Abstract
Background: Dissolvable Microneedle Patches (DMP) have emerged as a promising approach for improved topical delivery of skincare agents with dermatological values (dermo-cosmetics), effectively addressing the various skin concerns. These patches enable minimally invasive penetration of the skin’s outer layer, facilitating efficient transdermal delivery of actives by overcoming skin barrier for successful outcomes. Objectives: The aim of this work was to assess the efficacy and safety of hyaluronic acid-based microneedle patches (HA-MNP) with agents for the managements of an inflammatory disorder of acne. A particular focus was on helping individuals with moderate inflammatory acne. Methods: A single-center clinical study was conducted over a period of four weeks on acne patients. Measurable skin properties, including sebum content, redness, and severity of inflammation, were evaluated to gauge the overall usefulness of the MN patches. Results: The application of the patches resulted in a significant decrease in sebum content, with reductions of −4.9% and −36.8% observed after two and four weeks of use, respectively. The redness of localized acne lesions also showed a marked decline, with reductions of −47.2% and −65.5% observed after two and four weeks of use, respectively. Additionally, the severity of inflammatory signs in acne lesions showed significant improvements, with reductions of −68.8% and −83.3% observed for the application periods. The patches utilized in this investigation exhibited highly encouraging results, displaying a notable synergistic effect in the context of combating acne without adverse effects. Conclusions: The patches have the potential to be broadly applied as a modular and adaptable approach for therapeutic delivery of actives for various skin diseases and concerns.
1. Introduction
The disorder of acne vulgaris is probably the most common dermatological condition that affects over 80% of adolescents during the teenage years [1]. The prevalence of acne vulgaris among adolescents and young adults has been rising globally since the 1990s [2]. It has a substantial burden of disease and influence on quality of life and mental wellness including self-esteem and social stigmatization, that do not directly correlate with the severity of disease. The disorder is a persistent, relapsing inflammatory disease developed in the pilosebaceous component, a distinctive skin unit that consists of the sebaceous gland and the erector pili muscle along with the hair follicle and shaft (Figure 1A) [3]. The disorder is initiated once hair follicles are blocked with oil and lifeless skin cells and elevated when interaction between resident bacteria and the host cells occur (Figure 1B). The pathogenesis of the condition is multifactorial, involving genetic tendency, hormone levels, metabolism, immunity, environment, and diet. Currently, there are four main factors that are commonly accepted to contribute to acne formation, namely elevated sebum production, follicular hyperkeratinization, proliferation of skin bacteria, and/or disruption to the balance of C. acnes phylotypes (also known as Propionibacterium acnes) in the skin microbiota [4]. Overall, the imbalances of skin microbial variety together with initiation of nonspecific (innate) immunity are supposed to be crucial factors stimulating the prolonged inflammatory conditions [5].
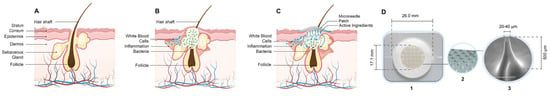
Figure 1.
Diagrammatic sketch of the acne lesion model and MNs patch application (A) Normal skin, (B) Skin with acne lesion, (C) HA-MNs application on the acne lesion and (D) HA-MNs patches used by the subjects: (1) the manufactured HA-MNs patches with its dimensions, (2) a graphical presentation of the patch surface, and (3) a scanning electron microscopic image of an individual microneedle with its dimensions.
Although treatments are available, clinicians and patients alike are continuously searching for ways to expand acne management and prevent relapse. Therefore, a comprehensive management that targets different aspects of acne pathogenesis is of paramount importance. Its lengthy course and recurrence pattern require prolonged and uninterrupted treatment [3], highlighting the need for more targeted, efficacious, and easy to use interventions to mitigate its impact. A comprehensive therapeutic approach, targeting multiple facets of acne pathogenesis requires close and urgent attention. The therapeutic interventions should possess characteristics that facilitate convenient utilization, manipulation, and administration, while concurrently being well received and tolerated by patients. Since the acne pathogenesis has several characteristics, the treatments should also be comprehensive to target all the factors. The multidimensionality of the acne process commands that any anti-acne product must be devised wisely based on targeting the underlying factors associated with acne. It is generally accepted that for any substance to function as a management preference for acne, it must counteract the factors of acne formation. Currently, a few drugs are recognized to target multiple pathological processes [6]. Isotretinoin is one of the universally accepted and prescribed drugs in acne treatment despite its teratogenicity, liver enzyme abnormalities and dyslipidemia [7]. There are also topical other retinoids such as tretinoin, adapalene, and tazarotene and antibiotics including clindamycin and erythromycin used currently for acne treatment, even though their known side effects including burning and irritation for the formers, and more importantly bacterial resistance [8]. On the other hand, selected cosmetic ingredients, known as dermo-cosmetics and being described as skincare agents with dermatologically active ingredients that directly support or care for the symptoms of various skin conditions, are gradually being recognized in recent years as an integral part of the acne management [5,9]. For optimal management of acne, products with active ingredients can have positive effects both with and without prescription products. An international survey has revealed a relation between enhanced adherence to acne therapy and recommendation of dermo-cosmetics [10]. They could provide not only synergistic effects to improve the efficacy of other treatments but also provide good patient adherence–maintenance phase of management and a high level of patient satisfaction along with reducing the demands on health services.
What is more, the integration of anti-acne actives into an advanced delivery system could further enhance the efficacy and precision of treatment by ensuring targeted delivery to the affected areas, improving bioavailability, and minimizing potential side effects. In recent decades, microneedle patch technology has emerged as a promising platform for the transdermal delivery of therapeutics and cosmeceuticals. This innovative approach has garnered significant attention for its potential in a wide range of applications, including the administration of vaccines, pharmaceutical agents, and bioactive molecules targeting dermatological conditions [11]. Microneedle patches are composed of arrays of microscale needles, typically measuring between 50 and 900 μm in length, designed to penetrate the stratum corneum—the outermost barrier of the skin—without reaching the underlying nerve-rich or vascularized layers. This minimally invasive penetration facilitates the enhanced transdermal transport of active compounds, overcoming the inherent limitations posed by the skin’s barrier function. Among the various types of microneedle systems, dissolvable microneedle patches (fabricated from water-soluble and biodegradable polymers) have gained particular interest due to their ability to deliver encapsulated agents directly into the skin in a controlled and biocompatible manner [11,12]. Thus, dissolvable microneedle patches (DMP) with dermo-cosmetics offers targeted and efficient managements of acne, addressing acne pathogenesis while enhancing patient convenience and compliance. Such integration may optimize the therapeutic outcomes by increasing the stability and sustained release of active ingredients, thereby providing more consistent and long-term benefits in acne management. DMP are submillimeter-sized polymeric needles (50–900 μm long) arrayed on a patch, which bypasses the stratum corneum (SC), and enables the delivery of dermo-cosmetics including peptides and proteins into the epidermis in a minimally invasive manner [11] (Figure 1C). Hyaluronic acid-based dissolving microneedles (HA-DMP) has attracted substantial attention in the last decade not only for drugs delivery means but also for cosmetic applications including anti-wrinkle and anti-hyperpigmentation applications. Microneedles have been described as an appealing and promising transdermal drug delivery way due to the assets of safety, painlessness, minimally invasive and high delivery efficiency [13,14]. Furthermore, excellent biocompatibility with all types of skins and its viscoelastic properties for mass manufacturing have presented great opportunities. Due to the complexity of the acne vulgaris, the importance of patient adherence to the treatment regimen and required patients’ satisfaction, DMP with anti-acne dermo-cosmetics could present an attractive option for acne management. In this context, we have evaluated derma-cosmetics with DMP delivery system for evaluation of acne management. In this study, a mixture of ingredients including peptides, antioxidants, and extracts with known anti-acne properties are applied to subjects with common acne using HA-MNP. The assessment of the potentially synergistic mixture for its success on the acne-prone skin including sebum production, redness, and inflammatory signs (response) was studied to gather new data using human subjects over a course of four weeks by a monocentric clinical study. This study aimed to explore the evidence supporting the use of microneedle patches (MNP) in combination with selected dermo-cosmetic formulations for acne management, with a particular focus on their effects on sebum production, redness, and inflammatory signs.
2. Materials and Methods
2.1. Study Design and Participants
The study was designed as a monocentric clinical study over a period of four weeks with interim examination after two weeks and conducted between April 2021 and October 2021. The skin tolerability and efficacy of the test product were investigated precisely according to clinical-dermatological test criteria. Primary outcomes of the application study were the assessment of skin tolerability and possibly sensitization potential. The secondary outcomes were the assessment of efficacy of the test product, which includes skin sebum content (Sebumetry), digital recording and analysis with the VISIA® system (Canfield Scientific, Inc., Parsippany, NJ, USA) digital photographs, and a query of the subjective impression by questionnaire and dermatological assessment.
In the first phase of application, the patches were applied daily to the concerned area for two weeks and twice a week application for another two weeks. Twenty-one male and female subjects participated in the study with a circumscribed severe acne inflammation, severe redness, or multiple confluent pimples. They were 10 adolescents and 11 adults, aged between 13 and 31 years old and having any skin type. Participation in the study was voluntary and signed consent statements of their free will and accord were obtained. The consents and agreements for adolescents were acquired from their legal guardians.
The attending dermatologist or the attending study nurse went through the terms of the study with each participant, as well as their obligations over the course of the investigation. Only individuals who did not have any pathological skin changes in the application region signed the permission form of their own free choice or with the approval of their legal guardians, and they met all other inclusion and exclusion requirements that were included in the research. In the event of any objective or subjective skin changes throughout the study, all individuals were free to visit the dermatologist or the study nurse on duty. All the dermatological examinations were completed on time. If any of the following circumstances took place, the researcher could decide to exclude a subject from the clinical-dermatological application study: application of products with active substance and care products 7–10 days prior to the start of the test, serious allergies, any serious side effects of cosmetic preparations that have ever occurred, sunbathing or solarium visits during the study, as well as the use of medications that may impede with skin reactions (antiallergics, topical immunomodulators, glucocorticoids, etc.), recognized neoplastic illness, being pregnant or nursing, worsening acne symptoms, and the need for urgent medical care. The International Medical and Dental Ethics Commission GmbH (Freiburg, Germany; IRB Ref. No.: 2021/105) approved the study. The investigator obtained written informed consents before admission of the participants. The study was carried out in compliance with the Declaration of Helsinki, and other national and international regulations and guidelines (Regulation (EU) No: 1223/2009 on cosmetic products).
2.2. Design, Formulation and Manufacturing of the Test Product
The product was designed in a circular shape with a diameter of 17.1 mm (Figure 1D) (Supplied by IB-Labs, London, UK). Each patch contains 76 dissolving microneedles 500 μm in length and arrayed on a patch with an area of 2.3 cm2. For optimal application, the patch comprises a hydrocolloid base and a water-resistant backing that adheres securely to the target area. It features self-dissolving micro-cones, which are dissolvable microstructures embedded with active ingredients. The droplet-born air blowing (DAP) technique was used for the manufacturing of the patches at industrial scale [15]. The microneedle patches were manufactured commercially (Raphas Co. Ltd., Seoul, Republic of Korea). The quality control measures were satisfactorily achieved as standards such as the uniformity, peel test (≥150 g/12 mm), mechanical strength (≥0.058 N), stability (stable over 2 years), and dissolving properties (95% dissolution is achieved within 2 h). The ingredients of the test product included sodium hyaluronate (mixture of MMW and HMW with molecular weights of 1.8–2.5 MDa and 1.3–1.8 MDa, respectively), ascorbic acid 2-glucoside (AA2G), oligopeptide-76, oligopeptide-10, vitamin A palmitate (retinyl palmitate, helianthus annuus seed oil and DL-alpha-tocopherol), bakutrol (bakuchiol), lupeol (crataeva nurvula extract), and acetyl dipeptide-3 aminohexanoate. Acne pathogenesis involves four primary processes: excessive sebum production, abnormal keratinization (leading to pore clogging), Cutibacterium acnes colonization, and inflammation. The test product was specifically formulated to target these key pathogenic factors, with each active ingredient addressing at least one aspect of acne development. The roles of the active ingredients are as follows: sodium hyaluronate (enhancing hydration, supporting skin barrier function, and forming the microneedle core structure); ascorbic acid 2-glucoside (AA2G) (providing antioxidant activity, anti-inflammatory effects, and improvement of post-acne hyperpigmentation); oligopeptide-76 and oligopeptide-10 (both exhibiting antimicrobial properties); vitamin A palmitate complex (comprising retinyl palmitate, Helianthus annuus seed oil, and DL-α-tocopherol) (regulating keratinization, reducing comedone formation, and normalizing epidermal turnover); bakutrol (bakuchiol) (exerting anti-inflammatory effects); lupeol (derived from Crataeva nurvula extract) (exhibiting anti-inflammatory activity and regulating sebum production); and acetyl dipeptide-3 aminohexanoate (possessing anti-inflammatory properties). Further details regarding the specific mechanisms of action and targeted pathways for each active ingredient are provided in the Section 3.
2.3. Measurement of Skin Sebum Level (Sebumetry)
Skin sebum content was quantified using the Sebumeter® SM 815 (Courage + Khazaka electronic GmbH, Köln, Germany), which employs grease spot photometry [16]. A specialized mat tape was applied to the skin surface, and sebum absorption causes the tape to become transparent. The degree of transparency, proportional to sebum content, was measured via a photodiode. The device was specific to sebum and does not respond to skin moisture. Measurements were expressed in relative arbitrary units (AU), ranging from 0 to 350, with an accuracy of ±5%. For each time point, a minimum of three measurements were taken at distinct locations within the test area to ensure reproducibility and reliability. To minimize fluctuations caused by external influences such as room temperature and relative humidity, all measurements were always carried out at the same physical ambient conditions in rested status (~20 °C, humidity 40–60%).
2.4. Dermatological Assessment of Skin Redness and Inflamattory Signs
In vivo dermatological assessment of skin redness and inflammatory signs were conducted through both visual inspection and tactile evaluation. A trained assessor performed the examination using a numerical rating scale to quantify the severity of redness. The scale ranged from “no intensity/excellent condition” (0) to “maximum intensity/poor condition” (10). Intensity levels were recorded using an analogue scale. The evaluation focused on an inflammatory acne lesion, specifically assessing the redness parameter. A negative value in the post-application assessment indicated a reduction in redness intensity compared to baseline, signifying an improvement in the skin condition. Each parameter was assessed independently according to the defined scale [17].
2.5. Digital Imaging with the VISIA® System
High-resolution digital facial imaging was performed using the VISIA® system (Canfield Scientific, Inc., Parsippany, NJ, USA) under standardized conditions to ensure consistency in distance, exposure, perspective, and facial positioning. Three lighting techniques were utilized for facial imaging: IntelliFlash® (standardized light), cross-polarized light, and ultraviolet (UV) light (365 nm) (Canfield Scientific, Inc., Parsippany, NJ, USA). These lighting modalities enabled the visualization and analysis of various skin parameters. UV imaging facilitated the detection of porphyrins, which were indicative of bacterial activity and sun-induced skin damage. Cross-polarized imaging enhanced contrast and saturation while minimizing surface reflections and glare. The RBX® technology within the VISIA® system (Canfield Scientific, Inc., Parsippany, NJ, USA) was employed to differentiate between reddish and brownish pigmentation, allowing for the precise visualization of skin conditions such as telangiectasia (spider veins), hyperpigmentation, rosacea, and acne. Automated image analysis was conducted using two distinct approaches, namely feature count analysis and absolute rating analysis. Each subject underwent imaging in three standardized positions: left profile, right profile, and frontal view. Measurements were repeated for accuracy and consistency across subjects. An algorithm considered an element’s size, intensity, and impact on the state of the skin in addition to its frequency.
2.6. The Skin Topography Using Visiopor® PP 34 System
The Visiopor® PP 34 system (Courage + Khazaka electronic GmbH, Köln, Germany) allowed for the precise recording of fluorescence signals and lesion morphology over an 8 × 10 mm skin surface area. Image analysis included lesion size assessment (measurement of the surface area affected by acne lesions before and after treatment) and porphyrin quantification (evaluation of porphyrin fluorescence intensity), serving as an indirect marker for C. acnes activity and bacterial burden [18]. These analyses facilitated the objective monitoring of acne progression and response to treatment throughout the study. Corynebacterium acnes produced fluorescent porphyrins as a byproduct of its metabolic activity within follicular openings. The intensity of follicular fluorescence was correlated with the quantity of C. acnes, and thus with acne severity [19].
2.7. Digital Imaging
Digital images (macro images) were captured with the Nikon D200 (Nikon Europe B.V., Düsseldorf, Germany) and the AF-S Micro Nikkor lens (focal length 60 mm; light intensity 1:2.8; type G ED glass; exposure time 1:200; aperture F/22; ISO 200) to visually assess the affected skin areas. One image for each subject before application and one image after the application period was taken and provided in digital form. To minimize fluctuations caused by external influences such as room temperature and relative humidity, all measurements were always carried out at the same physical ambient conditions in rested status (~20 °C, humidity 40–60%).
2.8. Statistics
The values of the individual measurements were averaged, and the differences of the before and after values and the relative change of the mean values were calculated. MS Excel add-in STAT was used to analyze the data. A paired t-test was performed after testing normal distribution.
3. Results and Discussion
3.1. Formulation Rationale and Production of the Test Product
Since acne is an inflammatory disorder of multifactorial nature, the composition of the test product was designed to target factors of acne pathophysiology including hyperseborrhea, altered keratinization, proliferation of C. acnes and inflammation. The product mainly consists of anti-seboratic actives, actives for hyperkeratinization, anti-bacterial peptides such as oligopeptide-76 and oligopeptide-10, and anti-inflammatory ingredients of vitamin A palmitate, bakuchiol, and lupeol. A stable form of vitamin C (ascorbic acid 2-glucoside, AA2G) was also included in the formulation to provide defense against Uv-A and Uv-B together with helping to prevent post-acne discoloration. Dissolving microneedles based on hyaluronic acid (HA-MNs) have provided innovative and effective way of delivering active ingredients by overcoming the skin’s diffusion limitations.
3.2. Skin Tolerability
The examinations were performed using clinical-dermatological assessment criteria. The test product was used over an extended period of four weeks in the area of acne concern. Potential hazards and risks that had to be averted were analyzed and identified. All the test persons’ skin looked healthy before, during, and after the application study. There was no interruption of any test, no referral to a dermatologist, and no treatment in any case. No skin pathology was found in any form and there were no adverse skin reactions attributable to the subjects. The test products were well tolerated by all.
3.3. Skin Sebum Level Analysis
Three skin sebum content measurements were carried out at distinct points in the test area-a circumscribed area of severe acne inflammation at the time of 14 days (T2) and 28 days (T4), and the values were averaged. The efficacy of the test product with respect to the sebum content are shown in Figure 2.
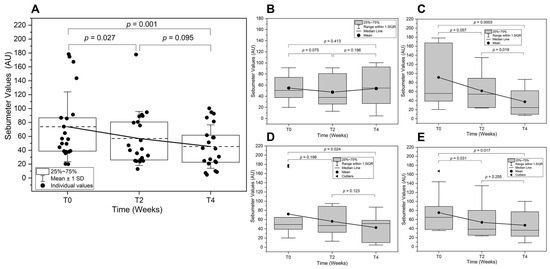
Figure 2.
Skin sebum analysis over time. Skin sebum level was assessed at baseline (T0), two weeks (T2), and four weeks (T4) post-treatment. (A) Overall sebum analysis comparison across all subjects is shown with the mean lines (---). Subgroup analyses are also presented for (B) Adolescent, (C) Adult, (D) Male, and (E) Female subjects. (AU: Arbitrary Units).
The overall change of the sebum content for all subjects was calculated to be −4.9% and −36.8% after 14 days and 28 days of application, respectively (Figure 2A). At the start of the study, the sebum levels exhibited substantial inter-individual variability, with some participants presenting high values indicative of excessive sebaceous gland activity. A statistically significant reduction in mean sebum levels was observed at T2 compared to T0 (p = 0.043), suggesting an early treatment effect on sebaceous gland function. The effect was sustained at T4. The sebum levels remained lower than at T0; however, the difference between T2 and T4 did not reach statistical significance (p = 0.085). The overall decrease from T0 to T4 (p = 0.001) confirms that the treatment effectively reduced sebum levels over time.
The adolescent and adult subgroup analysis (Figure 2B,C) showed that the sebum levels followed a similar trend. The adolescent subgroup exhibited more inter-individual differences in response, potentially reflecting age-related variations in sebaceous gland activity- implications for conditions associated with excessive sebaceous activity. A modest decrease was observed at T2, though it did not reach statistical significance. A further reduction occurred at T4, mirroring the overall cohort trend but with slightly greater variability. The adult subgroup, however, demonstrated a distinct pattern. More pronounced reduction in sebum levels was observed at T2 compared to T0, with a near-significant trend. By T4, adults showed a statistically significant decline in sebum levels, suggesting a stronger and possibly more consistent response to the intervention due to differences in hormonal regulation of sebaceous gland activity. These subgroup findings highlight how fluctuations in sebaceous gland activity—particularly when pronounced—may influence certain individuals to dermatological conditions linked to excess sebum production. In this context, understanding how hormonal and age-related factors modulate sebum levels provides a crucial foundation for exploring the downstream effects of hyperseborrhoea and altered sebum composition, both of which have been implicated in acne pathogenesis. The disruption in sebaceous gland activity associated with excessive sebum production (hyperseborrhoea) and alterations in sebum fatty acid composition and skin surface lipids (i.e., the oxidant/antioxidant ratio) are considered the primary concurrent events associated with the development of acne [20].
The subgroup sebum level analysis between male and female are shown in Figure 2D,E. In male subjects, a significant reduction in sebum levels was observed between T0 and T2 (p = 0.024), suggesting an early treatment effect on sebaceous gland activity. However, the change between T2 and T4 did not reach statistical significance, indicating that while sebum levels remained lower than baseline, additional reductions beyond T2 were not substantial. Similarly, in female subjects, a statistically significant reduction was observed at T2 compared to T0 (p = 0.031). However, the difference between T2 and T4 was not significant, suggesting a stabilization of the treatment effect. Interestingly, the overall decline in sebum levels from T0 to T4 was statistically significant in females, whereas in males, the overall reduction did not reach this threshold. This suggests that female subjects may have exhibited a more sustained response to treatment over the four-week period. The observed sex-specific differences in treatment response may be attributed to hormonal influences on sebaceous gland activity [21]. Males generally have higher baseline sebum production due to increased androgen stimulation, particularly testosterone and dihydrotestosterone (DHT), which strongly regulate sebaceous gland function. Androgen hormones, specifically testosterone together with Insulin-like Growth Factor (IGF-1), were reported to increase sebum synthesis and secretion [22]. Testosterone is converted into the more potent androgen DHT within sebaceous glands by the enzyme 5α-reductase. This conversion enhances sebaceous gland activity, leading to increased sebum production. Similarly, elevated IGF-1 levels, commonly observed during puberty, are associated with increased sebum production by stimulating cell growth, differentiation, and survival. IGF-1 enhances sebaceous gland activity and lipid synthesis, contributing to excessive sebum secretion and the development of acne. The higher variability in male sebum levels at T0, as seen in the first graph, may reflect these hormonal fluctuations. While the initial reduction at T2 was significant in both sexes, the relatively greater sustained effect in females suggests that the treatment may be more effective in reducing sebum production in hormonally responsive female sebocytes. Females, on the other hand, experience cyclical hormonal variations due to estrogen and progesterone, which also modulate sebaceous gland function [20].
The lower baseline sebum levels and the observed more consistent response in females may be due to greater sensitivity of sebocytes to retinoid-related mechanisms or differences in skin barrier function between sexes [23].
The results align with subgroups indicating that retinoids, as well as sebum-regulating compounds such as lupeol, modulate sebaceous activity through retinoid receptor-mediated pathways. The inclusion of retinyl palmitate and lupeol to the actives of HA-MNP for the acne management could represent a particularly promising strategy [24]. That is because topical treatment with retinoids is typically the initial course of action for mild-to-moderate acne cases. Both retinol and retinyl esters have the capacity to undergo oxidation to retinaldehyde, ultimately leading to the production of retinoic acid cis/trans isomers within keratinocytes, and then they act as systemic sebum-suppressive and long-term remissive effects [25]. Similarly, lupeol, as a pentacyclic triterpene with favorable physicochemical properties, has been shown to significantly reduce lipid production in SEB-1 sebocytes in a dose-dependent manner, with substantial reductions in palmitic, stearic, and oleic acids, the major components of human sebum, observed in cellular models [26]. In addition, bakuchiol (a meroterpene) may also serve as a valuable therapeutic addition to the armamentarium of acne treatments by downregulating 5-alpha-reductase—an enzyme responsible for converting testosterone to DHT, a potent stimulator of oil glands. Preferably, an effective acne therapy should act as an inhibitor of 5-α-reductase activity or down-regulate the synthesis of 5-α-reductase. A prior study has demonstrated that bakuchiol at a concentration of 10 μg/mL is capable of effectively downregulating the formation of 5-α reductase by nearly 40%. When combined with 2% salicylic acid, 1% bakuchiol exhibited a reduction in acne lesions and inflammation of nearly 70% [27].
With the effective delivery of MNP, such skincare actives (retinyl palmitate, lupeol and bakuchiol) could potentially become available in the deeper skin layers modulating the biological function of sebocytes through the expression of retinoic acid receptor (RAR) and retinoid X receptor (RXR) in sebocytes [28] and could modulate androgen-related pathways and influence lipid metabolism, correspondingly. Moreover, the synergy that exists between the various retinoid associates appears to further potentiate the targeted stimulation of retinoid receptors, while simultaneously enhancing the overall tolerability profile due to the reduced retinoid concentration in these combined formulations [29].
3.4. Dermatological Assessments
The efficacy of the test product regarding the parameters of redness and inflammatory signs in a circumscribed severe acne inflammation with severe redness or multiple confluent pimples was determined using a numerical rating scale. The results were summarized and discussed in the following sections.
3.4.1. Skin Redness (Erythema)
The overall improvements of the redness parameters after two weeks (T2) and four weeks (T4) of application were 47.2% and by 65.5%, respectively. Improvements in skin redness were shown in Figure 3.
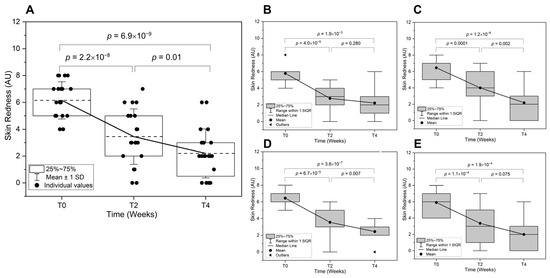
Figure 3.
Reduction in skin redness over time. Skin redness is assessed at baseline (T0), two weeks (T2), and four weeks (T4) post-treatment. (A) Overall redness comparison across all subjects is shown with mean line (---). Subgroup analyses are also presented for (B) Adolescent, (C) Adult, (D) Male, and (E) Female subjects. Redness severity was measured using a numerical scale (0 = no redness, 10 = maximum redness, AU: Arbitrary Units).
Over a two-week period, there was overall significant reduction in skin redness (p = 2.2 × 10−8), indicating an early anti-inflammatory effect of the test product (Figure 3A). This reduction continued at T4 (p = 0.010), confirming a progressive decline in acne-associated erythema. The overall change from T0 to T4 (p = 6.9 × 10−8) suggests a sustained treatment effect. The age-related differences in the treatment response is illustrated in Figure 3B,C. The adolescents exhibited a significant reduction in skin redness from T0 to T2 (p = 4.0 × 10−5), though the decline between T2 and T4 was not statistically significant. This suggests that the initial treatment effect was pronounced, but the response plateaued over time. In contrast, the adults showed a significant reduction from T0 to T2 (p = 1.5 × 10−4) and continued improvement at T4 (p = 0.002). The overall reduction suggests that adults experienced a more prolonged response, potentially due to greater sebocyte sensitivity to treatment or hormonal differences influencing inflammation resolution.
For the sex-related differences in redness reduction (Figure 3D,E), males exhibited a rapid and significant reduction from T0 to T2 (p = 6.7 × 10−5), with further improvement at T4 (p = 0.007), indicating a consistent anti-inflammatory effect. The overall change (p = 3.6 × 10−7) suggests a robust response to the treatment. Females also experienced a significant reduction at T2 (p = 1.1 × 10−4), but the continued decrease to T4 did not reach statistical significance. This slower rate of improvement may reflect hormonal modulation of sebaceous activity and inflammation, requiring a longer duration for optimal outcomes. The significant reduction in skin redness across all groups underscores the treatment’s efficacy in mitigating acne-associated inflammation. However, individual variability—particularly in age and sex-specific responses—suggests that personalized treatment regimens may optimize long-term outcomes [21]. Further research into hormonal influences, skin barrier function, and sebocyte receptor expression could refine therapeutic strategies for acne-prone skin.
3.4.2. Inflammatory Signs (Response)
The study demonstrated a significant reduction in inflammatory signs across all subject groups following the treatment, with notable differences in response patterns based on age and gender. The overall improvements in the inflammatory signs in the test area after two and four weeks of application were by 68.8% and 83.3%, respectively. A significant reduction was observed from T0 to T2 (p = 2.39 × 10−7) and from T0 to T4 (p = 1.51 × 10−9) (Figure 4A).
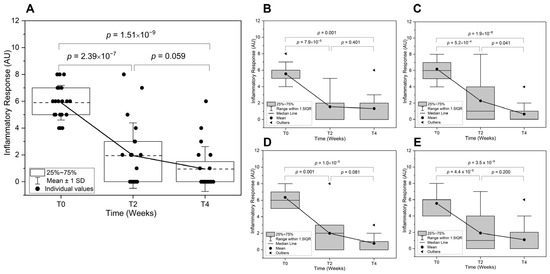
Figure 4.
Improvements in inflammatory response over time. The inflammatory response is shown at baseline (T0), and two weeks (T2) and four weeks (T4) treatments with the MNs acne patches on selected spots. (A) Overall inflammatory response comparison across all subjects is shown. Subgroup analyses are also presented for (B) Adolescent, (C) Adult, (D) Male, and (E) Female subjects. The severity of inflammatory signs (response) was measured using a numerical scale (0 to 10), AU: Arbitrary Units).
The difference between T2 and T4 is not statistically significant, indicating that the major improvement occurs within the first two weeks, with minimal further reduction afterward. For the age-related differences in inflammatory signs (Figure 4B,C), the adolescents showed rapid improvement early on, but inflammation plateaus after two weeks, aligning with the fact that their acne is largely driven by hormonal fluctuations and excessive sebum production. In younger individuals, the rapid turnover of skin cells and a stronger immune response may contribute to a faster reduction in inflammation. Therefore, the results are consistent with the literature as such adolescent acne responds rapidly to treatment, while adult acne is more resistant and frequent relapses are seen, requiring longer maintenance therapy [30]. On the other hand, the adults showed a more gradual decline, with continued significant improvement even after T2. This suggests that acne inflammation in adults might take longer to resolve due to the testosterone-driven sebaceous gland activity, which is more dominant in males and contributes to deeper nodular lesions. For the sex-related differences in inflammatory signs, (Figure 4D,E), males exhibited a trend of sustained improvement beyond T2 (borderline significance), whereas females experience most of their reduction within the first two weeks with no further significant change. This could be attributed to hormonal or skin structure differences [31]. While females may experience a more rapid response due to hormonal fluctuations, especially if they are undergoing hormonal treatments (e.g., oral contraceptives), males tend to have thicker skin and produce more sebum, potentially prolonging the response to treatment as discussed earlier. More persistent inflammatory response may be experienced in certain demographics, potentially influenced by hormonal factors and skin physiology.
The mechanisms underlying inflammatory acne involve the activation of Toll-like receptor 2 (TLR-2), pro-inflammatory cytokines (IL-6, IL-12, TNF-α, IFN-γ), and transcription factor AP-1, leading to persistent inflammation and lesion formation. Retinoids, widely used for their anti-inflammatory and comedolytic effects, suppress TLR-2 expression, cytokine release, and AP-1 activity [32]. However, their use is often limited by side effects, including skin irritation, photosensitivity, erythema, and peeling, making them intolerable for some patients. Bakuchiol, a natural meroterpenoid derived from P. corylifolia, has emerged as an alternative with “retinol-like” properties but with reduced adverse effects. It exerts its anti-inflammatory effects through inhibition of the cyclooxygenase (COX-1, COX-2) and lipoxygenase (5-LOX) pathways, effectively modulating prostaglandin synthesis—a key driver of acne-related inflammation. Higher epidermal COX-2 levels have been correlated with increased acne severity [33], making COX inhibition a promising therapeutic target. Bakuchiol has shown IC50 values of 2.34 µM (COX-1), 3.41 µM (COX-2), and 2.34 µM (5-LOX), comparable to aspirin’s activity in COX inhibition [34]. Additionally, bakuchiol stimulates collagen synthesis and extracellular matrix remodeling, contributing to post-inflammatory repair and barrier integrity [35]. The findings indicate that anti-inflammatory intervention effectively reduces acne severity, particularly within the first two weeks, with age- and gender-related differences in response time. Given retinoids’ efficacy but high irritation potential, bakuchiol represents a viable alternative with a favorable safety profile, targeting inflammatory and non-inflammatory acne lesions through COX/LOX inhibition and immunomodulation. Future research should explore long-term outcomes and combination therapies to optimize acne management while minimizing adverse effects.
3.5. Skin Actives with Anti-Bacterial Properties
There is a substantial body of evidence demonstrating that the skin microbiome plays a paramount role in skin health, appearance, and physiological functions [36]. Any disturbance in the skin microbiome leads to dysbiosis, which can disrupt the microbiome community [37]. Acne pathogenesis was conventionally linked with the over-proliferation of C. acnes accompanied by excess sebum production within the blockage of pilosebaceous follicles, even though the exact nature of the skin microbiome perturbation is not completely understood [38,39] However, C. acnes hypercolonization is not a key factor by itself in acne pathogenesis. Although studies have found that the magnitude of bacteria is equally distributed in both acne patients and normal individuals, C. acnes with antibiotic resistance (including biofilm formation) and virulence properties are dominant types on the skin of acne patients [40]. The loss of skin’s microbial diversity is more likely the main contributor to pathogenesis. More detailed analysis demonstrated that some particular C. acnes subspecies or phylotype (IA1) are more likely related to acne pathogenesis than others such as type II and III [41]. Other microorganisms such as Staphylococcus and Streptococcus are the subject of notice in the case of acne-prone skin. Despite the advancing science and better understanding of pathogenesis, there has been no consensus on that a single bacterial view is sufficient. Thus, the overall balance of bacteria with their subspecies should be studied in the blemished skin. A balanced approach rather than broad-spectrum antibacterial strategies must be considered to preserve the microbiota benefits while restraining a specific harmful component. As the increasing occurrence of antibiotic resistance in C. acnes and other skin commensal bacteria becomes increasingly alarming, topical antibiotic monotherapy is not recommended for mild-to-moderate acne. In this work, two antibacterial peptides, namely oligopeptide 10 and oligopeptide 76, were incorporated into the test product. Oligopeptide 10 is a relatively short bioactive peptide comprising fifteen naturally occurring amino acids that offer anti-microbial activity against C. acnes [42]. It was used in many acne products to improve natural defense systems. Clinical studies have demonstrated a significant reduction in the appearance of blemishes and red, blotchy spots when products contain oligopeptide-10. It has potent activity against C. acnes by causing rapid cell death via creating a lethal osmotic imbalance within the bacteria by binding lipoteichoic acids of bacteria enabling access to its key target of the cytoplasmic membrane. In this way, bacteria have a very low probability of becoming resistant. As a result, it neutralizes pro-inflammatory bacterial components and reduces redness. Oligopeptide-10 was shown to be non-sensitizing, non-irritating, non-cytotoxic, and non-mutagenic. Additionally, oligopeptide-76 was also widely used in antibacterial products for acne patients, and in vitro experiments indicate that it has antibacterial properties towards various strains of C. acnes. Acetyl Dipeptide-3 Aminohexanoate was one of the other peptides in the formulation of the test product and synthesized from acetic acid and dipeptide-3 with 6-aminohexanoic acid [43]. It is a tripeptide invention that maintains the balance between commensal microbes and pathogens in the skin. Lastly, bakuchiol was also demonstrated to have antibacterial effects against certain microorganisms including C. acnes [44].
3.6. Digital Imaging with the VISIA® System
A series of photographs with standard, ultraviolet, and cross-polarized lighting by VISIA® Analysis System (Canfield Scientific, Inc., Parsippany, NJ, USA) were used to evaluate the effectiveness of the test product for acne-prone skin. Representative examples for both adolescents and adult subjects with acne concerns are shown in Figure 5 and Figure 6, respectively.
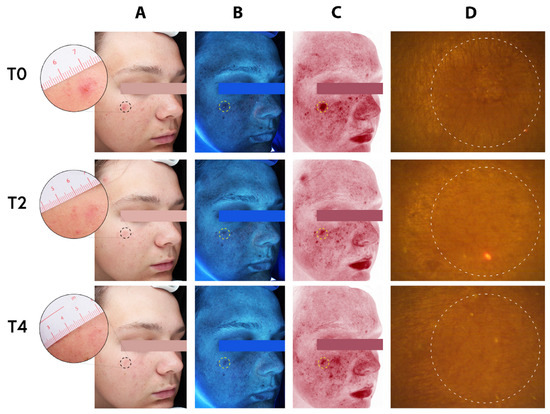
Figure 5.
Complexion analysis of a 17-year-old adolescent male. (A) Standard clinical photography (B) erythema-directed digital images, (C) excitation images, and (D) fluorescence images of porphyrin measurements, at baseline (T0), two weeks (T2), and four weeks (T4) of treatment with the MNs acne patches on selected spots (dotted circles). Assessment of inflammatory lesions and followed by period of two and four weeks of treatment (T2 and T4) with the test patches showing visible improvement of both inflammatory and non-inflammatory lesions.
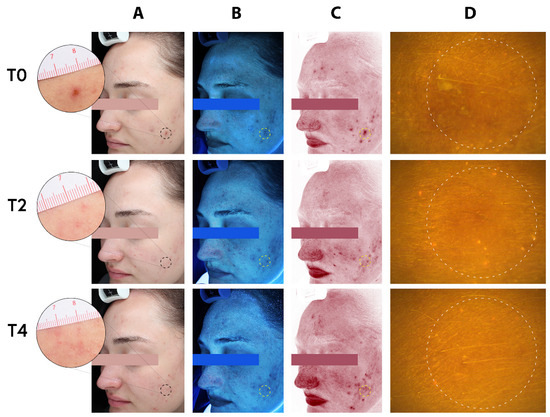
Figure 6.
Complexion analysis of a 23-year-old adult female. (A) Standard clinical photography (B) erythema-directed digital images, (C) excitation images, and (D) fluorescence images of porphyrin measurements, at baseline (T0), two weeks (T2), and four weeks (T4) of treatment with the MNs acne patches (the test product) on selected spots (dotted circles). Assessment of inflammatory lesions and followed by period of two and four weeks of treatment (T2 and T4) with the test patches showing visible improvement of both inflammatory and non-inflammatory lesions.
The photographs have provided visuals to assess the effectiveness of the test product by providing quantification of acne-related skin chances. For a representative adolescent subject, the standard clinical photograph with the erythema-directed digital image allows for visualization of inflammatory lesions of the patients.
Before the treatment, the patient’s inflammatory lesions were clearly visible on both the standard clinical photograph (Figure 5, T0/A) and the erythema-directed digital image (Figure 5, T0/B). The corresponding images after two (T2) and four weeks (T4) of treatments clearly showed significant improvements in the inflammatory lesions (Figure 5; T2 and T4, respectively). The photographs showed that there was important improvement of the patient with acne using the test product (HA based MNs-patch). The applied area becomes smoother with an overall reduced appearance of lesion (depth, area, volume). No noteworthy adverse effect was noted in all skin types. Similarly, for a representative adult subject, the standard clinical photograph and the erythema-directed digital image showed the significant improvements in the subject’s inflammatory lesions. The lesions where the patch was applied are clearly visible on both the standard clinical photograph (Figure 6, T0/A) and the erythema-directed digital image (Figure 6, T0/B) before the treatment (T0). After the treatments, however, the corresponding images showed important improvements (Figure 6; T2 and T4). The applied area became smoother with largely reduced appearance of lesion (area, depth, volume). The porphyrins content (bacterial pigments from C. acnes creating comedones and later acne) using Visiopor® PP34N probe (Courage + Khazaka electronic GmbH, Köln, Germany) for fluorescence diagnosis was inconclusive for both adolescents and adult subjects (Figure 5 and Figure 6/Ds). Nevertheless, it appeared that the surface texture of the skin was improved.
4. Conclusions
The management of acne-prone skin poses a challenge and necessitates long-term treatment. For an anti-acne remedy utilizing skincare active to qualify as a viable treatment option, it must at the very least target and mitigate one of the pathogenic factors. The test products utilizing an innovative MN delivery system in combination with skincare ingredients (dermo-cosmetics) have shown potential for managing mild-to-moderate acne vulgaris. The actives targeting all four factors in the development of acne may synergistically enhance the efficacy of the formulation without compromising the safety. The clinical study yielded substantial efficacy in reducing sebum production, as well as substantial decreases in redness and inflammatory signs, confirmed by spectroscopic measurements and dermatological assessments. This study could usher in distinct prospects for incorporating proteins, peptides, and other biologically active molecules into dissolving MNs that target specific pathways, thus facilitating the expansion of multi-functional, highly effective MN patches for various skin concerns. This pilot study provides preliminary information. To obtain a more comprehensive understanding of the test product, a larger number of subjects from diverse regions and skin types will be necessary along with a head-to-head comparison with the existing treatments.
Author Contributions
Conceptualization, M.A. and A.C.; methodology, M.A.; software, A.C.; validation, M.A., J.K. and D.J.; formal analysis, J.K. and M.A.; investigation, J.K. and M.A.; resources, A.C. and M.A.; data curation, A.C. and J.K.; writing—original draft preparation, M.A. and A.C.; writing—review and editing, J.K. and D.J.; visualization, J.K.; supervision, A.C.; project administration, A.C.; funding acquisition, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Imperial Bioscience Ltd. (Grant Number: 2020BR2Y).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (Ethics Committee) of the International Medical and Dental Ethics Commission GmbH (IRB Ref. No.: 2021/105, Date of Approval: 3 April 2021).
Informed Consent Statement
Informed consent for participation and publication was obtained from all subjects involved in the study. For underage participants, written informed consent was obtained from the legal guardians.
Data Availability Statement
Data supporting the reported results can be obtained from the corresponding author upon reasonable request.
Acknowledgments
We thank all participants for their time and interest in the study. We would like to thank Nuriye I. Celik (C&R) for her help in the preparation of the figures.
Conflicts of Interest
All authors are employees or affiliates of Imperial Bioscience Ltd. (London, UK) (M.A. and A.C.), Dermatest GmbH (Germany) (J.K.), or Raphas Co., Ltd. (Korea) (D.J.). Two authors (M.A. and A.C.) are employees of Imperial Bioscience Ltd. and were directly involved in the design of the study, data analysis, and the decision to publish the results. However, no additional external influence was exercised by the sponsor regarding data interpretation or the final manuscript content. All authors confirm that the data were analyzed and reported objectively. The authors declare that they have no additional conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript: DMP: Dissolvable Microneedle Patches, HA-DMP: Hyaluronic acid-based Dissolvable Microneedle Patches.
References
- Rademaker, M.; Garioch, J.J.; Simpson, N.B. Acne in schoolchildren: No longer a concern for dermatologists. BMJ 1989, 298, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhong, X.; Luo, Z.; Liu, M.; Zhang, H.; Zheng, H.; Li, J. Global, regional and national burdens of acne vulgaris in adolescents and young adults aged 10–24 years from 1990 to 2021: A trend analysis. Br. J. Dermatol. 2025, 192, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Moradi Tuchayi, S.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.C. Acne vulgaris. Nat. Rev. Dis. Primers. 2015, 1, 15029–15049. [Google Scholar] [CrossRef]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018, 32 (Suppl. S2), 5–14. [Google Scholar] [CrossRef]
- Araviiskaia, E.; Lopez Estebaranz, J.L.; Pincelli, C. Dermocosmetics: Beneficial adjuncts in the treatment of acne vulgaris. J. Dermatol. Treat. 2021, 32, 3–10. [Google Scholar] [CrossRef]
- Eichenfield, D.Z.; Sprague, J.; Eichenfield, L.F. Management of Acne Vulgaris: A Review. JAMA 2021, 326, 2055–2067. [Google Scholar] [CrossRef]
- Fallah, H.; Rademaker, M. Isotretinoin in the management of acne vulgaris: Practical prescribing. Int. J. Dermatol. 2021, 60, 451–460. [Google Scholar] [CrossRef]
- Habeshian, K.A.; Cohen, B.A. Current Issues in the Treatment of Acne Vulgaris. Pediatrics 2020, 145, S225–S230. [Google Scholar] [CrossRef]
- Varcin, M.; Knapen, C. Focus on: Cosmeceuticals Definitions, regulations and a review of the market. PMFA News 2016, 3, 36–37. [Google Scholar]
- Dreno, B.; Thiboutot, D.; Gollnick, H.; Finlay, A.Y.; Layton, A.; Leyden, J.J.; Leutenegger, E.; Perez, M.; Global Alliance to Improve Outcomes in Acne. Large-scale worldwide observational study of adherence with acne therapy. Int. J. Dermatol. 2010, 49, 448–456. [Google Scholar] [CrossRef]
- Avcil, M.; Celik, A. Microneedles in Drug Delivery: Progress and Challenges. Micromachines 2021, 12, 1321. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kumar, S.; Kim, S.H.; Seong, K.Y.; Lee, H.; Kim, C.; Jung, Y.S.; Yang, S.Y. Odorless Glutathione Microneedle Patches for Skin Whitening. Pharmaceutics 2020, 12, 100. [Google Scholar] [CrossRef]
- Ingrole, R.S.J.; Azizoglu, E.; Dul, M.; Birchall, J.C.; Gill, H.S.; Prausnitz, M.R. Trends of microneedle technology in the scientific literature, patents, clinical trials and internet activity. Biomaterials 2021, 267, 120491. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xia, D.; Prausnitz, M.R. Efficient Drug Delivery into Skin Using a Biphasic Dissolvable Microneedle Patch with Water-Insoluble Backing. Adv. Funct. Mater. 2021, 31, 2103359. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Kim, M.; Yang, H.; Lee, K.; Jung, H. Droplet-born air blowing: Novel dissolving microneedle fabrication. J. Control. Release 2013, 170, 430–436. [Google Scholar] [CrossRef]
- Meng, H.; Lin, W.; Dong, Y.; Li, L.; Yi, F.; Meng, Q.; Li, Y.; He, Y. Statistical analysis of age-related skin parameters. Technol. Health Care 2021, 29, 65–76. [Google Scholar] [CrossRef]
- Hayes, M.H.S.; Patterson, D.G. Experimental development of the graphic rating method. Psychol. Bull. 1921, 18, 98–99. [Google Scholar]
- Richter, C.; Trojahn, C.; Dobos, G.; Blume-Peytavi, U.; Kottner, J. Follicular fluorescence quantity to characterize acne severity: A validation study. Skin. Res. Technol. 2016, 22, 451–459. [Google Scholar] [CrossRef]
- McGinley, K.J.; Webster, G.F.; Leyden, J.J. Facial follicular porphyrin fluorescence: Correlation with age and density of Propionibacterium acnes. Br. J. Dermatol. 1980, 102, 437–441. [Google Scholar] [CrossRef]
- Zouboulis, C.C. Acne and sebaceous gland function. Clin. Dermatol. 2004, 22, 360–366. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, H.; Cunliffe, W.; Berson, D.; Dreno, B.; Finlay, A.; Leyden, J.J.; Shalita, A.R.; Thiboutot, D.; Global Alliance to Improve Outcomes in Acne. Management of acne: A report from a Global Alliance to Improve Outcomes in Acne. J. Am. Acad. Dermatol. 2003, 49, S1–S37. [Google Scholar] [CrossRef] [PubMed]
- Lagace, F.; D’Aguanno, K.; Prosty, C.; Laverde-Saad, A.; Cattelan, L.; Ouchene, L.; Oliel, S.; Genest, G.; Doiron, P.; Richer, V.; et al. The Role of Sex and Gender in Dermatology—From Pathogenesis to Clinical Implications. J. Cutan. Med. Surg. 2023, 27, NP1–NP36. [Google Scholar] [CrossRef]
- Vaidya, T.; Hoffman, L.; Chapas, A. Evaluating Common Ingredients Contained in Dietary Acne Supplements: An Evidence-Based Review. J. Clin. Aesthet. Dermatol. 2024, 17, 34–41. [Google Scholar]
- Kurlandsky, S.B.; Xiao, J.H.; Duell, E.A.; Voorhees, J.J.; Fisher, G.J. Biological activity of all-trans retinol requires metabolic conversion to all-trans retinoic acid and is mediated through activation of nuclear retinoid receptors in human keratinocytes. J. Biol. Chem. 1994, 269, 32821–32827. [Google Scholar] [CrossRef]
- Kwon, H.H.; Yoon, J.Y.; Park, S.Y.; Min, S.; Kim, Y.I.; Park, J.Y.; Lee, Y.S.; Thiboutot, D.M.; Suh, D.H. Activity-guided purification identifies lupeol, a pentacyclic triterpene, as a therapeutic agent multiple pathogenic factors of acne. J. Investig. Dermatol. 2015, 135, 1491–1500. [Google Scholar] [CrossRef]
- Chaudhuri, R.K.; Marchio, F. Bakuchiol in the management of acne-affected Skin. Cosmet. Toiletries® 2011, 126, 502–510. [Google Scholar]
- Reichrath, J.; Mittmann, M.; Kamradt, J.; Müller, S.M. Expression of retinoid-X receptors (-alpha,-beta,-gamma) and retinoic acid receptors (-alpha,-beta,-gamma) in normal human skin: An immunohistological evaluation. Histochem. J. 1997, 29, 127–133. [Google Scholar] [CrossRef]
- Barros, B.S.; Zaenglein, A.L. The Use of Cosmeceuticals in Acne: Help or Hoax? Am. J. Clin. Dermatol. 2017, 18, 159–163. [Google Scholar] [CrossRef]
- Kutlu, O.; Karadag, A.S.; Wollina, U. Adult acne versus adolescent acne: A narrative review with a focus on epidemiology to treatment. An. Bras. Dermatol. 2023, 98, 75–83. [Google Scholar] [CrossRef]
- Rahrovan, S.; Fanian, F.; Mehryan, P.; Humbert, P.; Firooz, A. Male versus female skin: What dermatologists and cosmeticians should know. Int. J. Womens Dermatol. 2018, 4, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Cong, T.X.; Hao, D.; Wen, X.; Li, X.H.; He, G.; Jiang, X. From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Bakry, O.A.; El Farargy, S.M.; El Kady, N.; Dawy, H.F.A. Immunohistochemical Expression of Cyclo-oxygenase 2 and Liver X Receptor-alpha in Acne Vulgaris. J. Clin. Diagn. Res. 2017, 11, WC01–WC07. [Google Scholar]
- Blanco, F.J.; Guitian, R.; Moreno, J.; de Toro, F.J.; Galdo, F. Effect of antiinflammatory drugs on COX-1 and COX-2 activity in human articular chondrocytes. J. Rheumatol. 1999, 26, 1366–1373. [Google Scholar] [PubMed]
- Chen, H.; Du, X.; Tang, W.; Zhou, Y.; Zuo, J.; Feng, H.; Li, Y. Synthesis and structure-immunosuppressive activity relationships of bakuchiol and its derivatives. Bioorganic Med. Chem. 2008, 16, 2403–2411. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef]
- Dreno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The Skin Microbiome: A New Actor in Inflammatory Acne. Am. J. Clin. Dermatol. 2020, 21, 18–24. [Google Scholar] [CrossRef]
- Huang, C.; Zhuo, F.; Han, B.; Li, W.; Jiang, B.; Zhang, K.; Jian, X.; Chen, Z.; Li, H.; Huang, H.; et al. The updates and implications of cutaneous microbiota in acne. Cell Biosci. 2023, 13, 113. [Google Scholar] [CrossRef]
- Xu, H.; Li, H. Acne, the Skin Microbiome, and Antibiotic Treatment. Am. J. Clin. Dermatol. 2019, 20, 335–344. [Google Scholar] [CrossRef]
- Moreau, M.; Zhen, Y. Skin Microbiome: General Overview and Application Perspectives. In Cosmetic Dermatology: Products and Procedures; Draelos, Z.D., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022. [Google Scholar]
- De Paoli Ambrosi, G. Antibacterial Composition for Topical Use. International Patent Application No. PCT/IB2013/056199, 1 August 2012. [Google Scholar]
- Van Den Nest, W.; Domenech, N.A.; Puche, J.C.; Serraïma, C.C. Peptides Useful in the Treatment and/or Care of Skin, Mucous Membranes, Scalp and/or Hair and Their Use in Cosmetic or Pharmaceutical Compositions. South Korea Patent KR101988675B1, 10 April 2014. [Google Scholar]
- Katsura, H.; Tsukiyama, R.I.; Suzuki, A.; Kobayashi, M. In vitro antimicrobial activities of bakuchiol against oral microorganisms. Antimicrob. Agents Chemother. 2001, 45, 3009–3013. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).