Abstract
Background: Recombinant monoclonal antibodies represent a vital category of biologics, constituting the largest class of molecules used to treat autoimmune disorders, cancers, rheumatoid arthritis, and other chronic conditions. The IgG1 subclass is the most potent among all the immunoglobulin gamma (IgG) antibodies, inducing Fc-related effector functions. N-linked glycan distribution of therapeutic IgG1s affects Fc-related effector functions such as CDC (complement-dependent cytotoxicity) and ADCC (antibody dependent cell-mediated cytotoxicity) biological activities and efficacy in vivo. Hence, as a critical quality attribute (CQA), the glycosylation profile of therapeutic IgG1s must be consistently preserved, which is primarily influenced by manufacturing process factors. In the era of biosimilars, it is challenging for biopharmaceutical manufacturers to not only obtain the desired glycan distribution consistently but also to meet the innovator molecule specifications as per the regulatory agencies. Methods: This study investigates the CHO fed-batch process parameters that affect the titer and terminal galactosylation of the TNF-α blocker-IgG1. It was hypothesized that galactose supplementation would enhance the galactosylation of TNF-α blocker-IgG1. Results: It was observed that such in-cultivation process shift does not affect cell culture parameters yet significantly enhances the galactosylation of TNF-α blocker-IgG1. Interestingly, the results indicate that supplementing D-galactose from the exponential phase of the CHO fed-batch process had the greatest effect on Fc galactosylation, increasing the amount of total galactosylated TNF-α blocker-IgG1 from 7.7% to 15.8%. Conclusions: Our results demonstrate a relatively easy and viable technique for cell culture engineering that is more appropriate for industrial production than costly in vitro glycoengineering.
1. Introduction
Monoclonal antibodies (mAbs) have transformed contemporary therapy, offering focused treatment alternatives for various illnesses, such as cancers, autoimmune conditions, and infectious diseases. With no clear indications of deceleration, these protein biopharmaceuticals are anticipated to take up over 30% of the entire pharmaceutical market [1]. Their clinical achievement is therefore evident in the continuous commitment of the biopharmaceutical sector to this specific category of molecules [2,3,4,5]. The cost of biopharmaceutical treatments is significantly higher than that of traditional small molecule treatments [6,7]. However, many healthcare systems have no choice but to enforce rationing of expensive therapies due to the rising costs of biologic pharmaceuticals, which keeps patients from receiving the right therapy [8,9,10,11]. The expiration of patents for therapeutic proteins facilitates the creation and production of biosimilar products by various biopharmaceutical manufacturers [12,13]. The recent expiration of patent protections for several major biotherapeutics, such as adalimumab, infliximab, and trastuzumab, has led to a notable transformation within the biopharmaceutical sector, emphasizing the development of more affordable alternatives [14]. As a result, biosimilars could improve access to these transformative therapies for a broader population of patients and might help to reduce total healthcare costs [1,15].
Therapeutic mAbs present several advantages over small molecule drugs, including a higher degree of specificity, reduced side effects, and an extended half-life in human circulation, typically lasting several weeks [12]. The primary drawback, in contrast to small molecules, is not only in the complex production and purification processes but also in the significant heterogeneity resulting from post-translational modifications [6,11]. Nevertheless, these modifications present opportunities to incorporate additional functionalities, thereby enabling further refinement of the efficacy of certain therapeutic monoclonal antibodies [10]. Among the mAbs created for therapeutic applications, IgG1-based TNF-α inhibitors are especially effective in treating conditions like rheumatoid arthritis, Crohn’s disease, and psoriasis, as they work by neutralizing the pro-inflammatory cytokine TNF-α, thereby reducing disease symptoms and enhancing patient outcomes [16]. While the therapeutic efficacy of these antibodies is primarily attributed to their ability to bind TNF-α, the pharmacological properties of IgG1-based blockers are also significantly influenced by their glycosylation patterns, which can modulate antibody stability, immunogenicity, and effector functions [17,18].
Glycosylation represents the most prevalent and complex post-translational modification found in both natural and recombinant proteins, with approximately 50% of human proteins experiencing this modification [9,19,20,21]. Mammalian cell-produced therapeutic IgG-type antibodies undergo N-glycosylation at asparagine 297 located on both heavy chains within the Fc region [22,23]. Each glycan of IgG1 typically belongs to the categories of high-mannose or complex biantennary families, characterized by an N, N-diacetylchitobiose core [20]. This core is linked to different amounts of mannose, galactose, fucose, sialic acid, and N-acetyl glucosamine residues [24]. The therapeutic efficacy of therapeutic IgG1s is closely linked to their total glycosylation profile, which can significantly influence their efficacy, stability, and immunogenicity, primarily due to their influence on Fc-mediated interactions with various receptors [9,25,26]. Among the various glycosylation modifications, galactosylation, the addition of galactose residues to the oligosaccharide chains in the Fc region, plays a critical role in modulating complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), and improving pharmacokinetic properties [19,22,27,28,29]. Alterations in the galactosylation pattern can enhance the mAb’s ability to trigger immune responses, making it a key factor in therapeutic optimization. Specifically, IgG1 antibodies with higher levels of galactose in their glycan chains exhibit enhanced interactions with Fcγ receptors on immune cells, improving their ability to recruit effector cells for target cell killing [22]. Galactosylation increases binding to FcγRIIIa [19]. It was demonstrated that terminal galactosylation substantially improved the FcγRIIIa binding affinity in both non-fucosylated and fucosylated species [30]. Also, IgG1 with terminal galactosylation exhibited a longer retention time in pH gradient human FcRn affinity chromatography and clearly de-accelerated the speed of aggregate formation compared to agalactosylated variants [30]. In another study, galactose concentrations were enzymatically adjusted for four different CHO-derived monoclonal antibodies, verifying that the existence of terminal galactose improves ADCC activity [26]. Furthermore, increased galactosylation has been associated with reduced immunogenicity [31] and improved therapeutic efficacy by prolonging the serum half-life of the IgG antibody [19,26]. Galactosylation serves to prolong the circulation half-life of IgG by inhibiting the binding of terminal GlcNAc residues to the mannose receptor [32]. The deficiency in galactosylation could be the likely reason for the decreased sialic acid level of the recombinant protein [33], indicating the importance of galactosylation in therapeutic antibodies. It was reported that anti-CD20-IgG1 drug product samples with a total galactose content of >38% showed stable CDC bioactivity under temperature stress conditions, while those with 16% galactose content showed reduced CDC activity [27]. Thus, glycosylation is widely regarded as a critical quality attribute (CQA) in therapeutic protein [34]. The recent expansion of the biosimilar sector has garnered significant focus on enhancing galactosylation, since biosimilar products must successfully mimic this critical quality attribute present in the reference biologics [35]. Currently, regulatory agencies anticipate that biosimilar products should exhibit structural and functional similarity to their reference counterparts, and based on this expectation, they are contemplating the possibility of waiving clinical studies [36].
Despite the critical role of galactosylation in modulating antibody function, the natural glycosylation profile of IgG1 antibodies expressed in mammalian cells often results in suboptimal levels of galactose incorporation, which may limit the full therapeutic potential of the antibody [23,37]. To overcome this challenge, strategies to enhance galactosylation, such as the addition of media supplements during the cell culture process, have been proposed as a means to fine-tune glycan composition and improve the biological activity of recombinant monoclonal antibodies [10,37,38,39,40]. However, the optimal timing and concentration of galactose supplementation for maximum impact on glycosylation remains poorly understood. In addition, it is a challenge for the biopharmaceutical industry to obtain consistent glycosylation patterns between batches of biopharmaceuticals, even when the same production process is used, as biological systems are inherently variable [12]. Furthermore, drug regulatory agencies have particular standards for glycosylation patterns, and it is crucial to guarantee adherence to regulatory obligations [34,35,41]. To address this challenge, it was hypothesized that supplementing the growth media with optimum glycosylation precursors during the exponential phase of the CHO fed-batch process would lead to a higher degree of galactosylation of TNF-α blocker-IgG1 while maintaining cell viability and product yield.
This study aims to assess the impact of time-dependent galactose supplementation on the overall terminal galactosylation of a TNF-α blocker-IgG1 monoclonal antibody and any potential batch-to-batch variation. The results of the present study are intended to serve as a preliminary guideline for creating an efficient galactose supplementation approach focused on obtaining a specific glycan profile without compromising the titer. The findings of this study could enable swift application and reduce development timelines, which may lead to decreased overall development costs.
2. Materials and Methods
2.1. Cell Line, Pre-Culture, and Reagents
A stable Chinese Hamster Ovary (CHO) cell line expressing recombinant human TNF-α blocker-IgG1 was cultured in a chemically defined growth medium supplemented with Efficient Feed™ B+. Cells were maintained in shake flasks (Corning Inc., Corning, NY, USA) as suspension cultures in a CO2 gas (5%), rotation (150 rpm), and temperature (37 °C)-controlled humidified incubator (ISF1-Z incubator shaker, Kuhner Shaker, San Carlos, CA, USA) before being used in the production. L-glutamine, galactose, and bicarbonate were purchased from Sigma. Other chemicals and reagents were purchased as described in our earlier studies [27,42].
2.2. Fed-Batch Culture Media for Expression of TNF-α Blocker-IgG1 with Higher Galactose
A CHO-derived clone for expressing TNF-α blocker-IgG1 was grown in suspension format in a chemically defined media free of animal components, enriched with 4 mM L-glutamine. The fed-batch culture was initiated with cells that were exponentially growing, inoculated at a density of 0.5 × 106 cells/mL, and kept at 37 °C. Different concentrations of Efficient Feed B plus were evaluated to improve the cell growth as well as product titer. The starting concentration of glutamine was 4.5 mM in every medium that was examined. The feed medium was prepared from powder at various concentrations (i.e.,1.5×, 2.0×, 2.5×, and 3.0× feed) and then filtered using a 0. 2 µm filter (Pall corporation, Port Washington, NY, USA). Dissolved oxygen concentrations were kept at 30% air saturation by sparging with air and pure oxygen gas via a micro-sparger. Starting from the second day, the feeding medium was added to the bioreactors at a rate of 3% of the original culture volume and continued until the fed-batch process was completed.
Furthermore, to enhance the TNF-α blocker-IgG1 protein quality in terms of galactosylation, 1.5 g/L galactose (=8.33 mM)—the key precursor of the galactosylation—was added to the fed-batch bioreactor on different days with the optimized feed (Table 1 and Table 2). Feed and galactose were dissolved in the growth medium and subjected to sterile filtration prior to being added to the shake flasks or spiked into the 5 L bioreactor.

Table 1.
Mammalian cell culture glycoengineering: feed regimes for enhanced Fc galactosylation of TNF-α blocker-IgG1.

Table 2.
CHO cell fed-batch process parameters for 5 L bioreactor.
2.3. Monitoring of Cell Growth, Osmolality, Metabolites, and Antibody Titer
Viable cell density and percentage of cell viability were assessed using the Vi -CELL™ X cell viability analyzer (Beckmann Coulter, Indianapolis, IN, USA) using the Trypan blue dye exclusion method. The osmolality of the culture samples was assessed utilizing the Osmometry kit (Osmopro, Advanced Instruments, Norwood, MA, USA), and the cell metabolites (Glucose and Lactate) were analyzed using the YSI Auto analyzer (YSI, Yellow Springs, OH, USA) every 24 h as, per the recommendations of the manufacturer. The concentration of the TNF-α blocker-IgG1 was assessed using the spectrometry-based UV absorbance technique, which gauges the optical density of the antibody at 280 nm with a quartz cuvette employing a Synergy H1 hybrid reader (BioTek, Winooski, VT, USA) [43].
2.4. Protein A Chromatography for Purification of TNF-α Blocker-IgG1
Following the fed-batch protocol for 14 days, the conditioned media was separated from cells and subjected to purification using Protein A chromatography. Briefly, conditioned media was centrifuged at 4000× g in a fixed-angle rotor maintained at 4 °C for 15 min, and the obtained supernatant was filtered using a 0.22 μm filter (Pall corporation). The filtered supernatant containing the TNF-α blocker-IgG1 was purified using affinity chromatography using MabSelectSuRe LX resin (Cat# 17-5474, Cytiva, Maharashtra, India). The column was equilibrated using a Tris/sodium chloride buffer, pH 7.4. Filtered supernatant samples were directly loaded on to the column, followed by a wash with the Tris/sodium chloride buffer. The elution was performed using a citrate buffer at pH 3.6, which was subsequently neutralized with a Tris buffer at pH 8.0.
2.5. HILIC-UPLC of AB-Labeled N-Glycans for Glycan Profile Analysis
Glycan analysis of the acquired TNF-α blocker-IgG1 was conducted to investigate the glycosylation variability, which is a typical post-translational modification in recombinant monoclonal antibodies produced in mammalian cell lines. A total of 200 µg of TNF-α blocker-IgG1 from each experimental condition was subjected to glycan profile analysis. Preparation of sample and glycan labeling was done as per the Prozyme Glyco®signal™2-AB Labelling kit (Catalog no: GKK404, Osaka, Japan) as per the manufacturer’s instructions. N-glycans were liberated from the Fc region of the cleaved antibody samples following enzymatic digestion with PNGase enzyme (QA bio, San Mateo, CA, USA). The liberated N-glycans were isolated utilizing S-cartridges (Prozyme, Hayward, Charlotte, NC, USA) and concentrated using a rotational vacuum concentrator (RVC 2-25 CD plus, CHRIST). Completely dried glycan samples were derivatized and labelled using 2-AB (2-aminobenzamide) fluorescent dye. An Acquity® H–Class Bio UPLC system (Waters, Milford, CT, USA) with a fluorescence detector (excitation, 330 nm; emission, 420 nm) was employed to separate and monitor 2-AB-labelled glycans using an Acquity UPLC® Glycan BEH glycan column (2.1 mm× 100 mm, 1.7 µm; Waters) with a run time of 55 min in gradient elution with mobile phase-A (100 mM ammonium formate buffer, pH 4.5), mobile Phase-B (100% acetonitrile), and mobile Phase-C (water for injection). Resultant data was acquired, and chromatograms were integrated and analyzed using Empower 3.0 software (Waters, Milford, MA, USA). The glycan results are presented as the mean area percentage of the replicate injection peaks for each glycan variant.
The overall galactose content was determined by adding the peak percentages of different types of galactose structures, including the G1, G2, G1FA, G1FB, G1F-GN, and G2F forms. Likewise, total mannose and total fucose were derived by summing the peak percentages of mannose structures (M5 and M6) and fucose structures (G0F, G1FA, G1FB, G2F, G0F+GN, and G1F-GN), respectively.
2.6. Statistics
Statistical analyses were executed within GraphPad Prism 8.0 for windows 64-bit. The data were tested for normality distribution using normality testing in GraphPad prism. For parametric data, an unpaired t-test was conducted between the two groups, while the Mann–Whitney test was employed for non-parametric data. The level of significance was set at p ≤ 0.05. Statistical significance is denoted by * p ≤ 0.05, ** p ≤ 0.01.
3. Results
3.1. Description of Approach
CHO cells stably expressing IgG1 monoclonal antibody against TNF-α were cultured using different concentrations of Efficient Feed B+ (Section 2.2) to find the optimum feed composition. Furthermore, the media composition was optimized to obtain the TNF-α blocker-IgG1 with increased galactosylation by introducing the galactosylation precursor (D-Galactose) on different days of the fed-batch process. Upon completion of fed-batch cultivation, TNF-α blocker-IgG1 batches were purified using Protein-A affinity chromatography, and affinity eluents were analyzed for glycan content (Figure 1).
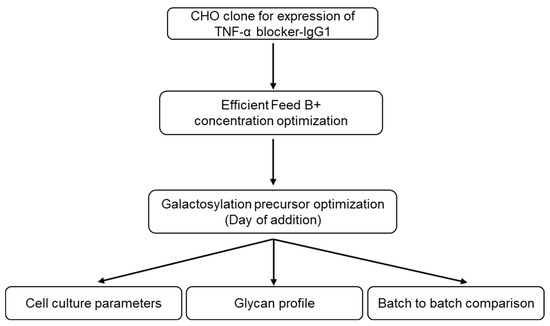
Figure 1.
Schematic presentation of the research workflow. TNF-α blocker-IgG1 clone was cultured under different feed concentrations to optimize the feed concentration. To enhance the galactosylation of TNF-α blocker-IgG1 fragment crystallizable (Fc) region, fed-batch cultures were also supplemented with a galactosylation precursor. Obtained TNF-α blocker-IgG1 batches were purified using protein A chromatography and compared for their glycan composition using HILIC-UPLC and batch-to-batch variation, if any.
3.2. Effect of Feed Concentration on Cell Culture Parameters and Titer
It was intriguing to assess the influence of the Efficient Feed B+ concentration on cell culture factors along with the titer of TNF-α blocker-IgG1. For each condition of Feed B+, the maximum viable cell count was observed on day 7, and cell viability was above 90% until the harvest except for the batch fed with 1× feed (Figure 2A,B). As expected, the residual glucose and lactate accumulation was high during the early exponential phase, but the same lactate was consumed by the rapidly growing cells (Figure 2C,D). The experimental cells were harvested on the 14th day, and the titer was measured. As the concentration of the Efficient Feed B+ increased, the titer of the TNF-α blocker-IgG1 also increased, especially for concentrations of 2× and above, yielding maximum TNF-α blocker-IgG1 protein (Table 3).
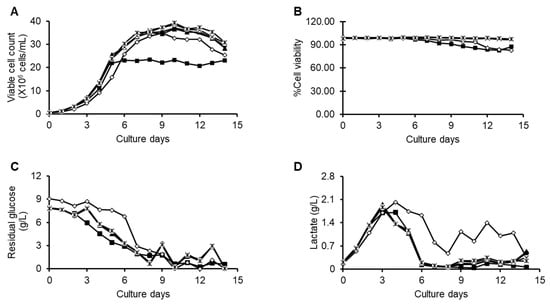
Figure 2.
Cell culture performances of the fed-batch processes that were fed with 1× feed (solid square), 1.5× (open square), 2× feed (circle), 2.5× (crossmark), and 3× feed (strikethrough multiplication sign). Time profiles of (A) viable cell count, (B) viability, (C) glucose, and (D) lactate.

Table 3.
TNF-α blocker-IgG1 titer using different concentrations of the Efficient Feed B+.
3.3. Effects of Galactose Supplementation on Bioprocess, Titer, and Glycan Profile
The cell culture media was supplemented with concentrated D-galactose (1.5 g/L) from day 4, day 6, or day 8 of the fed-batch process until the completion of the process. The effects of galactose supplementation on cell growth, percent viability, metabolism, and titer of all the batches were analyzed. The addition of the galactose slightly reduced the cell density, especially when added from day 4 of the fed-batch; however, it improved the titer quality in terms of total galactosylation (Table 4; Figure 3A,B). Similar glucose profiles were observed in all the batches (Figure 3C). Glucose was maintained at 3–4 g/L from day 6 onwards using 50% glucose solution. Peak lactate concentrations were reached during the initial 3 or 4 days of the fed-batch process, especially in the no galactose and day 4 batches, and later, it went to consumption mode (Figure 3D). Osmolality was similar across all the batches, and harvest osmolality was to be found in the range of 220–250 mOsm/kg (Figure 3E). The results demonstrate that the titer obtained across all the batches was similar (Table 5), indicating that galactose feeding had little impact on titer.

Table 4.
The proportion of TNF-α blocker-IgG1 glycan species from different culture conditions.
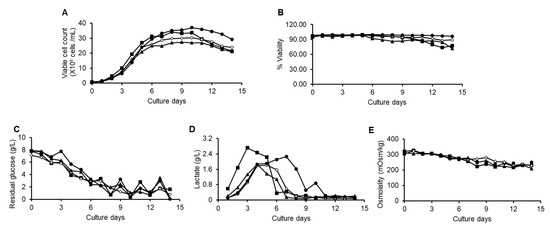
Figure 3.
Cell culture performances of the fed-batch processes that were introduced with galactose starting from 4th day (triangle), 6th day (open square), 8th day (solid circle), and no galactose (solid square). Time profiles of (A) viable cell count, (B) percent cell viability, (C) residual glucose, (D) lactate, and (E) osmolality.

Table 5.
Titer values of resultant TNF-α blocker-IgG1 upon supplementing the media with galactose on different days.
Further, to analyze the impact of various culture conditions such as galactose supplementation on antibody Fc galactosylation, the glycan content of each batch was quantified. Glycans were cleaved from TNF-α blocker-IgG1 protein backbone with PNGase, labelled and separated using HILIC-UPLC as described in Section 2.5. Table 4 summarizes the quantitative glycan distribution. Figure 4 illustrates the galactose processing pathway within the cell, leading to the production of six galactosylated variants of IgG1. It is interesting to note that TNF-α blocker-IgG1 Fc galactosylation was increased from 7.7% (no galactose batch) to 15.8% (day 4 galactose batch) without affecting the other glycan structures significantly (Figure 5).
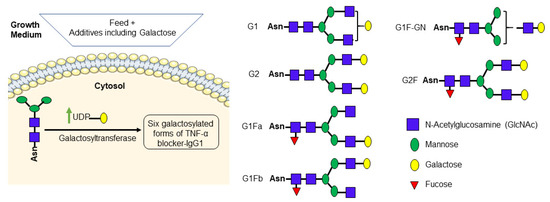
Figure 4.
Simplified galactose processing pathway. Galactose supplementation increases the UDP-galactose pool in the cell (Butler 2005). UDP-galactose was transferred to the glycan by galactosyltransferase. UDP-galactose serves as a precursor for the synthesis of galactose-containing proteins, which are essential for various biological functions, including building cellular structures and chemical signaling. This process resulted in the formation of six galactosylated forms of IgG1. The galactosylated structures represented above are drawn according to the symbol nomenclature defined in [44] 2015a.
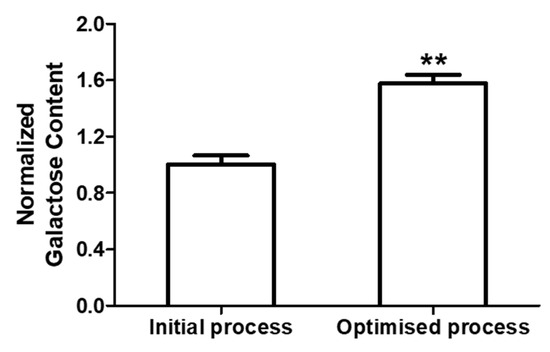
Figure 5.
Total galactose content of the initial (no galactose addition) and optimized (galactose addition) processes. The data are represented as mean ± SEM of five independent batches performed on different days. ** p < 0.01.
3.4. Comparison of Batch-to-Batch Bioprocess Parameters and Glycan Profile
In order to compare the variability in the bioprocess parameters and glycan profiles among the different batches, the experiments were set up on different days with the optimized fed-batch process parameters in 5 L bioreactors. Cell growth, percent viability, metabolite consumption, and IgG1 titer (1.5 to 1.7 g/L) were highly reproducible in our batches (Figure 6A–C). Figure 6D compares the relative glycan profiles of the five independent batches performed on different days, where the narrow error bars confirm that the fed-batch process conditions were optimal and the glycan relative quantification was highly comparable, including the galactosylation.
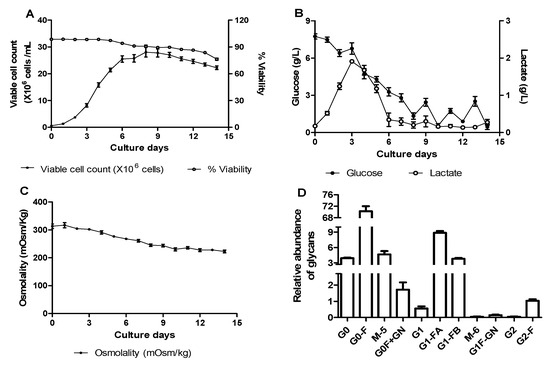
Figure 6.
Batch-to-batch comparison. Time profiles of cell culture parameters: (A) viable cell count and percent cell viability, (B) residual glucose and lactate, and (C) osmolality, and (D) relative abundance of 2-AB-labeled glycans of TNF-α blocker-IgG1 obtained from optimized (galactose addition) fed-batch process. The data are represented as mean ± SEM of five independent batches performed on different days.
4. Discussion
The global market for biosimilars is poised to grow over the next decade, with more than 30 blockbuster innovator drugs expected to lose their exclusivity by 2030 [13,14,45]. Currently, cost, safety, and speed are the primary considerations for process improvements in the manufacturing of recombinant therapeutic proteins, including the monoclonal antibodies. This can be achieved through advances in genetic engineering of cell lines, cell culture process development, development of chemically defined media, and greater focus on product characterization. The influence of various media supplements on the modulation of critical quality attributes of monoclonal antibodies, especially concerning the glycan distribution profile, has garnered significant attention in recent times [10]. The growing need for enhanced regulation of antibody glycosylation is accompanied by a lack of affordable methods, which ultimately limits the accessibility of these therapeutics to populations in need.
The present study investigated the impact of fed-batch process conditions, specifically supplementation of D-galactose, on cell culture parameters, total galactosylation, and titer of the TNF-α blocker-IgG1. Owing to the several advantages reviewed in [46], Chinese hamster ovary (CHO) cells were employed in the present study for recombinant TNF-α blocker-IgG1 monoclonal antibody production in fed-batch mode to achieve maximum product titer. These cells were cultured in suspension mode using serum-free medium where the initial growth was supported by a basal medium followed by supplementing the concentrated feed to replenish nutrients as well as to prolong the culture time [47,48]. Our results support the hypothesis that galactose supplementation during the exponential phase of the CHO fed-batch process significantly enhances the total galactosylation of the TNF-α blocker-IgG1 antibody. A key advantage of our approach is that it did not negatively affect cell culture parameters, such as viability, growth rate, and titer (Figure 3 and Table 5). These results are consistent with other studies that have shown minimal negative impact on cell metabolism when sugars like galactose are supplemented during the fed-batch culture [33,39]. By optimizing the timing and quantity of galactose supplementation, enhancement of galactosylation without sacrificing productivity, a critical consideration for industrial-scale manufacturing, was achieved. The increase in total galactosylation from 7.7% to 15.8% (Table 4) of the total antibody pool is a notable improvement and suggests that in-cultivation process modifications can be used to optimize glycosylation profiles in industrial cell culture systems. Batch-to batch comparison data (Figure 5 and Figure 6) suggests that galactose supplementation is a viable strategy to enhance the galactosylation of TNF-α blocker-IgG1 antibody reproducibly.
The presence of intracellular nucleotide sugars plays a crucial role in regulating N-glycosylation [39,49]. It was anticipated that the feed additives or specific glycosylation precursors would enhance the intracellular pool of nucleotide sugars by promoting their biosynthetic pathways [50,51]. Galactose is a key sugar in the terminal glycosylation of antibodies, and its supplementation has been reported to enhance the expression levels of transcripts for the UDP-galactose transporter and several galactosyltransferases, which play a crucial role in the glycosylation of monoclonal antibodies, particularly the incorporation of galactose into the Fc region [33,52,53]. Based on the results, it could be speculated that the biosynthesis of UDP-Gal and the activity of galactosylation processing enzymes may serve as critical limiting factors for terminal galactosylation of the IgG1 in our cell line, as shown in Figure 4. Therefore, the introduction of galactose into the culture medium could have elevated the intracellular UDP-Gal and promoted the expression of galactosylation processing enzymes, thereby potentially increasing the abundance of terminal galactose residues in the TNF-α blocker IgG1 glycan (Table 4). Our findings align with previous studies that have explored the impact of carbohydrate supplementation on protein glycosylation profiles [33,37,51,52]. While a variety of glycoengineering strategies have been developed to improve antibody galactosylation [54,55,56], the approach described in this study offers a simpler, more cost-effective alternative for enhancing galactosylation of CHO-derived monoclonal antibodies.
Previous studies have demonstrated that uridine or arginine, ammonia, glucosamine, Mn2+, or galactose supplementation to the culture media or the synergistic effects of these components enhances galactosylation of monoclonal antibodies [38,49,54,57,58,59,60,61]. Except for galactose (below 20 mM), other components previously used for galactosylation significantly affected either cell viability or titer quality and quantity [39,49,58]. Therefore, galactose supplementation as the primary component of the test was chosen in the present study. Galactose supplementation in the exponential phase of fed-batch culture has been previously shown to be key for glycosylation modulation, likely due to its interaction with the cell’s metabolic state [39]. In mammalian cell cultures, the enhanced availability of galactose during the exponential phase may promote its incorporation into the glycans of recombinant antibodies, thus leading to higher galactosylation [50]. Our study further corroborates this by demonstrating that the most significant effect on IgG1 galactosylation occurs when D-galactose is added during the early culture days, especially in the exponential phase. We have challenged the present method with another recombinant human protein—anti-CD20-IgG1. Supplementing D-galactose to the bioreactor at regular intervals starting from the exponential phase consistently enhanced galactosylation of anti-CD20-IgG1 protein without affecting cell culture performances, titer, or other glycans (Figures S1 and S2). Further experiments and adjustments will still be necessary to customize strategies for individual cell lines and products.
It has been reported that Fc galactosylation leads to conformational changes in the CH2 domain and enhances the hexamerization potential of IgG1 and therefore C1q binding, subsequently leading to increased CDC activity [62,63]. To assess the effects of TNF-α blocker-IgG1 galactosylation, the CDC activity of various batches with differing levels of galactosylation was analyzed. No alterations were observed in the CDC activities for the 11% and 21% galactosylated TNF-α blocker-IgG1. Nevertheless, it was reported that the relative CDC activities for the de-galactosylated and the TNF-α blocker-IgG1 with 16% galactose content were 26.2% and 108.0% respectively [27]. Similar galactose effects can be attributed to another antibody, anti-CD-20-IgG1, which exhibited a low CDC activity in de-galactosylated form. A comparable pattern in C1q binding was observed for both the antibodies, highlighting the vital role of galactosylation for IgG1 antibodies [27]. Based on the evidence so far, we believe that a minimum difference of 15.0% in total galactose content between batches is necessary to observe the effect of IgG1 galactosylation on CDC activity.
Overall, our study also highlights the practical advantages of in-culture glycosylation engineering over more complex, costly in vitro glycoengineering techniques. While the results from this study are promising, several aspects of the time-dependent galactose supplementation strategy warrant further investigation depending on the cell line and product.
5. Conclusions
The results of this study envisage that a relatively simple modification to fed-batch processes—specifically the supplementation of galactose during the exponential phase—can significantly enhance the galactosylation of TNF-α blocker-IgG1 without compromising bioprocess performance. This approach offers a cost-effective alternative to glycoengineering methods and holds potential for tailoring the glycosylation profile of therapeutic antibodies in industrial production settings. Future research should investigate the applicability of this technique for heterologously expressed biopharmaceuticals with different combinations of glycosylation precursors in a statistical design of experiment approach to gain a comprehensive understanding of the specific enzymes involved in galactosylation pathways and interactions between additives, if any.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biologics5020016/s1, Figure S1: Viable cell count and % Viability of the (A) initial (No galactose addition) and (B) optimized (Galactose addition) fed-batch processes for an anti-CD20-IgG1. The data are represented as mean ± SEM of three to four independent batches performed on different days.; Figure S2. Relative abundance of the (A) Galactose, (B) Mannose and (C) Fucose obtained from the initial (No galactose addition) and optimized (Galactose addition) fed-batch processes for an anti-CD20-IgG1. The data are represented as mean ± SEM of three to four independent batches performed on different days. * p < 0.01; ns: non-significant.
Author Contributions
Conceptualization, M.P. and P.K.I.; data curation, M.P., P.K.I., R.K.P. and N.P.; formal analysis, M.P., P.K.I., R.K.P. and N.P.; investigation, M.P., R.P. and S.R.C.; methodology, M.P. and P.K.I.; project administration, S.R.C.; resources, R.P. and S.R.C.; supervision, R.P. and S.R.C.; validation, P.K.I. and R.K.P.; visualization, N.P.; writing—original draft, N.P.; writing—review and editing, M.P., P.K.I., R.K.P., N.P., R.P. and S.R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Hetero Biopharma Limited.
Data Availability Statement
Data supporting the reported results of this article will be made available by the authors upon reasonable request.
Acknowledgments
The authors wish to express their gratitude to Hetero Biopharma Limited for the administrative, financial and instrumental assistance provided to them. They also extend their gratitude to KL University for the essential support received throughout the duration of this study.
Conflicts of Interest
All authors except R.P. are Hetero Biopharma Employees. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Altamirano, C.; Illanes, A.; Becerra, S.; Cairó, J.J.; Gòdia, F. Considerations on the lactate consumption by CHO cells in the presence of galactose. J. Biotechnol. 2006, 125, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, C.; Paredes, C.; Cairó, J.J.; Gòdia, F. Improvement of CHO cell culture medium formulation: Simultaneous substitution of glucose and glutamine. Biotechnol. Prog. 2000, 16, 69–75. [Google Scholar] [CrossRef]
- Anderson, R.; Hock, L.; Yao, R.; Ozturk, S. Enhanced Galactosylation of Monoclonal Antibodies: Using Medium Supplements and Precursors of UDP-Galactose, Part 1. 2018. Available online: https://www.bioprocessintl.com/cell-culture-media/enhanced-galactosylation-of-monoclonal-antibodies-using-medium-supplements-and-precursors-of-udp-galactose-part-1 (accessed on 27 December 2024).
- Babul, J.; Stellwagen, E. Measurement of protein concentration with interferences optics. Anal. Biochem. 1969, 28, 216–221. [Google Scholar] [CrossRef]
- Bheemareddy, B.R.; Pulipeta, M.; Iyer, P.; Dirisala, V.R. Effect of the total galactose content on complement-dependent cytotoxicity of the therapeutic anti-CD20 IgG1 antibodies under temperature stress conditions. J. Carbohydr. Chem. 2019, 38, 1–19. [Google Scholar] [CrossRef]
- Bheemareddy, B.R.; Reddy, P.N.; Vemparala, K.; Dirisala, V.R. Enhancement of effector functions of anti-CD20 monoclonal antibody by increased afucosylation in CHO cell line through cell culture medium optimization. J. Genet. Eng. Biotechnol. 2022, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Boune, S.; Hu, P.; Epstein, A.L.; Khawli, L.A. Principles of N-Linked Glycosylation Variations of IgG-Based Therapeutics: Pharmacokinetic and Functional Considerations. Antibodies 2020, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Butler, M. Optimisation of the cellular metabolism of glycosylation for recombinant proteins produced by Mammalian cell systems. Cytotechnology 2006, 50, 57–76. [Google Scholar] [CrossRef]
- Conte, F.; van Buuringen, N.; Voermans, N.C.; Lefeber, D.J. Galactose in human metabolism, glycosylation and congenital metabolic diseases: Time for a closer look. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129898. [Google Scholar] [CrossRef]
- Crowell, C.K.; Grampp, G.E.; Rogers, G.N.; Miller, J.; Scheinman, R.I. Amino acid and manganese supplementation modulates the glycosylation state of erythropoietin in a CHO culture system. Biotechnol. Bioeng. 2007, 96, 538–549. [Google Scholar] [CrossRef]
- Datta-Mannan, A. Mechanisms Influencing the Pharmacokinetics and Disposition of Monoclonal Antibodies and Peptides. Drug Metab. Dispos. 2019, 47, 1100–1110. [Google Scholar] [CrossRef]
- Derbyshire, M.; Shina, S. Patent Expiry Dates for Biologicals: 2018 Update. GaBI J. 2019, 8, 24–31. Available online: https://gabi-journal.net/patent-expiry-dates-for-biologicals-2018-update.html (accessed on 27 December 2024).
- EMA. Reflection Paper on a Tailored Clinical Approach in Biosimilar Development; European Medicines Agency: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Fan, Y.; Del Val, I.J.; Müller, C.; Sen, J.W.; Rasmussen, S.K.; Kontoravdi, C.; Weilguny, D.; Andersen, M.R. Amino acid and glucose metabolism in fed-batch CHO cell culture affects antibody production and glycosylation. Biotechnol. Bioeng. 2015, 112, 521–535. [Google Scholar] [CrossRef] [PubMed]
- GaBI. Comparison of the Cost of Development of Biologicals and Biosimilars. 2022. Available online: https://www.gabionline.net/reports/comparison-of-the-cost-of-development-of-biologicals-and-biosimilars (accessed on 27 December 2024).
- GaBI Online. Role of Biologicals and Biosimilars in Cancer Treatment Amidst Rising Cases. In Generics and Biosimilars Initiative. 2024. Available online: https://gabionline.net/reports/role-of-biologicals-and-biosimilars-in-cancer-treatment-amidst-rising-cases (accessed on 27 December 2024).
- Gandhi, S.; Kashiramka, S.; Rathore, A.S. Emerging themes and factors influencing the prices of biotherapeutics. World Med. Health Policy 2023, 15, 613–637. [Google Scholar] [CrossRef]
- Gandhi, S.; Patankar, D.; Kashiramka, S.; Rathore, A.S. The economics of translating a biosimilar from lab to market in India. Ann. N. Y. Acad. Sci. 2024, 1541, 219–229. [Google Scholar] [CrossRef]
- Gessner, J.E.; Heiken, H.; Tamm, A.; Schmidt, R.E. The IgG Fc receptor family. Ann. Hematol. 1998, 76, 231–248. [Google Scholar] [CrossRef]
- Grainger, R.K.; James, D.C. CHO cell line specific prediction and control of recombinant monoclonal antibody N-glycosylation. Biotechnol. Bioeng. 2013, 110, 2970–2983. [Google Scholar] [CrossRef]
- Gramer, M.J.; Eckblad, J.J.; Donahue, R.; Brown, J.; Shultz, C.; Vickerman, K.; Priem, P.; Bremer, E.T.v.D.; Gerritsen, J.; van Berkel, P.H. Modulation of antibody galactosylation through feeding of uridine, manganese chloride, and galactose. Biotechnol. Bioeng. 2011, 108, 1591–1602. [Google Scholar] [CrossRef]
- Gupta, K.; Modi, D.; Jain, R.; Dandekar, P. A Stable CHO K1 Cell Line for Producing Recombinant Monoclonal Antibody Against TNF-α. Mol. Biotechnol. 2021, 63, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Chaudari, P.S.; Nath, R. Opportunities and Challenges in Biosimilar Development. 2017. Available online: https://www.bioprocessintl.com/biosimilars/opportunities-and-challenges-in-biosimilar-development (accessed on 27 December 2024).
- Hills, A.E.; Patel, A.; Boyd, P.; James, D.C. Metabolic control of recombinant monoclonal antibody N-glycosylation in GS-NS0 cells. Biotechnol. Bioeng. 2001, 75, 239–251. [Google Scholar] [CrossRef]
- Houde, D.; Peng, Y.; Berkowitz, S.A.; Engen, J.R. Post-translational Modifications Differentially Affect IgG1 Conformation and Receptor Binding. Mol. Cell Proteom. 2010, 9, 1716–1728. [Google Scholar] [CrossRef]
- ICH Q6B Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products; European Medicines Agency: Amsterdam, The Netherlands, 1999.
- Jefferis, R. Glycosylation of recombinant antibody therapeutics. Biotechnol. Prog. 2005, 21, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, R. Recombinant antibody therapeutics: The impact of glycosylation on mechanisms of action. Trends Pharmacol. Sci. 2009, 30, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Jennewein, M.F.; Alter, G. The Immunoregulatory Roles of Antibody Glycosylation. Trends Immunol. 2017, 38, 358–372. [Google Scholar] [CrossRef]
- Kamiya, Y.; Satoh, T.; Kato, K. Recent advances in glycoprotein production for structural biology: Toward tailored design of glycoforms. Curr. Opin. Struct. Biol. 2014, 26, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Kildegaard, H.F.; Fan, Y.; Sen, J.W.; Larsen, B.; Andersen, M.R. Glycoprofiling effects of media additives on IgG produced by CHO cells in fed-batch bioreactors. Biotechnol. Bioeng. 2016, 113, 359–366. [Google Scholar] [CrossRef]
- Kirchhoff, C.F.; Wang, X.M.; Conlon, H.D.; Anderson, S.; Ryan, A.M.; Bose, A. Biosimilars: Key regulatory considerations and similarity assessment tools. Biotechnol. Bioeng. 2017, 114, 2696–2705. [Google Scholar] [CrossRef]
- Liu, L. Antibody Glycosylation and Its Impact on the Pharmacokinetics and Pharmacodynamics of Monoclonal Antibodies and Fc-Fusion Proteins. J. Pharm. Sci. 2015, 104, 1866–1884. [Google Scholar] [CrossRef]
- Maverakis, E.; Kim, K.; Shimoda, M.; Gershwin, M.E.; Patel, F.; Wilken, R.; Raychaudhuri, S.; Ruhaak, L.R.; Lebrilla, C.B. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: A critical review. J. Autoimmun. 2015, 57, 1–13. [Google Scholar] [CrossRef]
- McCracken, N.A.; Kowle, R.; Ouyang, A. Control of galactosylated glycoforms distribution in cell culture system. Biotechnol. Prog. 2014, 30, 547–553. [Google Scholar] [CrossRef]
- Fontanillo, M.; Körs, B.; Monnard, A. Three Imperatives for R&D in Biosimilars|McKinsey. 2022. Available online: https://www.mckinsey.com/industries/life-sciences/our-insights/three-imperatives-for-r-and-d-in-biosimilars (accessed on 27 December 2024).
- O’flaherty, R.; Bergin, A.; Flampouri, E.; Mota, L.M.; Obaidi, I.; Quigley, A.; Xie, Y.; Butler, M. Mammalian cell culture for production of recombinant proteins: A review of the critical steps in their biomanufacturing. Biotechnol. Adv. 2020, 43, 107552. [Google Scholar] [CrossRef]
- Pfizer. What are Biosimilars and Biologics?|Pfizer Biosimilars. In Biosimilars and Biologics|Pfizer Biosimilars. 2024. Available online: https://www.pfizerbiosimilars.com/characteristics-of-biosimilars/ (accessed on 27 December 2024).
- Raju, T.S. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr. Opin. Immunol. 2008, 20, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.M. Global antibody development trends. MAbs 2009, 1, 86–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reichert, J.M. Metrics for antibody therapeutics development. MAbs 2010, 2, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.M.; Rosensweig, C.J.; Faden, L.B.; Dewitz, M.C. Monoclonal antibody successes in the clinic. Nat. Biotechnol. 2005, 23, 1073–1078. [Google Scholar] [CrossRef]
- Ritacco, F.V.; Wu, Y.; Khetan, A. Cell culture media for recombinant protein expression in Chinese hamster ovary (CHO) cells: History, key components, and optimization strategies. Biotechnol. Prog. 2018, 34, 1407–1426. [Google Scholar] [CrossRef]
- Spearman, M.; Butler, M. Glycosylation in Cell Culture. In Animal Cell Culture; Al-Rubeai, M., Ed.; Springer International Publishing: Cham, Swizerland, 2015; pp. 237–258. [Google Scholar]
- St Amand, M.M.; Radhakrishnan, D.; Robinson, A.S.; Ogunnaike, B.A. Identification of manipulated variables for a glycosylation control strategy. Biotechnol. Bioeng. 2014, 111, 1957–1970. [Google Scholar] [CrossRef]
- Thomann, M.; Reckermann, K.; Reusch, D.; Prasser, J.; Tejada, M.L. Fc-galactosylation modulates antibody-dependent cellular cytotoxicity of therapeutic antibodies. Mol. Immunol. 2016, 73, 69–75. [Google Scholar] [CrossRef]
- Thomann, M.; Schlothauer, T.; Dashivets, T.; Malik, S.; Avenal, C.; Bulau, P.; Rüger, P.; Reusch, D. In vitro glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity. PLoS ONE 2015, 10, e0134949. [Google Scholar] [CrossRef]
- Tsuruta, L.R.; Lopes dos Santos, M.; Moro, A.M. Biosimilars advancements: Moving on to the future. Biotechnol. Prog. 2015, 31, 1139–1149. [Google Scholar] [CrossRef]
- US FDA. Guidance for Industry: Quality Considerations in Demonstrating Biosimilarity of a Therapeutic Protein Product to a Reference Protein Product; U.S. Food and Drug Administration: Washington, DC, USA, 2015. [Google Scholar]
- van Osch, T.L.J.; Nouta, J.; Derksen, N.I.L.; van Mierlo, G.; van der Schoot, C.E.; Wuhrer, M.; Rispens, T.; Vidarsson, G. Fc Galactosylation Promotes Hexamerization of Human IgG1, Leading to Enhanced Classical Complement Activation. J. Immunol. 2021, 207, 1545–1554. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. (Eds.) Essentials of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2015. [Google Scholar]
- Voruganti, S.; Xu, J.; Li, X.; Balakrishnan, G.; Singh, S.M.; Kar, S.R.; Das, T.K. A Detailed Protocol for Generation of Therapeutic Antibodies with Galactosylated Glycovariants at Laboratory Scale Using In-Vitro Glycoengineering Technology. J. Pharm. Sci. 2021, 110, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Wada, R.; Matsui, M.; Kawasaki, N. Influence of N-glycosylation on effector functions and thermal stability of glycoengineered IgG1 monoclonal antibody with homogeneous glycoforms. mAbs 2019, 11, 350–372. [Google Scholar] [CrossRef]
- Walsh, G.; Jefferis, R. Post-translational modifications in the context of therapeutic proteins. Nat. Biotechnol. 2006, 24, 1241–1252. [Google Scholar] [CrossRef]
- Wei, B.; Gao, X.; Cadang, L.; Izadi, S.; Liu, P.; Zhang, H.-M.; Hecht, E.; Shim, J.; Magill, G.; Pabon, J.R.; et al. Fc galactosylation follows consecutive reaction kinetics and enhances immunoglobulin G hexamerization for complement activation. mAbs 2021, 13, 1893427. [Google Scholar] [CrossRef]
- Wolf, B.; Piksa, M.; Beley, I.; Patoux, A.; Besson, T.; Cordier, V.; Voedisch, B.; Schindler, P.; Stöllner, D.; Perrot, L.; et al. Therapeutic antibody glycosylation impacts antigen recognition and immunogenicity. Immunology 2022, 166, 380–407. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-H. Protein Glycosylation: New Challenges and Opportunities. J. Org. Chem. 2005, 70, 4219–4225. [Google Scholar] [CrossRef]
- Wong, N.S.; Wati, L.; Nissom, P.M.; Feng, H.; Lee, M.; Yap, M.G. An investigation of intracellular glycosylation activities in CHO cells: Effects of nucleotide sugar precursor feeding. Biotechnol. Bioeng. 2010, 107, 321–336. [Google Scholar] [CrossRef]
- Wright, A.; Sato, Y.; Okada, T.; Chang, K.H.; Endo, T.; Morrison, S.L. In vivo trafficking and catabolism of IgG1 antibodies with Fc associated carbohydrates of differing structure. Glycobiology 2000, 10, 1347–1355. [Google Scholar] [CrossRef]
- Xu, W.-J.; Lin, Y.; Mi, C.-L.; Pang, J.-Y.; Wang, T.-Y. Progress in fed-batch culture for recombinant protein production in CHO cells. Appl. Microbiol. Biotechnol. 2023, 107, 1063–1075. [Google Scholar] [CrossRef]
- Chen, Y.; Monnard, A.; da Silva, J.S. An Inflection Point for Biosimilars|McKinsey. 2021. Available online: https://www.mckinsey.com/industries/life-sciences/our-insights/an-inflection-point-for-biosimilars (accessed on 27 December 2024).
- Zhang, H.; Shi, N.; Diao, Z.; Chen, Y.; Zhang, Y. Therapeutic potential of TNFα inhibitors in chronic inflammatory disorders: Past and future. Genes. Dis. 2021, 8, 38–47. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).