Abstract
Background: Patients living with chronic obstructive pulmonary disease (COPD) are at risk for lower respiratory tract infections caused by respiratory syncytial virus (RSV). The first RSV vaccines were approved in 2023 for adults ages 60 years and older. The safety and efficacy of the RSV vaccines and their clinical implications in patients living with COPD, apart from composite comorbidity results, are under-reported. Methods: This rapid review aimed to collect and report data pertaining to RSV vaccine safety and efficacy in patients living with COPD. Resources searched included Ovid MEDLINE, EMBASE, International Pharmaceutical Abstracts, published peer-reviewed abstracts, ClinicalTrials.gov, and the United States Food and Drug Administration (FDA) website. Results: Seven records were included: five research manuscripts and two ongoing clinical trials. Patients living with COPD were included in RSV vaccine clinical trials, but outcomes of RSV vaccine safety and efficacy in patients living with COPD were grossly unreported. Conclusions: Future clinical trials of patients living with COPD and subgroup analyses of patients living with COPD within existing studies evaluating RSV vaccine safety and efficacy are necessary to substantiate outcomes in this population.
1. Introduction
One in ten patients living with COPD may develop symptomatic RSV infection [1]. The chief complaint of symptomatic RSV infection in patients living with COPD is dyspnea, which may persist between 4 and 5 days before patients seek medical care [2]. The 30-day mortality rate attributable to RSV infection in patients living with COPD is approximately 10%, which is nominally higher than that in immunocompetent older adults (7%) [2,3]. Between 50% and 100% of patients living with COPD may require hospitalization to treat RSV infection, and older adults living with COPD are hospitalized 3 to 14 times more frequently than patients without a history of COPD [1,2,4]. Patients living with COPD may thus benefit from vaccines protecting against symptomatic and severe RSV.
Research developing and assessing candidate vaccines against RSV has been chronicled over 60 years [5,6]. Initial vaccine candidates raised concerns because infants immunized with formalin-inactivated vaccines developed more severe RSV following immunization [6]. The development of safe and effective RSV vaccines remained elusive over the late 20th century. As of 2023, between 30 and 40 candidate vaccines encompassing 31 potential pharmacologic targets to provide immunoprophylaxis against RSV are undergoing clinical trials [6,7]. Presently, vaccines targeting the pre-fusion (pre-F) conformation of the fusion glycoprotein have elicited robust immunity and RSV-neutralizing antibodies [6].
The US Food and Drug Administration (FDA) approved two pre-F RSV vaccines in 2023 after clinical trials determined these vaccines were efficacious in preventing symptomatic and severe RSV in adults 60 years and older [8,9,10]. In June 2023, the Advisory Committee on Immunization Practices (ACIP) recommended shared clinical decision making for the use of RSV vaccines in adults 60 years and older [11]. This shared clinical decision-making consideration followed the Committee’s Evidence to Recommendation Framework and also accounted for the rare but serious inflammatory neurologic events that occurred more often than expected in the RSV vaccine intervention groups [10,11]. Patients living with COPD are mentioned by the ACIP as a high-risk group that may benefit from RSV immunization to prevent severe RSV disease [11]. The efficacy and safety of the RSV vaccines in patients living with COPD, apart from composite comorbidity results, remain unknown.
Previous reviews address treatment and prevention considerations for RSV infection, but their methodologies are limited by narrative review designs [12,13], and others were published almost two decades ago [14,15], well before the newly approved RSV vaccines. One systematic review on the use of palivizumab to prevent RSV in children exists; however, palivizumab is considered passive immunoprophylaxis and not active immunization [16]. Thus, the body of evidence describing RSV vaccine safety and efficacy in patients living with COPD is uncharted and begets systematic review. The aim of this rapid review is to identify the safety and efficacy of RSV vaccines in patients living with COPD.
2. Materials and Methods
2.1. Rapid Review Reporting and Registration
This was a rapid review on the safety and efficacy of RSV vaccines in patients living with COPD. The PRISMA extension for rapid reviews (PRISMA-RR) is under development, but interim suggestions are available, and they were used to prepare this review [17]. This rapid review also adhered to the rapid review checklist published by the Cochrane Rapid Reviews Methods Group [18]. This rapid review was registered in both the International Prospective Register of Systematic Reviews (PROSPERO) and Open Science Frameworks registries (PROSPERO registration CRD42023474010, registered 29 October 2023; Open Science Frameworks digital object identifier 10.17605/OSF.IO/PCFH3, registered 3 October 2023).
2.2. Data Sources and Search
An electronic literature search was performed using Ovid MEDLINE, EMBASE, International Pharmaceutical Abstracts, ClinicalTrials.gov, and the US Food and Drug Administration website on 8 September 2023. A health sciences librarian was consulted for the design and execution of the search. Preliminary results from a search of Ovid MEDLINE were reviewed by the health sciences librarian and one investigator (PB), and then the search was refined and executed across the databases, websites, and registries. Search strings varied depending on the resource searched; exact search strategies for the databases are provided in Appendix A, but keywords generally included “respiratory syncytial virus” and “chronic obstructive pulmonary disease.” The abstracts and full-text reports were screened by one investigator (PB).
2.3. Report Selection
Reports were eligible for inclusion if they described an RSV vaccine and the study population was patients living with COPD or if the general study population included subgroups of patients living with COPD. Humanized monoclonal antibodies, including palivizumab and nirsevimab, provide passive immunity against RSV but are not considered vaccines, and thus, reports on monoclonal antibodies were excluded [19]. Reports published from 1 January 1960 to 8 September 2023 were eligible for inclusion in this rapid review. Systematic reviews, meta-analyses, newsletters, editorials, theses, commentaries, and citations without abstracts were excluded. Non-English language articles were ineligible for inclusion; however, no articles meeting inclusion criteria were published in languages other than English, and thus, no articles were excluded from this review because they were not published in English.
2.4. Data Charting and Synthesis
Results from the searches were exported as a Research Information Systems file and imported into Covidence (Covidence, Melbourne, VI, Australia) for abstract screening, full-text review and assessment, and data extraction. Abstracts were independently screened for inclusion by one author (PB). Full-text reports were independently screened for inclusion by one author (PB). One author (PB) completed data extraction. Data were extracted using an existing template in Covidence. A bias assessment of the reports was performed using the Cochrane Risk of Bias Tool [20]. Narrative data synthesis was completed by all investigators (P.M.B., M.E.F., N.P., J.A.W. and A.W); disagreements were resolved through discussion and consensus.
3. Results
Two hundred forty records, published between 1960 and 2023, that describe RSV and COPD were collected via database, website, and registry searching. Figure 1 depicts the flow diagram for searching, screening, assessment, and extraction. Eleven studies were identified as duplicate records, and thus, two hundred twenty-nine unique study abstracts were screened. Thirty-four studies were deemed eligible for full-text retrieval and appraisal; reasons full texts were excluded are listed in Figure 1.
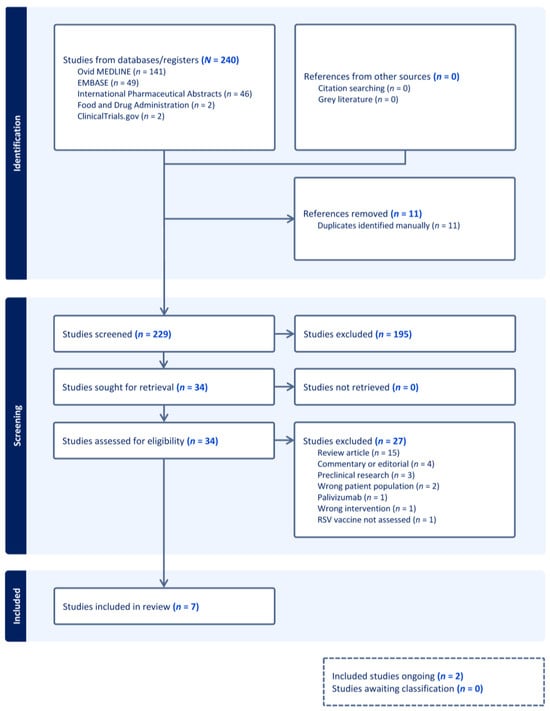
Figure 1.
PRISMA flow diagram of study search, screen, assessment, and extraction.
Seven studies met eligibility criteria and were included in this rapid review [21,22,23,24,25,26,27]. Five studies were published as peer-reviewed research papers [21,22,23,24,25], and two studies included ongoing clinical trials registered in ClinicalTrials.gov [26,27]. Results from these studies are charted in Table 1. Patients living with COPD were eligible for enrollment in all seven studies evaluating RSV vaccine immunogenicity, safety, and efficacy [21,22,23,24,25,26,27]. Across the five published clinical trials, there were 57,365 total study participants [21,22,23,24,25]. Among four studies that provided details on counts and frequencies of patients living with COPD enrolled in the clinical trials, 2216 patients were reported as having COPD [21,22,24,25].

Table 1.
Studies evaluating RSV vaccine safety and efficacy in patients living with COPD [21,22,23,24,25,26,27].
3.1. Experimental Studies
This rapid review identified three randomized controlled trials reporting on landmark RSV vaccine studies: the Confirmed RSV-Mediated Lower Respiratory Tract Disease in Adults Aged 65 Years and Older (CYPRESS) study [22], Adult Respiratory Syncytial Virus (AReSVi-006) study [23], and RSV Vaccine Efficacy Study in Older Adults Immunized against RSV Disease (RENOIR) [25]. Of the two FDA-approved RSV vaccines, Arexvy was approved based on findings from the AResVi-006 study [10,23], and Abrysvo was approved based on results from RENOIR [9,25]. The RSV vaccine candidate investigated in the CYPRESS study is no longer under investigation [22,28].
CYPRESS was a phase 2b, randomized, double-blind, placebo-controlled trial of over 5700 participants [22]. Four hundred twenty-seven participants (29.1% of all enrollees) categorized as livi with COPD were aggregated into a subgroup analysis with other study participants deemed to be at high risk for severe lower respiratory tract infection (LRTI). Vaccine efficacy ranged between approximately 70 and 80%, depending on three different case definitions of RSV infection [22]. Solicited adverse events were more common in the vaccine arm versus placebo (51.4% vs. 20.2%, p-value unreported), and unsolicited and serious adverse events were nominally similar between groups (16.7% vs. 14.4% and 4.6% vs. 4.7%, respectively, p-values unreported) [22]. A safety subpopulation analysis, including patients living with COPD among other participants with high-risk criteria, demonstrated similar findings [22].
AReSVi-006 is an ongoing, international, phase 3, randomized, placebo-controlled trial [27], and preliminary results from one 8-month RSV season were published [23]. A total of 26,664 participants were enrolled, and 24,966 participants ultimately received an RSV vaccine or a placebo. Patients living with COPD were eligible for inclusion in the AReSVi-006 study, but its prevalence among participants was unreported [23]. RSV vaccine efficacy in this trial was calculated as 82.6%. Solicited adverse events were common and occurred frequently in participants who received the RSV vaccine versus placebo (71.9% vs. 27.9%, respectively, p-value unreported). Study investigators reported the RSV vaccine was more reactogenic than the placebo (33% vs. 17.8%, respectively, p-value unreported), and serious adverse events were nominally similar between groups (4.2% vs. 4%, respectively, p-value unreported) [23]. Subgroup analyses of vaccine efficacy and safety specific to patients living with COPD were unreported [23].
RENOIR is an ongoing, international, phase 3, randomized, placebo-controlled trial [26], and preliminary results from one 12-month RSV season were published [25]. A total of 35,971 participants were enrolled, and 34,284 participants received a vaccine or placebo. Two hundred sixty-five participants (3.7% of all enrollees) living with COPD were eligible for inclusion. Vaccine efficacy ranged between approximately 67 and 86%, depending on two different case definitions of RSV infection [25]. Among 7169 participants reporting adverse reactions, this RSV vaccine was well tolerated, and the incidence of adverse events was nominally similar to that for the placebo: systemic events (27% vs. 26%, respectively, p-value unreported), local reactions (12% vs. 7%, respectively, p-value unreported), and severe reactions (less than 0.7% in both groups, p-value unreported). Two patients in the vaccine arm experienced Guillain–Barré syndrome [25]. Subgroup analyses of vaccine efficacy and safety specific to patients living with COPD were unreported [25].
Only one study, published as a conference abstract, specifically evaluated RSV vaccine efficacy in patients living with COPD [24]. Stratified results from the phase 2, randomized, controlled trial of 1599 participants presented a vaccine efficacy of 64% in preventing RSV LRTI, but these findings did not meet the threshold for statistical significance in the subsequent phase 3, randomized, controlled trial of 11,856 participants [24].
Only one study reported on COPD-related safety issues [21]. Therein, one patient experienced an exacerbation of chronic obstructive pulmonary disease (ECOPD) within four hours following one dose of a non-adjuvanted RSV vaccine [21]. In a second-year extension within the same study, one additional patient experienced ECOPD between 33 and 286 days following non-adjuvanted RSV vaccination [21].
Risk of Bias in Experimental Studies
The Cochrane Risk of Bias Tool was used for qualitative assessment [20]. The bias assessment results for the five experimental studies are charted in Table 2 [21,22,23,24,25]. The articles and their online supplementary materials, if available, were screened for the bias assessment.

Table 2.
Risk of bias within experimental studies [20,21,22,23,24,25].
The CYPRESS, AReSVi-006, and RENOIR studies provided sufficient details in their articles and supplementary documents such that their risk for selection, performance, detection, attrition, and reporting bias was categorized as low [22,23,25]. The study by Falsey and colleagues in 2008 [21] completely and clearly reported on study outcomes, and thus, the risks for attrition and reporting bias were categorized as low; however, details regarding allocation sequencing, participant and personnel blinding, and outcome blinding were not described in sufficient detail, and thus, there may be high potential for selection, performance, and detection bias, respectively [21]. The details of the randomized controlled trials by Shinde and colleagues [24] were published in abstract form only and thus constrained in information provided; however, of importance, the abstract did not mention blinding, and thus, there is potential for a high risk of performance and detection bias [20].
3.2. Clinical Trials in Progress
One ongoing clinical trial, NCT05035212, is an extension of the RENOIR study [25,26]. NCT05035212 includes two substudies that aim to evaluate the safety and immunogenicity of a second RSV vaccine dose after 1 year (Substudy B) or 2 years (Substudy A) [26]. Patients living with COPD are eligible for inclusion in these analyses, the study is actively recruiting participants, and the estimated completion date is 21 March 2025 [26].
One ongoing clinical trial, NCT04886596, is an extension of the AReSVi-006 study [23,27]. Outcome measures of this ongoing trial include LRTI-RSV prevention for three RSV seasons following immunization and LRTI-RSV prevention following RSV revaccination [27]. Patients living with COPD are eligible for inclusion, the study is no longer actively recruiting, and the estimated completion date is 31 May 2024 [27]. Vaccine efficacy in NCT04886596 will be reported and assessed in terms of baseline comorbidities, including COPD, in the revaccination arm [27].
4. Discussion
This rapid review identified seven studies reporting on the immunogenicity, safety, and efficacy of RSV vaccines in patients living with COPD [21,22,23,24,25,26,27]. Approximately 7% of subjects enrolled in RSV vaccine clinical trials were labeled as having COPD [21,22,24,25]. Two clinical trials included extensions of the RENOIR and AReSVi-006 trials, patients living with COPD were eligible for enrollment, and these trials are ongoing into 2024 and 2025 [26,27].
The 2024 GOLD Report recommends six vaccines for patients living with COPD: influenza, pneumococcal, coronavirus disease 2019 (COVID-19), tetanus–diphtheria–acellular pertussis, RSV, and zoster vaccines [29]. The GOLD Report acknowledges there is a lack of high-quality evidence supporting these vaccines in patients living with COPD but advocates for influenza and pneumococcal vaccines, specifically, because they reduced ECOPD and were well tolerated [29]. In our rapid review, only the phase 2 and phase 3 study abstracts published by Shinde and colleagues [24] reported on RSV vaccine efficacy in the prevention of ECOPD, wherein vaccine efficacy varied: 100% in phase 2 and 46% in phase 3. Ongoing clinical trials will be informative on RSV vaccine efficacy in preventing ECOPD. Notably, the 3-year extension of the AReSVi-006 trial (NCT04886596) plans to report vaccine efficacy within the COPD subgroup [27]. Upcoming GOLD Reports are likely to address considerations for RSV vaccines in patients living with COPD. Until then, patients and clinicians should adhere to local vaccine guidance, such as the ACIP recommendations for shared clinical decision making, to balance vaccine efficacy against safety [9,10,11].
Across the AReSVi, RENOIR, and CYPRESS clinical trials, RSV vaccine efficacy ranged between 69.8 and 85.7%, depending on each study’s definition of RSV-LRTI [22,23,25]. These efficacy rates, however, are representative of the entire study population and not specific to patients living with COPD [22,23,25]. Vaccine efficacy in patients with COPD could not be determined in these trials because the COPD population was aggregated with other co-existing or high-risk cardiopulmonary comorbidities [22,23,25]. Moreover, there was heterogeneity across these three major trials in the representativeness of patients living with COPD: 29.1% COPD prevalence in the CYPRESS trial [22], 3.7% COPD prevalence in the RENOIR trial [25], and unreported prevalence in the AReSVi-006 trial [23]. Falsey and colleagues [21] conducted a randomized, double-blind, placebo-controlled study of adjuvanted and non-adjuvanted candidate RSV vaccines, with a representative sample of patients living with COPD (54% of all subjects enrolled), but acknowledged their research was underpowered to assess vaccine efficacy. Unadjusted, crude estimates of vaccine efficacy are calculable based on their data provided, given vaccine efficacy is equal to 1 minus the risk ratio, as defined in the RENOIR protocol [25]. For Falsey and colleagues’ [21] non-adjuvanted RSV vaccine to prevent any RSV-related acute respiratory illness (versus placebo), vaccine efficacy at 1 year was 7% (1 − [12 events in intervention arm]/[13 events in control arm]) and at 2 years was −167% (1 − [8 events in intervention arm/[3 events in control arm]). Between the data specific to patients living with COPD from Shinde, Falsey, and colleagues [21,24], RSV vaccine efficacy in patients living with COPD ranges from −167% (unprotective) to 100% (protective). Ongoing and future clinical trials should strive for increased transparency and report RSV vaccine efficacy in subgroups of trial participants living with COPD because of the dichotomy of existing data in addition to this population’s susceptibility to RSV LRTI.
Only one study in this rapid review implied RSV vaccine safety in patients living with COPD. In this study, one patient notably experienced ECOPD within 4 h of one RSV vaccine dose, and one patient experienced ECOPD between 33 and 286 days following RSV vaccine dose [21]. Four clinical trials did not specifically provide safety information in COPD subgroups [22,23,24,25]. Not only should ongoing and future clinical trials strive to report on vaccine tolerability and safety in patients living with COPD, but also active and passive surveillance programs (at community, state, and federal levels) should be established to determine if RSV vaccination is associated with more adverse events, including ECOPD, in patients living with COPD.
Limitations
Our review is not without limitations. This was a rapid review, which is a type of systematic review that aims to quickly collect and synthesize evidence [17,18]. Rapid reviews utilize a flexible approach to provide a synthesis of evidence for timely decision making in healthcare, whereas systematic reviews involve a more rigorous process that limits the ability to meet the time-sensitive needs of stakeholders [18]. A fair critique of rapid reviews is that their rigor pales compared to the iterative, exhaustive, and time-consuming methods associated with systematic reviews over two-year periods, and the PRISMA-RR protocol is under development [17]. To improve our project’s methodologic rigor, we adhered to preliminary guidance from the Cochrane Group on rapid reviews’ essential conduct and reporting requirements [18]. The CYPRESS study was a phase 2B clinical trial identified in our rapid review, and it paved the way for the EVERGREEN phase 3 clinical trial (NCT04908683) [30]. Compared to the CYPRESS protocol (NCT03982199), EVERGREEN does not specifically list patients living with COPD as eligible for inclusion in its published eligibility criteria, but it appears patients living with COPD could be eligible for enrollment if their condition is stable per an investigator’s assessment [30]; hence, EVERGREEN was neither identified nor included in our rapid review. Furthermore, EVERGREEN was discontinued, and that RSV vaccine was not submitted for approval [28]. Over 30 RSV vaccine clinical trials are underway but were not captured in our search of ClinicalTrials.gov [5,6]. A scoping review of clinical trial registries (i.e., ClinicalTrials.gov, International Clinical Trials Registry Platform, Open Science Frameworks, etc.) is necessary and should be undertaken. Patients living with COPD, as diagnosed by a clinical trial investigator or with a pre-existing history of mild–moderate COPD, were eligible for inclusion in all seven studies in this rapid review [21,22,23,24,25,26,27]; however, an accurate diagnosis of COPD must be affirmed by spirometry [29]. Thus, there is the potential that patients living with COPD within the clinical trials are susceptible to misclassification bias [31]. One author (PB) screened abstracts and full texts for inclusion in this rapid review, while the Cochrane Group recommends two authors perform these tasks [18]. This author (PB) completed advanced coursework in systematic review research and has previously published systematic and scoping reviews in peer-reviewed journals, but nonetheless, we acknowledge there is potential for selection and information bias because only one author screened the abstracts and full texts in this rapid review.
5. Conclusions
Patients living with COPD have been enrolled in RSV vaccine clinical trials evaluating immunogenicity, safety, and efficacy. RSV vaccine outcomes specific to patients living with COPD, beyond composite endpoint results, are under-reported in clinical trials, and reporting transparency concerning RSV vaccine immunogenicity, safety, and efficacy that are attributable to patients living with COPD enrolled within clinical trials is necessary.
Author Contributions
Conceptualization, P.M.B.; methodology, P.M.B. and A.W.; software, P.M.B.; validation, P.M.B., M.E.F., N.P., J.A.W. and A.W.; formal analysis, P.M.B., M.E.F., N.P., J.A.W. and A.W.; investigation, P.M.B.; resources, P.M.B.; data curation, P.M.B.; writing—original draft preparation, P.M.B., M.E.F., N.P., J.A.W. and A.W.; writing—review and editing, P.M.B., M.E.F., N.P., J.A.W. and A.W.; visualization, P.M.B.; supervision, P.M.B.; project administration, P.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data was created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors acknowledge Shari Clifton for her assistance in performing and executing the database searches.
Conflicts of Interest
Paul M. Boylan has received extramural funding from Pfizer Inc. He declares no relevant conflicts of interest relevant to this research. The remaining authors declare no conflicts of interest.
Appendix A. Database Search Strategies
Search Executed: 14 August 2023
Search Sets Included for Review: #18|#19 (references also forwarded in an EndNote library)
Database: Ovid MEDLINE(R) ALL <1946 to 11 August 2023>
Search Strategy:
1 exp Pulmonary Disease, Chronic Obstructive/(67,074)
2 (chronic$ obstruct$ adj2 (pulm$ or air$ or lung$)).mp. (82,751)
3 copd$.mp. (59,098)
4 or/1–3 (105,792)
5 Respiratory Syncytial Virus Infections/(8710)
6 Respiratory Syncytial Virus, Human/(3954)
7 Respiratory Syncytial Virus Vaccines/(908)
8 (respirat$ sync$ vir$ or rsv$).mp. (24,752)
9 or/5–8 (24752)
10 4 and 9 (253)
11 ..l/10 lg = en (235)
12 ..l/11 yr = 2013-current (141)
13 remove duplicates from 12 (141)
14 exp *Pulmonary Disease, Chronic Obstructive/(56,367)
15 (chronic$ obstruct$ adj2 (pulm$ or air$ or lung$)).ti,kf. (30,874)
16 copd$.ti,kf. (29,070)
17 or/14–16 (71,921)
18 13 and 17 (76)
19 13 not 18 (65)
Search Executed: 8 September 2023
Search Set Included for Review: #5
Database: International Pharmaceutical Abstracts <1970 to August 2023>
Search Strategy:
1 ((respirat$ syncytial$ vir$ or rsv$) adj3 (vaccin$ or immuniz$)).mp. (50)
2 (nirsevimab$ or beyfortus$).mp. (4)
3 1 or 2 (53)
4 ..l/3 lg = en (47)
5 remove duplicates from 4 (46)
Search Executed: 8 September 2023
Search Set Included for Review: #11
Database: Embase Classic + Embase <1947 to 7 September 2023>
Search Strategy:
1 exp respiratory syncytial virus vaccine/(1998)
2 ((respirat$ syncytial$ vir$ or rsv$) adj3 (vaccin$ or immuniz$)).mp. (2920)
3 (nirsevimab$ or beyfortus$).mp. (148)
4 or/1–3 (3011)
5 chronic obstructive lung disease/(176,576)
6 (chronic$ obstruct$ adj2 (pulm$ or air$ or lung$)).mp. (189,834)
7 copd$.mp. (112,675)
8 or/5–7 (207,162)
9 4 and 8 (50)
10 ..l/9 lg = en (49)
11 remove duplicates from 10 (49)
References
- Mehta, J.; Walsh, E.E.; Mahadevia, P.J.; Falsey, A.R. Risk Factors for Respiratory Syncytial Virus Illness among Patients with Chronic Obstructive Pulmonary Disease. COPD 2013, 10, 293–299. [Google Scholar] [CrossRef]
- Anderson, N.W.; Binnicker, M.J.; Harris, D.M.; Chirila, R.M.; Brumble, L.; Mandrekar, J.; Hata, D.J. Morbidity and Mortality among Patients with Respiratory Syncytial Virus Infection: A 2-Year Retrospective Review. Diagn. Microbiol. Infect. Dis. 2016, 85, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Wongsurakiat, P.; Sunhapanit, S.; Muangman, N. Respiratory Syncytial Virus-Associated Acute Respiratory Illness in Adult Non-Immunocompromised Patients: Outcomes, Determinants of Outcomes, and the Effect of Oral Ribavirin Treatment. Influenza Other Respir. Viruses 2022, 16, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Branche, A.R.; Saiman, L.; Walsh, E.E.; Falsey, A.R.; Sieling, W.D.; Greendyke, W.; Peterson, D.R.; Vargas, C.Y.; Phillips, M.; Finelli, L. Incidence of Respiratory Syncytial Virus Infection among Hospitalized Adults, 2017–2020. Clin. Infect. Dis. 2022, 74, 1004–1011. [Google Scholar] [CrossRef]
- Mazur, N.I.; Higgins, D.; Nunes, M.C.; Melero, J.A.; Langedijk, A.C.; Horsley, N.; Buchholz, U.J.; Openshaw, P.J.; McLellan, J.S.; Englund, J.A.; et al. The Respiratory Syncytial Virus Vaccine Landscape: Lessons from the Graveyard and Promising Candidates. Lancet Infect. Dis. 2018, 18, e295–e311. [Google Scholar] [CrossRef]
- Mazur, N.I.; Terstappen, J.; Baral, R.; Bardaji, A.; Beutels, P.; Buchholz, U.J.; Cohen, C.; Crowe, J.E., Jr.; Cutland, C.L.; Eckert, L.; et al. Respiratory Syncytial Virus Prevention within Reach: The Vaccine and Monoclonal Antibody Landscape. Lancet Infect. Dis. 2023, 23, e2–e21. [Google Scholar] [CrossRef]
- Walsh, E.E. Respiratory Syncytial Virus Infection: An Illness for All Ages. Clin. Chest Med. 2017, 38, 29–36. [Google Scholar] [CrossRef]
- Linder, K.A.; Malani, P.N. Rsv Infection in Older Adults. JAMA 2023, 330, 1200. [Google Scholar] [CrossRef] [PubMed]
- Abrysvo. Package Insert; Pfizer Inc.: New York, NY, USA, 2023. [Google Scholar]
- Arexvy. Package Insert; GlaxoSmithKline: Durham, NC, USA, 2023. [Google Scholar]
- Melgar, M.; Britton, A.; Roper, L.E.; Talbot, H.K.; Long, S.S.; Kotton, C.N.; Havers, F.P. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices-United States, 2023. MMWR Morb. Mortal Wkly. Rep. 2023, 72, 793–801. [Google Scholar] [CrossRef]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef]
- Verwey, C.; Madhi, S.A. Review and Update of Active and Passive Immunization against Respiratory Syncytial Virus. BioDrugs 2023, 37, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Chidgey, S.M.; Broadley, K.J. Respiratory Syncytial Virus Infections: Characteristics and Treatment. J. Pharm. Pharmacol. 2005, 57, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B. Respiratory Syncytial Virus and Parainfluenza Virus. N. Engl. J. Med. 2001, 344, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Mac, S.; Sumner, A.; Duchesne-Belanger, S.; Stirling, R.; Tunis, M.; Sander, B. Cost-Effectiveness of Palivizumab for Respiratory Syncytial Virus: A Systematic Review. Pediatrics 2019, 143, e20184064. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.; Garritty, C.; Hersi, M.; Moher, D. Developing PRISMA-RR a Reporting Guideline for Rapid Reviews of Primary Studies (Protocol). 2018. Available online: https://www.equator-network.org/wp-content/uploads/2018/02/PRISMA-RR-protocol.pdf (accessed on 21 January 2024).
- Garritty, C.; Gartlehner, G.; Nussbaumer-Streit, B.; King, V.J.; Hamel, C.; Kamel, C.; Affengruber, L.; Stevens, A. Cochrane Rapid Reviews Methods Group Offers Evidence-Informed Guidance to Conduct Rapid Reviews. J. Clin. Epidemiol. 2021, 130, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.H.; Ison, M.G. Respiratory Syncytial Virus Infection in Adults. BMJ 2019, 366, l5021. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. Group Cochrane Bias Methods, and Group Cochrane Statistical Methods. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Falsey, A.R.; Walsh, E.E.; Capellan, J.; Gravenstein, S.; Zambon, M.; Yau, E.; Gorse, G.J.; Edelman, R.; Hayden, F.G.; McElhaney, J.E.; et al. Comparison of the Safety and Immunogenicity of 2 Respiratory Syncytial Virus (RSV) Vaccines-Nonadjuvanted Vaccine or Vaccine Adjuvanted with Alum-Given Concomitantly with Influenza Vaccine to High-Risk Elderly Individuals. J. Infect. Dis. 2008, 198, 1317–1326. [Google Scholar] [CrossRef]
- Falsey, A.R.; Williams, K.; Gymnopoulou, E.; Heijnen, E. Investigators Cypress, and et al. Efficacy and Safety of an Ad26.Rsv.Pref-Rsv Pref Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 609–620. [Google Scholar] [CrossRef]
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.-G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef]
- Shinde, V.; Cho, I.; Thomas, N.; Fries, L.; Glenn, G. Post-Hoc Analyses of a Phase 2 and Phase 3 Efficacy Trial of an Unadjuvanted Respiratory Syncytial Virus (Rsv) F-Glycoprotein Vaccine in Older Adults in the US: A Consistent Signal of Efficacy Against Hospitalizations for Acute Exacerbation of COPD (AECOPD). Am. J. Respir. Crit. Care Med. 2019, 199, A7094. [Google Scholar]
- Walsh, E.E.; Marc, G.P.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Pfizer. Study to Evaluate the Efficacy, Immunogenicity, and Safety of RSVpref in Adults. Available online: https://classic.clinicaltrials.gov/show/NCT05035212 (accessed on 5 December 2023).
- GlaxoSmithKline. Efficacy Study of Gsk’s Investigational Respiratory Syncytial Virus (RSV) Vaccine in Adults Aged 60 Years and Above. 2021. Available online: https://classic.clinicaltrials.gov/show/NCT04886596 (accessed on 5 December 2023).
- Gallagher, A. Johnson & Johnson Discontinues Phase 3 Study Evaluating Respiratory Syncytial Virus Vaccine. Pharmacy Times. Available online: https://www.pharmacytimes.com/view/johnson-johnson-discontinues-phase-3-study-evaluating-respiratory-syncytial-virus-vaccine (accessed on 5 December 2023).
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2024 Report. 2024. Available online: www.goldcopd.org (accessed on 5 December 2023).
- ClinicalTrials.gov. A Study of an Adenovirus Serotype 26 Pre-Fusion Conformation-Stabilized F Protein (Ad26. Rsv. Pref) Based Respiratory Syncytial Virus (Rsv) Vaccine in the Prevention of Lower Respiratory Tract Disease in Adults Aged 60 Years and Older (EVERGREEN). In EVERGREEN; Janssen: Titusville, NJ, USA, 2023. [Google Scholar]
- Celentano, D.D.; Szklo, M. (Eds.) Gordis Epidemiology; Elsevier: Philadelphia, PA, USA, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


