Abstract
The measurement of rabies-neutralizing antibody is important for monitoring the response to rabies vaccination. For all the purposes of measurement, such as routine monitoring of vaccine response in humans and animals, serosurveys, and biologics qualification, accurate and precise results are necessary. The risks associated with sample handling variation, which may impact the test results, can be overlooked within a laboratory. To determine the robustness of rabies-neutralizing antibodies in human and animal serum, samples were treated to mimic various possible deviations in the sample handling protocols. Potential deviations were designed to investigate common client inquiries and possible sample conditions experienced during shipping, storage, and laboratory processes. The treatments included the duration that sera were kept at a temperature greater than that of a refrigerator (room temperature, zero hours to two weeks), the number and duration of heat inactivation treatments (i.e., heat inactivation directly from freezer storage, etc.), the number of freeze–thaw cycles (zero, four, or six cycles), and the storage duration of sample dilutions in chamber slides before the addition of virus (zero hours to overnight). The results provided evidence for the robustness of rabies antibodies and the antibodies’ neutralizing function in uncontaminated, clear human and animal serum. In addition, prolonged heat exposure was identified as exerting the greatest impact on the measurement of rabies antibodies.
1. Introduction
Rabies antibody assays provide information consequential to clinical and pharmaceutical decisions. No one method is ideal for all testing purposes; yet, serum neutralization (SN) assays, such as the rapid fluorescent focus inhibition test (RFFIT), are globally recognized as the gold standard for rabies antibody measurement due to the fact that they measure functional antibody as a correlate of protection [1,2]. SN assays, as are most laboratory methods, are subject to assay variables that will affect the measurement of precision and the accuracy of rabies virus-neutralizing antibodies (RVNAs) [3]. Quality control of the assay (biological reagents, equipment, training, etc.) will control variability, but the quality of the serum sample is an uncontrolled component. Proper handling of samples can preserve the quality of antibodies and prevent the growth of infectious agents, which could produce less accurate results [4]. Assay validation usually includes the verification of expected storage conditions. Published validation of the RFFIT has demonstrated limits of refrigerated (2 to 8 °C) and frozen storage (−20 and −80 °C), as well as of freeze–thaw cycles (two and five cycles) [5,6].
In addition to providing rabies titer results to inform whether a booster vaccine is needed (result of less than 0.5 IU/mL) for those at increased risk of rabies exposure, rabies serology is used for pet export, epidemiology serosurveys, and clinical studies on vaccines and rabies immunoglobulin. The sample quality and quantity are key factors in obtaining accurate results. Samples shipped longer distances or from areas with limited storage capabilities, as well as shipping delays are all real-life possibilities. In addition, as the number of samples tested within a laboratory increases, so does the potential for sample handling deviations and inquiries into how these variations may affect a sample’s result. Extended room temperature storage and errors in heat inactivation handling are possible. Establishing the limits of handling errors allows for informed decision-making when out-of-specification events occur, especially for rare or low-volume samples.
To determine the robustness of rabies virus-neutralizing antibodies (RVNAs) in human and animal serum using the RFFIT, samples were treated to mimic potential deviations in sample handling protocols. Potential deviations were designed to investigate common client inquiries and possible sample conditions experienced during shipping, processing, and setup. These potential deviations include the duration of serum storage (up to two weeks) at a temperature greater than that of refrigeration (room temperature, 18 to 25 °C), the number and duration of heat inactivation (HI) treatments, the number of freeze–thaw cycles (above three cycles was previously validated), and the storage duration of sample dilutions in chamber slides before the addition of virus.
Samples with results below and between the RFFIT-validated linear range of 0.1 to 15.0 IU/mL were tested and analyzed. In light of the (prior to 2022) recommended cut-off of the Advisory Committee on Immunization Practices (ACIP), defined as the complete neutralization of rabies virus at a serum dilution of 1:5 (~0.1 IU/mL) and the current ACIP and World Health Organization (WHO) guideline of 0.5 IU/mL as an adequate response [1,7,8], this study examined how the above conditions affected samples, specifically with results around these two target levels.
2. Materials and Methods
A panel of 15 human and 15 animal (feline, canine, and equine) serum samples was created (n = 30, unless otherwise stated). Sample aliquots (220 µL) were prepared to create panels for all testing scenarios (see below). An untreated control panel was tested in parallel with the test panels. Testing was performed by the rapid fluorescent focus inhibition test (RFFIT) method, with each sample tested in duplicate. All samples were heat inactivated (55–57 °C for 30 min) 1–3 days prior to testing, except for the heat inactivation panel (see Section 2.3 below).
2.1. Samples
Panels were prepared by creating pools of clinical human serum or clinical animal serum submitted for rabies titer testing. Samples were prepared to represent RVNA IU/mL across the linear range of the assay and results below the level of quantitation, as defined by the assay validation. Serum samples were pooled by species and by measured IU/mL range (see Supplemental File Table S1). As needed, some samples were further diluted in assay media. Four samples in the panels were less than 0.1 IU/mL, including two human and two animal samples. The results (by treatment) of all samples in the test panels can be found in Supplemental Files Tables S2–S5.
2.2. Room Temperature Stability
Panels were stored at room temperature (RT) for 0 (control), 4, 12, 24, 48, or 72 h or for 1 or 2 weeks. Panels were stored at 2 to 8 °C until RT incubation (acceptable range, 18 to 25 °C), if applicable, and after heat inactivation, prior to testing.
2.3. Heat Inactivation (HI) Variables
Panels were treated based on potential lab-based variables in heat inactivation. The variables tested included standard HI conditions (30 min, control), situations to mimic serum samples being left in the water bath overnight (16 h, overnight HI), HI directly from −80 °C storage (45 min, HI from −80 °C), and two rounds of HI (30 min each) with different storage temperatures between rounds (2 × HI with 4 °C storage or 2 × HI with −80 °C storage).
2.4. Eight-Well Chamber Slide Incubation (2 to 8 °C)
Panels, standards, and references were appropriately diluted and transferred to 8-well chamber slides (n = 36). Chamber slides were incubated at 2–8 °C for 0 (control), 1, 2, 4, or 6 h or overnight prior to the next steps of the RFFIT procedure.
2.5. Freeze–Thaw Stability
Panels were subjected to 4 or 6 freeze–thaw cycles prior to testing. One cycle consisted of 48 h at −80 °C, followed by 2 h at room temperature (time for complete thaw).
2.6. RFFIT Serological Testing
The RFFIT, using CVS-11 as the challenge virus strain, was used to test all serum samples for the baseline rabies virus-neutralizing antibody (RVNA) titer value, as previously described [9]. The RFFIT assay has been routinely validated in the Kansas State University Rabies Laboratory [5]. Briefly, 100 μL of each serum sample was serially diluted five-fold in 96-well microplates, and 100 µL of each serum dilution was transferred to 8-well Lab-Tek chamber slides (Nunc™ Lab-Tek™ Chamber Slide System, catalog# 177445), and 100 μL of the challenge virus (ATCC), at a concentration of 50 TCID50, was added. The resulting serial dilution represented serum dilutions of 1:5, 1:25, 1:125, and 1:625. After a 90-min incubation period to allow for virus neutralization by any RVNAs present in the samples, 200 μL of DEAE-treated BHK cells (5 × 105/mL) was added, followed by further incubation for 20 to 24 h. Residual virus detection under fluorescence microscopy was achieved by staining acetone-fixed slides with FITC-conjugated anti-N rabies antibody (Millipore, Temecula, CA, USA). Counts of virus-detected fields were transformed to IU/mL results using the Reed and Muench formula for endpoint titer, and the results were compared to an RVNA in-house reference serum titer tested alongside the samples. A panel of internal standards, including a negative control, were tested alongside the samples for assay monitoring. The in-house reference serum was qualified against an international rabies immunoglobulin standard sample. High-titer sera were pre-diluted in assay media to obtain readable results within the linear range of the assay (0.1 IU/mL to 15.0 IU/mL) as defined during validation; the lower limit of quantitation was validated as 0.1 IU/mL, and the upper limit of quantitation was validated as 15.0 IU/mL in the human (as well as animal) serum matrix.
2.7. Data Analysis
The sample IU/mL results were calculated by comparing the sample titer to the titer of the untreated reference serum sample for each experiment, as appropriate; see Table S1. Duplicate IU/mL values per sample were averaged and compared across treatments within each experiment. Percent recovery (%R = experimental result/theoretical result × 100) and the Coefficient of Variance (CV% = standard deviation/sample mean × 100) were calculated for each sample relative to its respective experimental control. Acceptance criteria for the accuracy and precision of the results of the same sample across treatments was set at a percent recovery (%R) between 50% and 150% and a precision (CV%) less than or equal to 50%. Furthermore, standard laboratory practice dictates that greater than 90% of samples must meet these criteria for both CV% and %R for a treatment group to be accepted. These acceptance criteria were selected based on the expectation for antibody titer variation [10,11]. The acceptance CV% for the RFFIT per the assay validation is 30% CV% [5,6].
3. Results
3.1. Room Temperature Stability of RFFIT IU/mL Results
The test panel IU/mL results and comparison of the results to the control panel can be seen in Figure 1a,b, respectively. Across treatments, the average CV% ranged from 7.6–10%, and the average percent recovery ranged from 95.5–102% (Table 1). When test panel results were compared to the control samples, all samples passed the laboratory acceptance criteria for less than 50% CV%, with 93.3–100% of samples having a CV% less than 30%. Additionally, between 96.7 and 100% of samples passed the acceptance criteria for %R. All samples maintained result consistency at the 0.1 and 0.5 IU/mL levels.
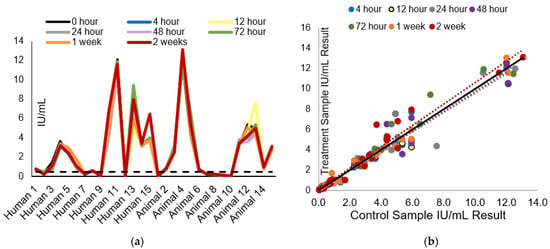
Figure 1.
Influence of room temperature storage time on RFFIT IU/mL result: (a) The effect of room temperature storage by number of hours on the measured rabies-neutralizing antibody (IU/mL) is displayed. The dotted line represents the acceptable level for rabies vaccination seroconversion. (b) The comparison of room temperature-stored samples’ results (IU/mL) to the control sample results (stored at 2 to 8 °C until testing). The black line is the control data trendline line; the colored dotted trendlines correspond to the data sets per treatment by color in the legend.

Table 1.
The average result precision (CV%) and accuracy (%R) comparison, the percentage of samples meeting the acceptance criteria, and the ratio of samples reflecting the two seroconversion rates (0.1 and 0.5 IU/mL) by the sample handling variable. n = 30 for all variables, except 8-well chamber slide incubation with n = 36.
3.2. Influence of Sample Serum Heat Inactivation Time on Stability of RFFIT IU/mL Results
The HI treatment panel and control panel IU/mL results and the comparison of each test sample to its control sample results are displayed in Figure 2a,b, respectively. Across treatments, the average CV% compared with the control samples ranged from 7.9–15.5%, and the average %R ranged from 85.8–112.3% (Table 1). All samples had results that met the laboratory acceptance criteria of less than 50% CV% in all groups except the overnight (16 h) HI panel sample results. Only 93.1% of samples had a CV% less than 50%; additionally, only 82.8% of samples had a CV% less than 30%. Between 93.3 and 96.7% of the sample results passed the acceptance criteria for %R in all treatments, except the overnight HI test sample results. The overnight HI treatment sample results failed the laboratory acceptance criteria for percent recovery, with only 89.7% of results passing acceptance. Overall, all samples maintained result consistency at the 0.1 and 0.5 IU/mL levels.
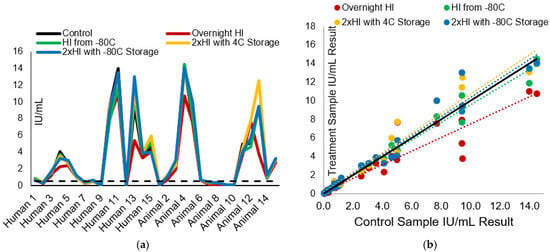
Figure 2.
The influence of heat inactivation time on RFFIT IU/mL result: (a) The effect of different HI and storage schemes on the measured rabies-neutralizing antibody (IU/mL) is displayed. The dotted line represents the acceptable level for rabies vaccination seroconversion. (b) The comparison of the heat inactivation scheme sample results (IU/mL) to the control sample results. The black line is the control data trendline line; the colored dotted trendlines correspond to the data sets per treatment by color in the legend.
3.3. Influence of 2 to 8 °C Storage of Sample Dilutions in Chamber Slide Wells on the RFFIT IU/mL Results
The sample IU/mL results and comparison to the control panel IU/mL results can be seen in Figure 3a,b, respectively. Across treatments, the average CV% compared with the control samples ranged from 7.2–13.7%, and the average %R ranged from 94.5–116.7% (Table 1). When the test panel results were compared to the control panel results, all test panel results passed the laboratory acceptance criteria of less than 50% CV%; 94.4–100% of samples had a CV% less than 30%. Additionally, between 91.7 and 100% of the results passed the acceptance criteria for %R. All test panel samples maintained result consistency at the 0.1 IU/mL level; however, one sample in the 6-h incubation group did not maintain result consistency at the 0.5 IU/mL level. The control treatment result average (to two decimal places) was 0.55 IU/mL, compared to the 0.40 IU/mL result after the 4-h chamber slide storage treatment.
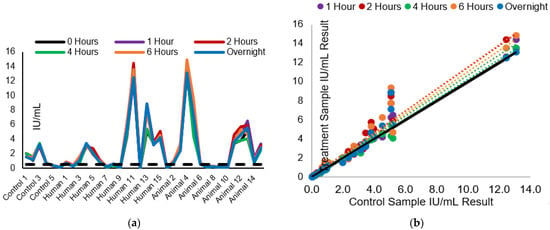
Figure 3.
The influence of 2 to 8 °C storage time of serum sample dilutions in chamber slides on RFFIT IU/mL result: (a) The effect of different storage times on the measured rabies-neutralizing antibody (IU/mL) is displayed. The dotted line represents the acceptable level for rabies vaccination seroconversion. (b) The comparison of the chamber slide storage time sample results (IU/mL) to the control sample results. The black line is the overall data trendline line; colored dotted trendlines correspond to the data sets per treatment by color in the legend.
3.4. Freeze-Thaw Stability of RFFIT IU/mL Results
The test and control panel sample IU/mL results and test panel result comparison to the control panel IU/mL results can be seen in Figure 4a,b, respectively. Across treatments, the average percent CV compared with the control samples was 8.8 and 8.9% for 4 and 6 cycles, and the average percent recovery was 96.9 and 98.7% for 4 and 6 cycles, respectively (Table 1). When the test panel results were compared to the control samples, all samples passed the laboratory acceptance criteria for less than 50% CV, with 93.9–96.7% of samples having a CV% less than 30%. Additionally, all samples passed the acceptance criteria for percent recovery and maintained result consistency at the 0.1 and 0.5 IU/mL levels.
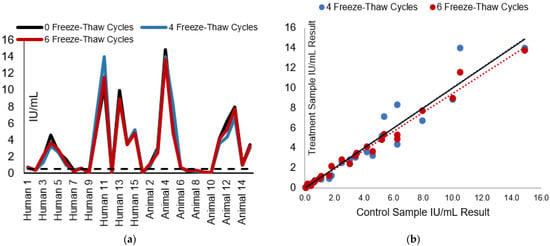
Figure 4.
RFFIT IU/mL results of test panels after 4 and 6 freeze–thaw cycles compared to the control panel (no freeze–thaw cycle): (a) The effect of freeze–thaw cycles on the measured rabies-neutralizing antibody (IU/mL) is displayed. The dotted line represents the acceptable level for rabies vaccination seroconversion. (b) The comparison of the freeze–thaw test panel results (IU/mL) to the control samples. The black line is the overall data trendline line; colored dotted trendlines correspond to the data sets per treatment by color in the legend.
3.5. Intra-Assay Variability
The duplicate results of all samples in all treatments and their associated controls were calculated to evaluate intra-assay variability; see Table 2. The CV% range of all control samples was 8.5 to 13.3%.

Table 2.
Duplicate result precision (CV%) and percentage of samples meeting CV% acceptance criteria by sample handling variable.
4. Discussion
SN methods rely on healthy cells for sufficient replication of viral proteins, which are the target of the conjugated antibody used for the detection of the rabies virus (RABV) in the cells. Thus, any factor or condition that affects cell health, such as infection, substandard reagents, and adverse temperature/pH, will affect the performance of the assay [12]. Similarly, any condition that influences the binding of RABV to the cell receptor will play a part in the assay performance. The conditions of the test environment can also impact the neutralizing capability of the antibodies, to various degrees, depending on the mechanism of neutralization. Interfering factors can be biologic (as in contamination with bacteria or viruses) or chemical (as in the use of tubes or labware made from plastic, which is toxic to the cells or causes excess binding of antibody to the plastic). Specimen quality alone can interfere with attaining accurate results [4]. Lipids in lipemic samples may interfere with the measurement of neutralizing antibodies through interference/non-specific reactions [13]. Serum from patients acutely ill with other viral diseases has produced non-specific reactions in both SN and ELISA rabies serology methods [14].
Shipping preparations and conditions can be complex and difficult to manage, especially for a larger number of samples [15]. Some components measured in blood samples can be very sensitive to shipping and storage conditions, whereas others are not [16]. In this study, RVNAs in clean human and animal serum samples tested using the RFFIT assay were shown to be very stable when handled outside of standard handling protocols. Clean samples are samples that are not hemolyzed, icteric, lipemic, or contain bacterial contamination. All test conditions, except the 16-h heat inactivation treatment, produced sample results within 50% CV% of the control results; furthermore, 90% or greater were within 30% CV%. Freeze–thaw cycles, which can affect the stability of proteins, have been previously studied for rabies antibodies up to four cycles [5,6]. This study expands the extent of RVNA stability to six cycles. The stability of frozen samples is particularly important in research and clinical trial studies, where the reliability of both frozen shipment and sample storage is a requirement; however, both conditions are vulnerable to unexpected temperature excursion due to various environments during shipment and the length of the storage time. Antibody stability within serum samples was investigated in a previous study by Hodgkinson et al., which looked at the effects of the time/temperature to fractionation (centrifugation of blood sample), the time/temperature of storage prior to testing, and the collection tube type on infectious disease antibodies [15]. The authors concluded that there was no difference in the blood collection tube types and that the samples can be stored for up to six days at room temperature or 4 °C with no difference in measured antibody level, demonstrating the robustness of sample stability in preanalytical handling.
Adequate seroconversion for rabies vaccination per the World Health Organization and currently by the Advisory Committee on Immunization Practices (ACIP) is 0.5 IU/mL [8,17]. Prior to the new ACIP recommendations published in 2022, the acceptable level representing an adequate response to rabies vaccination was described as complete neutralization of virus at a 1:5 dilution of serum, which is approximately 0.1 to 0.3 IU/mL, as tested in the RFFIT. The level 0.1 IU/mL is the lower limit of quantitation per the method validation at the time of these experiments; this level can vary per individual laboratory or assay type. In this study, samples at these two levels were particularly evaluated, as the levels most consequential for decision-making for clinicians, occupational health professionals, researchers, and in clinical trial studies. All samples tested with an initial RVNA level of 0.1 IU/mL maintained this result in all treatments. The same was true for samples with an initial RVNA level of 0.5 IU/mL in all treatments, except for one; this sample had an antibody level of 0.4 IU/mL in the 4-h chamber slide incubation condition. A 0.1 IU/mL difference is within the normal variability for the RFFIT assay; further, the result was within two standard deviations of the control result (0.5 IU/mL). All other results of this sample in the chamber slide incubation experiments (longer than 4 h) were 0.5 IU/mL.
Serum neutralization tests are inherently variable; however, variability can be controlled with good quality-assurance measures. The overall average of duplicate samples of controls was 11.1%, ranging from 8.5 to 13.3% (see Table 2). Comparing this (expected) range to the range of CV% in each treatment group in Table 1, we see that only the overnight HI treatment groups produces a notably higher CV% (25.6%).
Establishing the limits of sample stability for rabies antibody testing provides guidance for shipping decisions, as well as follow-up actions when sample storage conditions fall outside the stated requirements or accepted practice guidelines. A limitation of this study is that the samples used to prepare the panels were clinical samples shipped to the laboratory for rabies titer testing and, as such, the sample handling prior to and during shipment are unknown. Additionally, some samples were diluted to reach the target IU/mL for the experiment design and may not represent non-diluted samples.
5. Conclusions
The evidence from the sample handling experiments in this study demonstrates that a clean serum sample tested to determine a sufficient antibody level per the ACIP (~0.1 IU/mL) or WHO (0.5 IU/mL) can withstand deviations from standard handling protocols and still produce results within the established assay variability. Importantly, the sample handling deviations investigated did not affect the results at the cut-off level for the determination of adequate vaccination response to rabies vaccination for those at risk of rabies exposure. Similarly, for samples at the level of quantitation, 0.1 IU/mL, sample robustness was demonstrated. Knowledge of the limits of sample handling deviation is of utmost importance when samples are difficult to obtain or unable to be reproduced (i.e., time point sample or wild animal sample).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biologics4030016/s1, Table S1: Sample preparation; Tables S2–S5: IU/mL results of samples per treatment.
Author Contributions
Conceptualization, S.J.P., S.K.G. and S.M.M.; methodology, S.J.P., S.K.G. and S.M.M.; validation, S.J.P. and S.K.G.; formal analysis, S.J.P. and S.K.G.; investigation, C.C.K.; data curation, C.C.K.; writing—original draft preparation, S.J.P. and S.K.G.; writing—review and editing, S.M.M. and C.C.K. visualization, S.J.P. and S.K.G.; supervision, S.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study proposal was reviewed by the Institutional Review Board of Kansas State University (protocol code 9819, 23 July 2019) and found to be exempt under the criteria set forth in the Federal Policy for the Protection of Human Subjects, 45 CFR 46.101, paragraph b. category: 4, subsection: ii.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the reported results can be found in the Supplemental Tables; see Supplementary Materials above for the link.
Acknowledgments
We express our gratitude for the excellent technical support from Lindsey (Howard) Gose and Brenda Waters.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. WHO Expert Consultation on Rabies, Third Report; World Health Organization: Geneva, Switzerland, 2018; pp. 1–195. [Google Scholar]
- Moore, S.M.; Hanlon, C.A. Rabies-Specific Antibodies: Measuring Surrogates of Protection against a Fatal Disease. PLoS Neglect. Trop. Dis. 2010, 4, e595. [Google Scholar] [CrossRef] [PubMed]
- Wandeler, A.I. Rabies vaccinology and immunology. Dev. Biol. 2006, 125, 181–184. [Google Scholar]
- Sturgeon, C.M.; Viljoen, A. Analytical error and interference in immunoassay: Minimizing risk. Ann. Clin. Biochem. Int. J. 2011, 48 Pt. 5, 418–432. [Google Scholar] [CrossRef]
- Kostense, S.; Moore, S.; Companjen, A.; Bakker, A.B.; Marissen, W.E.; von Eyben, R.; Weverling, G.J.; Hanlon, C.; Goudsmit, J. Validation of the rapid fluorescent focus inhibition test (RFFIT) for rabies virus neutralizing antibodies in clinical samples. Antimicrob. Agents Chemother. 2012, 56, 3524–3530. [Google Scholar] [CrossRef] [PubMed]
- Timiryasova, T.M.; Luo, P.; Zheng, L.; Singer, A.; Zedar, R.; Garg, S.; Petit, C.; Moore, S.; Hu, B.T.; Brown, M. Rapid fluorescent focus inhibition test optimization and validation: Improved detection of neutralizing antibodies to rabies virus. J. Immunol. Methods 2019, 474, 112626. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, C.E.; Briggs, D.; Brown, C.M.; Franka, R.; Katz, S.L.; Kerr, H.D.; Lett, S.M.; Levis, R.; Meltzer, M.I.; Schaffner, W.; et al. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: Recommendations of the advisory committee on immunization practices. MMWR Recomm. Rep. 2010, 59, 1–9. [Google Scholar] [PubMed]
- Rao, A.K.; Briggs, D.; Moore, S.M.; Whitehill, F.; Campos-Outcalt, D.; Morgan, R.L.; Wallace, R.M.; Romero, J.R.; Bahta, L.; Frey, S.E.; et al. Use of a Modified Preexposure Prophylaxis Vaccination Schedule to Prevent Human Rabies: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. Mmwr. Morb. Mortal. Wkly. Rep. 2022, 71, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Laboratory Techniques in Rabies. The Rapid Fluorescent Focus Inhibition Test (RFFIT), 5th ed.; World Health Organization: Geneva, Switzerland, 2018; p. 288. [Google Scholar]
- Kostense, S.; Hendriks, J. Challenges of Immunogenicity Assays for Vaccines. Bioanalysis 2012, 4, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.J.; Durham, T.M. Reproducibility of serological titers. J. Clin. Microbiol. 1980, 11, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.M.; Gilbert, A.; Vos, A.; Freuling, C.M.; Ellis, C.; Kliemt, J.; Muller, T. Rabies Virus Antibodies from Oral Vaccination as a Correlate of Protection against Lethal Infection in Wildlife. Trop. Med. Infect. Dis. 2017, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Myler, H.; Pedras-Vasconcelos, J.; Lester, T.; Civoli, F.; Xu, W.; Wu, B.; Vainshtein, I.; Luo, L.; Hassanein, M.; Liu, S.; et al. Neutralizing Antibody Validation Testing and Reporting Harmonization. AAPS J. 2023, 25, 69. [Google Scholar] [CrossRef] [PubMed]
- Rudd, R.J.; Appler, K.A.; Wong, S.J. Presence of Cross-Reactions with Other Viral Encephalitides in the Indirect Fluorescent-Antibody Test for Diagnosis of Rabies. J. Clin. Microbiol. 2013, 51, 4079–4082. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, V.S.; Egger, S.; Betsou, F.; Waterboer, T.; Pawlita, M.; Michel, A.; Baker, M.S.; Banks, E.; Sitas, F. Preanalytical Stability of Antibodies to Pathogenic Antigens. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Youngman, L.D.; Palmer, A.; Parish, S.; Peto, R.; Collins, R. Stability of plasma analytes after delayed separation of whole blood: Implications for epidemiological studies. Int. J. Epidemiol. 2003, 32, 125–130. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Rabies vaccines: WHO position paper, April 2018—Recommendations. Vaccine 2018, 36, 5500–5503. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).