Abstract
The solute carrier family 35 (SLC35) comprises multiple members of transporters, including a group of proteins known as nucleotide sugar transporters (NSTs), an adenosine triphosphate (ATP) transporter, 3′-phosphoadenosine 5′-phosphosulfate (PAPS) transporters, and transporters of unknown function. To date, seven subfamilies (A to G) and 32 members have been classified into this large SLC35 family. Since the majority of glycosylation reactions occur within the lumen of the endoplasmic reticulum (ER) and Golgi apparatus, the functions of NSTs are indispensable for the delivery of substrates for glycosylation. Recent studies have revealed the diverse functions of this family of proteins in the regulation of numerous biological processes, including development, differentiation, proliferation, and disease progression. Furthermore, several congenital disorders of glycosylation (CDGs) resulting from variations in the SLC35 family member genes have been identified. To elucidate the pathology of these diseases, a variety of knockout mice harboring mutations in the family member genes have been generated and employed as animal models for CDGs. This review presents a historical overview of the SLC35 family, with a particular focus on recent advances in research on the functions of this family and their relationship to human diseases.
1. Introduction
The solute carrier (SLC) superfamily of membrane proteins represents a large and diverse family of transporters that facilitate the transport of a wide range of solutes across biological membranes. The SLC families and their constituent members are defined by the HUGO Gene Nomenclature Committee (HGNC) of the Human Genome Organization (HUGO), based on protein sequence similarity and phylogenetic analyses [1]. The substrates of SLC transporters include a variety of ions, sugars, amino acids, nucleotides, and other small molecules that are essential for cellular functions. The SLC35 family comprises multiple members, including a group of proteins known as nucleotide sugar transporters (NSTs), as a kind of the drug/metabolite transporter superfamily [2].
The majority of this family of proteins are involved in the transport of nucleotide sugars, which serve as essential substrates for glycosylation reactions in the endoplasmic reticulum (ER) and Golgi apparatus. In addition, the SLC35 family includes an adenosine triphosphate (ATP) transporter (SLC35B1), 3′-phosphoadenosine 5′-phosphosulfate (PAPS) transporters (SLC35B2 and SLC35B3), and a thiamine transporter (SLC35F3), in addition to transporters with unknown function (i.e., orphan transporters). To date, seven subfamilies (A to G) and 32 members (including pseudogene E2A) have been classified into this large SLC35 family (Figure 1 and Table 1). Among them, the functions of the A-D subfamily members have been well characterized, whereas little knowledge has been obtained regarding the functions of the members in the E-G subfamilies. The majority of NSTs are multiple transmembrane proteins localized in the ER or Golgi apparatus and contribute to the delivery of substrates for glycosylation [3].
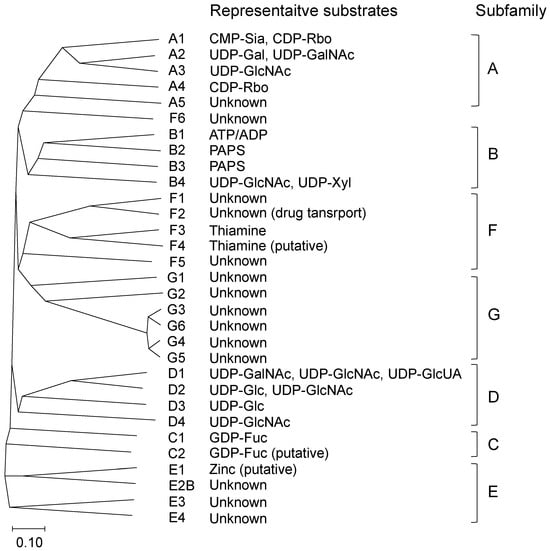
Figure 1.
A phylogenetic tree of human solute carrier family 35 (SLC35). Phylogenetic tree of sequences was constructed using the neighbor-joining (NJ) method with Clustal Omega. The scale at the bottom represents evolutionary distance. ADP, adenosine diphosphate; ATP, adenosine triphosphate; CDP-Rbo, cytidine diphosphate ribitol; CMP-Sia, cytidine monophosphate sialic acid; GDP-Fuc, guanosine diphosphate fucose; GDP-Man, GDP-mannose; PAPS, 3′-phosphoadenosine 5′-phosphosulfate; UDP-Gal, uridine diphosphate galactose; UDP-GalNAc, UDP-N-acetylgalactosamine; UDP-Glc, UDP-glucose; UDP-GlcNAc, UDP-N-acetylglucosamine; UDP-GlcUA, UDP-glucuronic acid; UDP-Xyl, UDP-xylose.
Glycosylation is a common modification of proteins and lipids that is catalyzed by glycosyltransferases. In eukaryotes, sugars are activated into nucleotide sugars, including cytidine monophosphate sialic acid (CMP-Sia), guanosine diphosphate fucose (GDP-Fuc), GDP-mannose (GDP-Man), uridine diphosphate galactose (UDP-Gal), UDP-N-acetylgalactosamine (UDP-GalNAc), UDP-glucose (UDP-Glc), UDP-N-acetylglucosamine (UDP-GlcNAc), and UDP-xylose (UDP-Xyl), as the substrates for glycosylation reaction. Glycosyltransferases utilize these nucleotide sugars as donor substrates for the enzymatic attachment of sugar residues. These nucleotide sugars are synthesized in the cytosol (in the case of CMP-Sia, the nucleus), and most glycosylation reactions occur in the ER and Golgi lumens, where the glycosyltransferases are localized [4]. In the ER lumen, UDP-glucuronic acid (UDP-GlcUA) serves as a substrate for glucuronidation, which is catalyzed by UDP-glucuronosyltransferases.
As the ER and Golgi apparatus are separated from the cytosol by a membrane, the functions of NSTs are essential for the translocation of these nucleotide sugars across the membrane. Furthermore, ATP and PAPS transporters are also localized and function in the membrane of these organelles [3]. In early studies, the transport functions of NSTs, PAPS transporters, and ATP transporters have been demonstrated using Golgi vesicles isolated from mammalian tissues and cell lines [3]. In addition, mutant cell lines that lack CMP-Sia or UDP-Gal transport activity have been identified and utilized for the cloning of NSTs through a complementation approach [5].
The first molecular cloning and identification of NSTs were reported in 1996. The milk yeast Kluyveromyces lactis UDP-GlcNAc transporter [6], murine CMP-Sia transporter [7], and human UDP-Gal transporters [8,9] were identified and characterized. Following these discoveries, numerous studies have identified NST genes in a range of eukaryotic organisms. Initially, it was hypothesized that a single monospecific NST would be sufficient for the transport of each corresponding nucleotide sugar. However, subsequent studies demonstrated that the majority of NSTs are multisubstrate-specific transporters, and multiple NSTs exhibit functional redundancy.
NSTs employ an antiport mechanism to facilitate the transport of nucleotide sugars. This process entails the utilization of a corresponding luminal nucleoside monophosphate as an antiport molecule, including CMP, GMP, and UMP, which are generated through a glycosyltransferase reaction and subsequent luminal nucleoside diphosphatase reaction [10] (Figure 2). However, recent studies have proposed more variable antiport mechanisms. Furthermore, a number of studies have demonstrated that the functions of NSTs are interrelated and that NSTs and glycosyltransferases form complexes and regulate nucleotide sugar supply in a coordinated manner [5].
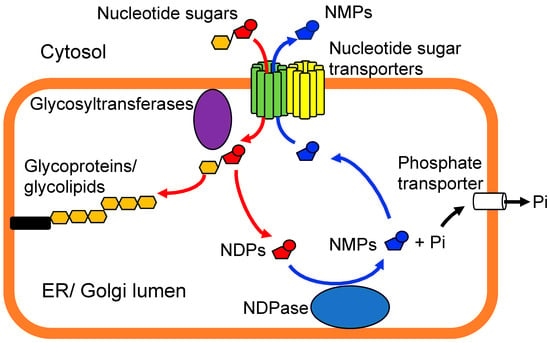
Figure 2.
A schematic illustration of the function of typical nucleotide-sugar transporters (NSTs). NSTs translocate specific nucleotide sugars from the cytosol into the endoplasmic reticulum (ER) or Golgi lumens using an antiport mechanism. Glycosyltransferases transfer a sugar residue from corresponding donor substrate to a specific hydroxyl group of its acceptor molecules. Nucleoside diphosphatase (NDPase) catalyzes the hydrolysis of nucleoside diphosphate (NDP) to yield nucleoside monophosphate (NMP) and inorganic phosphate (Pi). NSTs utilize MNPs as their antiport molecules. NSTs form homodimers or heterodimers with themselves or other NSTs and interact with specific glycosyltransferases. It should be noted that in the case of certain NSTs, alternative antiport mechanisms have been proposed, including UDP-sugar/UDP-sugar countertransport.
The first crystal structure of an NST, the yeast Vrg4, was published in 2017. Parker and Newstead presented the crystal structures of the GDP-mannose transporter of S. cerevisiae in both the substrate-free and substrate-bound states [11]. The typical NST has a conserved architecture comprising 10 transmembrane domains, arranged in the form of two inverted topology repeats of five transmembrane helices around a central ligand-binding site [12]. Additionally, the researchers demonstrated the indispensable functions of short-chain lipids in the activation of the transporter in the Golgi membrane. The lipids facilitate the dimerization of NSTs in the membrane by forming an integral part of the dimer [11,13]. The crystal structures of NSTs also revealed the crucial roles of conserved sequence motifs in substrate recognition and transport, such as the consensus motifs for Man (GALNK), Fuc (GTAKA), and guanine (FYNN). Subsequently, the structures of the mouse and Zea mays CMP-Sia transporters have been reported [14,15]. These three-dimensional structural analyses yielded insights into the mechanisms underlying the diverse functions of NSTs and disease-causing mutations. For a more comprehensive understanding of the mechanisms of the structure and function of NSTs, we recommend other excellent reviews [5,12,16].
Because the structures and functions of SLC35 family members have been conserved in a variety of eukaryotes throughout evolution, numerous studies have investigated the biological functions of each NST using invertebrate mutant models, such as Caenorhabditis elegans and Drosophila melanogaster. More recently, congenital disorders of glycosylation (CDGs) caused by variations of the SLC35 family member genes have been identified, including SLC35A1-CDG, SLC35A2-CDG, SLC35A3-CDG, SLC35B2-CDG, SLC35C1-CDG, and SLC35D1-CDG. Consequently, knockout mice of the family member genes have been created and analyzed as animal models for the study of the pathology of these CDG and the elucidation of the functions of these transporters. This review presents a historical overview of the SLC35 family, with a particular focus on recent advances in research on the functions of this family and their relationship to human diseases.

Table 1.
Human SLC35 family members.
Table 1.
Human SLC35 family members.
| Member | Aliases | Known Substrates | Chromosome | Cellular Localization | Tissue Specificity 1 | CDG Name and MIM Number 2 | Identified Drosophila Genes 3 | Identified C. elegans Genes 4 | |
|---|---|---|---|---|---|---|---|---|---|
| A | 1 | CST | CMP-Sia CDP-Rbo | 6q15 | Golgi | Low tissue specificity | SLC35A1-CDG (CDG-IIf) MIM: 603585 | ugalt/dmugt (CG2675) UDP-Gal UDP-GalNAc | srf-3 (CE43555) UDP-Gal UDP-GlcNAc nstp-4 (CE32110) UDP-GalNAc UDP-GlcNAc nstp-5 (CE15465) UDP-Gal UDP-Glc UDP-GalNAc UDP-GlcNAc |
| 2 | UGT | UDP-Gal UDP-GalNAc | Xp11.23 | Golgi (UGT1) ER (UGT2) | Low tissue specificity | SLC35A2-CDG (CDG-IIm) MIM: 300896 | |||
| 3 | UGTREL2 | UDP-GlcNAc | 1p21.2 | Golgi | Low tissue specificity | SLC35A3-CDG MIM: 615553 | |||
| 4 | CLRP UGTREL3 | CDP-Rbo | 5q31.3 | Golgi | Low tissue specificity | senju (CG14040) UDP-Gal | |||
| 5 | UGTREL5 | orphan | 3q13.2 | Golgi | Low tissue specificity | ||||
| B | 1 | UGTREL1 AXER | ATP/ADP (UDP-GlcUA) | 17q21.33 | ER | Low tissue specificity | meigo (CG5802) Unknown | hut-1 (CE24129) UDP-Gal UDP-Glc | |
| 2 | PAPST1 | PAPS | 6p21.1 | Golgi | Low tissue specificity | SLC35B2-CDG MIM: 620269 | sll (CG7623) PAPS | pst-1 (CE12374) PAPS | |
| 3 | PAPST2 | PAPS | 6p24.3 | Golgi | Low tissue specificity | dpapst2 (CG7853) PAPS | pst-2 (CE01312) PAPS | ||
| 4 | YEA4 | UDP-GlcNAc, UDP-Xyl (UDP-GlcUA) | 7q33 | ER/Golgi | Low tissue specificity | efr (CG3774) GDP-Fuc UDP-GlcNAc UDP-Xyl | |||
| C | 1 | FUCT1 | GDP-Fuc | 11p11.2 | Golgi | Tissue enhanced (liver) | SLC35C1-CDG (CDG-IIc, LADII) MIM: 266265 | nac/gfr (CG9620) GDP-Fuc | nstp-10 (CE30910) GDP-Fuc |
| 2 | OVCOV1 | GDP-Fuc (putative) | 20q13.12 | ER/Golgi | Low tissue specificity | CG14971 Unknown | |||
| D | 1 | UGTREL7 | UDP-GalNAc UDP-GlcNAc UDP-GlcUA | 1p31.3 | ER | Tissue enhanced (liver) | SLC35D1-CDG MIM: 269250 | frc (CG3874) UDP-sugars | sqv-7 (CE04263) UDP-Gal UDP-GalNAc UDP-GlcUA |
| 2 | HFRC1 | UDP-Glc UDP-GlcNAc GDP-Man * (* not human) | 9q22.32 | Golgi | Low tissue specificity | ||||
| 3 | FRCL1 | UDP-Glc | 6q23.3 | Early endosome/ Dense granule | Tissue enriched (brain) | ||||
| 4 | TMEM241 C18orf45 | UDP-GlcNAc | 18q11.2 | Golgi | Low tissue specificity | ||||
| E | 1 | Zinc (putative) | 19p13.11 | Low tissue specificity | |||||
| 2A 2B | 2A: pseudogene 2B: orphan | 1p36.33 | Low tissue specificity | ||||||
| 3 | BLOV1 | orphan | 12q15 | Low tissue specificity | |||||
| 4 | orphan | 22q12.2 | Tissue enhanced (testis) | ||||||
| F | 1 | C6orf169 | orphan | 6q22.2–q22.31 | Recycling endosome | Group enriched (brain, retina) | |||
| 2 | HSNOV1 | orphan | 11q22.3 | Plasma membrane | Tissue enhanced (salivary gland) | ||||
| 3 | Thiamine | 1q42.2 | Plasma membrane | Tissue enriched (brain) | |||||
| 4 | C14orf36 | Thiamine (putative) | 14q22.3–q23.1 | Group enriched (brain, retina, seminal vesicle) | |||||
| 5 | orphan | 2q14.1 | Low tissue specificity | ||||||
| 6 | C2orf18 ANT2BP | orphan | 2p23.3 | Mitochondria | Low tissue specificity | ||||
| G | 1 | TMEM20 POST | orphan | 10q23.33 | ER | Tissue enriched (intestine) | |||
| 2 | TMEM22 | orphan | 3q22.3 | Low tissue specificity | |||||
| 3 | TMEM21A | orphan | 17q12 | Tissue enriched (testis) | |||||
| 4 | AMAC1L1 | orphan | 18p11.21 | Not detected | |||||
| 5 | AMAC1L2 | orphan | 8p23.1 | Tissue enriched (testis) | |||||
| 6 | AMAC1L3 | orphan | 17p13.1 | Tissue enriched (testis) | |||||
1 The data were obtained from the Human Protein Atlas (HPA) database (https://www.proteinatlas.org, accessed on 18 June 2024). The tissue specificity is based on mRNA expression levels in the analyzed samples, which were derived from a combination of data from the HPA and the Genotype-Tissue Expression (GTEx) projects. The classification includes four categories: tissue enriched, group enriched, tissue enhanced, low tissue specificity, and not detected [17]. It should be noted that the expression profiles presented here are not entirely consistent with those previously reported [18]. 2 Disease name and OMIM (Online Mendelian Inheritance in Man, https://omim.org, accessed on 18 June 2024) ID. 3 Gene name, FlyBase (https://flybase.org, accessed on 18 June 2024) ID, and known substrates. 4 Gene name, WormBase (https://wormbase.org, accessed on 18 June 2024) protein ID for a representative isoform, and known substrates.
2. SLC35A Subfamily
The SLC35A subfamily comprises five members. Since the first cloning of mammalian NSTs in 1996, numerous studies have extensively characterized the function of SLC35A subfamily members. All members of this subfamily are considered to be assigned to NSTs, which have a variety of substrate specificities. These include CMP-Sia, UDP-Gal, UDP-GalNac, UDP-GlcNac, and CDP-ribitol (CDP-Rbo). To date, no substrate specificity has been identified for SLC35A5. However, studies have suggested that this protein is also involved in glycosylation and nucleotide sugar transport into the Golgi apparatus.
2.1. SLC35A1
The SLC35A1 protein functions as a CMP-Sia transporter. Additionally, it has been proposed that SLC35A1 may also transport other cytosine nucleotide conjugates, such as CDP-Rbo [19].
In 1996, Eckhardt et al. reported the molecular cloning of mouse Slc35a1 cDNA by complementation of Lec2 mutant cells [7]. Lec2 and its subclone 6B2 are mutant cell lines derived from Chinese hamster ovary (CHO) cells that exhibit a significant reduction in sialylation of glycoproteins and glycolipids due to a defect in the CMP-Sia transport activity [20,21]. The expressed mouse SLC35A1 protein was localized in the Golgi apparatus and restored the glycosylation defects of the 6B2 mutant [7]. The CMP-Sia transport activity of the mouse SLC35A1 was confirmed by utilizing Golgi vesicles overexpressed with this protein in Saccharomyces cerevisiae [22]. Subsequently, the human homolog was cloned and identified as a CMP-Sia transporter by the complementation of the Lec2 cell line and its CMP-Sia transport activity into microsomal vesicles [23].
The biosynthesis of CMP-Sia occurs within the nucleus [24], and the CMP-Sia transporter facilitates its translocation from the cytosol into the Golgi apparatus for glycosyltransferases. The SLC35A1 is primarily localized in the medial Golgi and possesses two specific ER export motifs in its carboxyl-terminal cytoplasmic tail. The deletion of the C-terminal IIGV motif results in the retention of the SLC35A1 in the ER membrane [25].
NSTs have been predicted to be type III multi-transmembrane proteins with an even number of transmembrane domains and both N- and C-termini directed towards the cytosolic side of the ER or Golgi membrane. An initial computer-based structural analysis of hamster SLC35A1 predicted an eight-transmembrane-helix model [26]. However, a detailed topological analysis determined that the mouse SLC35A1 contains 10 transmembrane domains with N- and C-termini facing on the cytosolic side of the Golgi membrane [27]. It is proposed that SLC35A1 forms a homodimer in the Golgi membrane and that this dimerization is important for its transport activity. The fifth and tenth transmembrane domains are involved in the dimer formation, while the other domains are involved in the formation of transport bundles [15,28]. The seventh transmembrane domain is the key player for the CMP-Sia transport activity [29,30]. The Tyr214 and Ser216 residues located in the seventh transmembrane domain of the human SLC35A1 are of critical importance for the recognition of CMP-Sia as a transporting substrate [30]. In 2019, Ahuja and Whorton reported the first crystal structures of the mouse SLC35A1 in complex with its physiological substrates, CMP and CMP-sialic acid [14]. The analysis of these structures elucidated the fundamental principles of the structure–function relationship of NSTs, including substrate recognition and selectivity as well as the mechanism of substrate accessibility between the NST and the Golgi lumen.
In 2005, Martinez-Duncker et al. identified that a mutation in the SLC35A1 gene is associated with the development of a syndrome characterized by macrothrombocytopenia and neutropenia [31]. The patient completely lacked sialyl Lewis X (sLeX) antigen on the surface of polymorphonuclear cells [31]. In the patient, the inactivation of the CMP-Sia transporter gene was due to genetic alterations caused by a double microdeletion in one allele and a 130-bp deletion in the other allele, resulting in the partial skipping of exon 6. The autosomal recessive disorder was designated as CDG-IIf (currently, SLC35A1-CDG) [31], and subsequently several cases of SLC35A1-CDG have been reported [32,33,34]. The affected individuals displayed bleeding diathesis, macrothrombocytopenia, proteinuria, and neurological symptoms including ataxia, intellectual disability, and epilepsy. Kauskot et al. proposed that the function of SLC35A1 is not essential for proplatelet formation but is crucial for platelet lifespan [34]. Ma et al. generated Slc35a1 conditional knockout mice and analyzed their Slc35a1–/– megakaryocytes and platelets. The Slc35a1–/– platelets exhibited thrombocytopenia, and the number of bone marrow megakaryocytes was reduced. Furthermore, impaired megakaryocyte maturation and the excessive clearance of desialylated platelets by liver Küpffer cells were observed [35]. Sialic acid is a common terminal residue of glycans on proteins and acidic sphingolipids such as gangliosides and has important biological functions. Szulc et al. reported that SLC35A1 knockout HEK293T cells had reduced but still detectable sialylated N-glycans, although sialylation of O-glycans appeared to be completely absent. The researchers postulated the existence of an SLC35A1-independent Golgi CMP-Sia uptake route [28].
A study utilizing haploid screening for Lassa virus entry suggested that SLC35A1 is one of the candidate genes for α-dystroglycanopathy [36]. Although glycosylation of α-dystroglycan does not require CMP-Sia, the inactivation of SLC35A1 in the haploid cell line HAP1 prevented functional glycosylation of α-dystroglycan and Lassa virus entry, which requires fully glycosylated α-dystroglycan. Moreover, the loss-of-function mutations in SLC35A1 resulted in aberrant α-dystroglycan O-mannosylation, whereas the removal of Sia from wild-type HAP1 cells or the prevention of sialylation did not affect α-dystroglycan O-mannosylation [36,37].
In 2021, Ury et al. reported that the SLC35A1 can transport CDP-Rbo, a nucleotide-pentose alcohol, as well as CMP-Sia [19]. It is known that the functional O-mannosyl glycan on α-dystroglycan contains a tandem repeat of Rbo 5-phosphate, which is required to prime the addition of a repeated disaccharide (-Xyl-α1,3-GlcUA-β1,3-)n, called matriglycan, to the glycan chain [38] (Figure 3). This unique glycan structure of α-dystroglycan is of paramount importance in the interaction with extracellular matrix proteins, such as laminin. CDP-Rbo is synthesized by an enzyme called CDP-L-ribitol pyrophosphorylase A (CRPPA; also known as isoprenoid synthase domain-containing protein, ISPD) in the cytosol. Subsequently, enzymes FUKUTIN (FKTN) and FUKUTIN-related protein (FKRP) transfer Rbo 5-phosphate to the core M3 structure (GalNAc-β1-3-GlcNAc-β1-4-Man) on the α-dystroglycan in the Golgi apparatus [39,40] (Figure 3). The defects in CRPPA [41,42], FKTN [43], and FKRP [44,45] have been associated with muscular dystrophy due to defective glycosylation of α-dystroglycan. Although the translocation of CMP-Rbo from the cytosol into the Golgi apparatus is an essential process for the Rbo 5-phosphate modification, patients with SLC35A1-CDG did not exhibit symptoms of α-dystroglycanopathy. Another CDP-ribitol transporter, SLC35A4, is proposed to be involved in the transport of CDP-ribitol into the Golgi apparatus in a redundant manner [19].
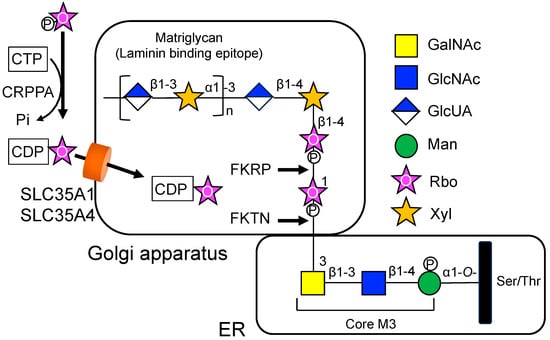
Figure 3.
Potential involvement of SLC35A1 and SLC35A4 in the synthesis of O-mannosyl glycans on α-dystroglycan. CDP-Rbo is synthesized from CTP and Rbo-5P via the CDP-L-ribitol pyrophosphorylase A (CRPPA) enzyme and transported into the Golgi apparatus via SLC35A1 and SLC35A4. Subsequently, the enzymes FUKUTIN (FKTN) and FUKUTIN-related protein (FKRP) transfer Rbo 5-phosphate to the core M3 structure on the α-dystroglycan in a sequential manner. These modifications are essential for the addition of a repeated disaccharide matriglycan.
2.2. SLC35A2
SLC35A2, also known as the UDP-Gal transporter, is one of the most extensively studied NSTs. A multitude of studies have demonstrated a correlation between the expression of SLC35A2 and the progression and malignancy of various cancers, including colon cancer [46]. Its mutations are responsible for a rare type of CDG, CDG-IIm (currently, SLC35A2-CDG) [47,48]. The SLC35A2 and its Drosophila ortholog, dmUGT, transport UDP-Gal and UDP-GalNAc into the Golgi lumen [49]. The homologs of SLC35A2 are present in a variety of species, including nematode C. elegans [50], yeasts S. cerevisiae [51] and Schizosaccharomyces pombe [52,53], and protozoan parasites Entamoeba histolytica [54] and Leishmania major [55].
The human SLC35A2 was identified as the first mammalian NST based on its ability to complement the galactosylation defect of a mouse mutant cell line, Had-1 [8,9]. In 1996, Miura et al. cloned the cDNA of SLC35A2 (hUGT1) from a human cDNA library [8]. Subsequently, they also cloned hUGT2, an alternative splicing variant of SLC35A2 [9]. In microsomal vesicles derived from Had-1 cells, both UGT1 (SLC35A2v1) and UGT2 (SLC35A2v2) exhibited equivalent UDP-Gal transport activity [9]. The substrate specificity of SLC35A2 (hUGT1 and hUGT2) was confirmed using microsomal vesicles isolated from the yeast expression system. The SLC35A2 was found to transport UDP-GalNAc in addition to UDP-Gal [49]. A CHO mutant cell line Lec8 [56,57] and a ricin-resistant mutant MDCK cell line (MDCK-RCAr) [57,58] also lack UDP-Gal transport activity and galactosylated glycans. Both hUGT1 and hUGT2 have the potential to restore glycosylation defects in these mutant cells [59].
The amino acid sequences of these two variants differ only at their C-terminus (UGT1, SVLVK; UGT2, LLTKVKGS) [60]. The UGT1 protein exhibits Golgi localization [60,61], whereas UGT2 exhibits dual localization in the ER and Golgi apparatus due to its dilysine ER-retention motif, KVKGS [60]. The subcellular localization of SLC35A2 is also influenced by its interaction with other proteins, including glycosyltransferases and other NSTs. The localization of hUGT1 shifted from the Golgi apparatus to the ER as a consequence of the association with UDP-galactose:ceramide galactosyltransferase, a protein localized in the ER [62]. In addition, the formation of a heterodimeric complex between hUGT1 and hUGT2 proteins resulted in the relocation of hUGT1 from the Golgi apparatus to the ER due to the presence of hUGT2 [63].
A number of studies have demonstrated that SLC35A2 and SLC35A3 function in a coordinated manner. SLC35A2 and SLC35A3 are known to tightly interact with each other [64]. In SLC35A2-deficient cells, overexpression of SLC35A3 partially restored galactosylation of N-glycans [65]. The SLC35A2 and SLC35A3 can substitute for each other’s function if appropriate regions of these transporters are conserved. The short N-terminal region of SLC35A2, comprising 35 amino acid residues, is crucial for galactosylation of N-glycans [66].
The SLC35A2 gene mutations are responsible for a rare genetic disorder, SLC35A2-CDG (CDG-IIm). In 2013, Ng et al. identified mutations in the SLC35A2 gene from three unrelated patients as an undiagnosed X-linked CDG [47]. The patients exhibited a range of symptoms, including developmental delay, hypotonia, seizures, ocular anomalies, skeletal abnormalities, and brain malformations. Each mutation in the patients resulted in reduced UDP-Gal transport and Gal-deficient glycoproteins [47]. Additionally, Kodera et al. identified two de novo frameshift mutations and one missense mutation in SLC35A2 in three unrelated Japanese females diagnosed with early-onset epileptic encephalopathy (EOEE), a group of epileptic encephalopathies characterized by frequent seizures accompanied by developmental retardation [48]. The missense mutant protein was correctly localized in the Golgi apparatus, whereas the two frameshift mutant proteins did not properly express [48]. Following these discoveries, researchers have identified additional mutations in SLC35A2 that are associated with this rare form of CDG, SLC35A2-CDG.
The phenotypic manifestations of SLC35A2-CDG are variable; however, the patients diagnosed with SLC35A2-CDG commonly exhibit severe neurological symptoms. In many cases, developmental and epileptic encephalopathy with hypsarrhythmia were observed [67,68]. It is well known that a variety of CDGs present with a range of neurological symptoms, including psychomotor retardation, cognitive impairment, epileptic seizures, and hypotonia [69,70,71]. In particular, CDGs with aberrant N-glycosylation have been linked to the pathogenesis of neurological disorders and the associated symptoms [71]. Since SLC35A2 is indispensable for glycosylation processes, including those involving N-glycans, any disruption to its function will inevitably result in a multitude of neurological symptoms. In contrast, the majority of patients show normal glycosylation patterns of serum transferrin protein, which is a common biomarker for most types of CDGs [68,72]. Ng et al. developed an assay for the diagnosis of the disease by evaluating UDP-Gal transport into the Golgi apparatus using permeabilized patient fibroblast cells. In primary fibroblasts from affected individuals, the UDP-Gal transport activity was directly correlated with the ratio of wild-type to mutant alleles [72].
Defects in UDP-Gal transport can affect almost all galactosylated glycans, including N- and O-linked glycans, GAGs, and glycosphingolipids [73]. As the SLC35A2 gene is located on the X chromosome (Xp11.23), the majority of SLC35A2-CDG patients are female. They have heterozygous mutant alleles of SLC35A2, and almost all male patients are somatic mosaics. This indicates that a wild-type SLC35A2 allele is essential for their viability. Witters et al. reported that oral supplementation with D-Gal resulted in clinical and biochemical improvement in SLC35A2-CDG. The supplementation of Gal increased levels of a fully galactosylated N-glycan and decreased the ratio of incompletely formed glycans to fully formed glycans in patients [74].
2.3. SLC35A3
SLC35A3 was initially described as UDP-Gal transporter (UGT)-related protein 2 (UGTrel2) [9] and subsequently identified to be a UDP-GlcNAc transporter [75,76]. GlcNAc is a sugar found in the majority of glycoconjugates, including N- and O-linked glycoproteins, proteoglycans, and glycolipids. UDP-GlcNAc is synthesized in the cytosol and incorporated into the Golgi lumen via several transporters, including SLC35A3.
In 1998, Guillen et al. cloned the cDNA of the mammalian SLC35A3 gene from Madin-Darby canine kidney (MDCK) cells. They discovered that this gene encodes a UDP-GlcNAc transporter by using a phenotypic correction of the K. lactis mnn2-2 mutant [75]. Mnn2-2 is a mutant line of K. lactis, an aerobic milk yeast, lacking terminal GlcNAc in its mannan chains. The mnn2-2 mutant’s defective features were rescued by transformation with the canine SLC35A3 gene, but not by the SLC35A1 or SLC35A2 genes [75]. Subsequently, the human SLC35A3 gene was identified based on nucleotide sequence similarity to the human SLC35A2 gene and was demonstrated to have UDP-GlcNAc transport activity [76]. The SLC35A3 gene is ubiquitously expressed in organs, and the protein is localized to the Golgi apparatus in CHO cells [76]. The amino acid sequence of the SLC35A3 protein exhibits 53% and 40% identity to the mammalian SLC35A2 (UDP-Gal transporter) and SLC35A1 (CMP-Sia transporter) sequences, respectively.
In 2006, Thomsen et al. reported that a missense mutation in the SLC35A3 gene causes complex vertebral malformation (CMV) in bovine [77]. CMV is an autosomal recessive inherited disease of Holstein Friesian cattle, which is globally found in the world and causes intra-uterine or perinatal mortality [78,79]. This bovine disease is characterized by malformation of the cervical and thoracic vertebral column, craniofacial dysmorphism, malformed ribs, arthrogryposis of the lower limb joints, and cardiac anomalies. Thomsen et al. demonstrated that the aberrant protein glycosylation in tissues is associated with the bovine CMV [77]. The transformation with the wild-type SLC35A3 gene fully complemented the defect of the mnn2-2 mutant, whereas the mutated SLC35A3 gene did not increase surface expression of GlcNAc. The heterozygous carriers of the missense mutation (Val180Phe) were asymptomatic, whereas its homozygous mutation typically caused death in utero [77].
In 2013, Edvardson et al. identified deleterious mutations in SLC35A3 in patients with arthrogryposis, impaired intellectual development, and seizures [80]. The Golgi vesicles isolated from the fibroblasts of SLC35A3-CDG patients exhibited significantly reduced UDP-GlcNAc transport activity, resulting in a substantial reduction in highly branched N-glycans on the cell surface. Subsequently, studies have identified that mutations in SLC35A3 cause skeletal dysplasia in patients with a range of skeletal deformities, including CMV-like vertebral anomalies [81,82]. The presence of neurological symptoms and skeletal abnormalities in patients with SLC35A3-CDG indicates that the function of SLC35A3 is essential for the glycosylation of molecules involved in neural and skeletal development.
Saito et al. generated mutant mice lacking the Slc35a3 gene using the CRISPR/Cas9 genome editing system [83]. The Slc35a3–/– mice exhibited chondrodysplasia accompanied by CVM-like vertebral anomalies and were perinatal lethal. In the Slc35a3–/– embryos, the extracellular matrix (ECM) in the growth plate cartilage was drastically reduced, and many flat proliferative chondrocytes were reshaped. The significant reduction in the levels of proteoglycans, including heparan sulfate (HS), keratan sulfate (KS), and chondroitin sulfate (CS)/dermatan sulfate (DS), suggests that the chondrodysplasia phenotypes observed in Slc35a3–/– mice were at least partially caused by the decreased glycosaminoglycan (GAG) synthesis [83].
Cartilage tissues are composed primarily of type II collagen, hyaluronan (HA), and a cartilage-specific proteoglycan, aggrecan. Aggrecan is a large proteoglycan composed of a core protein and numerous GAG chains of CS/DS and KS. In cartilage tissues, the major components of the ECM are produced by chondrocytes, which contribute to water retention and provide resistance to compression [84,85]. GAGs in proteoglycans receive varying degrees of sulfation and acquire a negative charge, enabling them to interact with a diverse array of molecules, including epidermal growth factor (EGF), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), Wnt, Hedgehog, and bone morphogenic protein (BMP) [86]. These GAGs are known to play crucial roles in skeletal development and bone homeostasis [87]. The decreased GAG synthesis may deteriorate the ECM quality, leading to chondrodysplasia phenotypes in CMV and Slc35a3–/– mice [83].
SLC35A3 is also involved in the biosynthesis of highly branched N-glycans [88]. Several studies have suggested that NSTs and glycosyltransferases form glycosylation-related complexes in the Golgi apparatus [58,64,89,90]. In the Golgi membrane, SLC35A2 and SLC35A3 form heterologous complexes and cooperate in the delivery of donor substrates for glycosyltransferases located in the Golgi apparatus [58,64]. These transporters also interact with multiple mannoside acetylglucosaminyltransferases (Mgats) [89] and N-acetylglucosaminyltransferase IV (GnT-IV) [91] in the Golgi membranes, and the multi-enzyme/multi-transporter complexes facilitate the efficient synthesis of complex N-glycans [90].
However, despite the clear demonstration of the UDP-Gal-providing activity of SLC35A2, the significance of SLC35A3 in the N- and O-glycan synthesis remains unclear. Szulc et al. analyzed SLC35A2 and SLC35A3 knockout cell lines and demonstrated that SLC35A3-knockout CHO cells showed only minor alterations in GlcNAc incorporation into N-glycans, whereas SLC35A2-deficient cells displayed significant changes in N- and O-glycan synthesis [73]. Since SLC35B4, SLC35D1, and SLC35D2 also exert UDP-GlcNAc transport activity, Szulc et al. propose that SLC35A3 may not be the primary UDP-GlcNAc transporter involved in N- and O-glycan synthesis [73].
2.4. SLC35A4
The SLC35A4 gene encodes a Golgi-resident CDP-Rbo transporter protein [19]. Additionally, it has been postulated that SLC35A4 may play a role in regulating the intracellular trafficking of other NSTs [92].
In 2002, Pérez-Márquez et al. cloned the Slc35a4 (clrp) cDNA from rat brain tissue [93]. The sequence of SLC35A4 exhibited high homology to SLC35A1 and SLC35A2, and its GFP-tagged protein was observed to localize to the Golgi apparatus. In the brain, Slc35a4 mRNA was detected in a limited set of neurons, including the pyramidal cells of the cortex, Purkinje cells of the cerebellum, and the motoneurons of the brainstem [93]. Sosicka et al. proposed that SLC35A4 is not involved in glycosylation but rather plays a role in the intracellular trafficking of SLC35A2/SLC35A3 complexes [92]. SLC35A4 is primarily localized in the intermediate ER-Golgi compartment, which is involved in trafficking between these two organelles. In HepG2 cells, knockout of the SLC35A4 gene did not result in glycosylation changes, but it did result in a disturbed subcellular distribution of SLC35A2/SLC35A3 complexes [92].
In 2021, Ury et al. demonstrated that the SLC35A4 protein plays a role in transporting CDP-Rbo into the Golgi apparatus. SLC35A1 has the capacity to transport both CMP-Sia and CDP-Rbo, whereas SLC35A4 only exerts CDP-Rbo transport and lacks CMP-Sia transport activity [19]. These two transporters exhibit a high degree of structural similarity, yet the amino acid sequence of the SLC35A4 protein shares no substantial sequence conservation with that of SLC35A1 within the binding site for CMP-Sia. The structural homology model indicated that SLC35A1 has a large binding pocket that can accommodate both bulky CMP-Sia and smaller CDP-Rbo, whereas SLC35A4 only accepts the CDP-Rbo [19]. In SLC35A1 knockout cells, the deficiency of Rbo 5-phosphate in the glycan on α-dystroglycan was rescued by SLC35A4, while it did not complement the loss of CMP-Sia transport activity. The researchers proposed that CDP-Rbo transport is mainly reliant on the function of SLC35A1, whereas SLC35A4 plays a redundant role in the CDP-Rbo incorporation [19].
It has been reported that SLC35A4 mRNA contains an upstream open reading frame (uORF) that encodes a functional microprotein [94,95,96,97]. uORF is a cis-acting element within the 5′-untranslated region upstream (5′-UTR) of the main protein coding sequence. It has a regulatory function in the translation of the downstream open reading frames (ORFs). In the 5′-UTR of SLC35A4, the uORF encodes a 103 amino acid peptide sequence that contains the PFAM domain DUF4535 (PFAM ID: PF15054) and is highly conserved across vertebrates [95]. Moreover, the uORF of SLC35A4 mRNA produces a single-pass transmembrane microprotein that is targeted to the inner mitochondrial membrane. The loss of function of the microprotein significantly diminished maximal cellular respiration and ATP generation in the mitochondria [96,97].
2.5. SLC35A5
To date, the substrate specificity of SLC35A5 has not been identified. However, it is postulated that this orphan transporter protein modulates the functions of other NSTs by forming complexes [98].
Sosicka et al. reported that the SLC35A5 protein is a Golgi-resident multiprotein complex member involved in nucleotide sugar transport. Inactivation of the SLC35A5 gene resulted in a decreased uptake of UDP-GlcUA, UDP-GlcNAc, and UDP-GalNAc in the Golgi apparatus, with no effect on UDP-Gal transport activity [98]. Nevertheless, in HepG2 cells, the deletion of SLC35A5 did not significantly alter glycosylation. The SLC35A5 protein formed homomers as well as heteromers with other members of the SLC35A protein subfamily [92,98].
In the fruit fly Drosophila, a homolog of SLC35A4 and SLC35A5, senju (CG14040), encodes a UDP-Gal-specific transporter [99]. In senju mutants, the reduction of Gal-containing glycans resulted in the hyperactivation of immune responses via the Toll signaling pathway in the absence of immune challenges [99].
3. SLC35B Subfamily
The SLC35B subfamily comprises four member genes. Due to the high homology of these members with the UDP-Gal transporter (UGT, SLC35A2), they were initially considered to be putative NSTs. Since the first identification of SLC35B2 as a PAPS transporter, studies have revealed a diverse array of their substrate specificity, including ATP and PAPS. However, the functions and substrate specificities of some members in this group have not yet been fully elucidated.
3.1. SLC35B1
SLC35B1 is proposed to act as a transporter, facilitating the import of UDP-GlcUA into the ER. This process contributes to glucuronidation by providing substrate for UDP-glucuronosyltransferases [100,101]. Additionally, recent studies have demonstrated that SLC35B1 functions as an ATP/ADP exchanger within the ER and is involved in protein folding and ER quality control (ERQC) [102,103].
In 1996, Ishida et al. first reported the sequence of human SLC35B1 as a putative NST with unknown function and named it UGT-related protein 1 (UGTrel1) [9]. The SLC35B1 gene is conserved in various eukaryotic species, including yeast, nematode, plant, and fruit fly. Yeasts have highly homologous genes of human SLC35B1, namely hut-1. The overexpression of hut-1 in S. cerevisiae and S. pombe resulted in increased galactosyl modification, and the proteins exhibited UDP-Gal and UDP-Glc transport activity [104,105]. The function of HUT-1 is involved in protein folding in the ER under stress conditions, although it is not essential for cell growth under normal conditions [105]. In addition, ceHUT-1 in the C. elegans is involved in maintaining ER homeostasis and is essential for its development [106]. The cehut-1 deletion mutant worms exhibited larval growth defects and lethality with disrupted intestinal morphology. Of greater significance, the lethality and the ER stress phenotype of the mutant were rescued by the introduction of the human SLC35B1 gene [106]. In Drosophila, MEIGO (CG5802) is an ER-resident protein that regulates ER folding capacity and protein N-glycosylation. MEIGO modulates neuronal dendrite targeting specificity by regulating the protein level and N-glycosylation of ephrin, a regulator of axon guidance [107]. Moreover, MEIGO is involved in regulating the dendrite targeting of projection neurons by modulating the amount and localization of cell surface receptors, including Toll-6 [108].
In human cells, Kobayashi et al. reported that the SLC35B1 protein exhibits counter-transport activity of UDP-GlcUA:UDP-GlcNAc in the ER membrane [100]. Ondo et al. proposed that SLC35B1 is a principal transporter for the translocation of UDP-GlcUA into the ER, thereby contributing to glucuronidation through the delivery of substrate for UDP-glucuronosyltransferases [101]. In contrast, Klein et al. recently demonstrated that human SLC35B1 functions as an ATP/ADP exchanger in the ER membrane and plays a role in ERQC and protein folding [102]. The protein, designated AXER, was proposed to provide ATP to BiP, a molecular chaperone in the ER [102]. Schwarzbaum et al. reported that SLC35B1 did not transport any mononucleotides or nucleotide sugars directly [103]. Furthermore, they identified di- and tri-nucleotides as potential suitable counter-substrates for the ATP transport process, in addition to ATP/ADP exchange. The researchers proposed that the reduction in UDP-GlcUA-dependent glucuronosyltransferase activity observed in the ER lumen following the knockdown of SLC35B1 might be attributable, at least in part, to an impaired UDP export mechanism [103].
3.2. SLC35B2
The SLC35B2 gene encodes a protein that transports PAPS from the cytosol into the Golgi apparatus [109]. Impairment of its function results in a severe autosomal recessive form of chondrodysplasia (SLC35B2-CDG) [110].
PAPS is a high-energy sulfate donor substrate for enzymes called sulfotransferases, which catalyze sulfation reactions by transferring sulfate groups from PAPS to acceptor molecules. Sulfation is an essential modification for various biomolecules, including proteoglycans, glycoproteins, glucolipids, and protein tyrosine residues, and regulates numerous biological processes of the cells. PAPS is synthesized from ATP and inorganic sulfate through a series of enzymatic reactions in the nuclear and cytosol. As sulfotransferases for proteins, glycoproteins, and proteoglycans are localized in the Golgi lumen, the presence of saturated PAPS transporters in the Golgi apparatus has long been postulated in mammalian cells [3,111,112,113,114].
In 2003, Kamiyama et al. first cloned the cDNA of human SLC35B2 and identified its substrate specificity, tissue distribution, and functions [109]. The SLC35B2 protein, which they termed PAPST1, is a PAPS-specific transporter with a Km value of 0.8 µM and no transport activity for nucleotide sugars. SLC35B2 is found to be localized to the Golgi apparatus and is expressed ubiquitously in human organs [109]. Furthermore, its single Drosophila ortholog, SLALOM (SLL, CG7623), was identified as a PAPS-specific transporter with a Km value similar to that of human SLC35B2. The SLL protein plays a role in sulfating the GAG chain of HS, and its functionality is essential for viability [109]. Lüders et al. independently reported that the Drosophila sll gene encodes a PAPS transporter [115]. Drosophila sll mutants exhibited segment polarity defects as a result of abnormalities in multiple signaling pathways, including Wingless and Hedgehog [115]. In C. elegans, an SLC35B2 ortholog, pst-1, has also been identified as a gene that encodes a PAPS transporter and demonstrated to be essential for viability and involved in HS sulfation [116].
It is well known that the sulfated state of GAGs is a key factor in determining the function of PGs [117]. PAPS synthesis and transport are essential for the sulfation process. The initial step involves the transport of sulfate ions into the cell by sulfate transporters. This is followed by the conversion of the sulfate ion to an activated sulfate donor, PAPS, by PAPS synthases. The subsequent step involves the translocation of PAPS from the cytosol into the Golgi apparatus by PAPS transporters. Finally, sulfotransferases transfer sulfate from PAPS to GAGs (Figure 4). In humans, it is known that anomalies in the PAPS synthesis cause a range of dysplasias. SLC26A2 (DTDST) is a plasma-membrane sulfate transporter that is responsible for four different osteochondrodysplasias: diastrophic dysplasia (DTD) [118], achondrogenesis type IB [119], atelosteogenesis type II [120], and multiple epiphyseal dysplasia (MED) [121]. DTD is a disease that is characterized by clinical features of dysplasia, including dwarfism, spinal deformation, and abnormalities of the joints [118]. In addition, it has been demonstrated that mutations in the human PAPS synthase 2 (PAPSS2) gene are associated with a specific form of spondyloepimetaphyseal dysplasia (SEMD) [122]. In a study using zebrafish, Wiweger et al. reported that the mutation of SLC35B2 ortholog pinscher (pic) resulted in defective sulfation of GAGs and other molecules and was potentially involved in skeletal dysplasia [123,124]. The homozygote pic mutant displayed a complete absence of sulfation of proteoglycans in the ECM and other sulfated molecules. Furthermore, the mutant displayed dwarfism and severe cartilage and bone defects that closely resembled those observed in human skeletal dysplasia, as well as pronounced ultrastructural abnormalities [123].
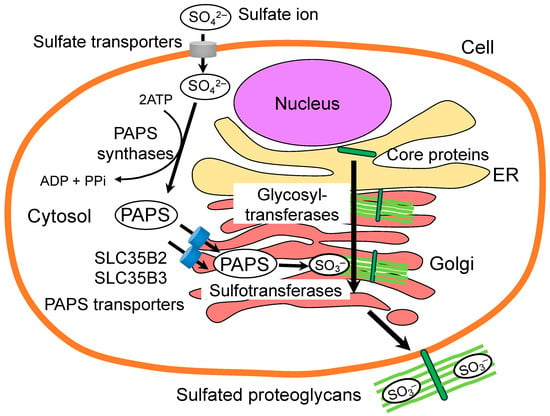
Figure 4.
The role of PAPS transporters in the sulfation of proteoglycan GAGs. PAPS is utilized as a universal sulfate donor for the sulfation modification. PAPS is synthesized in the cytosol or nucleus with ATP by PAPS synthases. Subsequently, PAPS transporters translocate PAPS from the cytosol into the Golgi lumen, where the sulfotransferases are localized. Sulfotransferases transfer sulfate from PAPS to the defined positions on the sugar residues of GAGs.
In 2022, Guasto et al. first reported that functional impairment of human SLC35B2 causes a severe autosomal recessive form of chondrodysplasia (SLC35B2-CDG) [110]. They identified homozygous variants in SLC35B2 that cause a novel syndromic chondrodysplasia with severe intellectual disability and hypomyelinating leukodystrophy in two patients. The loss-of-function variants in SLC35B2 resulted in decreased CS sulfation due to decreased gene expression and protein mislocalization of SLC35B2 [110].
It is notable that patients with chondrodysplasia and biallelic SLC35B2 variants exhibit neurological features, including a severe psychomotor delay and a hypomyelinating leukodystrophy with thin corpus callosum [110]. It is known that sulfated proteoglycans play a critical role in the development of the nervous system [125,126]. In C. elegans, the SLC35B2 ortholog pst-1 is essential for neuronal connectivity and specific aspects of nervous system development [116]. The pst-1 mutants exhibited highly specific neuronal defects in the formation of collateral axonal branches and motor synapses. These defects were correlated with reduced complexity of sulfation patterns in HS [116]. Shimazu et al. reported that dsm-1 (D-serine modulator-1), the rat ortholog of SLC35B2, is predominantly expressed in the forebrain and involved in the translocation of D-serine, an endogenous co-agonist for the N-methyl-D-aspartate (NMDA) receptor [127]. Furthermore, Uezato et al. proposed the potential involvement of human SLC35B2 in the pathophysiology of bipolar disorder and schizophrenia [128]. They identified significant associations between single nucleotide polymorphisms (SNPs) in the SLC35B2 and these disorders. The mRNA expression of SLC35B2 was found to be significantly reduced in the postmortem dorsolateral prefrontal cortex of bipolar disorder patients, whereas no change was observed in schizophrenia [128].
A number of studies have indicated that SLC35B2 is associated with cancer progression. Kamiyama et al. demonstrated that SLC35B2 plays a role in the proliferation of colorectal carcinoma cells by controlling their sulfation status [129]. In the colorectal carcinoma tissues, the expression of SLC35B2 was increased in fibroblasts in the vicinity of invasive cancer cells. The silencing of PAPS transporter genes resulted in a reduction in FGF signaling and the proliferation of colorectal DLD-1 cells [129]. Chim-ong et al. reported that the upregulation of SLC35B2 serves as a prognostic biomarker for breast cancer, and its expression is associated with a poor prognosis of invasive ductal breast carcinoma [130].
The function of SLC35B2 has also been implicated in tyrosine sulfation [131], a post-translational modification that regulates a variety of cellular functions [132]. High expressions of SLC35B2 and tyrosylprotein sulfotransferase 2 (TPST2) were found to be correlated with poor survival rates among patients diagnosed with pancreatic ductal adenocarcinoma [133]. Furthermore, the knockdown of SLC35B2 or TPST2 expression was observed to attenuate the growth and metastasis of pancreatic ductal adenocarcinoma cells [133]. In hepatocellular carcinoma, oxidative stress has been shown to upregulate SLC35B2 expression and increase tyrosine sulfation of SAV1, a scaffold protein involved in the Hippo signaling pathway [134]. The sulfation of SAV1 resulted in the subsequent inactivation of Hippo signaling, which in turn fostered the growth of hepatocellular carcinoma cells [134].
3.3. SLC35B3
In 2006, Kamiyama et al. identified human SLC35B3 as another PAPS transporter and named it PAPST2 [135]. Similar to SLC35B2, the SLC35B3 protein exhibited Golgi localization, while it was highly expressed in the colon, where SLC35B2 is less expressed. The overexpression of the SLC35B3 protein in the HCT116 colon cancer cell line resulted in an increase in PAPS transport activity [135]. In human colorectal carcinoma DLD-1 cells, RNA interference (RNAi) of either SLC35B2 or SLC35B3 genes resulted in a reduction in the level of sulfation of cellular proteins and a suppression of cell proliferation [129]. Furthermore, both SLC35B2 and SLC35B3 have been demonstrated to play a role in the maintenance and differentiation of mouse embryonic stem cells [136]. These observations indicate that SLC35B3 may serve a redundant function to SLC35B2 in sulfation modification.
SLC35B3 has a single Drosophila ortholog gene (dpapst2, CG7853), which was identified to encode a PAPS-specific transporter with a Km similar to that of the human SLC35B3 [137]. Drosophila dpapst2 exhibited a similar expression pattern to Drosophila sll, an ortholog of the human SLC35B2 gene, although dpapst2 showed low levels of expression in most tissues. Unlike sll, RNAi-mediated knockdown of the dpapst2 gene resulted in a weak phenotype. However, it genetically interacted with genes involved in HS synthesis during wing or eye formation [137]. In contrast, the C. elegans PST-1 (SLC35B2 ortholog) and PST-2 (SLC35B3 ortholog) proteins have distinct and non-redundant functions under physiological conditions, despite their similar PAPS transport activities in the Golgi apparatus [116]. The pst-1 deletion mutant exhibited defects in cuticle formation, post-embryonic seam cell development, vulval morphogenesis, cell migration, and embryogenesis, whereas the pst-2 mutant exhibited a wild-type phenotype. These C. elegans PAPS transporters exhibited distinct tissue distributions and subcellular localizations, and the phenotypes of double mutants of these genes were similar to those of the pst-1 single mutant [138].
3.4. SLC35B4
SLC35B4 was initially reported as a human gene homologous to yeast Yea4, a gene for an ER-resident UDP-GlcNAc transporter required for cell wall chitin (poly β1,4-GlcNAc) synthesis in S. cerevisiae [139].
In 2005, Ashikov et al. identified the human SLC35B4 protein as a bifunctional transporter with specificity for UDP-Xyl and UDP-GlcNAc [140]. They showed that SLC35B4 is localized to the Golgi apparatus and proposed a role in the delivery of substrates for proteoglycan synthesis [140]. However, subsequent studies have reported that endogenous SLC35B4 is also localized to the ER [63,141], and that its C-terminal dilysine motif (KDSKKN) is critical for ER localization [142]. In the HepG2 cell line, knockout of the SLC35B4 gene did not affect the major UDP-Xyl- and UDP-GlcNAc-dependent glycosylation pathways [142]. Moreover, studies have indicated that SLC35B4 also transports UDP-GlcUA, in addition to UDP-Xyl and UDP-GlcNAc. Kobayashi et al. showed that both SLC35B1 and SLC35B4 could transport UDP-GlcUA via UDP-GlcUA:UDP-GlcNAc countertransport in the ER membrane [100,101]. The expression of SLC35B4 in human liver tissues was found to be significantly lower than that of SLC35B1. This indicates that SLC35B4 may play only a minor role in the supply of UDP-GlcUA [101].
In Drosophila, an SLC35B4 homolog protein, EFR (CG3774), is found to be capable of transporting GDP-Fuc in addition to UDP-GlcNAc and UDP-Xyl [143]. EFR was observed to localize specifically to the ER and demonstrated functional redundancy with GFR, a GDP-Fuc transporter homologous to human SLC35C1, despite their different cellular localizations [143]. However, human SLC35B4 lacks both GDP-Fuc transport activity and the ability to complement GDP-Fuc transporter-deficient fibroblast cells [140].
A number of studies have indicated a possible association between the SLC35B4 gene and both obesity and type 2 diabetes mellitus [141,144,145,146]. In humans, an SNP in the SLC35B4 gene (rs1619682) has been identified that is significantly associated with waist circumference [144]. Moreover, an elevated SLC35B4 expression in subcutaneous adipose tissue was observed in obese humans [146]. A quantitative trait locus analysis of congenic mouse strains identified that Slc35b4 is a regulator of obesity, insulin resistance, and gluconeogenesis in mice [145]. A reduction in hepatic Slc35b4 expression was associated with decreased gluconeogenesis in the liver, which may contribute to hepatic insulin resistance [145]. Wex et al. reported that SLC35B4 acts as an inhibitor of gluconeogenesis in response to glucose stimulation [141]. The expression of SLC35B4 protein was increased by glucose stimulation, while the knockdown of SLC35B4 resulted in a decrease in the expression of several proteins implicated in the pathogenesis of diabetes, including HSP60, which is known to have O-GlcNAc modification [147]. The researchers postulated that the uptake of UDP-GlcNAc into the ER and Golgi apparatus by SLC35B4 reduces O-linked glycosylation in the cytosol or nuclear by depleting the substrate for the glycosyltransferases, thereby leading to a decrease in glucose production [141].
4. SLC35C Subfamily
The SLC35C subfamily comprises two members, SLC35C1 and SLC35C2. SLC35C1 is a GDP-Fuc transporter, while SLC35C2 is a putative GDP-Fuc transporter. Fuc is a terminal residue widely found in N- and O-glycans, and GDP-Fuc is a nucleotide sugar that acts as a donor substrate for fucosyltransferases. Defects in the GDP-Fuc transporter are known to cause an inherited recessive disorder, leukocyte adhesion deficiency type II (LADII, currently SLC35C1-CDG) [148,149]. Mice lacking Slc35c1 exhibit a phenotype similar to that observed in human LAD II patients [150,151].
4.1. SLC35C1
SLC35C1 encodes a Golgi-localized GDP-Fuc transporter. In mammalian cells, GDP-Fuc is synthesized by two pathways in the cytosol. The de novo pathway produces GDP-Fuc from L-Man, and the salvage pathway uses exogenous or recovered L-Fuc via lysosomal degradation of glycoconjugates [152,153,154]. Subsequently, the synthesized GDP-Fuc is translocated from the cytosol into the Golgi lumen, where the fucosyltransferases are localized. The existence of a Golgi GDP-Fuc transporter has long been suggested, and the protein has been partially purified from rat liver Golgi membranes [155]. The identification of the GDP-Fuc transporter was achieved in 2001 as the gene responsible for the human hereditary recessive disorder, LADII [150,151].
LADII (CDG-IIc, currently SLC35C1-CDG) is a disorder that is characterized by a deficiency of fucosylated glycoconjugates on proteins. Individuals with this disorder exhibit features of severe mental retardation, slow growth, and facial and skeletal dysplasia. Additionally, they suffer from immunodeficiency and recurrent infections due to reduced leukocyte adhesion caused by the absence of fucosylated selectin ligands on leukocytes. In 1999, Lübke et al. demonstrated that LADII is caused by a generalized fucosylation defect resulting from impaired GDP-fucose transport into the Golgi apparatus [156]. Subsequently, two independent research groups reported in 2001 that SLC35C1 encodes a GDP-Fuc transporter and that its mutations are responsible for LADII [148,149]. Lübke et al. cloned SLC35C1 cDNA by complementation of the fucosylation defect in skin fibroblasts from LADII patients and verified its GDP-fucose transport activity. They identified two missense mutations in SLC35C1 in the patients and classified the disease as a new type of CDG [148]. Lühn et al. also reported that SLC35C1 encodes a Golgi-localized GDP-fucose transporter protein [149]. The LADII patient had an R147C amino acid change in the conserved fourth transmembrane region of the SLC35C1 protein. The SLC35C1 gene was conserved in diverse species, and its C. elegans ortholog could restore fucosylation in the fibroblast cells derived from the LADII patient. In contrast, overexpression of the mutant protein from the LADII patient could not rescue its fucosylation defects [149]. Subsequently, several mutations in SLC35C1 have been identified in LADII patients [157,158,159,160,161,162]. Helmus et al. classified SLC35C1 mutations in LADII patients into two groups [163]. Patients in one group had single amino acid substitutions that only affected its transport function, and another group lost two important regions of the protein, causing defects in both function and Golgi localization [163].
The existence of SLC35C1-independent GDP-Fuc transport pathways in the Golgi apparatus has been discussed. It is known that high-dose oral Fuc supplementation partially rescues the symptoms of certain types of LADII patients [158,164,165]. Lübke et al. demonstrated that Fuc supplementation in the culture medium of fibroblasts from LADII patients results in restoration of fucosylation [148]. Furthermore, LADII patient fibroblasts did not exhibit impaired protein O-fucosylation, including on Notch proteins, although fucosylation on N-linked glycans was severely reduced [165]. Hellbusch et al. reported that Slc35c1 knockout mice exhibited reduced fucosylation and abnormalities such as growth retardation and impaired lymphocyte assembly and adhesion, which are similar to those observed in human LADII patients [150,151]. As in human LADII patients, oral supplementation of Fuc ameliorated the fucosylation defect in Slc35c1 knockout mice. Nevertheless, the elimination of the Slc35c1 gene did not result in any notable defects in Notch signaling during development [150]. In addition, neither LADII patients nor Slc35c1 knockout mice exhibit alterations in T cell development, which is dependent on Notch signaling.
Notch signaling is a conserved pathway in animals that is involved in a multitude of biological processes, including embryonic development, cell differentiation, and cell proliferation [166,167]. The O-fucosylation of epidermal growth factor-like (EGF) tandem repeats in the extracellular domain of Notch is essential for Notch protein folding and its cell surface presentation. In mice, targeted mutation of the protein O-fucosyltransferase 1 (Pofut1) gene leads to global Notch signaling defects and causes mid-gestation embryonic lethality [168]. In Drosophila, the removal of the O-fucosyltransferase 1 (ofut1) gene results in global Notch signaling defects [169,170]. In contrast, the Drosophila null mutants of gfr (CG9620), an SLC35C1 ortholog gene, exhibit only mild Notch signaling defects [171], although the cDNA can recover the defects in GDP-fucose transport in fibroblast cells from the LADII patient [171,172]. Skurska et al. reported that fucosylation was significantly reduced but not completely abolished in the SLC35C1-deficient cell lines [173]. Supplementation with high concentrations of Fuc restored fucosylation of N- and O-glycans in the SLC35C1-deficient cells. The SLC35C1-dependent pathway appears to primarily use GDP-Fuc derived from the de novo pathway, whereas the SLC35C1-independent pathway preferentially uses GDP-Fucose derived from the salvage pathway [173].
4.2. SLC35C2
SLC35C2 is closely related to the GDP-Fuc transporter SLC35C1 and has been proposed to be a putative GDP-Fuc transporter. However, the function and substrate specificity of the protein remain unclear.
SLC35C2 was initially identified as a gene that is overexpressed in ovarian cancer (OVCOV1) [174]. In 2010, Lu et al. reported that SLC35C2 promotes Notch1 fucosylation and is required for optimal Notch signaling in cultured mammalian cells [175]. SLC35C2 is shown to localize predominantly to the Golgi apparatus, but a small subset is found in the ER and the ER-Golgi intermediate compartment. Lu et al. proposed that SLC35C2 is a potential transporter specifically required for O-fucosylation of certain proteins, including the Notch receptor [175].
However, the Slc35c2 null mice were viable and fertile and exhibited no evidence of defective Notch signaling during skeletal or T cell development [176]. Furthermore, the deletion of both the Slc35c1 and Slc35c2 genes did not result in any defects in Notch signaling or Notch1 afucosylation [176]. Furthermore, Skurska et al. observed that SLC35C1 and SLC35C2 double knockout HEK293T cells exhibit fucosylated glycans that are comparable to those in SLC35C1 single knockout cells [173]. SLC35C2 may serve as a non-redundant GDP-Fuc supplier for the fucosylation of N- and O-glycans in the Golgi lumen and may also exist as an additional GDP-Fuc transport pathway in the mammalian Golgi membrane [5,173].
In a study, Gao M. et al. reported that SLC35C2 is a specific transporter for the uptake of CDyB (Compound of Designation yellow for B cell), a small molecule probe used for the selective detection of live B lymphocytes [177]. The knockout of the SLC35C2 transporter eliminated the ability of target cells to internalize the molecule into the ER or Golgi apparatus. Moreover, SLC35C2 expression was found to be positively correlated with the B cell maturation process, whereas it was not involved in the development of T and NK cells [177].
5. SLC35D Subfamily
The SLC35D subfamily comprises four member genes. Among them, SLC35D1 and SLC35D2 are closely related and encode multisubstrate-specific transporters involved in GAG synthesis. Both C. elegans and Drosophila have single corresponding genes encoding for UDP-sugar transporters, which are involved in proteoglycan synthesis and essential for development [178,179,180,181]. It is known that loss-of-function mutations in the SLC35D1 gene are associated with Schneckenbecken dysplasia (SLC35D1-CDG) [182].
5.1. SLC35D1
SLC35D1 is a multisubstrate-specific transporter for UDP-GalNAc, UDP-GlcNAc, and UDP-GlcUA [183,184]. As these nucleotide sugars are substrates for GAG synthesis, SLC35D1 has been considered to be essential for the production of proteoglycans and skeletal development. Loss-of-function mutations in this gene are responsible for the human disorder known as Schneckenbecken dysplasia (SLC35D1-CDG) [182].
In 2001, Muraoka et al. first reported the SLC35D1 gene as UDP-Gal transporter-related 7 (UGTrel7) and identified that it encodes a transporter for UDP-GalNAc and UDP-GlcUA [183]. Subsequently, the researchers reported that SLC35D1 also transports UDP-GlcNAc via an UDP-sugar/UDP-sugar antiport mechanism [184]. SLC35D1 protein shows an ER localization in mammalian cells [183,185], suggesting a potential role in glucuronidation via the supply of UDP-GlcUA into the ER lumen, where glucuronosyltransferases are localized [186,187,188]. Furthermore, SLC35D1 can provide the donor substrates for the synthesis of the repeating disaccharide unit of CS, namely UDP-GlcUA and UDP-GalNAc [184], although the elongation of repeating disaccharides of GAG chains occurs within the Golgi lumen [189,190] (Figure 5).
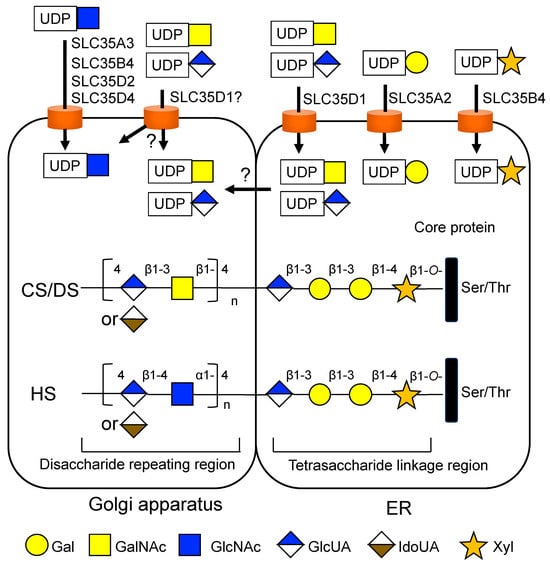
Figure 5.
Potential involvement of NSTs in the synthesis of chondroitin sulfate (CS), dermatan sulfate (DS), and heparan sulfate (HS) glycosaminoglycans (GAGs). The biosynthesis of GAG is initiated by the formation of the O-linked tetrasaccharide structure, which is common to CS/DS and HS. CS/DS and HS contain different types of repeated disaccharide units. HS and DS contain a high content of iduronic acid (IdoUA), which is converted from glucuronic acid (GlcUA) by C5 epimerization. SLC35D1 can provide the donor substrates for the elongation of CS/DS-type disaccharide units; however, SLC35D1 is localized to the ER, and the elongation of repeating disaccharides occurs within the Golgi lumen. SLC35A3, SLC35B4, SLC35D2, and SLC35D4 can transport UDP-GlcNAc, which serves as a donor substrate for the elongation of HS-type disaccharide units.
In 2007, Hiraoka et al. demonstrated that SLC35D1 plays a critical role in CS synthesis in cartilage and is indispensable in skeletal development in mice and humans [182]. The Slc35d1–/– homozygous knockout mice exhibited a skeletal dysplasia with severe shortening of limbs and facial structures, as well as neonatal lethality. The Slc35d1–/– mice also exhibited hypoplastic craniofacial bones, vertebral bone flattening, longitudinally short ilia, and extremely short long bones. Moreover, epiphyseal cartilage in Slc35d1–/– mice showed a decreased proliferating zone with round chondrocytes, scarce matrices, and reduced proteoglycan aggregates. The immunohistological examination of the epiphyseal cartilage in the mutant mice revealed a markedly reduced content of CS. The production of short, sparse CS chains due to a defect in CS biosynthesis resulted in the disorganization of a functional ECM in epiphyseal cartilage [182].
The researchers also revealed that loss-of-function mutations of SLC35D1 are associated with Schneckenbecken dysplasia in humans [182]. Schneckenbecken dysplasia is an autosomal recessive skeletal dysplasia classified as a severe spondylodysplastic dysplasia group disease. The disease is characterized by the presence of snail-shaped hypoplastic iliac bones, limbs with extremely short, long bones, and flat vertebral bodies. These defects are associated with perinatal lethality. In two typical cases of Schneckenbecken dysplasia, three mutations were identified that were predicted to produce truncated proteins. These truncated proteins exhibited loss of function with respect to transport activity toward UDP-GalNAc, UDP-GlcUA, and UDP-GlcNAc [182]. Moreover, the skeletal and histological phenotypes observed in Slc35d1–/– mice were analogous to those observed in human patients with Schneckenbecken dysplasia.
Subsequently, several additional mutations in SLC35D1 have been identified in patients with Schneckenbecken dysplasia [191,192,193]. These loss-of-function mutations of SLC35D1 appear to be exclusively associated with Schneckenbecken dysplasia and not in other severe spondylodysplastic dysplasia-related diseases [191]. Rautengarten et al. reported that SLC35D1 acts as a general UDP-sugar transporter [194]. They observed that its hypomorphic missense variants of Pro133Leu and Thr65Pro resulted in a significant decrease in transport activity and Schneckenbecken-like dysplasia. The researchers also proposed that SLC35D1 may have an expanded role beyond CS biosynthesis, participating in a variety of important glycosylation reactions occurring in the ER [194].
5.2. SLC35D2
In 2004, Suda et al. identified human SLC35D2 as a Drosophila frc homolog and named it hfrc1 (human fringe connection 1) [185]. SLC35D2 is closely related to human SLC35D1, with 54.3% identity of their amino acid sequences. However, in contrast to SLC35D1, SLC35D2 was found to localize to the Golgi apparatus [185]. The SLC35D2 protein exhibited multi-substrate transport activity towards UDP-GlcNAc, UDP-Glc, and GDP-Man [185]. However, the GDP-Man transport activity was only observed in the yeast expression system, which contains a high level of the antiport molecule GMP in the Golgi apparatus but not in the mammalian cells [185].
Both SLC35D1 and SLC35D2 mRNAs are expressed in all adult and fetal tissues [18,185]. The tissue distribution of SLC35D2 is highly similar to that of SLC35D1, while the expression level of SLC35D2 is consistently higher than that of SLC35D1 in most tissues [185]. Ishida et al. independently reported that SLC35D2 can transport UDP-GlcNAc to the Golgi apparatus [195]. Sesma et al. demonstrated that overexpressed SLC35D2 protein is localized to the Golgi apparatus in human bronchial epithelial cells and enhances the expression of cell surface GlcNAc-rich glycans, as well as the cellular release of UDP-sugar signaling molecules [196]. Suda et al. also demonstrated that overexpression of the SLC35D2 gene in HCT116 cells modulates cell surface HS levels by providing UDP-GlcNAc for GAG synthesis in the Golgi lumen [185]. In MDA-MB-231 breast cancer cells, the expression of SLC35D1 and SLC35D2 affected breast cancer cell behavior in conjunction with the expression of glycosyltransferases for HS synthesis [197]. However, unlike SLC35A3, no disease associated with SLC35D2 impairment has yet been identified.
SLC35D2 also exerts a UDP-Glc transport activity in the Golgi apparatus [185]. The Golgi-localized UDP-Glc transporter has been postulated to be involved in glycosylation processes in the Golgi apparatus, such as the addition of O-linked glucose [198]. Gehre et al. demonstrated that human SLC35D2 exhibits UDP-Glc transport activity in glycogen-rich vacuoles [199]. In cells infected with the pathogen Chlamydia trachomatis, SLC35D2 was recruited to the inclusion membrane and translocated UDP-Glc into the lumen for de novo glycogen synthesis of the bacteria. The knockdown of the SLC35D2 gene resulted in a significant reduction of intraluminal glycogen staining [199]. However, because the reduction was only partial, the researchers proposed the existence of other UDP-Glc transporter(s) in the Golgi apparatus. Indeed, UDP-Glc transport activity has also been found in another subfamily member, SLC35D3 [200] (see the next section).
In both Drosophila and C. elegans, the human SLC35D1 and SLC35D2 genes share a single highly homologous gene, namely the fringe connection (frc, CG3874) and sqv-7, respectively. The sqv-7 gene encodes a multisubstrate-specific transporter for UDP-Gal, UDP-GalNAc, and UDP-GlcUA [179]. In C. elegans, sqv-7 mutants exhibit defects in both chondroitin and HS biosynthesis and display the squashed vulva (sqv) phenotype, which is characterized by a defect in epithelial invaginations of the vulva during early embryogenesis [178]. In Drosophila, the frc gene encodes a multisubstrate transporter that transfers UDP sugars, including UDP-GlcUA, UDP-GlcNAc, and UDP-Xyl, from the cytosol into the Golgi lumen [180,181]. The frc mutant embryos display defects in Wingless, Hedgehog, and FGF signaling [181], as well as a Notch signaling defect [180]. It has been proposed that FRC and SLC35D1 utilize UDP-sugars and UDP as well as UMP as antiport molecules for countertransport [184].
5.3. SLC35D3
SLC35D3 has been identified as a UDP-Glc transporter [200] and proposed to be involved in platelet dense granule formation, synaptic vesicular neurotransmitter transport, and adipocyte differentiation. SLC35D3 has been proposed to be involved in the pathogenesis of some recessive metabolic disorders, including Hermansky–Pudlak syndrome (HPS) and Chediak–Higashi syndrome (CHS). HPS is a rare genetic disorder that is characterized by albinism, which is the absence of pigment in the skin, hair, and eyes. It is also associated with bleeding problems due to a deficiency of the platelet δ-storage pool, which is caused by malformation of platelet dense granules and pigment cell melanosomes [201].
In 2007, Chintala et al. first reported that mutation of Slc35d3 causes platelet dysfunction by regulating the content of platelet dense granules in Roswell (ros) mutant mice [201]. The ros is a mouse model of HPS and CHS in which the lysosome-related organelles, including dense granules, are dysfunctional. Due to its genetic heterogeneity, several candidate genes have been identified in both human HPS and mouse models [202]. In hypopigmentation mouse models, the ashen strain exhibits typical features of HPS, including prolonged bleeding time accompanied by a substantial platelet-dense granule deficiency due to a mutation on the Ashen gene (Rab27a), which is a critical gene for organelle-specific protein trafficking in melanocytes and platelets [203]. The ros mutant mice possess an additional mutation on the Slc35d3 gene due to an insertion of an IAP element within exon 1. This mutation results in a possible alteration of membrane topology by amino acid substitutions at the N-terminus [201].
Chintala et al. demonstrated that the platelet-dense granule defect observed in ros–/– mice was effectively reversed in transgenic mice that expressed the normal Slc35d3 gene. However, the effect was specific to platelet-dense granules and had no effect on melanosomes (i.e., pigmentation defect) or lysosomes. Furthermore, no SLC35D3 mutations were identified in 22 HPS patients who presented with albinism and prolonged bleeding symptoms of unknown etiology [201]. In a study using ros–/– mutant mice, Meng et al. observed that the delivery of SLC35D3 protein from early endosomes of megakaryocytes is required for platelet dense granule biogenesis, which is differentially defective in HPS models [204]. Epitope-tagged and endogenous SLC35D3 was predominantly localized to early endosomes and subsequently delivered to nascent, dense granules. Alternatively, it may function in dense granule biogenesis directly from early endosomes [204].
In 2021, Qian et al. identified that SLC35D3 transports UDP-Glc as a major substrate by combining metabolite profiling with a radiolabeled substrate transport assay [200]. They also found that SLC35D3 is localized to synaptic vesicles and functions as a vesicular neurotransmitter transporter by ultrastructural analysis [200]. In the mouse brain, Slc35d3 mRNA is highly enriched in striatonigral medium spiny neurons (MSNs) [205,206]. Its expression is restricted to dopamine receptor D1 but not D2 in the brain [207]. Zhang et al. reported that the ros mutant mice exhibit obesity and metabolic syndrome, as well as reduced membrane dopamine receptor D1 [207]. The mice showed impaired dopamine signaling in striatal neurons, which are involved in metabolic control in the central nervous system by regulating dopamine signaling. Additionally, the researchers identified two mutations in SLC35D3 (K404X and insL201) in patients with metabolic syndrome. The mutations resulted in altered subcellular localization of SLC35D3 and impaired dopamine signaling in striatal neurons [207].
In humans, a locus near the sequence tagged site (STS) marker D6S1009, located in the intergenic region between SLC35D3 (55,419 bp apart on the centromeric side) and NHEG1 (867 bp apart on the telomeric side), has been associated with obesity or body mass index (BMI) [208,209,210]. Li et al. proposed that SLC35D3 is a specific transcriptional regulatory factor during porcine intramuscular preadipocyte differentiation [211]. In porcine tissues, SLC35D3 exhibited high expression in various tissues, including leaf fat and subcutaneous adipose tissue. Gene silencing with SLC35D3 siRNA resulted in increased adipogenic processes in intramuscular adipocytes [211]. Wang et al. also reported that SLC35D3 can promote white adipose tissue browning and ameliorate obesity by inhibiting Notch signaling in mice [212]. They showed that white adipocyte-specific Slc35d3 knockin protected against diet-induced obesity, whereas its knockout inhibited white adipose tissue browning and caused decreased energy expenditure and impaired insulin sensitivity. SLC35D3 directly interacted with the extracellular domain of Notch1, resulting in the accumulation of Notch1 protein in the ER and the inhibition of its transport to the plasma membrane [212].
5.4. SLC35D4
The SLC35D4 gene, previously known as TMEM241 (Transmembrane Protein 241, synonyms C18orf45 or hVVT), has been recently added to the SLC35D subfamily. The SLC35D4 gene encodes a UDP-GlcNAc transporter that is localized in the Golgi apparatus [213].
In 2023, Zhao et al. reported that the SLC35D4 (TMEM241) is required for the mannose-6-phosphate (Man-6P) modification of NPC2 and cholesterol transport from lysosomes [213]. This modification is necessary for the lysosomal targeting of proteins, including NPC2, which mediates cholesterol exit from lysosomes. In HeLa cells, the knockout of the SLC35D4 gene resulted in impaired sorting of NPC2 protein, which in turn caused cholesterol accumulation in lysosomes. The SLC35D4 protein is closely related to SLC35A3, and both proteins are localized to the cis-Golgi network. Cells lacking either SLC35A3 or SLC35D4 exhibited a 70–80% reduction in UDP-GlcNAc content in the Golgi apparatus compared to wild-type cells. In SLC35A3 knockout cells, Man-6P modification of total cellular proteins was also reduced, resulting in cholesterol accumulation in lysosomes. Furthermore, overexpression of SLC35A3 rescued intracellular cholesterol accumulation and NPC2 sorting abnormalities caused by SLC35D4 knockout. Mice deficient in SLC35D4 exhibited cholesterol accumulation in pulmonary cells and displayed pulmonary injury and hypokinesia, indicating the crucial role of this transporter protein in maintaining lysosome homeostasis and lung function [213].
6. SLC35E Subfamily
Five genes (E1, E2A, E2B, E3, and E4; E2A is a pseudogene) belong to the E subfamily, yet neither their transport substrates nor their biological functions have been elucidated.
In a recent study, Huang et al. demonstrated that SLC35E1 plays a role in regulating zinc-ion-mediated keratinocyte proliferation [214]. They observed that SLC35E1 is enriched in the suprabasal layer of psoriasis lesions and that its expression is increased in keratinocytes of patients with psoriasis. Furthermore, Slc35e1 knockout mice with imiquimod-induced psoriasis displayed a less severe psoriasis phenotype than wild-type mice. In keratinocytes, SLC35E1 regulates zinc ion concentrations and localization, and its deficiency inhibits the proliferation of keratinocytes. The researchers proposed that SLC35E1 promotes keratinocyte proliferation by regulating zinc levels, although they did not confirm its zinc ion transport activity [214].
7. SLC35F Subfamily
Six genes belong to the SLC35F subfamily. To date, no member of the SLC35F subfamily has been reported to be involved in nucleotide sugar transport. It has been proposed that these transporters may facilitate drug transport. In these members, SLC35F3 has been identified to have a thiamine (vitamin B1) transport activity [215]. Some of the SLC35F members have been proposed to be associated with a range of human diseases, such as hypertension [215] and neurodevelopmental disorders [216].
7.1. SLC35F1
Despite the absence of conclusive evidence regarding the function of SLC35F1, several studies have indicated that SLC35F1 may play a role in neurodevelopment and be associated with human neurodevelopmental disorders.
In humans, SLC35F1 mRNA is highly expressed in the fetal and adult brain [18]. Farenholtz et al. reported that Slc35f1 is highly expressed in a variety of brain structures, including the cortex, hippocampus, amygdala, thalamus, basal ganglia, and hypothalamus, in adult mouse forebrain [217]. The SLC35F1 protein is localized in the recycling endosome but not in the Golgi or ER, where the majority of NSTs are localized [217].
In 2015, Szafranski et al. reported that deletions in a chromosomal region, including the regulatory sequences of SLC35F1 (6q22.1q22.31) are associated with pediatric epilepsy [218]. Furthermore, Di Fede et al. reported that a patient carrying a heterozygous missense variant in SLC35F1 exhibited a phenotype similar to Rett syndrome (RTT) [216]. RTT is a severe neurodevelopmental disorder that results in psychomotor regression and the progressive development of a severe condition associated with motor, cognitive, and behavioral impairment. The patient with the SLC35F1 mutation exhibited refractory seizures, severe intellectual disability, limited speech, and an inability to walk independently [216].
However, unlike humans, the knockout mice lacking the Slc35f1 gene displayed no significant alterations in neurodevelopmental phenotypes [219]. Ehlers et al. reported that the elimination of Slc35f1 in mice did not result in any significant impairment of learning and memory, nor did it have a substantial impact on brain morphology [219]. Furthermore, their neuroanatomical parameters of neuronal plasticity (as dendritic spines and adult hippocampal neurogenesis) remain unaltered [219]. The researchers presume that this discrepancy may be attributed to the difference in Slc35f1 expression in astrocytes between mice and humans.
7.2. SLC35F2
SLC35F2 was initially identified as a lung squamous cell carcinoma-related gene HSNOV1 (homo sapiens novel protein 1) [220]. The physiological function of SLC35F2 remains unclear. However, it has been reported that this protein is involved in the uptake of the anticancer drug YM155.
The SLC35F2 mRNA is expressed at the highest levels in the adult salivary gland [18] and brain [221]. The SLC35F2 transcripts were found to be highly enriched in brain microvascular endothelial cells (BMEC), suggesting a potential role in the construction of the brain endothelial cell-selective blood–brain barrier [221]. Additionally, SLC35F2 is expressed at a high level in a variety of human cancers. A number of studies have reported that the expression and function of SLC35F2 are related to the development of malignant tumors in various cancers, including lung cancer [222,223], papillary thyroid cancer [224], bladder cancer [225], and pancreatic cancer [226].
In 2014, Winter et al. reported that SLC35F2 is a key factor in the biological function of an anticancer drug, YM155 (sepantronium bromide) [227]. YM155 is a drug that inhibits the transcription of the survivin promoter. The YM155 molecule is transported into cells via the SLC35F2 transporter, where it generates DNA damage through intercalation [227]. Winter et al. demonstrated a correlation between YM155 sensitivity and SLC35F2 expression and its drug-importing activity in several cancer cell lines. SLC35F2 is identified as the primary determinant of YM155-mediated DNA damage toxicity [227]. Voges et al. also reported that the drug-resistant cell lines displayed decreased sensitivity to YM155, accompanied by downregulation of SLC35F2 [228]. Neuroblastoma cells that have adapted to YM155 displayed reduced levels of survivin and SLC35F2, increased levels of a multidrug resistance transporter ABCB1, and decreased sensitivity to survivin depletion [228]. Furthermore, Nyquist et al. reported that androgen and its receptor signaling axis upregulate SLC35F2 transcription and enhance sensitivity to YM155 in castrate prostate cancer patients. Since SLC35F2 expression is correlated with androgen levels, high-dose androgens act in synergy with YM155 treatment by inducing SLC35F2 expression in prostate cancer cells [229,230].
YM155 is also utilized as a stemotoxic molecule to eliminate undifferentiated human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells for tumor-free cell therapy. In human ES cells lacking SLC35F2, the stemotoxic activity of YM155 was completely absent. The formation of hydrogen bonds involving the pyrazine ring structure is crucial for the recognition of YM155 by SLC35F2 [231]. Additionally, the high cytotoxicity of YM155 in human iPS cells is attributed to the high expression of SLC35F2 in these cells. The knockout of SLC35F2 with CRISPR-Cas9, or depletion with siRNAs, rendered the iPS cells highly resistant to YM155 [232].
7.3. SLC35F3 and F4
The SLC35F3 protein was shown to have thiamine (vitamin B1) transport activity. Furthermore, it has been reported that its SNPs are associated with an elevated risk of hypertension in humans [215]. SLC35F4 is a paralog of SLC35F3 with 92% sequence similarity and is proposed as a putative thiamine transporter [233].
In 2014, Zhang et al. identified that two SNPs (rs16842784 and rs17514104) at the SLC35F3 gene are associated with high blood pressure and an increased risk of hypertension in a primary care population of North America and Western Europe [215]. The SLC35F3 protein, which exhibits high homology with the yeast Thi74 thiamine transporter, was found to exert thiamine transport activity. Individuals homozygous for the risk allele at SLC35F3 (rs17514104 T/T) exhibited decreased erythrocyte thiamine content. Zang et al. also identified an association between a different missense variant of SLC35F3 (rs34032258) and hypertension risk in the Chinese Han population [234]. Additionally, Seo and Choi found that a genetic variation in rs10910387 T>C and rs12135117 T>A genotypes in SLC35F3 is associated with an increased risk for hypertension in females and males, respectively, in the Korean population [235]. In both studies, the genetic variation in SLC35F3 was not a decisive predictor of hypertension risk, indicating that allele frequency may vary across different ethnic populations [234,235].
SNPs in the SLC35F3 gene have also been identified as being associated with lifestyle-related diseases, including diabetes [236], obesity [237], and metabolic syndromes [238]. In 2016, Simonti et al. identified a replicating association between SNP rs12049593 in intron 2 of SLC35F3, which is one of the Neanderthal variants, and protein-calorie malnutrition [239]. Additionally, several studies have indicated that SLC35F3 has been positively selected in East Asian populations [233,240,241]. Cheng et al. postulated that SLC35F3 may have been an adaptive genetic variant in ancestral East Asian populations associated with the adaptation to eating polished grains [241]. Ma and Xu identified the adaptive haplotypes at three thiamine transporter genes, SLC19A2, SLC35F3, and SLC35F4, by analyzing genome data of 3823 individuals representing 223 global populations [233]. The putative adaptive haplotype, allele C of SLC35F3 rs61824595, is likely of archaic origins. It reached a high frequency at least 10,000 years ago and has changed little in the recent 10,000 years in East Asian populations [233].
7.4. SLC35F5
SLC35F5 mRNA is expressed in a wide range of adult and fetal tissues [18]. However, the function of SLC35F5 remains poorly understood.
Some studies have indicated that SLC35F5 may be associated with the sensitivity of cancers to antitumor drugs. Matsuyama et al. reported that SLC35F5 is associated with 5-fluorouracil (5-FU) sensitivity in colorectal cancers. The expression of SLC35F5 was significantly higher in 5-FU responding tumors than in nonresponding tumors [242]. Hao et al. proposed that SLC35F5 may be a key effector of chemoresistance-promoting activity [243]. In non-small cell lung cancer (NSCLC) cells, the upregulation of microRNA MIR369-3p and downregulation of SLC35F5 expression were found to be associated with cisplatin resistance and the magnitude of malignancy of the cells. MIR369-3p regulates cisplatin chemoresistance in NSCLC cells by directly targeting the 3′ untranslated region of the SLC35F5 gene. There was an inverse correlation between SLC35F5 expression levels and MIR369-3p expression levels. Furthermore, the inhibition of miR-369-3p or the reactivation of epigenetically silenced SLC35F5 in NSCLC cells resulted in sensitization to cisplatin treatment [243].
7.5. SLC35F6
SLC35F6 (formerly known as C2orf18 or ANT2BP) is an orphan transporter with a poorly understood function. Kashiwaya et al. reported that SLC35F6 is one of the key molecules involved in pancreatic carcinogenesis [244].
The SLC35F6 mRNA is shown to have limited expression in normal adult organs, whereas it is highly expressed in pancreatic ductal adenocarcinoma (PDAC) cells. SLC35F6 protein is localized in the mitochondria and interacts with adenine nucleotide translocase 2 (ANT2) protein, which is involved in the maintenance of the mitochondrial membrane potential and energy homeostasis. RNAi-mediated knockdown of SLC35F6 in PDAC cell lines resulted in the induction of apoptosis and suppression of cancer cell growth, indicating that this gene plays an essential role in maintaining the viability of PDAC cells [244].
8. SLC35G Subfamily
The SLC35G group has recently been classified as a member of the SLC35 family. At present, six member genes are known to belong to this subfamily, yet the functions of these genes remain largely unidentified.
In these members, SLC35G1 was reported to be involved in the store-operated calcium entry as a scaffold protein by interacting with stromal interaction molecule 1 (STIM1), an ER calcium sensor protein [245]. Krapivinsky et al. identified the SLC35G1 protein through its interaction ability with ORAI1, a calcium-selective plasma membrane ion channel, and named it POST (partner of stromal interaction molecule 1, previously called TMEM20) [245]. The researchers revealed that following the Ca2+ store depletion in the ER, the SLC35G1 protein binds to STIM1 within the ER and moves to the plasma membrane. The STIM1-POST complex results in sustained high cytosolic Ca2+ concentration through the activation of Orai1 and the inhibition of plasma membrane Ca2+ ATPase (PMCA) activity [245].
In a recent study, Wang et al. reported that the SLC35G1 gene is a novel candidate gene for autism spectrum disorder (ASD) in the East Asian population [246]. The researchers identified nine novel ASD candidate genes from a large Chinese ASD cohort. One of these genes, SLC35G1, was examined in a mouse model to assess its function. Heterozygous Slc35g1 deletion mice exhibited deficits in social interaction and increased stereotypic behaviors [246].
9. Conclusions
The study of NSTs has spanned approximately half a century. Following the first identification of NSTs in 1996, numerous studies have revealed the diverse and important functions of the transporters in this large family. The analysis of invertebrate model organisms, such as Drosophila and C. elegans, has yielded insights into the essential roles of these NSTs and PAPS transporters in glycosylation and biological functions during development. The subsequent identification of diseases associated with variations in SLC35 family genes has led to an increasing recognition of their importance in glycosylation over the past decade. Additionally, genome editing technology has enabled the generation of an extensive number of knockout mice and knockout cells, thereby facilitating a more comprehensive understanding of the functions of the SLC35 family members. Nevertheless, over half of the members belonging to the SLC35 family remain unelucidated with regard to their functional properties and transport substrates. Despite the considerable accumulation of knowledge about NSTs, several conflicting findings have been reported regarding their transport mechanisms, substrate specificities, subcellular localizations, and functions. It is anticipated that future investigations will provide further clarification of the mechanism underlying the functions of the SLC35 family transporters.
Author Contributions
Conceptualization, S.K.; investigation, S.K.; supervision, S.K. and H.S.; visualization, S.K.; writing—original draft preparation, S.K.; writing—review and editing, S.K. and H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pizzagalli, M.D.; Bensimon, A.; Superti-Furga, G. A guide to plasma membrane solute carrier proteins. FEBS J. 2021, 288, 2784–2835. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Kawakita, M. Molecular physiology and pathology of the nucleotide sugar transporter family (SLC35). Pflugers Arch. 2004, 447, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, C.B.; Robbins, P.W.; Abeijon, C. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu. Rev. Biochem. 1998, 67, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P. Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 2011, 3, a005199. [Google Scholar] [CrossRef] [PubMed]
- Maszczak-Seneczko, D.; Wiktor, M.; Skurska, E.; Wiertelak, W.; Olczak, M. Delivery of Nucleotide Sugars to the Mammalian Golgi: A Very Well (un)Explained Story. Int. J. Mol. Sci. 2022, 23, 8648. [Google Scholar] [CrossRef] [PubMed]
- Abeijon, C.; Robbins, P.W.; Hirschberg, C.B. Molecular cloning of the Golgi apparatus uridine diphosphate-N-acetylglucosamine transporter from Kluyveromyces lactis. Proc. Natl. Acad. Sci. USA 1996, 93, 5963–5968. [Google Scholar] [CrossRef]
- Eckhardt, M.; Mühlenhoff, M.; Bethe, A.; Gerardy-Schahn, R. Expression cloning of the Golgi CMP-sialic acid transporter. Proc. Natl. Acad. Sci. USA 1996, 93, 7572–7576. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Ishida, N.; Hoshino, M.; Yamauchi, M.; Hara, T.; Ayusawa, D.; Kawakita, M. Human UDP-galactose translocator: Molecular cloning of a complementary DNA that complements the genetic defect of a mutant cell line deficient in UDP-galactose translocator. J. Biochem. 1996, 120, 236–241. [Google Scholar] [CrossRef]
- Ishida, N.; Miura, N.; Yoshioka, S.; Kawakita, M. Molecular cloning and characterization of a novel isoform of the human UDP-galactose transporter, and of related complementary DNAs belonging to the nucleotide-sugar transporter gene family. J. Biochem. 1996, 120, 1074–1078. [Google Scholar] [CrossRef]
- Berninsone, P.M.; Hirschberg, C.B. Nucleotide sugar transporters of the Golgi apparatus. Curr. Opin. Struct. Biol. 2000, 10, 542–547. [Google Scholar] [CrossRef]
- Parker, J.L.; Newstead, S. Structural basis of nucleotide sugar transport across the Golgi membrane. Nature 2017, 551, 521–524. [Google Scholar] [CrossRef]
- Parker, J.L.; Newstead, S. Gateway to the Golgi: Molecular mechanisms of nucleotide sugar transporters. Curr. Opin. Struct. Biol. 2019, 57, 127–134. [Google Scholar] [CrossRef]
- Parker, J.L.; Corey, R.A.; Stansfeld, P.J.; Newstead, S. Structural basis for substrate specificity and regulation of nucleotide sugar transporters in the lipid bilayer. Nat. Commun. 2019, 10, 4657. [Google Scholar] [CrossRef]
- Ahuja, S.; Whorton, M.R. Structural basis for mammalian nucleotide sugar transport. eLife 2019, 8, e45221. [Google Scholar] [CrossRef] [PubMed]
- Nji, E.; Gulati, A.; Qureshi, A.A.; Coincon, M.; Drew, D. Structural basis for the delivery of activated sialic acid into Golgi for sialyation. Nat. Struct. Mol. Biol. 2019, 26, 415–423. [Google Scholar] [CrossRef]
- Hadley, B.; Litfin, T.; Day, C.J.; Haselhorst, T.; Zhou, Y.; Tiralongo, J. Nucleotide Sugar Transporter SLC35 Family Structure and Function. Comput. Struct. Biotechnol. J. 2019, 17, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Digre, A.; Lindskog, C. The Human Protein Atlas-Spatial localization of the human proteome in health and disease. Prot. Sci. 2021, 30, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Suzuki, S.; Satoh, T.; Naito, S. Tissue-specific mRNA expression profiles of human solute carrier 35 transporters. Drug Metab. Pharmacokinet. 2009, 24, 91–99. [Google Scholar] [CrossRef]
- Ury, B.; Potelle, S.; Caligiore, F.; Whorton, M.R.; Bommer, G.T. The promiscuous binding pocket of SLC35A1 ensures redundant transport of CDP-ribitol to the Golgi. J. Biol. Chem. 2021, 296, 100789. [Google Scholar] [CrossRef]
- Stanley, P.; Siminovitch, L. Complementation between mutants of CHO cells resistant to a variety of plant lectins. Somatic Cell Genet. 1977, 3, 391–405. [Google Scholar] [CrossRef]
- Deutscher, S.L.; Nuwayhid, N.; Stanley, P.; Briles, E.I.; Hirschberg, C.B. Translocation across Golgi vesicle membranes: A CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell 1984, 39, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Berninsone, P.; Eckhardt, M.; Gerardy-Schahn, R.; Hirschberg, C.B. Functional expression of the murine Golgi CMP-sialic acid transporter in saccharomyces cerevisiae. J. Biol. Chem. 1997, 272, 12616–12619. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Ito, M.; Yoshioka, S.; Sun-Wada, G.H.; Kawakita, M. Functional expression of human golgi CMP-sialic acid transporter in the Golgi complex of a transporter-deficient Chinese hamster ovary cell mutant. J. Biochem. 1998, 124, 171–178. [Google Scholar] [CrossRef]
- Münster, A.K.; Eckhardt, M.; Potvin, B.; Mühlenhoff, M.; Stanley, P.; Gerardy-Schahn, R. Mammalian cytidine 5′-monophosphate N-acetylneuraminic acid synthetase: A nuclear protein with evolutionarily conserved structural motifs. Proc. Natl. Acad. Sci. USA 1998, 95, 9140–9145. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, T.L.; Vertel, B.M.; Colley, K.J. The CMP-sialic acid transporter is localized in the medial-trans Golgi and possesses two specific endoplasmic reticulum export motifs in its carboxyl-terminal cytoplasmic tail. J. Biol. Chem. 2006, 281, 31106–31118. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, M.; Gerardy-Schahn, R. Molecular cloning of the hamster CMP-sialic acid transporter. Eur. J. Biochem. 1997, 248, 187–192. [Google Scholar] [CrossRef]
- Eckhardt, M.; Gotza, B.; Gerardy-Schahn, R. Membrane topology of the mammalian CMP-sialic acid transporter. J. Biol. Chem. 1999, 274, 8779–8787. [Google Scholar] [CrossRef] [PubMed]
- Szulc, B.; Zadorozhna, Y.; Olczak, M.; Wiertelak, W.; Maszczak-Seneczko, D. Novel Insights into Selected Disease-Causing Mutations within the SLC35A1 Gene Encoding the CMP-Sialic Acid Transporter. Int. J. Mol. Sci. 2020, 22, 304. [Google Scholar] [CrossRef]
- Aoki, K.; Ishida, N.; Kawakita, M. Substrate recognition by nucleotide sugar transporters: Further characterization of substrate recognition regions by analyses of UDP-galactose/CMP-sialic acid transporter chimeras and biochemical analysis of the substrate specificity of parental and chimeric transporters. J. Biol. Chem. 2003, 278, 22887–22893. [Google Scholar] [CrossRef]
- Takeshima-Futagami, T.; Sakaguchi, M.; Uehara, E.; Aoki, K.; Ishida, N.; Sanai, Y.; Sugahara, Y.; Kawakita, M. Amino acid residues important for CMP-sialic acid recognition by the CMP-sialic acid transporter: Analysis of the substrate specificity of UDP-galactose/CMP-sialic acid transporter chimeras. Glycobiology 2012, 22, 1731–1740. [Google Scholar] [CrossRef][Green Version]
- Martinez-Duncker, I.; Dupré, T.; Piller, V.; Piller, F.; Candelier, J.J.; Trichet, C.; Tchernia, G.; Oriol, R.; Mollicone, R. Genetic complementation reveals a novel human congenital disorder of glycosylation of type II, due to inactivation of the Golgi CMP-sialic acid transporter. Blood 2005, 105, 2671–2676. [Google Scholar] [CrossRef]
- Mohamed, M.; Ashikov, A.; Guillard, M.; Robben, J.H.; Schmidt, S.; van den Heuvel, B.; de Brouwer, A.P.; Gerardy-Schahn, R.; Deen, P.M.; Wevers, R.A.; et al. Intellectual disability and bleeding diathesis due to deficient CMP--sialic acid transport. Neurology 2013, 81, 681–687. [Google Scholar] [CrossRef]
- Ng, B.G.; Asteggiano, C.G.; Kircher, M.; Buckingham, K.J.; Raymond, K.; Nickerson, D.A.; Shendure, J.; Bamshad, M.J.; University of Washington Center for Mendelian Genomics; Ensslen, M.; et al. Encephalopathy caused by novel mutations in the CMP-sialic acid transporter, SLC35A1. Am. J. Med. Genet. A 2017, 173, 2906–2911. [Google Scholar] [CrossRef] [PubMed]
- Kauskot, A.; Pascreau, T.; Adam, F.; Bruneel, A.; Reperant, C.; Lourenco-Rodrigues, M.D.; Rosa, J.P.; Petermann, R.; Maurey, H.; Auditeau, C.; et al. A mutation in the gene coding for the sialic acid transporter SLC35A1 is required for platelet life span but not proplatelet formation. Haematologica 2018, 103, e613–e617. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Kondo, Y.; Shi, H.; Han, J.; Jiang, Y.; Bai, X.; Archer-Hartmann, S.A.; Azadi, P.; Ruan, C.; et al. Slc35a1 deficiency causes thrombocytopenia due to impaired megakaryocytopoiesis and excessive platelet clearance in the liver. Haematologica 2021, 106, 759–769. [Google Scholar] [CrossRef]
- Jae, L.T.; Raaben, M.; Riemersma, M.; van Beusekom, E.; Blomen, V.A.; Velds, A.; Kerkhoven, R.M.; Carette, J.E.; Topaloglu, H.; Meinecke, P.; et al. Deciphering the glycosylome of dystroglycanopathies using haploid screens for lassa virus entry. Science 2013, 340, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Riemersma, M.; Sandrock, J.; Boltje, T.J.; Büll, C.; Heise, T.; Ashikov, A.; Adema, G.J.; van Bokhoven, H.; Lefeber, D.J. Disease mutations in CMP-sialic acid transporter SLC35A1 result in abnormal α-dystroglycan O-mannosylation, independent from sialic acid. Hum. Mol. Genet. 2015, 24, 2241–2246. [Google Scholar] [CrossRef]
- Praissman, J.L.; Willer, T.; Sheikh, M.O.; Toi, A.; Chitayat, D.; Lin, Y.Y.; Lee, H.; Stalnaker, S.H.; Wang, S.; Prabhakar, P.K.; et al. The functional O-mannose glycan on α-dystroglycan contains a phospho-ribitol primed for matriglycan addition. Elife 2016, 5, e14473. [Google Scholar] [CrossRef]
- Gerin, I.; Ury, B.; Breloy, I.; Bouchet-Seraphin, C.; Bolsée, J.; Halbout, M.; Graff, J.; Vertommen, D.; Muccioli, G.G.; Seta, N.; et al. ISPD produces CDP-ribitol used by FKTN and FKRP to transfer ribitol phosphate onto α-dystroglycan. Nat. Commun. 2016, 7, 11534. [Google Scholar] [CrossRef] [PubMed]
- Kanagawa, M.; Kobayashi, K.; Tajiri, M.; Manya, H.; Kuga, A.; Yamaguchi, Y.; Akasaka-Manya, K.; Furukawa, J.I.; Mizuno, M.; Kawakami, H.; et al. Identification of a Post-translational Modification with Ribitol-Phosphate and Its Defect in Muscular Dystrophy. Cell Rep. 2016, 14, 2209–2223. [Google Scholar] [CrossRef]
- Willer, T.; Lee, H.; Lommel, M.; Yoshida-Moriguchi, T.; de Bernabe, D.B.; Venzke, D.; Cirak, S.; Schachter, H.; Vajsar, J.; Voit, T.; et al. ISPD loss-of-function mutations disrupt dystroglycan O-mannosylation and cause Walker-Warburg syndrome. Nat. Genet. 2012, 44, 575–580. [Google Scholar] [CrossRef]
- Roscioli, T.; Kamsteeg, E.J.; Buysse, K.; Maystadt, I.; van Reeuwijk, J.; van den Elzen, C.; van Beusekom, E.; Riemersma, M.; Pfundt, R.; Vissers, L.E.; et al. Mutations in ISPD cause Walker-Warburg syndrome and defective glycosylation of α-dystroglycan. Nat. Genet. 2012, 44, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Nakahori, Y.; Miyake, M.; Matsumura, K.; Kondo-Iida, E.; Nomura, Y.; Segawa, M.; Yoshioka, M.; Saito, K.; Osawa, M.; et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature 1998, 394, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Brockington, M.; Blake, D.J.; Prandini, P.; Brown, S.C.; Torelli, S.; Benson, M.A.; Ponting, C.P.; Estournet, B.; Romero, N.B.; Mercuri, E.; et al. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin alpha2 deficiency and abnormal glycosylation of alpha-dystroglycan. Am. J. Hum. Genet. 2001, 69, 1198–1209. [Google Scholar] [CrossRef]
- Brockington, M.; Yuva, Y.; Prandini, P.; Brown, S.C.; Torelli, S.; Benson, M.A.; Herrmann, R.; Anderson, L.V.; Bashir, R.; Burgunder, J.M.; et al. Mutations in the fukutin-related protein gene (FKRP) identify limb girdle muscular dystrophy 2I as a milder allelic variant of congenital muscular dystrophy MDC1C. Hum. Mol. Genet. 2001, 10, 2851–2859. [Google Scholar] [CrossRef]
- Kumamoto, K.; Goto, Y.; Sekikawa, K.; Takenoshita, S.; Ishida, N.; Kawakita, M.; Kannagi, R. Increased expression of UDP-galactose transporter messenger RNA in human colon cancer tissues and its implication in synthesis of Thomsen-Friedenreich antigen and sialyl Lewis A/X determinants. Cancer Res. 2001, 61, 4620–4627. [Google Scholar]
- Ng, B.G.; Buckingham, K.J.; Raymond, K.; Kircher, M.; Turner, E.H.; He, M.; Smith, J.D.; Eroshkin, A.; Szybowska, M.; Losfeld, M.E.; et al. Mosaicism of the UDP-galactose transporter SLC35A2 causes a congenital disorder of glycosylation. Am. J. Hum. Genet. 2013, 92, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Kodera, H.; Nakamura, K.; Osaka, H.; Maegaki, Y.; Haginoya, K.; Mizumoto, S.; Kato, M.; Okamoto, N.; Iai, M.; Kondo, Y.; et al. De novo mutations in SLC35A2 encoding a UDP-galactose transporter cause early-onset epileptic encephalopathy. Hum. Mutat. 2013, 34, 1708–1714. [Google Scholar] [CrossRef]
- Segawa, H.; Kawakita, M.; Ishida, N. Human and Drosophila UDP-galactose transporters transport UDP-N-acetylgalactosamine in addition to UDP-galactose. Eur. J. Biochem. 2002, 269, 128–138. [Google Scholar] [CrossRef]
- Höflich, J.; Berninsone, P.; Göbel, C.; Gravato-Nobre, M.J.; Libby, B.J.; Darby, C.; Politz, S.M.; Hodgkin, J.; Hirschberg, C.B.; Baumeister, R. Loss of srf-3-encoded nucleotide sugar transporter activity in Caenorhabditis elegans alters surface antigenicity and prevents bacterial adherence. J. Biol. Chem. 2004, 279, 30440–30448. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Yoko-o, T.; Ikenaga, H.; Jigami, Y. Functional evidence for UDP-galactose transporter in Saccharomyces cerevisiae through the in vivo galactosylation and in vitro transport assay. J. Biol. Chem. 1998, 273, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.; Tanaka, N.; Iwahara, S.; Takegawa, K. The Schizosaccharomyces pombe gms1+ gene encodes an UDP-galactose transporter homologue required for protein galactosylation. Biochem. Biophys. Res. Commun. 1997, 232, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Segawa, H.; Ishida, N.; Takegawa, K.; Kawakita, M. Schizosaccharomyces pombe UDP-galactose transporter: Identification of its functional form through cDNA cloning and expression in mammalian cells. FEBS Lett. 1999, 451, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Bredeston, L.M.; Caffaro, C.E.; Samuelson, J.; Hirschberg, C.B. Golgi and endoplasmic reticulum functions take place in different subcellular compartments of Entamoeba histolytica. J. Biol. Chem. 2005, 280, 32168–32176. [Google Scholar] [CrossRef] [PubMed]
- Capul, A.A.; Barron, T.; Dobson, D.E.; Turco, S.J.; Beverley, S.M. Two functionally divergent UDP-Gal nucleotide sugar transporters participate in phosphoglycan synthesis in Leishmania major. J. Biol. Chem. 2007, 282, 14006–14017. [Google Scholar] [CrossRef] [PubMed]
- Oelmann, S.; Stanley, P.; Gerardy-Schahn, R. Point mutations identified in Lec8 Chinese hamster ovary glycosylation mutants that inactivate both the UDP-galactose and CMP-sialic acid transporters. J. Biol. Chem. 2001, 276, 26291–26300. [Google Scholar] [CrossRef] [PubMed]
- Olczak, M.; Guillen, E. Characterization of a mutation and an alternative splicing of UDP-galactose transporter in MDCK-RCAr cell line. Biochim. Biophys. Acta 2006, 1763, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Olczak, M.; Maszczak-Seneczko, D.; Sosicka, P.; Jakimowicz, P.; Olczak, T. UDP-Gal/UDP-GlcNAc chimeric transporter complements mutation defect in mammalian cells deficient in UDP-Gal transporter. Biochem. Biophys. Res. Commun. 2013, 434, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Maszczak-Seneczko, D.; Olczak, T.; Wunderlich, L.; Olczak, M. Comparative analysis of involvement of UGT1 and UGT2 splice variants of UDP-galactose transporter in glycosylation of macromolecules in MDCK and CHO cell lines. Glycoconj. J. 2011, 28, 481–492. [Google Scholar] [CrossRef]
- Kabuss, R.; Ashikov, A.; Oelmann, S.; Gerardy-Schahn, R.; Bakker, H. Endoplasmic reticulum retention of the large splice variant of the UDP-galactose transporter is caused by a dilysine motif. Glycobiology 2005, 15, 905–911. [Google Scholar] [CrossRef]
- Yoshioka, S.; Sun-Wada, G.H.; Ishida, N.; Kawakita, M. Expression of the human UDP-galactose transporter in the Golgi membranes of murine Had-1 cells that lack the endogenous transporter. J. Biochem. 1997, 122, 691–695. [Google Scholar] [CrossRef]
- Sprong, H.; Degroote, S.; Nilsson, T.; Kawakita, M.; Ishida, N.; van der Sluijs, P.; van Meer, G. Association of the Golgi UDP-galactose transporter with UDP-galactose:ceramide galactosyltransferase allows UDP-galactose import in the endoplasmic reticulum. Mol. Biol. Cell 2003, 14, 3482–3493. [Google Scholar] [CrossRef] [PubMed]
- Maszczak-Seneczko, D.; Olczak, T.; Olczak, M. Subcellular localization of UDP-GlcNAc, UDP-Gal and SLC35B4 transporters. Acta Biochim. Pol. 2011, 58, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Maszczak-Seneczko, D.; Sosicka, P.; Majkowski, M.; Olczak, T.; Olczak, M. UDP-N-acetylglucosamine transporter and UDP-galactose transporter form heterologous complexes in the Golgi membrane. FEBS Lett. 2012, 586, 4082–4087. [Google Scholar] [CrossRef]
- Maszczak-Seneczko, D.; Olczak, T.; Jakimowicz, P.; Olczak, M. Overexpression of UDP-GlcNAc transporter partially corrects galactosylation defect caused by UDP-Gal transporter mutation. FEBS Lett. 2011, 585, 3090–3094. [Google Scholar] [CrossRef]
- Sosicka, P.; Jakimowicz, P.; Olczak, T.; Olczak, M. Short N-terminal region of UDP-galactose transporter (SLC35A2) is crucial for galactosylation of N-glycans. Biochem. Biophys. Res. Commun. 2014, 454, 486–492. [Google Scholar] [CrossRef]
- Yates, T.M.; Suri, M.; Desurkar, A.; Lesca, G.; Wallgren-Pettersson, C.; Hammer, T.B.; Raghavan, A.; Poulat, A.L.; Møller, R.S.; Thuresson, A.C.; et al. SLC35A2-related congenital disorder of glycosylation: Defining the phenotype. Eur. J. Paediatr. Neurol. 2018, 22, 1095–1102. [Google Scholar] [CrossRef]
- Vals, M.A.; Ashikov, A.; Ilves, P.; Loorits, D.; Zeng, Q.; Barone, R.; Huijben, K.; Sykut-Cegielska, J.; Diogo, L.; Elias, A.F.; et al. Clinical, neuroradiological, and biochemical features of SLC35A2-CDG patients. J. Inherit. Metab. Dis. 2019, 42, 553–564. [Google Scholar] [CrossRef]
- Freeze, H.H.; Eklund, E.A.; Ng, B.G.; Patterson, M.C. Neurology of inherited glycosylation disorders. Lancet Neurol. 2012, 11, 453–466. [Google Scholar] [CrossRef]
- Paprocka, J.; Jezela-Stanek, A.; Tylki-Szymańska, A.; Grunewald, S. Congenital Disorders of Glycosylation from a Neurological Perspective. Brain Sci. 2021, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, P.; Kang, H.; Lee, B. Glycosylation and behavioral symptoms in neurological disorders. Transl. Psychiatr. 2023, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.G.; Sosicka, P.; Agadi, S.; Almannai, M.; Bacino, C.A.; Barone, R.; Botto, L.D.; Burton, J.E.; Carlston, C.; Chung, B.H.; et al. SLC35A2-CDG: Functional characterization, expanded molecular, clinical, and biochemical phenotypes of 30 unreported Individuals. Hum. Mutat. 2019, 40, 908–925. [Google Scholar] [CrossRef] [PubMed]
- Szulc, B.; Sosicka, P.; Maszczak-Seneczko, D.; Skurska, E.; Shauchuk, A.; Olczak, T.; Freeze, H.H.; Olczak, M. Biosynthesis of GlcNAc-rich N- and O-glycans in the Golgi apparatus does not require the nucleotide sugar transporter SLC35A3. J. Biol. Chem. 2020, 295, 16445–16463. [Google Scholar] [CrossRef] [PubMed]
- Witters, P.; Tahata, S.; Barone, R.; Õunap, K.; Salvarinova, R.; Grønborg, S.; Hoganson, G.; Scaglia, F.; Lewis, A.M.; Mori, M.; et al. Clinical and biochemical improvement with galactose supplementation in SLC35A2-CDG. Genet. Med. 2020, 22, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Guillen, E.; Abeijon, C.; Hirschberg, C.B. Mammalian Golgi apparatus UDP-N-acetylglucosamine transporter: Molecular cloning by phenotypic correction of a yeast mutant. Proc. Natl. Acad. Sci. USA 1998, 95, 7888–7892. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Yoshioka, S.; Chiba, Y.; Takeuchi, M.; Kawakita, M. Molecular cloning and functional expression of the human Golgi UDP-N-acetylglucosamine transporter. J. Biochem. 1999, 126, 68–77. [Google Scholar] [CrossRef]
- Thomsen, B.; Horn, P.; Panitz, F.; Bendixen, E.; Petersen, A.H.; Holm, L.E.; Nielsen, V.H.; Agerholm, J.S.; Arnbjerg, J.; Bendixen, C. A missense mutation in the bovine SLC35A3 gene, encoding a UDP-N-acetylglucosamine transporter, causes complex vertebral malformation. Genome Res. 2006, 16, 97–105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agerholm, J.S.; Bendixen, C.; Andersen, O.; Arnbjerg, J. Complex vertebral malformation in holstein calves. J. Vet. Diagn. Investig. 2001, 13, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Agerholm, J.S.; Bendixen, C.; Arnbjerg, J.; Andersen, O. Morphological variation of “complex vertebral malformation” in Holstein calves. J. Vet. Diagn. Investig. 2004, 16, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Edvardson, S.; Ashikov, A.; Jalas, C.; Sturiale, L.; Shaag, A.; Fedick, A.; Treff, N.R.; Garozzo, D.; Gerardy-Schahn, R.; Elpeleg, O. Mutations in SLC35A3 cause autism spectrum disorder, epilepsy and arthrogryposis. J. Med. Genet. 2013, 50, 733–739. [Google Scholar] [CrossRef]
- Marini, C.; Hardies, K.; Pisano, T.; May, P.; Weckhuysen, S.; Cellini, E.; Suls, A.; Mei, D.; Balling, R.; Jonghe, P.D.; et al. Recessive mutations in SLC35A3 cause early onset epileptic encephalopathy with skeletal defects. Am. J. Med. Genet. A 2017, 173, 1119–1123. [Google Scholar] [CrossRef]
- Edmondson, A.C.; Bedoukian, E.C.; Deardorff, M.A.; McDonald-McGinn, D.M.; Li, X.; He, M.; Zackai, E.H. A human case of SLC35A3-related skeletal dysplasia. Am. J. Med. Genet. A 2017, 173, 2758–2762. [Google Scholar] [CrossRef]
- Saito, S.; Mizumoto, S.; Yonekura, T.; Yamashita, R.; Nakano, K.; Okubo, T.; Yamada, S.; Okamura, T.; Furuichi, T. Mice lacking nucleotide sugar transporter SLC35A3 exhibit lethal chondrodysplasia with vertebral anomalies and impaired glycosaminoglycan biosynthesis. PLoS ONE 2023, 18, e0284292. [Google Scholar] [CrossRef]
- Kiani, C.; Chen, L.; Wu, Y.J.; Yee, A.J.; Yang, B.B. Structure and function of aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamada, Y.; Kimata, K. Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J. Biochem. 1998, 124, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Glycosaminoglycan (GAG) biosynthesis and GAG-binding proteins. Prog. Mol. Biol. Transl. Sci. 2010, 93, 1–17. [Google Scholar] [CrossRef]
- Paganini, C.; Costantini, R.; Superti-Furga, A.; Rossi, A. Bone and connective tissue disorders caused by defects in glycosaminoglycan biosynthesis: A panoramic view. FEBS J. 2019, 286, 3008–3032. [Google Scholar] [CrossRef]
- Maszczak-Seneczko, D.; Sosicka, P.; Olczak, T.; Jakimowicz, P.; Majkowski, M.; Olczak, M. UDP-N-acetylglucosamine transporter (SLC35A3) regulates biosynthesis of highly branched N-glycans and keratan sulfate. J. Biol. Chem. 2013, 288, 21850–21860. [Google Scholar] [CrossRef]
- Maszczak-Seneczko, D.; Sosicka, P.; Kaczmarek, B.; Majkowski, M.; Luzarowski, M.; Olczak, T.; Olczak, M. UDP-galactose (SLC35A2) and UDP-N-acetylglucosamine (SLC35A3) Transporters Form Glycosylation-related Complexes with Mannoside Acetylglucosaminyltransferases (Mgats). J. Biol. Chem. 2015, 290, 15475–15486. [Google Scholar] [CrossRef]
- Khoder-Agha, F.; Sosicka, P.; Escriva Conde, M.; Hassinen, A.; Glumoff, T.; Olczak, M.; Kellokumpu, S. N-acetylglucosaminyltransferases and nucleotide sugar transporters form multi-enzyme-multi-transporter assemblies in golgi membranes in vivo. Cell. Mol. Life Sci. 2019, 76, 1821–1832. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Isaji, T.; Nakano, M.; Liang, C.; Fukuda, T.; Gu, J. O-GlcNAcylation regulates β1,4-GlcNAc-branched N-glycan biosynthesis via the OGT/SLC35A3/GnT-IV axis. FASEB J. 2022, 36, e22149. [Google Scholar] [CrossRef] [PubMed]
- Sosicka, P.; Maszczak-Seneczko, D.; Bazan, B.; Shauchuk, Y.; Kaczmarek, B.; Olczak, M. An insight into the orphan nucleotide sugar transporter SLC35A4. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 825–838. [Google Scholar] [CrossRef]
- Pérez-Márquez, J.; Reguillo, B.; Paniagua, R. Cloning of the cDNA and mRNA expression of CLRP, a complex leucine repeat protein of the Golgi apparatus expressed by specific neurons of the rat brain. J. Neurobiol. 2002, 52, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Vanderperre, B.; Lucier, J.F.; Bissonnette, C.; Motard, J.; Tremblay, G.; Vanderperre, S.; Wisztorski, M.; Salzet, M.; Boisvert, F.M.; Roucou, X. Direct detection of alternative open reading frames translation products in human significantly expands the proteome. PLoS ONE 2013, 8, e70698. [Google Scholar] [CrossRef]
- Andreev, D.E.; O’Connor, P.B.; Fahey, C.; Kenny, E.M.; Terenin, I.M.; Dmitriev, S.E.; Cormican, P.; Morris, D.W.; Shatsky, I.N.; Baranov, P.V. Translation of 5′ leaders is pervasive in genes resistant to eIF2 repression. Elife 2015, 4, e03971. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.L.; Pai, V.; Perkins, G.; Chang, T.; Ma, J.; De Souza, E.V.; Chu, Q.; Vaughan, J.M.; Diedrich, J.K.; Ellisman, M.H.; et al. An Inner Mitochondrial Membrane Microprotein from the SLC35A4 Upstream ORF Regulates Cellular Metabolism. J. Mol. Biol. 2024, 436, 168559. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, F.; Zhu, Y.; Qin, S.; Hou, Y.; Guo, H.; Lin, F.; Chen, P.R.; Fan, X. Bioorthogonal photocatalytic proximity labeling in primary living samples. Nat. Commun. 2024, 15, 2712. [Google Scholar] [CrossRef]
- Sosicka, P.; Bazan, B.; Maszczak-Seneczko, D.; Shauchuk, Y.; Olczak, T.; Olczak, M. SLC35A5 Protein-A Golgi Complex Member with Putative Nucleotide Sugar Transport Activity. Int. J. Mol. Sci. 2019, 20, 276. [Google Scholar] [CrossRef]
- Yamamoto-Hino, M.; Muraoka, M.; Kondo, S.; Ueda, R.; Okano, H.; Goto, S. Dynamic regulation of innate immune responses in Drosophila by Senju-mediated glycosylation. Proc. Natl. Acad. Sci. USA 2015, 112, 5809–5814. [Google Scholar] [CrossRef]
- Kobayashi, T.; Sleeman, J.E.; Coughtrie, M.W.; Burchell, B. Molecular and functional characterization of microsomal UDP-glucuronic acid uptake by members of the nucleotide sugar transporter (NST) family. Biochem. J. 2006, 400, 281–289. [Google Scholar] [CrossRef]
- Ondo, K.; Arakawa, H.; Nakano, M.; Fukami, T.; Nakajima, M. SLC35B1 significantly contributes to the uptake of UDPGA into the endoplasmic reticulum for glucuronidation catalyzed by UDP-glucuronosyltransferases. Biochem. Pharmacol. 2020, 175, 113916. [Google Scholar] [CrossRef]
- Klein, M.C.; Zimmermann, K.; Schorr, S.; Landini, M.; Klemens, P.A.W.; Altensell, J.; Jung, M.; Krause, E.; Nguyen, D.; Helms, V.; et al. AXER is an ATP/ADP exchanger in the membrane of the endoplasmic reticulum. Nat. Commun. 2018, 9, 3489. [Google Scholar] [CrossRef]
- Schwarzbaum, P.J.; Schachter, J.; Bredeston, L.M. The broad range di- and tri-nucleotide exchanger SLC35B1 displays asymmetrical affinities for ATP transport across the ER membrane. J. Biol. Chem. 2022, 298, 101537. [Google Scholar] [CrossRef]
- Kainuma, M.; Chiba, Y.; Takeuchi, M.; Jigami, Y. Overexpression of HUT1 gene stimulates in vivo galactosylation by enhancing UDP-galactose transport activity in Saccharomyces cerevisiae. Yeast 2001, 18, 533–541. [Google Scholar] [CrossRef]
- Nakanishi, H.; Nakayama, K.; Yokota, A.; Tachikawa, H.; Takahashi, N.; Jigami, Y. Hut1 proteins identified in Saccharomyces cerevisiae and Schizosaccharomyces pombe are functional homologues involved in the protein-folding process at the endoplasmic reticulum. Yeast 2001, 18, 543–554. [Google Scholar] [CrossRef]
- Dejima, K.; Murata, D.; Mizuguchi, S.; Nomura, K.H.; Gengyo-Ando, K.; Mitani, S.; Kamiyama, S.; Nishihara, S.; Nomura, K. The ortholog of human solute carrier family 35 member B1 (UDP-galactose transporter-related protein 1) is involved in maintenance of ER homeostasis and essential for larval development in Caenorhabditis elegans. FASEB J. 2009, 23, 2215–2225. [Google Scholar] [CrossRef]
- Sekine, S.U.; Haraguchi, S.; Chao, K.; Kato, T.; Luo, L.; Miura, M.; Chihara, T. Meigo governs dendrite targeting specificity by modulating ephrin level and N-glycosylation. Nat. Neurosci. 2013, 16, 683–691. [Google Scholar] [CrossRef]
- Kamemura, K.; Moriya, H.; Ukita, Y.; Okumura, M.; Miura, M.; Chihara, T. Endoplasmic reticulum proteins Meigo and Gp93 govern dendrite targeting by regulating Toll-6 localization. Dev. Biol. 2022, 484, 30–39. [Google Scholar] [CrossRef]
- Kamiyama, S.; Suda, T.; Ueda, R.; Suzuki, M.; Okubo, R.; Kikuchi, N.; Chiba, Y.; Goto, S.; Toyoda, H.; Saigo, K.; et al. Molecular cloning and identification of 3′-phosphoadenosine 5′-phosphosulfate transporter. J. Biol. Chem. 2003, 278, 25958–25963. [Google Scholar] [CrossRef]
- Guasto, A.; Dubail, J.; Aguilera-Albesa, S.; Paganini, C.; Vanhulle, C.; Haouari, W.; Gorría-Redondo, N.; Aznal-Sainz, E.; Boddaert, N.; Planas-Serra, L.; et al. Biallelic variants in SLC35B2 cause a novel chondrodysplasia with hypomyelinating leukodystrophy. Brain 2022, 145, 3711–3722. [Google Scholar] [CrossRef]
- Capasso, J.M.; Hirschberg, C.B. Mechanisms of glycosylation and sulfation in the Golgi apparatus: Evidence for nucleotide sugar/nucleoside monophosphate and nucleotide sulfate/nucleoside monophosphate antiports in the Golgi apparatus membrane. Proc. Natl. Acad. Sci. USA 1984, 81, 7051–7055. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.K.; Capasso, J.M.; Hirschberg, C.B. Translocation of adenosine 3′-phosphate 5′-phosphosulfate into rat liver Golgi vesicles. J. Biol. Chem. 1984, 259, 3554–3559. [Google Scholar] [CrossRef]
- Mandon, E.C.; Milla, M.E.; Kempner, E.; Hirschberg, C.B. Purification of the Golgi adenosine 3′-phosphate 5′-phosphosulfate transporter, a homodimer within the membrane. Proc. Natl. Acad. Sci. USA 1994, 91, 10707–10711. [Google Scholar] [CrossRef] [PubMed]
- Ozeran, J.D.; Westley, J.; Schwartz, N.B. Identification and partial purification of PAPS translocase. Biochemistry 1996, 35, 3695–3703. [Google Scholar] [CrossRef]
- Lüders, F.; Segawa, H.; Stein, D.; Selva, E.M.; Perrimon, N.; Turco, S.J.; Häcker, U. Slalom encodes an adenosine 3′-phosphate 5′-phosphosulfate transporter essential for development in Drosophila. EMBO J. 2003, 22, 3635–3644. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Townley, R.A.; Berry, K.L.; Bülow, H.E. The PAPS transporter PST-1 is required for heparan sulfation and is essential for viability and neural development in C. elegans. J. Cell Sci. 2009, 122, 4492–4504. [Google Scholar] [CrossRef]
- Soares da Costa, D.; Reis, R.L.; Pashkuleva, I. Sulfation of Glycosaminoglycans and Its Implications in Human Health and Disorders. Annu. Rev. Biomed. Eng. 2017, 19, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Hästbacka, J.; de la Chapelle, A.; Mahtani, M.M.; Clines, G.; Reeve-Daly, M.P.; Daly, M.; Hamilton, B.A.; Kusumi, K.; Trivedi, B.; Weaver, A. The diastrophic dysplasia gene encodes a novel sulfate transporter: Positional cloning by fine-structure linkage disequilibrium mapping. Cell 1994, 78, 1073–1087. [Google Scholar] [CrossRef]
- Superti-Furga, A.; Hästbacka, J.; Wilcox, W.R.; Cohn, D.H.; van der Harten, H.J.; Rossi, A.; Blau, N.; Rimoin, D.L.; Steinmann, B.; Lander, E.S.; et al. Achondrogenesis type IB is caused by mutations in the diastrophic dysplasia sulphate transporter gene. Nat. Genet. 1996, 12, 100–102. [Google Scholar] [CrossRef]
- Hästbacka, J.; Superti-Furga, A.; Wilcox, W.R.; Rimoin, D.L.; Cohn, D.H.; Lander, E.S. Atelosteogenesis type II is caused by mutations in the diastrophic dysplasia sulfate-transporter gene (DTDST): Evidence for a phenotypic series involving three chondrodysplasias. Am. J. Hum. Genet. 1996, 58, 255–262. [Google Scholar]
- Superti-Furga, A.; Neumann, L.; Riebel, T.; Eich, G.; Steinmann, B.; Spranger, J.; Kunze, J. Recessively inherited multiple epiphyseal dysplasia with normal stature, club foot, and double layered patella caused by a DTDST mutation. J. Med. Genet. 1999, 36, 621–624. [Google Scholar] [CrossRef]
- Faiyaz ul Haque, M.; King, L.M.; Krakow, D.; Cantor, R.M.; Rusiniak, M.E.; Swank, R.T.; Superti-Furga, A.; Haque, S.; Abbas, H.; Ahmad, W.; et al. Mutations in orthologous genes in human spondyloepimetaphyseal dysplasia and the brachymorphic mouse. Nat. Genet. 1998, 20, 157–162. [Google Scholar] [CrossRef]
- Wiweger, M.I.; Avramut, C.M.; de Andrea, C.E.; Prins, F.A.; Koster, A.J.; Ravelli, R.B.; Hogendoorn, P.C. Cartilage ultrastructure in proteoglycan-deficient zebrafish mutants brings to light new candidate genes for human skeletal disorders. J. Pathol. 2011, 223, 531–542. [Google Scholar] [CrossRef]
- de Andrea, C.E.; Prins, F.A.; Wiweger, M.I.; Hogendoorn, P.C. Growth plate regulation and osteochondroma formation: Insights from tracing proteoglycans in zebrafish models and human cartilage. J. Pathol. 2011, 224, 160–168. [Google Scholar] [CrossRef]
- Bülow, H.E.; Hobert, O. Differential sulfations and epimerization define heparan sulfate specificity in nervous system development. Neuron 2004, 41, 723–736. [Google Scholar] [CrossRef]
- Schwartz, N.B.; Domowicz, M.S. Proteoglycans in brain development and pathogenesis. FEBS Lett. 2018, 592, 3791–3805. [Google Scholar] [CrossRef]
- Shimazu, D.; Yamamoto, N.; Umino, A.; Ishii, S.; Sakurai, S.; Nishikawa, T. Inhibition of D-serine accumulation in the Xenopus oocyte by expression of the rat ortholog of human 3′-phosphoadenosine 5′-phosphosulfate transporter gene isolated from the neocortex as D-serine modulator-1. J. Neurochem. 2006, 96, 30–42. [Google Scholar] [CrossRef]
- Uezato, A.; Jitoku, D.; Shimazu, D.; Yamamoto, N.; Kurumaji, A.; Iwayama, Y.; Toyota, T.; Yoshikawa, T.; Haroutunian, V.; Bentea, E.; et al. Differential genetic associations and expression of PAPST1/SLC35B2 in bipolar disorder and schizophrenia. J. Neural Transm. 2022, 129, 913–924. [Google Scholar] [CrossRef]
- Kamiyama, S.; Ichimiya, T.; Ikehara, Y.; Takase, T.; Fujimoto, I.; Suda, T.; Nakamori, S.; Nakamura, M.; Nakayama, F.; Irimura, T.; et al. Expression and the role of 3′-phosphoadenosine 5′-phosphosulfate transporters in human colorectal carcinoma. Glycobiology 2011, 21, 235–246. [Google Scholar] [CrossRef]
- Chim-ong, A.; Thawornkuno, C.; Chavalitshewinkoon-Petmitr, P.; Punyarit, P.; Petmitr, S. SLC35B2 expression is associated with a poor prognosis of invasive ductal breast carcinoma. Asian Pac. J. Cancer Prev. 2014, 15, 6065–6070. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Kumar, A.; Huhn, S.; Hullinger, L.; Du, Z. Modulating tyrosine sulfation of recombinant antibodies in CHO cell culture by host selection and sodium chlorate supplementation. Biotechnol. J. 2021, 16, e2100142. [Google Scholar] [CrossRef]
- Moore, K.L. The biology and enzymology of protein tyrosine O-sulfation. J. Biol. Chem. 2003, 278, 24243–24246. [Google Scholar] [CrossRef]
- Cai, X.; Li, S.; Zeng, X.; Xu, M.; Wang, Z.; Singhi, A.D.; Tang, D.; Li, S.; Yates, N.A.; Yang, D.; et al. Inhibition of the SLC35B2-TPST2 Axis of Tyrosine Sulfation Attenuates the Growth and Metastasis of Pancreatic Ductal Adenocarcinom. Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 473–495. [Google Scholar] [CrossRef]
- He, B.; Huang, Z.; Qin, S.; Peng, P.; Duan, X.; Wang, L.; Ye, Q.; Wang, K.; Jiang, J.; Li, B.; et al. Enhanced SLC35B2/SAV1 sulfation axis promotes tumor growth by inhibiting Hippo signaling in HCC. Hepatology 2024. [Google Scholar] [CrossRef]
- Kamiyama, S.; Sasaki, N.; Goda, E.; Ui-Tei, K.; Saigo, K.; Narimatsu, H.; Jigami, Y.; Kannagi, R.; Irimura, T.; Nishihara, S. Molecular cloning and characterization of a novel 3′-phosphoadenosine 5′-phosphosulfate transporter, PAPST2. J. Biol. Chem. 2006, 281, 10945–10953. [Google Scholar] [CrossRef]
- Sasaki, N.; Hirano, T.; Ichimiya, T.; Wakao, M.; Hirano, K.; Kinoshita-Toyoda, A.; Toyoda, H.; Suda, Y.; Nishihara, S. The 3′-phosphoadenosine 5′-phosphosulfate transporters, PAPST1 and 2, contribute to the maintenance and differentiation of mouse embryonic stem cells. PLoS ONE 2009, 4, e8262. [Google Scholar] [CrossRef]
- Goda, E.; Kamiyama, S.; Uno, T.; Yoshida, H.; Ueyama, M.; Kinoshita-Toyoda, A.; Toyoda, H.; Ueda, R.; Nishihara, S. Identification and characterization of a novel Drosophila 3′-phosphoadenosine 5′-phosphosulfate transporter. J. Biol. Chem. 2006, 281, 28508–28517. [Google Scholar] [CrossRef]
- Dejima, K.; Murata, D.; Mizuguchi, S.; Nomura, K.H.; Izumikawa, T.; Kitagawa, H.; Gengyo-Ando, K.; Yoshina, S.; Ichimiya, T.; Nishihara, S.; et al. Two Golgi-resident 3′-Phosphoadenosine 5′-phosphosulfate transporters play distinct roles in heparan sulfate modifications and embryonic and larval development in Caenorhabditis elegans. J. Biol. Chem. 2010, 285, 24717–24728. [Google Scholar] [CrossRef]
- Roy, S.K.; Chiba, Y.; Takeuchi, M.; Jigami, Y. Characterization of Yeast Yea4p, a uridine diphosphate-N-acetylglucosamine transporter localized in the endoplasmic reticulum and required for chitin synthesis. J. Biol. Chem. 2000, 275, 13580–13587. [Google Scholar] [CrossRef]
- Ashikov, A.; Routier, F.; Fuhlrott, J.; Helmus, Y.; Wild, M.; Gerardy-Schahn, R.; Bakker, H. The human solute carrier gene SLC35B4 encodes a bifunctional nucleotide sugar transporter with specificity for UDP-xylose and UDP-N-acetylglucosamine. J. Biol. Chem. 2005, 280, 27230–27235. [Google Scholar] [CrossRef]
- Wex, B.; Safi, R.M.; Antonios, G.; Zgheib, P.Z.; Awad, D.B.; Kobeissy, F.H.; Mahfouz, R.A.; El-Sabban, M.M.; Yazbek, S.N. SLC35B4, an Inhibitor of Gluconeogenesis, Responds to Glucose Stimulation and Downregulates Hsp60 among Other Proteins in HepG2 Liver Cell Lines. Molecules 2018, 23, 1350. [Google Scholar] [CrossRef]
- Bazan, B.; Wiktor, M.; Maszczak-Seneczko, D.; Olczak, T.; Kaczmarek, B.; Olczak, M. Lysine at position 329 within a C-terminal dilysine motif is crucial for the ER localization of human SLC35B4. PLoS ONE 2018, 13, e0207521. [Google Scholar] [CrossRef]
- Ishikawa, H.O.; Ayukawa, T.; Nakayama, M.; Higashi, S.; Kamiyama, S.; Nishihara, S.; Aoki, K.; Ishida, N.; Sanai, Y.; Matsuno, K. Two pathways for importing GDP-fucose into the endoplasmic reticulum lumen function redundantly in the O-fucosylation of Notch in Drosophila. J. Biol. Chem. 2010, 285, 4122–4129. [Google Scholar] [CrossRef]
- Fox, C.S.; Heard-Costa, N.; Cupples, L.A.; Dupuis, J.; Vasan, R.S.; Atwood, L.D. Genome-wide association to body mass index and waist circumference: The Framingham Heart Study 100K project. BMC Med. Genet. 2007, 8 (Suppl. 1), S18. [Google Scholar] [CrossRef]
- Yazbek, S.N.; Buchner, D.A.; Geisinger, J.M.; Burrage, L.C.; Spiezio, S.H.; Zentner, G.E.; Hsieh, C.W.; Scacheri, P.C.; Croniger, C.M.; Nadeau, J.H. Deep congenic analysis identifies many strong, context-dependent QTLs, one of which, Slc35b4, regulates obesity and glucose homeostasis. Genome Res. 2011, 21, 1065–1073. [Google Scholar] [CrossRef]
- Chen, J.; Meng, Y.; Zhou, J.; Zhuo, M.; Ling, F.; Zhang, Y.; Du, H.; Wang, X. Identifying candidate genes for Type 2 Diabetes Mellitus and obesity through gene expression profiling in multiple tissues or cells. J. Diab. Res. 2013, 2013, 970435. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, X.; Wang, K.; Zhang, X.; Yu, Y.; Lv, Y.; Zhang, S.; Zhang, L.; Guo, Y.; Li, Y.; et al. A novel YAP1/SLC35B4 regulatory axis contributes to proliferation and progression of gastric carcinoma. Cell Death Dis. 2019, 10, 452. [Google Scholar] [CrossRef]
- Lübke, T.; Marquardt, T.; Etzioni, A.; Hartmann, E.; von Figura, K.; Körner, C. Complementation cloning identifies CDG-IIc, a new type of congenital disorders of glycosylation, as a GDP-fucose transporter deficiency. Nat. Genet. 2001, 28, 73–76. [Google Scholar] [CrossRef]
- Lühn, K.; Wild, M.K.; Eckhardt, M.; Gerardy-Schahn, R.; Vestweber, D. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat. Genet. 2001, 28, 69–72. [Google Scholar] [CrossRef]
- Hellbusch, C.C.; Sperandio, M.; Frommhold, D.; Yakubenia, S.; Wild, M.K.; Popovici, D.; Vestweber, D.; Gröne, H.J.; von Figura, K.; Lübke, T.; et al. Golgi GDP-fucose transporter-deficient mice mimic congenital disorder of glycosylation IIc/leukocyte adhesion deficiency II. J. Biol. Chem. 2007, 282, 10762–10772. [Google Scholar] [CrossRef]
- Yakubenia, S.; Frommhold, D.; Schölch, D.; Hellbusch, C.C.; Körner, C.; Petri, B.; Jones, C.; Ipe, U.; Bixel, M.G.; Krempien, R.; et al. Leukocyte trafficking in a mouse model for leukocyte adhesion deficiency II/congenital disorder of glycosylation IIc. Blood 2008, 112, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, V. Formation of guanosine diphosphate L-fucose from guanosine diphosphate D-mannose. J. Biol. Chem. 1960, 235, 2196–2201. [Google Scholar] [CrossRef]
- Yurchenco, P.D.; Atkinson, P.H. Equilibration of fucosyl glycoprotein pools in HeLa cells. Biochemistry 1977, 16, 944–953. [Google Scholar] [CrossRef]
- Ohyama, C.; Smith, P.L.; Angata, K.; Fukuda, M.N.; Lowe, J.B.; Fukuda, M. Molecular cloning and expression of GDP-D-mannose-4,6-dehydratase, a key enzyme for fucose metabolism defective in Lec13 cells. J. Biol. Chem. 1998, 273, 14582–14587. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, L.; Hirschberg, C.B. Reconstitution, identification, and purification of the rat liver golgi membrane GDP-fucose transporter. J. Biol. Chem. 1999, 274, 35596–35600. [Google Scholar] [CrossRef]
- Lübke, T.; Marquardt, T.; von Figura, K.; Körner, C. A new type of carbohydrate-deficient glycoprotein syndrome due to a decreased import of GDP-fucose into the golgi. J. Biol. Chem. 1999, 274, 25986–25989. [Google Scholar] [CrossRef]
- Etzioni, A.; Sturla, L.; Antonellis, A.; Green, E.D.; Gershoni-Baruch, R.; Berninsone, P.M.; Hirschberg, C.B.; Tonetti, M. Leukocyte adhesion deficiency (LAD) type II/carbohydrate deficient glycoprotein (CDG) IIc founder effect and genotype/phenotype correlation. Am. J. Med. Genet. 2002, 110, 131–135. [Google Scholar] [CrossRef]
- Hidalgo, A.; Ma, S.; Peired, A.J.; Weiss, L.A.; Cunningham-Rundles, C.; Frenette, P.S. Insights into leukocyte adhesion deficiency type 2 from a novel mutation in the GDP-fucose transporter gene. Blood 2003, 101, 1705–1712. [Google Scholar] [CrossRef]
- Dauber, A.; Ercan, A.; Lee, J.; James, P.; Jacobs, P.P.; Ashline, D.J.; Wang, S.R.; Miller, T.; Hirschhorn, J.N.; Nigrovic, P.A.; et al. Congenital disorder of fucosylation type 2c (LADII) presenting with short stature and developmental delay with minimal adhesion defect. Hum. Mol. Genet. 2014, 23, 2880–2887. [Google Scholar] [CrossRef]
- Cagdas, D.; Yilmaz, M.; Kandemir, N.; Tezcan, I.; Etzioni, A.; Sanal, Ö. A novel mutation in leukocyte adhesion deficiency type II/CDGIIc. J. Clin. Immunol. 2014, 34, 1009–1014. [Google Scholar] [CrossRef]
- Cooper, N.; Li, Y.T.; Möller, A.; Schulz-Weidner, N.; Sachs, U.J.; Wagner, F.; Hackstein, H.; Wienzek-Lischka, S.; Grüneberg, M.; Wild, M.K.; et al. Incidental diagnosis of leukocyte adhesion deficiency type II following ABO typing. Clin. Immunol. 2020, 221, 108599. [Google Scholar] [CrossRef]
- Knapp, K.M.; Luu, R.; Baerenfaenger, M.; Zijlstra, F.; Wessels, H.J.C.T.; Jenkins, D.; Lefeber, D.J.; Neas, K.; Bicknell, L.S. Biallelic variants in SLC35C1 as a cause of isolated short stature with intellectual disability. J. Hum. Genet. 2020, 65, 743–750. [Google Scholar] [CrossRef]
- Helmus, Y.; Denecke, J.; Yakubenia, S.; Robinson, P.; Lühn, K.; Watson, D.L.; McGrogan, P.J.; Vestweber, D.; Marquardt, T.; Wild, M.K. Leukocyte adhesion deficiency II patients with a dual defect of the GDP-fucose transporter. Blood 2006, 107, 3959–3966. [Google Scholar] [CrossRef]
- Marquardt, T.; Lühn, K.; Srikrishna, G.; Freeze, H.H.; Harms, E.; Vestweber, D. Correction of leukocyte adhesion deficiency type II with oral fucose. Blood 1999, 94, 3976–3985. [Google Scholar] [CrossRef]
- Sturla, L.; Rampal, R.; Haltiwanger, R.S.; Fruscione, F.; Etzioni, A.; Tonetti, M. Differential terminal fucosylation of N-linked glycans versus protein O-fucosylation in leukocyte adhesion deficiency type II (CDG IIc). J. Biol. Chem. 2003, 278, 26727–26733. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef]
- Kopan, R.; Ilagan, M.X. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef]
- Shi, S.; Stanley, P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc. Natl. Acad. Sci. USA 2003, 100, 5234–5239. [Google Scholar] [CrossRef]
- Okajima, T.; Irvine, K.D. Regulation of notch signaling by o-linked fucose. Cell 2002, 111, 893–904. [Google Scholar] [CrossRef]
- Sasamura, T.; Sasaki, N.; Miyashita, F.; Nakao, S.; Ishikawa, H.O.; Ito, M.; Kitagawa, M.; Harigaya, K.; Spana, E.; Bilder, D.; et al. neurotic, a novel maternal neurogenic gene, encodes an O-fucosyltransferase that is essential for Notch-Delta interactions. Development 2003, 130, 4785–4795. [Google Scholar] [CrossRef]
- Ishikawa, H.O.; Higashi, S.; Ayukawa, T.; Sasamura, T.; Kitagawa, M.; Harigaya, K.; Aoki, K.; Ishida, N.; Sanai, Y.; Matsuno, K. Notch deficiency implicated in the pathogenesis of congenital disorder of glycosylation IIc. Proc. Natl. Acad. Sci. USA 2005, 102, 18532–18537. [Google Scholar] [CrossRef] [PubMed]
- Lühn, K.; Laskowska, A.; Pielage, J.; Klämbt, C.; Ipe, U.; Vestweber, D.; Wild, M.K. Identification and molecular cloning of a functional GDP-fucose transporter in Drosophila melanogaster. Exp. Cell Res. 2004, 301, 242–250. [Google Scholar] [CrossRef]
- Skurska, E.; Szulc, B.; Maszczak-Seneczko, D.; Wiktor, M.; Wiertelak, W.; Makowiecka, A.; Olczak, M. Incorporation of fucose into glycans independent of the GDP-fucose transporter SLC35C1 preferentially utilizes salvaged over de novo GDP-fucose. J. Biol. Chem. 2022, 298, 102206. [Google Scholar] [CrossRef] [PubMed]
- Leach, R.E.; Duniec-Dmuchowski, Z.M.; Pesole, G.; Tanaka, T.S.; Ko, M.S.; Armant, D.R.; Krawetz, S.A. Identification, molecular characterization, and tissue expression of OVCOV1. Mamm. Genome 2002, 13, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hou, X.; Shi, S.; Körner, C.; Stanley, P. Slc35c2 promotes Notch1 fucosylation and is required for optimal Notch signaling in mammalian cells. J. Biol. Chem. 2010, 285, 36245–36254. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Varshney, S.; Yuan, Y.; Wei, H.X.; Tanwar, A.; Sundaram, S.; Nauman, M.; Haltiwanger, R.S.; Stanley, P. In vivo evidence for GDP-fucose transport in the absence of transporter SLC35C1 and putative transporter SLC35C2. J. Biol. Chem. 2023, 299, 105406. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Lee, S.H.; Das, R.K.; Kwon, H.Y.; Kim, H.S.; Chang, Y.T. A SLC35C2 Transporter-Targeting Fluorescent Probe for the Selective Detection of B Lymphocytes Identified by SLC-CRISPRi and Unbiased Fluorescence Library Screening. Angew. Chem. Int. Ed. Engl. 2022, 61, e202202095. [Google Scholar] [CrossRef]
- Bulik, D.A.; Wei, G.; Toyoda, H.; Kinoshita-Toyoda, A.; Waldrip, W.R.; Esko, J.D.; Robbins, P.W.; Selleck, S.B. sqv-3, -7, and -8, a set of genes affecting morphogenesis in Caenorhabditis elegans, encode enzymes required for glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 2000, 97, 10838–10843. [Google Scholar] [CrossRef] [PubMed]
- Berninsone, P.; Hwang, H.Y.; Zemtseva, I.; Horvitz, H.R.; Hirschberg, C.B. SQV-7, a protein involved in Caenorhabditis elegans epithelial invagination and early embryogenesis, transports UDP-glucuronic acid, UDP-N- acetylgalactosamine, and UDP-galactose. Proc. Natl. Acad. Sci. USA 2001, 98, 3738–3743. [Google Scholar] [CrossRef]
- Goto, S.; Taniguchi, M.; Muraoka, M.; Toyoda, H.; Sado, Y.; Kawakita, M.; Hayashi, S. UDP-sugar transporter implicated in glycosylation and processing of Notch. Nat. Cell Biol. 2001, 3, 816–822. [Google Scholar] [CrossRef]
- Selva, E.M.; Hong, K.; Baeg, G.H.; Beverley, S.M.; Turco, S.J.; Perrimon, N.; Häcker, U. Dual role of the fringe connection gene in both heparan sulphate and fringe-dependent signalling events. Nat. Cell Biol. 2001, 3, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, S.; Furuichi, T.; Nishimura, G.; Shibata, S.; Yanagishita, M.; Rimoin, D.L.; Superti-Furga, A.; Nikkels, P.G.; Ogawa, M.; Katsuyama, K.; et al. Nucleotide-sugar transporter SLC35D1 is critical to chondroitin sulfate synthesis in cartilage and skeletal development in mouse and human. Nat. Med. 2007, 13, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, M.; Kawakita, M.; Ishida, N. Molecular characterization of human UDP-glucuronic acid/UDP-N-acetylgalactosamine transporter, a novel nucleotide sugar transporter with dual substrate specificity. FEBS Lett. 2001, 495, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, M.; Miki, T.; Ishida, N.; Hara, T.; Kawakita, M. Variety of nucleotide sugar transporters with respect to the interaction with nucleoside mono- and diphosphates. J. Biol. Chem. 2007, 282, 24615–24622. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Kamiyama, S.; Suzuki, M.; Kikuchi, N.; Nakayama, K.; Narimatsu, H.; Jigami, Y.; Aoki, T.; Nishihara, S. Molecular cloning and characterization of a human multisubstrate specific nucleotide-sugar transporter homologous to Drosophila fringe connection. J. Biol. Chem. 2004, 279, 26469–26474. [Google Scholar] [CrossRef] [PubMed]
- Nuwayhid, N.; Glaser, J.H.; Johnson, J.C.; Conrad, H.E.; Hauser, S.C.; Hirschberg, C.B. Xylosylation and glucuronosylation reactions in rat liver Golgi apparatus and endoplasmic reticulum. J. Biol. Chem. 1986, 261, 12936–12941. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, X.; Blanckaert, N. Carrier-mediated transport of intact UDP-glucuronic acid into the lumen of endoplasmic-reticulum-derived vesicles from rat liver. Biochem. J. 1994, 302, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Meech, R.; Mackenzie, P.I. Structure and function of uridine diphosphate glucuronosyltransferases. Clin. Exp. Pharmacol. Physiol. 1997, 24, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, A.; Fryer, P.R.; Hardingham, T.E. Proteoglycan biosynthesis in chondrocytes: Protein A-gold localization of proteoglycan protein core and chondroitin sulfate within Golgi subcompartments. J. Cell Biol. 1985, 101, 2355–2365. [Google Scholar] [CrossRef]
- Velasco, A.; Hidalgo, J.; Pérez-Vilar, J.; García-Herdugo, G.; Navas, P. Detection of glycosaminoglycans in the Golgi complex of chondrocytes. Eur. J. Cell Biol. 1988, 47, 241–250. [Google Scholar]
- Furuichi, T.; Kayserili, H.; Hiraoka, S.; Nishimura, G.; Ohashi, H.; Alanay, Y.; Lerena, J.C.; Aslanger, A.D.; Koseki, H.; Cohn, D.H.; et al. Identification of loss-of-function mutations of SLC35D1 in patients with Schneckenbecken dysplasia, but not with other severe spondylodysplastic dysplasias group diseases. J. Med. Genet. 2009, 46, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Özer, L.; Aktuna, S.; Unsal, E.; Ünal, M.A.; Sahin, G.; Baltaci, V. A novel SLC35D1 variant causing milder phenotype of Schneckenbecken dysplasia in a large pedigree. Am. J. Med. Genet. A 2022, 188, 3078–3083. [Google Scholar] [CrossRef] [PubMed]
- Esen, T.E.; Uzun, Ö.Ü.; Ceylan, A.C. A Mild Skeletal Dysplasia Caused by a Biallelic Missense Variant in the SLC35D1 Gene. Mol. Syndromol. 2023, 14, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Rautengarten, C.; Quarrell, O.W.; Stals, K.; Caswell, R.C.; De Franco, E.; Baple, E.; Burgess, N.; Jokhi, R.; Heazlewood, J.L.; Offiah, A.C.; et al. A hypomorphic allele of SLC35D1 results in Schneckenbecken-like dysplasia. Hum. Mol. Genet. 2019, 28, 3543–3551. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Kuba, T.; Aoki, K.; Miyatake, S.; Kawakita, M.; Sanai, Y. Identification and characterization of human Golgi nucleotide sugar transporter SLC35D2, a novel member of the SLC35 nucleotide sugar transporter family. Genomics 2005, 85, 106–116. [Google Scholar] [CrossRef]
- Sesma, J.I.; Esther, C.R.; Kreda, S.M.; Jones, L.; O’Neal, W.; Nishihara, S.; Nicholas, R.A.; Lazarowski, E.R. Endoplasmic reticulum/golgi nucleotide sugar transporters contribute to the cellular release of UDP-sugar signaling molecules. J. Biol. Chem. 2009, 284, 12572–12583. [Google Scholar] [CrossRef] [PubMed]
- Viola, M.; Brüggemann, K.; Karousou, E.; Caon, I.; Caravà, E.; Vigetti, D.; Greve, B.; Stock, C.; De Luca, G.; Passi, A.; et al. MDA-MB-231 breast cancer cell viability, motility and matrix adhesion are regulated by a complex interplay of heparan sulfate, chondroitin-/dermatan sulfate and hyaluronan biosynthesis. Glycoconj. J. 2017, 34, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Moloney, D.J.; Shair, L.H.; Lu, F.M.; Xia, J.; Locke, R.; Matta, K.L.; Haltiwanger, R.S. Mammalian Notch1 is modified with two unusual forms of O-linked glycosylation found on epidermal growth factor-like modules. J. Biol. Chem. 2000, 275, 9604–9611. [Google Scholar] [CrossRef] [PubMed]
- Gehre, L.; Gorgette, O.; Perrinet, S.; Prevost, M.C.; Ducatez, M.; Giebel, A.M.; Nelson, D.E.; Ball, S.G.; Subtil, A. Sequestration of host metabolism by an intracellular pathogen. Elife 2016, 5, e12552. [Google Scholar] [CrossRef]
- Qian, C.; Wu, Z.; Sun, R.; Yu, H.; Zeng, J.; Rao, Y.; Li, Y. Localization, proteomics, and metabolite profiling reveal a putative vesicular transporter for UDP-glucose. Elife 2021, 10, e65417. [Google Scholar] [CrossRef]
- Chintala, S.; Tan, J.; Gautam, R.; Rusiniak, M.E.; Guo, X.; Li, W.; Gahl, W.A.; Huizing, M.; Spritz, R.A.; Hutton, S.; et al. The Slc35d3 gene, encoding an orphan nucleotide sugar transporter, regulates platelet-dense granules. Blood 2007, 109, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Novak, E.K.; Gautam, R.; Reddington, M.; Collinson, L.M.; Copeland, N.G.; Jenkins, N.A.; McGarry, M.P.; Swank, R.T. The regulation of platelet-dense granules by Rab27a in the ashen mouse, a model of Hermansky-Pudlak and Griscelli syndromes, is granule-specific and dependent on genetic background. Blood 2002, 100, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M.; Yip, R.; Swing, D.A.; O’Sullivan, T.N.; Zhang, Y.; Novak, E.K.; Swank, R.T.; Russell, L.B.; Copeland, N.G.; Jenkins, N.A. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc. Natl. Acad. Sci. USA 2000, 97, 7933–7938. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Wang, Y.; Yao, Y.; Zhang, Z.; Harper, D.C.; Heijnen, H.F.; Sitaram, A.; Li, W.; Raposo, G.; Weiss, M.J.; et al. SLC35D3 delivery from megakaryocyte early endosomes is required for platelet dense granule biogenesis and is differentially defective in Hermansky-Pudlak syndrome models. Blood 2012, 120, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.K.; Karsten, S.L.; Gray, M.; Geschwind, D.H.; Yang, X.W. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat. Neurosci. 2006, 9, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Révy, D.; Jaouen, F.; Salin, P.; Melon, C.; Chabbert, D.; Tafi, E.; Concetta, L.; Langa, F.; Amalric, M.; Kerkerian-Le Goff, L.; et al. Cellular and behavioral outcomes of dorsal striatonigral neuron ablation: New insights into striatal functions. Neuropsychopharmacology 2014, 39, 2662–2672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hao, C.J.; Li, C.G.; Zang, D.J.; Zhao, J.; Li, X.N.; Wei, A.H.; Wei, Z.B.; Yang, L.; He, X.; et al. Mutation of SLC35D3 causes metabolic syndrome by impairing dopamine signaling in striatal D1 neurons. PLoS Genet. 2014, 10, e1004124. [Google Scholar] [CrossRef] [PubMed]
- Atwood, L.D.; Heard-Costa, N.L.; Cupples, L.A.; Jaquish, C.E.; Wilson, P.W.; D’Agostino, R.B. Genomewide linkage analysis of body mass index across 28 years of the Framingham Heart Study. Am. J. Hum. Genet. 2002, 71, 1044–1050. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arya, R.; Lehman, D.; Hunt, K.J.; Schneider, J.; Almasy, L.; Blangero, J.; Stern, M.P.; Duggirala, R.; Framingham, H.S. Evidence for bivariate linkage of obesity and HDL-C levels in the Framingham Heart Study. BMC Genet. 2003, 4 (Suppl. 1), S52. [Google Scholar] [CrossRef]
- Fox, C.S.; Heard-Costa, N.L.; Wilson, P.W.; Levy, D.; D’Agostino, R.B.; Atwood, L.D. Genome-wide linkage to chromosome 6 for waist circumference in the Framingham Heart Study. Diabetes 2004, 53, 1399–1402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, W.; Wu, K.; Liu, Y.; Yang, Y.; Wang, W.; Li, X.; Zhang, Y.; Zhang, Q.; Zhou, R.; Tang, H. Molecular cloning of SLC35D3 and analysis of its role during porcine intramuscular preadipocyte differentiation. BMC Genet. 2020, 21, 20. [Google Scholar] [CrossRef]
- Wang, H.; Yu, L.; Wang, J.; Zhang, Y.; Xu, M.; Lv, C.; Cui, B.; Yuan, M.; Zhang, Y.; Yan, Y.; et al. SLC35D3 promotes white adipose tissue browning to ameliorate obesity by NOTCH signaling. Nat. Commun. 2023, 14, 7643. [Google Scholar] [CrossRef]
- Zhao, N.; Deng, G.; Yuan, P.X.; Zhang, Y.F.; Jiang, L.Y.; Zhao, X.; Song, B.L. TMEM241 is a UDP-N-acetylglucosamine transporter required for M6P modification of NPC2 and cholesterol transport. J. Lipid Res. 2023, 64, 100465. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Chen, S.; Ding, K.; Zheng, B.; Lv, W.; Wang, X.; Zhong, Y.; Huang, H.; Zhang, X.; Ma, S.; et al. SLC35E1 promotes keratinocyte proliferation in psoriasis by regulating zinc homeostasis. Cell Death Dis. 2023, 14, 354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Huentelman, M.J.; Rao, F.; Sun, E.I.; Corneveaux, J.J.; Schork, A.J.; Wei, Z.; Waalen, J.; Miramontes-Gonzalez, J.P.; Hightower, C.M.; et al. Genetic implication of a novel thiamine transporter in human hypertension. J. Am. Coll. Cardiol. 2014, 63, 1542–1555. [Google Scholar] [CrossRef] [PubMed]
- Di Fede, E.; Peron, A.; Colombo, E.A.; Gervasini, C.; Vignoli, A. SLC35F1 as a candidate gene for neurodevelopmental disorders resembling Rett syndrome. Am. J. Med. Genet. A 2021, 185, 2238–2240. [Google Scholar] [CrossRef]
- Farenholtz, J.; Artelt, N.; Blumenthal, A.; Endlich, K.; Kroemer, H.K.; Endlich, N.; von Bohlen Und Halbach, O. Expression of Slc35f1 in the murine brain. Cell Tissue Res. 2019, 377, 167–176. [Google Scholar] [CrossRef]
- Szafranski, P.; Von Allmen, G.K.; Graham, B.H.; Wilfong, A.A.; Kang, S.H.; Ferreira, J.A.; Upton, S.J.; Moeschler, J.B.; Bi, W.; Rosenfeld, J.A.; et al. 6q22.1 microdeletion and susceptibility to pediatric epilepsy. Eur. J. Hum. Genet. 2015, 23, 173–179. [Google Scholar] [CrossRef]
- Ehlers, J.S.; Bracke, K.; von Bohlen Und Halbach, V.; Siegerist, F.; Endlich, N.; von Bohlen Und Halbach, O. Morphological and behavioral analysis of Slc35f1-deficient mice revealed no neurodevelopmental phenotype. Brain Struct. Funct. 2023, 228, 895–906. [Google Scholar] [CrossRef]
- Shen, C.; Zhao, H.; Wang, D.; Jiang, G.; Wang, J.; Zhang, G. Molecular cloning, identification and analysis of lung squamous cell carcinoma-related genes. Lung Cancer 2002, 38, 235–241. [Google Scholar] [CrossRef]
- Bangsow, T.; Baumann, E.; Bangsow, C.; Jaeger, M.H.; Pelzer, B.; Gruhn, P.; Wolf, S.; von Melchner, H.; Stanimirovic, D.B. The epithelial membrane protein 1 is a novel tight junction protein of the blood-brain barrier. J. Cereb. Blood Flow. Metab. 2008, 28, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Jiang, G.; Yang, F.; Liu, J.; Wang, J. Highly expressed SLC35F2 in non-small cell lung cancer is associated with pathological staging. Mol. Med. Report. 2011, 4, 1289–1293. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, M.; Qiu, Y.; Yang, J.; Cao, Y. Overexpression of SLC35F2 is a potential prognostic biomarker for lung adenocarcinoma. Heliyon 2024, 10, e23828. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Jin, Y.; Zhou, M.; Li, X.; Chen, W.; Wang, Y.; Gu, S.; Cao, Y.; Chu, C.; Liu, X.; et al. Solute carrier family 35 member F2 is indispensable for papillary thyroid carcinoma progression through activation of transforming growth factor-β type I receptor/apoptosis signal-regulating kinase 1/mitogen-activated protein kinase signaling axis. Cancer Sci. 2018, 109, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Kotolloshi, R.; Hölzer, M.; Gajda, M.; Grimm, M.O.; Steinbach, D. SLC35F2, a Transporter Sporadically Mutated in the Untranslated Region, Promotes Growth, Migration, and Invasion of Bladder Cancer Cells. Cells 2021, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Q.; Yuan, H.; Ren, L.; Liang, X.; Li, S.; Lv, S.; Jiang, H. Solute Carrier Family 35 Member F2 Regulates Cisplatin Resistance and Promotes Malignant Progression of Pancreatic Cancer by Regulating RNA Binding Motif Protein 14. J. Oncol. 2022, 2022, 5091154. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.E.; Radic, B.; Mayor-Ruiz, C.; Blomen, V.A.; Trefzer, C.; Kandasamy, R.K.; Huber, K.V.; Gridling, M.; Chen, D.; Klampfl, T.; et al. The solute carrier SLC35F2 enables YM155-mediated DNA damage toxicity. Nat. Chem. Biol. 2014, 10, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Voges, Y.; Michaelis, M.; Rothweiler, F.; Schaller, T.; Schneider, C.; Politt, K.; Mernberger, M.; Nist, A.; Stiewe, T.; Wass, M.N.; et al. Effects of YM155 on survivin levels and viability in neuroblastoma cells with acquired drug resistance. Cell Death Dis. 2016, 7, e2410. [Google Scholar] [CrossRef] [PubMed]
- Nyquist, M.D.; Corella, A.; Burns, J.; Coleman, I.; Gao, S.; Tharakan, R.; Riggan, L.; Cai, C.; Corey, E.; Nelson, P.S.; et al. Exploiting AR-Regulated Drug Transport to Induce Sensitivity to the Survivin Inhibitor YM155. Mol. Cancer Res. 2017, 15, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Nyquist, M.D.; Prasad, B.; Mostaghel, E.A. Harnessing Solute Carrier Transporters for Precision Oncology. Molecules 2017, 22, 539. [Google Scholar] [CrossRef]
- Go, Y.H.; Lim, C.; Jeong, H.C.; Kwon, O.S.; Chung, S.; Lee, H.; Kim, W.; Suh, Y.G.; Son, W.S.; Lee, M.O.; et al. Structure-Activity Relationship Analysis of YM155 for Inducing Selective Cell Death of Human Pluripotent Stem Cells. Front. Chem. 2019, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Park, J.C.; Jang, H.K.; Lee, H.; Park, S.; Kim, J.; Kwon, O.S.; Go, Y.H.; Jin, Y.; Kim, W.; et al. Safe scarless cassette-free selection of genome-edited human pluripotent stem cells using temporary drug resistance. Biomaterials 2020, 262, 120295. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, S. Archaic introgression contributed to the pre-agriculture adaptation of vitamin B1 metabolism in East Asia. iScience 2022, 25, 105614. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.L.; Han, W.Q.; Yang, F.P.; Ji, K.D.; Wang, J.G.; Gao, P.J.; He, G.; Wu, S.N. Association of a SNP in SLC35F3 Gene with the Risk of Hypertension in a Chinese Han Population. Front. Genet. 2016, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Choi, J.H. Genetic Variations in Thiamin Transferase SLC35F3 and the Risk of Hypertension in Koreans. Clin. Nutr. Res. 2021, 10, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Alba-Linares, J.J.; Pérez, R.F.; Tejedor, J.R.; Bastante-Rodríguez, D.; Ponce, F.; Carbonell, N.G.; Zafra, R.G.; Fernández, A.F.; Fraga, M.F.; Lurbe, E. Maternal obesity and gestational diabetes reprogram the methylome of offspring beyond birth by inducing epigenetic signatures in metabolic and developmental pathways. Cardiovasc. Diabetol. 2023, 22, 44. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, D.; Ouidir, M.; Workalemahu, T.; Zeng, X.; Tekola-Ayele, F. Placental DNA methylation changes associated with maternal prepregnancy BMI and gestational weight gain. Int. J. Obes. 2020, 44, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Shin, D. Effects of Interaction between SLC35F3 and Carbohydrate Intake on the Incidence of Metabolic Syndrome in Korean Middle-Aged Adults. Nutrients 2023, 15, 469. [Google Scholar] [CrossRef] [PubMed]
- Simonti, C.N.; Vernot, B.; Bastarache, L.; Bottinger, E.; Carrell, D.S.; Chisholm, R.L.; Crosslin, D.R.; Hebbring, S.J.; Jarvik, G.P.; Kullo, I.J.; et al. The phenotypic legacy of admixture between modern humans and Neandertals. Science 2016, 351, 737–741. [Google Scholar] [CrossRef]
- Racimo, F. Testing for Ancient Selection Using Cross-population Allele Frequency Differentiation. Genetics 2016, 202, 733–750. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, C.; DeGiorgio, M. Fast and robust detection of ancestral selective sweeps. Mol. Ecol. 2017, 26, 6871–6891. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, R.; Togo, S.; Shimizu, D.; Momiyama, N.; Ishikawa, T.; Ichikawa, Y.; Endo, I.; Kunisaki, C.; Suzuki, H.; Hayasizaki, Y.; et al. Predicting 5-fluorouracil chemosensitivity of liver metastases from colorectal cancer using primary tumor specimens: Three-gene expression model predicts clinical response. Int. J. Cancer 2006, 119, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.J.; Ding, Y.H.; Wen, H.; Li, X.F.; Zhang, W.; Su, H.Y.; Liu, D.M.; Xie, N.L. Attenuation of deregulated miR-369-3p expression sensitizes non-small cell lung cancer cells to cisplatin via modulation of the nucleotide sugar transporter SLC35F5. Biochem. Biophys. Res. Commun. 2017, 488, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, K.; Hosokawa, M.; Eguchi, H.; Ohigashi, H.; Ishikawa, O.; Shinomura, Y.; Nakamura, Y.; Nakagawa, H. Identification of C2orf18, termed ANT2BP (ANT2-binding protein), as one of the key molecules involved in pancreatic carcinogenesis. Cancer Sci. 2009, 100, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Krapivinsky, G.; Krapivinsky, L.; Stotz, S.C.; Manasian, Y.; Clapham, D.E. POST, partner of stromal interaction molecule 1 (STIM1), targets STIM1 to multiple transporters. Proc. Natl. Acad. Sci. USA 2011, 108, 19234–19239. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.; Wang, M.; Zhang, L.; Yang, K.; Du, X.; Wu, J.; Wang, X.; Li, F.; Qiu, Z. Discovery and Validation of Novel Genes in a Large Chinese Autism Spectrum Disorder Cohort. Biol. Psychiatr. 2023, 94, 792–803. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).