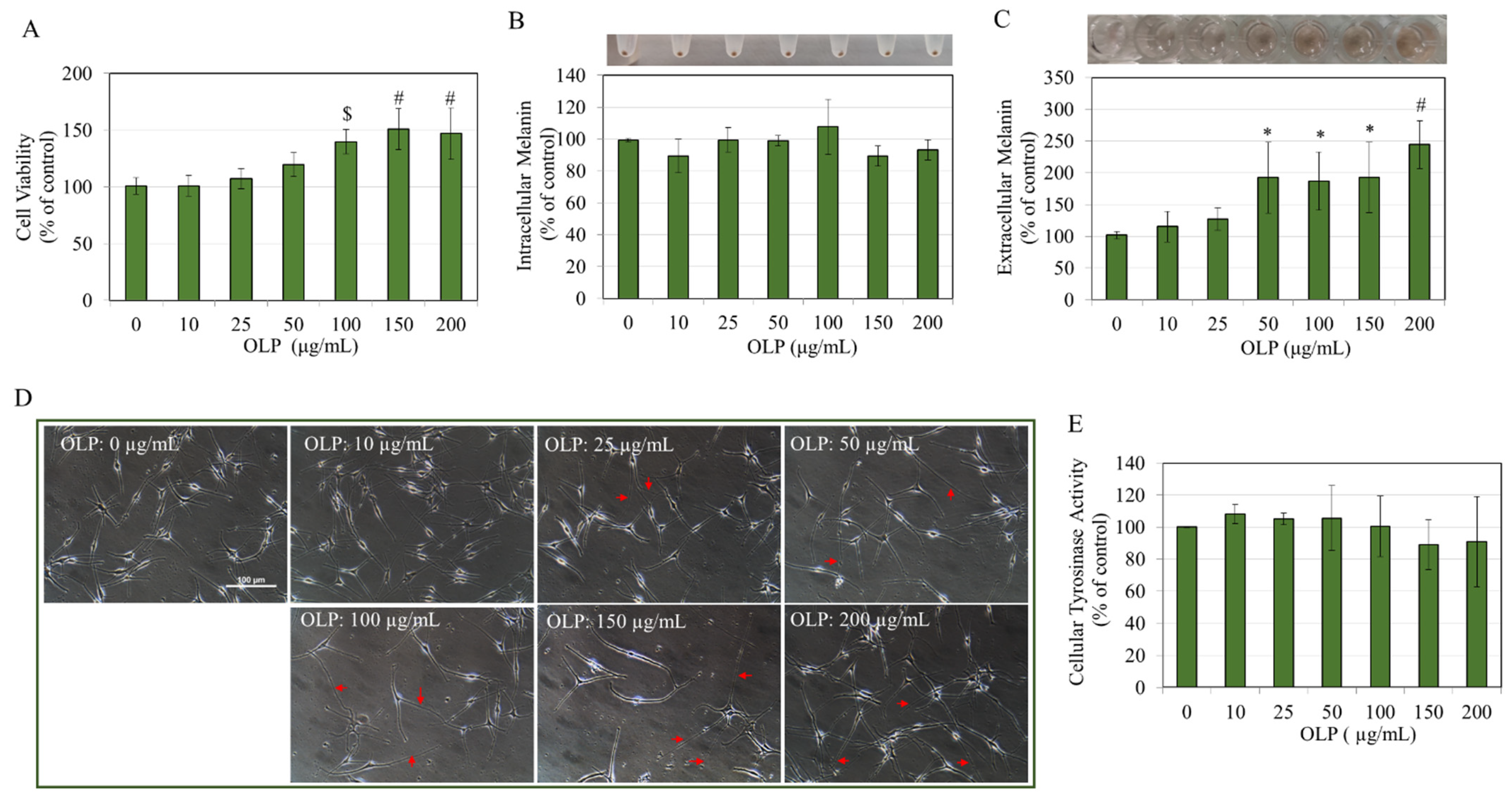
3.1. Effects of OLP in B16F10 Cells
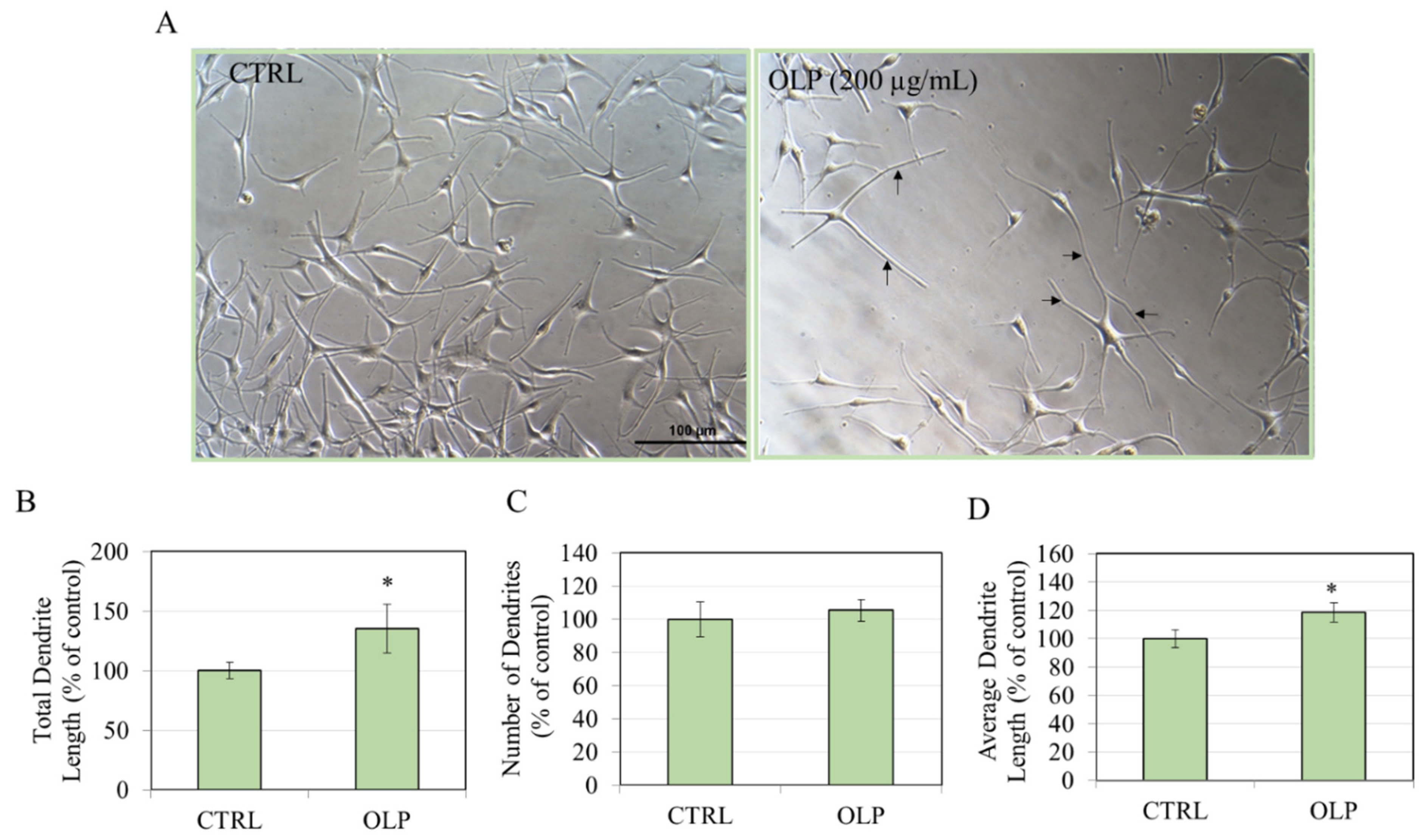
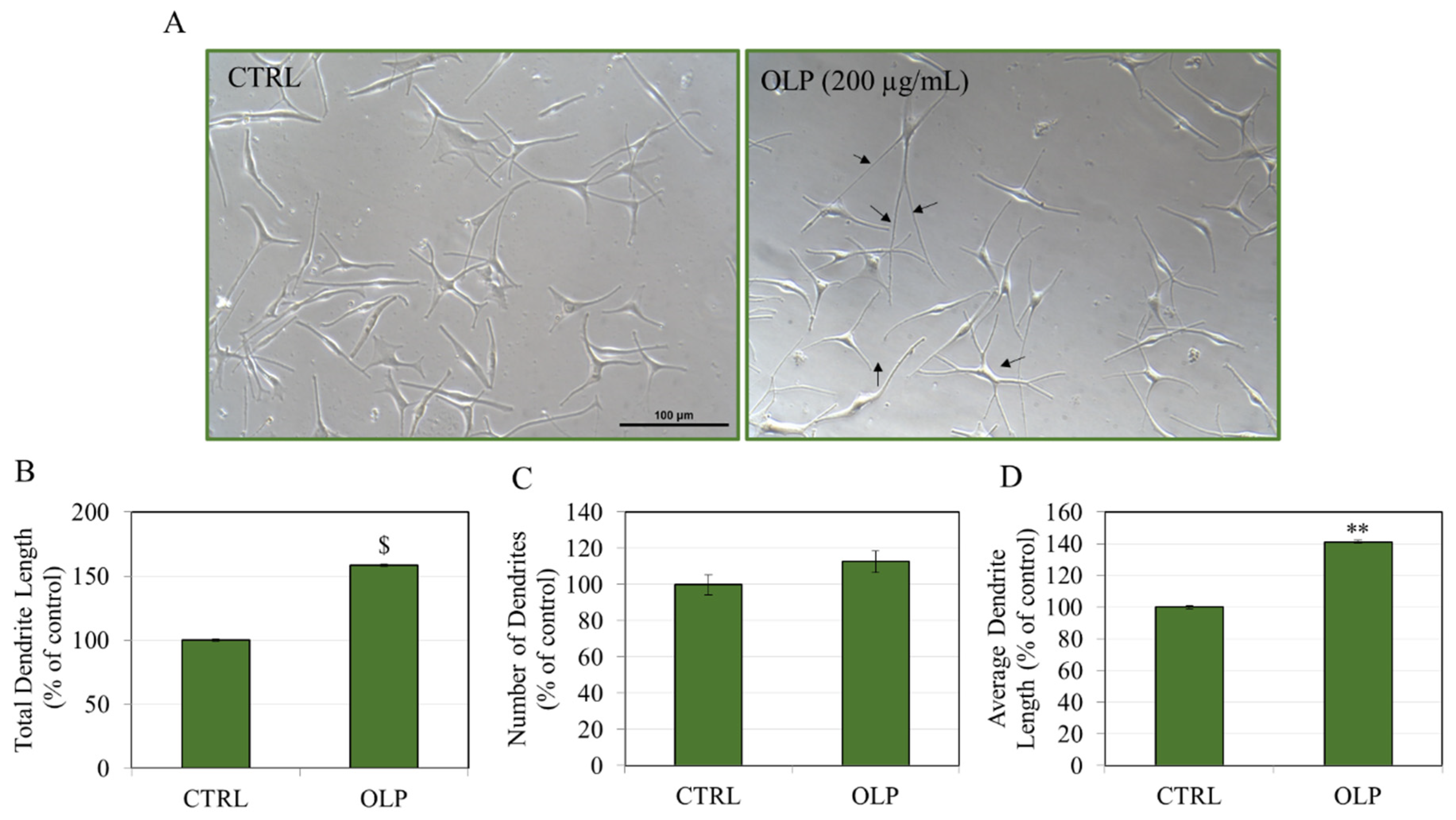
3.2. Effects of OLP in HEMn-LP Cells
3.3. Effects of OLP in HEMn-MP Cells
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costin, G.-E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar]
- Benito-Martínez, S.; Salavessa, L.; Raposo, G.; Marks, M.S.; Delevoye, C. Melanin transfer and fate within keratinocytes in human skin pigmentation. Integr. Comp. Biol. 2021, 61, 1546–1555. [Google Scholar] [PubMed]
- Lupon, E.; Berkane, Y.; Bertheuil, N.; Cetrulo, C.L.; Vaillant, C.; Chaput, B.; Camuzard, O.; Lellouch, A.G. Nonsurgical treatment of postburn hypopigmentation: A literature review. J. Burn Care Res. 2024, 45, 601–607. [Google Scholar] [CrossRef]
- Rao, M.; Young, K.; Jackson-Cowan, L.; Kourosh, A.; Theodosakis, N. Post-inflammatory Hypopigmentation: Review of the etiology, clinical manifestations, and Treatment options. J. Clin. Med. 2023, 12, 1243. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, R.; Caelenberg, E.V.; Belpaire, A.; Speeckaert, M.M.; Geel, N.v. Vitiligo: From Pathogenesis to Treatment. J. Clin. Med. 2024, 13, 5225. [Google Scholar] [CrossRef] [PubMed]
- Dabas, G.; Vinay, K.; Parsad, D.; Kumar, A.; Kumaran, M. Psychological disturbances in patients with pigmentary disorders: A cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 392–399. [Google Scholar]
- Rajatanavin, N.; Suwanachote, S.; Kulkollakarn, S. Dihydroxyacetone: A safe camouflaging option in vitiligo. Int. J. Dermatol. 2008, 47, 402–406. [Google Scholar] [CrossRef]
- Petersen, A.B.; Wulf, H.C.; Gniadecki, R.; Gajkowska, B. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2004, 560, 173–186. [Google Scholar]
- Levine, J.A.; Sorace, M.; Spencer, J.; Siegel, D.M. The indoor UV tanning industry: A review of skin cancer risk, health benefit claims, and regulation. J. Am. Acad. Dermatol. 2005, 53, 1038–1044. [Google Scholar] [CrossRef]
- Ando, H.; Niki, Y.; Ito, M.; Akiyama, K.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Investig. Dermatol. 2012, 132, 1222–1229. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B.; Breathnach, A. The epidermal melanin unit system. Dermatol. Wochenschr. 1963, 147, 481–489. [Google Scholar]
- Shah, A.N.; Marfatia, R.K.; Saikia, S.S. A study of noncultured extracted hair follicle outer root sheath cell suspension for transplantation in vitiligo. Int. J. Trichology 2016, 8, 67–72. [Google Scholar]
- Tobin, D.J.; Paus, R. Graying: Gerontobiology of the hair follicle pigmentary unit. Exp. Gerontol. 2001, 36, 29–54. [Google Scholar] [PubMed]
- Cao, Y.; Lv, J.; Tan, Y.; Chen, R.; Jiang, X.; Meng, D.; Zou, K.; Pan, M.; Tang, L. Tribuloside acts on the PDE/cAMP/PKA pathway to enhance melanogenesis, melanocyte dendricity and melanosome transport. J. Ethnopharmacol. 2024, 323, 117673. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yang, L.; Wu, L.; Wang, H.; Chen, K.; Wu, H.; Li, Y. Kaempferol, the melanogenic component of Sanguisorba officinalis, enhances dendricity and melanosome maturation/transport in melanocytes. J. Pharmacol. Sci. 2021, 147, 348–357. [Google Scholar] [PubMed]
- Lv, J.; Fu, Y.; Gao, R.; Li, J.; Kang, M.; Song, G.; Yun, C. Diazepam enhances melanogenesis, melanocyte dendricity and melanosome transport via the PBR/cAMP/PKA pathway. Int. J. Biochem. Cell Biol. 2019, 116, 105620. [Google Scholar] [CrossRef]
- Yamauchi, K.; Mitsunaga, T.; Inagaki, M.; Suzuki, T. Quercetin derivatives regulate melanosome transportation via EPI64 inhibition and elongate the cell shape of B16 melanoma cells. Biomed. Pharmacother. 2015, 70, 206–212. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, N.-H.; Koo, B.-S.; Lee, H.-J.; Lee, A.-Y. Bee venom stimulates human melanocyte proliferation, melanogenesis, dendricity and migration. Exp. Mol. Med. 2007, 39, 603–613. [Google Scholar]
- Venkatasamy, R.; Faas, L.; Young, A.R.; Raman, A.; Hider, R.C. Effects of piperine analogues on stimulation of melanocyte proliferation and melanocyte differentiation. Bioorganic Med. Chem. 2004, 12, 1905–1920. [Google Scholar] [CrossRef]
- Goenka, S. Cyclocurcumin, a Minor Curcuminoid, is a Novel Candidate for Hypopigmentary Skin Disorders with Melanogenesis-Stimulating Capacity. Drugs Drug Candidates 2024, 3, 410–436. [Google Scholar] [CrossRef]
- Goenka, S. Effects of a standardized hydrogenated extract of curcumin (curowhite™) on melanogenesis: A pilot study. Nutraceuticals 2023, 3, 421–437. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. A novel pro-melanogenic effect of standardized dry olive leaf extract on primary human melanocytes from lightly pigmented and moderately pigmented skin. Pharmaceuticals 2021, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the olive-derived polyphenol oleuropein on human health. Int. J. Mol. Sci. 2014, 15, 18508–18524. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a bioactive compound from Olea europaea L., as a potential preventive and therapeutic agent in non-communicable diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar]
- Gonçalves, M.; Aiello, A.; Rodríguez-Pérez, M.; Accardi, G.; Burgos-Ramos, E.; Silva, P. Olive oil components as novel antioxidants in neuroblastoma treatment: Exploring the therapeutic potential of oleuropein and hydroxytyrosol. Nutrients 2024, 16, 818. [Google Scholar] [CrossRef]
- Yılmaz, G.; Özdemir, F. Novel anti-tumor strategy for breast cancer: Synergistic role of oleuropein with paclitaxel therapeutic in MCF-7 cells. Anti-Cancer Agents Med. Chem. 2024, 24, 224–234. [Google Scholar]
- Li, H.; He, H.; Liu, C.; Akanji, T.; Gutkowski, J.; Li, R.; Ma, H.; Wan, Y.; Wu, P.; Li, D. Dietary polyphenol oleuropein and its metabolite hydroxytyrosol are moderate skin permeable elastase and collagenase inhibitors with synergistic cellular antioxidant effects in human skin fibroblasts. Int. J. Food Sci. Nutr. 2021, 73, 460–470. [Google Scholar]
- Visioli, F.; Bellomo, G.; Galli, C. Free radical-scavenging properties of olive oil polyphenols. Biochem. Biophys. Res. Commun. 1998, 247, 60–64. [Google Scholar] [CrossRef]
- El-Gogary, R.I.; Ragai, M.H.; Moftah, N.; Nasr, M. Oleuropein as a novel topical antipsoriatic nutraceutical: Formulation in microemulsion nanocarrier and exploratory clinical appraisal. Expert Opin. Drug Deliv. 2021, 18, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Zoric, N.; Kopjar, N.; Bobnjaric, I.; Horvat, I.; Tomic, S.; Kosalec, I. Antifungal activity of oleuropein against Candida albicans-the in vitro study. Molecules 2016, 21, 1631. [Google Scholar] [CrossRef]
- Bharathy, H.; Fathima, N.N. Exploiting oleuropein for inhibiting collagen fibril formation. Int. J. Biol. Macromol. 2017, 101, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Achour, I.; Arel-Dubeau, A.M.; Renaud, J.; Legrand, M.; Attard, E.; Germain, M.; Martinoli, M.G. Oleuropein Prevents Neuronal Death, Mitigates Mitochondrial Superoxide Production and Modulates Autophagy in a Dopaminergic Cellular Model. Int. J. Mol. Sci. 2016, 17, 1293. [Google Scholar] [CrossRef] [PubMed]
- Mehraein, F.; Sarbishegi, M.; Aslani, A. Evaluation of effect of oleuropein on skin wound healing in aged male BALB/c mice. Cell J. 2014, 16, 25–30. [Google Scholar]
- Ahmadi, I.; Foruozandeh, H.; Yekke, F. Evaluation the healing potential of oleuropein on second-degree burn wounds in a rat model. Jundishapur J. Nat. Pharm. Prod. 2022, 17, e114568. [Google Scholar] [CrossRef]
- Huang, W.-C.; Liou, C.-J.; Shen, S.-C.; Hu, S.; Chao, J.; Huang, C.-H.; Wu, S.-J. Oleuropein attenuates inflammation and regulates immune responses in a 2, 4-dinitrochlorobenzene-induced atopic dermatitis mouse model. Asian Pac. J. Allergy Immunol 2022, 10, 1–12. [Google Scholar]
- Tong, T.; Kim, N.; Park, T. Topical application of oleuropein induces anagen hair growth in telogen mouse skin. PLoS ONE 2015, 10, e0129578. [Google Scholar] [CrossRef][Green Version]
- Kimura, Y.; Sumiyoshi, M. Olive leaf extract and its main component oleuropein prevent chronic ultraviolet B radiation-induced skin damage and carcinogenesis in hairless mice. J. Nutr. 2009, 139, 2079–2086. [Google Scholar] [CrossRef]
- Busto, F.; Licini, C.; Luccarini, A.; Damiani, E.; Mattioli-Belmonte, M.; Cometa, S.; De Giglio, E. Oleuropein-rich gellan gum/alginate films as innovative treatments against photo-induced skin aging. Molecules 2023, 28, 4352. [Google Scholar] [CrossRef]
- Bansal, S.; Sahoo, B.; Garg, V. Psoralen–narrowband UVB phototherapy for the treatment of vitiligo in comparison to narrowband UVB alone. Photodermatol. Photoimmunol. Photomed. 2013, 29, 311–317. [Google Scholar] [PubMed]
- Hussain, I. The safety of medicinal plants used in the treatment of vitiligo and hypermelanosis: A systematic review of use and reports of harm. Clin. Cosmet. Investig. Dermatol. 2021, 14, 261–284. [Google Scholar] [PubMed]
- Stern, R.S.; Lange, R. Non-melanoma skin cancer occurring in patients treated with PUVA five to ten years after first treatment. J. Investig. Dermatol. 1988, 91, 120–124. [Google Scholar] [PubMed]
- Lueder, M. The Active Ingredients Mixture of Olives Provides Skin Whitening and Age Spot Reduction. Cosmet. Sci. Technol. 2011, 1–6. [Google Scholar]
- Cho, J.; Bejaoui, M.; Tominaga, K.; Isoda, H. Comparative analysis of olive-derived phenolic compounds’ pro-melanogenesis effects on B16F10 cells and epidermal human melanocytes. Int. J. Mol. Sci. 2024, 25, 4479. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Hu, J.; Si, J.; Xie, Y.; Wei, J.; Liu, Y.; Pei, D. Tyrosinase inhibitor screened from Olea europaea L. leaves: Identification, molecular docking analysis and molecular mechanisms. Ind. Crops Prod. 2024, 210, 118112. [Google Scholar]
- Kishikawa, A.; Ashour, A.; Zhu, Q.; Yasuda, M.; Ishikawa, H.; Shimizu, K. Multiple biological effects of olive oil by-products such as leaves, stems, flowers, olive milled waste, fruit pulp, and seeds of the olive plant on skin. Phytother. Res. 2015, 29, 877–886. [Google Scholar]
- Goenka, S.; Simon, S.R. Inhibitory effects of the bioactive thermorubin isolated from the fungus thermoactinomyces antibioticus on melanogenesis. Cosmetics 2020, 7, 61. [Google Scholar] [CrossRef]
- Goupy, P.; Fleuriet, A.; Amiot, M.J.; Macheix, J.J. Enzymic browning, oleuropein content, and diphenol oxidase activity in olive cultivars (Olea europaea L.). J. Agric. Food Chem. 1991, 39, 92–95. [Google Scholar]
- Konno, K.; Hirayama, C.; Yasui, H.; Nakamura, M. Enzymatic activation of oleuropein: A protein crosslinker used as a chemical defense in the privet tree. Proc. Natl. Acad. Sci. USA 1999, 96, 9159–9164. [Google Scholar]
- Gentile, L.; Uccella, N.A.; Sivakumar, G. Soft-MS and computational mapping of oleuropein. Int. J. Mol. Sci. 2017, 18, 992. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Bravo, K.A.; Jarén-Galán, M.; García-García, P.; Garrido-Fernández, A. Browning reactions in olives: Mechanism and polyphenols involved. Food Chem. 2009, 114, 1380–1385. [Google Scholar] [CrossRef]
- Goenka, S.; Nagabhushanam, K.; Majeed, M. Study of the Effects of Novel Analogs of Calebin-A on Melanogenesis. Drugs Drug Candidates 2024, 3, 471–487. [Google Scholar] [CrossRef]
- Yamauchi, K.; Mitsunaga, T. Methylquercetins stimulate melanin biosynthesis in a three-dimensional skin model. J. Nat. Med. 2018, 72, 563–569. [Google Scholar] [CrossRef]
- Faas, L.; Venkatasamy, R.; Hider, R.; Young, A.; Soumyanath, A. In vivo evaluation of piperine and synthetic analogues as potential treatments for vitiligo using a sparsely pigmented mouse model. Br. J. Dermatol. 2008, 158, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Soumyanath, A.; Venkatasamy, R.; Joshi, M.; Faas, L.; Adejuyigbe, B.; Drake, A.F.; Hider, R.C.; Young, A.R. UV irradiation affects melanocyte stimulatory activity and protein binding of piperine. Photochem. Photobiol. 2006, 82, 1541–1548. [Google Scholar] [CrossRef]
- Longo, E.; Morozova, K.; Scampicchio, M. Effect of light irradiation on the antioxidant stability of oleuropein. Food Chem. 2017, 237, 91–97. [Google Scholar] [CrossRef]
- Sachdeva, S. Fitzpatrick skin typing: Applications in dermatology. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 93. [Google Scholar] [CrossRef]
- Fukuda, M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic 2013, 14, 949–963. [Google Scholar] [CrossRef]
- Jordens, I.; Westbroek, W.; Marsman, M.; Rocha, N.; Mommaas, M.; Huizing, M.; Lambert, J.; Naeyaert, J.M.; Neefjes, J. Rab7 and Rab27a control two motor protein activities involved in melanosomal transport. Pigment Cell Res. 2006, 19, 412–423. [Google Scholar] [CrossRef]
- Kuroda, T.S.; Fukuda, M. Rab27A-binding protein Slp2-a is required for peripheral melanosome distribution and elongated cell shape in melanocytes. Nat. Cell Biol. 2004, 6, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, N.; Yatsu, A.; Tamura, K.; Fukuda, M. The Rab21-GEF activity of Varp, but not its Rab32/38 effector function, is required for dendrite formation in melanocytes. Mol. Biol. Cell 2012, 23, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Ohbayashi, N.; Maruta, Y.; Kanno, E.; Itoh, T.; Fukuda, M. Varp Is a Novel Rab32/38-binding Protein That Regulates Tyrp1 Trafficking in Melanocytes. Mol. Biol. Cell. 2009, 20, 2900–2908. [Google Scholar] [CrossRef]
- Ranieri, M.; Di Mise, A.; Difonzo, G.; Centrone, M.; Venneri, M.; Pellegrino, T.; Russo, A.; Mastrodonato, M.; Caponio, F.; Valenti, G. Green olive leaf extract (OLE) provides cytoprotection in renal cells exposed to low doses of cadmium. PLoS ONE 2019, 14, e0214159. [Google Scholar]
- Shin, Y.-H.; Seo, Y.-K.; Yoon, H.-H.; Song, K.-Y.; Park, J.-K. Effect of keratinocytes on regulation of melanogenesis in culture of melanocytes. Biotechnol. Bioprocess Eng. 2012, 17, 203–210. [Google Scholar] [CrossRef]
- Chen, N.; Hu, Y.; Li, W.H.; Eisinger, M.; Seiberg, M.; Lin, C.B. The role of keratinocyte growth factor in melanogenesis: A possible mechanism for the initiation of solar lentigines. Exp. Dermatol. 2010, 19, 865–872. [Google Scholar]
- Yan, X.; Wang, T.-M.; Ming, Y.-C.; Yeh, Y.-M.; Chen, T.-Y.; Pang, J.-H.S. Melanin uptake reduces cell proliferation of human epidermal keratinocytes. J. Cosmet. Dermatol. Sci. Appl. 2015, 5, 300–310. [Google Scholar]
- Potter, B.; Medenica, M. Ultramicroscopic phagocytosis of synthetic melanin by epidermal cells in vivo. J. Invest. Dermatol. 1968, 51, 300–303. [Google Scholar] [CrossRef][Green Version]
- Barygina, V.; Becatti, M.; Lotti, T.; Moretti, S.; Taddei, N.; Fiorillo, C. ROS-challenged keratinocytes as a new model for oxidative stress-mediated skin diseases. J. Cell. Biochem. 2019, 120, 28–36. [Google Scholar]
- Chang, H.; Choi, H.; Joo, K.M.; Kim, D.; Lee, T.R. Manassantin B inhibits melanosome transport in melanocytes by disrupting the melanophilin–myosin Va interaction. Pigment Cell Melanoma Res. 2012, 25, 765–772. [Google Scholar]
- Wang, Y.; Viennet, C.; Robin, S.; Berthon, J.-Y.; He, L.; Humbert, P. Precise role of dermal fibroblasts on melanocyte pigmentation. J. Dermatol. Sci. 2017, 88, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Asghariazar, V.; Vahidian, F.; Karimi, A.; Abbaspour-Ravasjani, S.; Mansoori, B.; Safarzadeh, E. The role of oleuropein, derived from olives, in human skin fibroblast cells: Investigating the underlying molecular mechanisms of cytotoxicity and antioxidant and anti-Inflammatory activities. Int. J. Clin. Pract. 2024, 2024, 8827501. [Google Scholar] [CrossRef]
- Babich, H.; Visioli, F. In vitro cytotoxicity to human cells in culture of some phenolics from olive oil. Il Farm. 2003, 58, 403–407. [Google Scholar] [CrossRef]
- Yang, J.; Wu, S.; Xu, H.; Yan, Y.; Ju, B.; Zhu, D.; Liang, X.; Hu, J. Inhibitory effects of phenylethanoid glycosides on melanin synthesis in cultured human epidermal melanocytes. Int. J. Clin. Exp. Med. 2016, 9, 18019–18025. [Google Scholar]
- Chaari, A. Inhibition of human islet amyloid polypeptide aggregation and cellular toxicity by oleuropein and derivatives from olive oil. Int. J. Biol. Macromol. 2020, 162, 284–300. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Zheng, M.; Xi, X.; Zhang, X.; Han, C. Structure properties, acquisition protocols, and biological activities of oleuropein aglycone. Front. Chem. 2018, 6, 239. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Natsume, M.; Yamaguchi, K.; Batubara, I.; Mitsunaga, T. Structure-activity relationship for vanilloid compounds from extract of Zingiber officinale var. rubrum rhizomes: Effect on extracellular melanogenesis inhibitory activity. Med. Chem. Res. 2019, 28, 1402–1412. [Google Scholar] [CrossRef]
- Lemonakis, N.; Mougios, V.; Halabalaki, M.; Skaltsounis, A.L.; Gikas, E. A novel bioanalytical method based on UHPLC-HRMS/MS for the quantification of oleuropein in human serum. Application to a pharmacokinetic study. Biomed. Chromatogr. 2016, 30, 2016–2023. [Google Scholar] [CrossRef]
- De Bock, M.; Thorstensen, E.B.; Derraik, J.G.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; Cosco, D.; Dini, L.; Di Marzio, L.; Fresta, M.; Paolino, D. Oleuropein-laded ufasomes improve the nutraceutical efficacy. Nanomaterials 2021, 11, 105. [Google Scholar] [CrossRef]
- Oi-Kano, Y.; Kawada, T.; Watanabe, T.; Koyama, F.; Watanabe, K.; Senbongi, R.; Iwai, K. Oleuropein supplementation increases urinary noradrenaline and testicular testosterone levels and decreases plasma corticosterone level in rats fed high-protein diet. J. Nutr. Biochem. 2013, 24, 887–893. [Google Scholar] [PubMed]
- Liamin, M.; Lara, M.P.; Michelet, O.; Rouault, M.; Quintela, J.C.; Le Bloch, J. Olive juice dry extract containing hydroxytyrosol, as a nontoxic and safe substance: Results from pre-clinical studies and review of toxicological studies. Toxicol. Rep. 2023, 10, 245–260. [Google Scholar]
- Clewell, A.E.; Béres, E.; Vértesi, A.; Glávits, R.; Hirka, G.; Endres, J.R.; Murbach, T.S.; Szakonyiné, I.P. A comprehensive toxicological safety assessment of an extract of Olea europaea L. leaves (bonolive™). Int. J. Toxicol. 2016, 35, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Speroni, E.; Guerra, M.; Minghetti, A.; Crespi-Perellino, N.; Pasini, P.; Piazza, F.; Roda, A. Oleuropein evaluated in vitro and in vivo as an antioxidant. Phytother. Res. 1998, 12, S98–S100. [Google Scholar]
- Asawanonda, P.; Klahan, S.-O. Tetrahydrocurcuminoid cream plus targeted narrowband UVB phototherapy for vitiligo: A preliminary randomized controlled study. Photomed. Laser Surg. 2010, 28, 679–684. [Google Scholar]
- Park, S.; Choi, H.; Kim, Y.J. Inhibition of isorhamnetin on β-Catenin/Tcf signaling and β-catenin-activated melanogenesis. J. Basic Appl. Sci. 2013, 9, 401–409. [Google Scholar]
- Cardinali, G.; Ceccarelli, S.; Kovacs, D.; Aspite, N.; Lotti, L.V.; Torrisi, M.R.; Picardo, M. Keratinocyte growth factor promotes melanosome transfer to keratinocytes. J. Investig. Dermatol. 2005, 125, 1190–1199. [Google Scholar]
- Wanitphakdeedecha, R.; Ng, J.N.C.; Junsuwan, N.; Phaitoonwattanakij, S.; Phothong, W.; Eimpunth, S.; Manuskiatti, W. Efficacy of olive leaf extract–containing cream for facial rejuvenation: A pilot study. J. Cosmet. Dermatol. 2020, 19, 1662–1666. [Google Scholar]
- SuperHeal® O-Live Cream 60g, Phyto-C Skin Care. Available online: https://www.phyto-c.com/products/superheal-o-live-cream-1?srsltid=AfmBOor19IiUJv-LxNVzbC6Rsp0ExipXO_d8HrRQe9WJFFNTLA6UvkVB (accessed on 15 January 2025).
- Kim, Y.C.; Choi, S.Y.; Park, E.Y. Anti-melanogenic effects of black, green, and white tea extracts on immortalized melanocytes. J. Vet. Sci. 2015, 16, 135–143. [Google Scholar]
- Yamakoshi, J.; Otsuka, F.; Sano, A.; Tokutake, S.; Saito, M.; Kikuchi, M.; Kubota, Y. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res. 2003, 16, 629–638. [Google Scholar]
- Oliveira, K.B.; Palú, É.; Weffort-Santos, A.M.; Oliveira, B.H. Influence of rosmarinic acid and Salvia officinalis extracts on melanogenesis of B16F10 cells. Rev. Bras. Farmacogn. 2013, 23, 249–258. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goenka, S. Oleuropein Is a Stimulator of Melanocyte Dendricity: Potential for Treatment of Hypopigmentation. Biologics 2025, 5, 8. https://doi.org/10.3390/biologics5020008
Goenka S. Oleuropein Is a Stimulator of Melanocyte Dendricity: Potential for Treatment of Hypopigmentation. Biologics. 2025; 5(2):8. https://doi.org/10.3390/biologics5020008
Chicago/Turabian StyleGoenka, Shilpi. 2025. "Oleuropein Is a Stimulator of Melanocyte Dendricity: Potential for Treatment of Hypopigmentation" Biologics 5, no. 2: 8. https://doi.org/10.3390/biologics5020008
APA StyleGoenka, S. (2025). Oleuropein Is a Stimulator of Melanocyte Dendricity: Potential for Treatment of Hypopigmentation. Biologics, 5(2), 8. https://doi.org/10.3390/biologics5020008