Abstract
High altitude illness in its most severe form can lead to high altitude cerebral edema (HACE). Current strategies have focused on prevention with graduated ascents, pharmacologic prophylaxis, and descent at first signs of symptoms. Little is understood regarding treatment with steroids and oxygenation being commonly utilized. Pre-clinical studies with turmeric derivatives have offered promise due to its anti-inflammatory and antioxidant properties, but they warrant validation clinically. Ongoing work is focused on better understanding the disease pathophysiology with an emphasis on the glymphatic system and venous outflow obstruction. This review highlights what is known regarding diagnosis, treatment, and prevention, while also introducing novel pathophysiology mechanisms warranting further investigation.
1. Introduction
High altitude illness (HAI) encompasses a group of conditions that are thought to occur secondary to hypoxemia, which develops at high altitude environments [1,2]. High altitude cerebral edema (HACE) is considered to be a HAI in one of its most severe forms [1,3]. It is a rare, yet potentially fatal, neurologic condition that warrants prompt attention and medical management [1].
Established risk factors for HACE include rapid ascent, inadequate acclimatization, extreme altitudes, physical exertion, and a prior history of HAI [4]. Among those traveling to high altitudes, such as tourists, trekkers and mountain climbers, the estimated incidence of HACE is reported to be approximately 0.5–1% [4,5,6]. Although less common, HACE also occurs in high-altitude residents and well-acclimatized climbers, most often in the setting of increased ascension, heavy physical exertion, or ingestion of substances, such as alcohol [7]. In addition to such risk factors, the presence of HACE in well-acclimatized populations could also be secondary to a “re-entry” phenomenon, as postulated to occur in high altitude pulmonary edema [8,9]. Although not yet studied in the setting of HACE, individuals well acclimatized to high altitudes may be primed to undergo abrupt physiologic adaptions that predispose to HACE upon return from excursions to lower altitude environments.
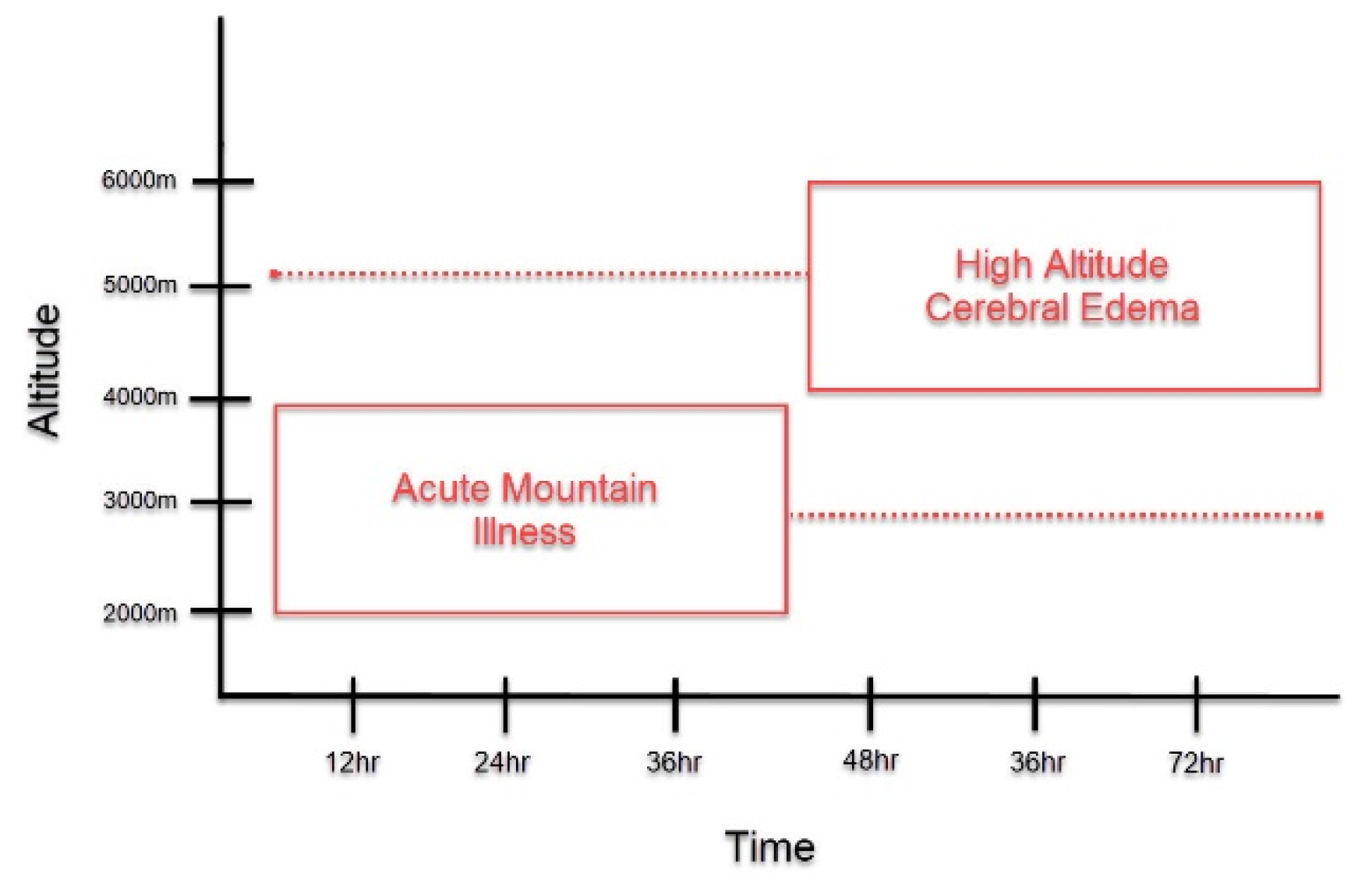
Patients with HACE present with encephalopathy and declining neurologic function, such as altered mental status, slurred speech, cranial nerve palsies, papilledema, and ataxia [3,10]. It most often occurs following prolonged exposure to altitudes greater than 4000 m, although HACE at lower altitudes have been reported [3,6,10]. Acute mountain sickness (AMS) is another important form of HAI that is often found to precede HACE (Figure 1) [4,11]. It is characterized by non-specific symptoms, such as headache, confusion, dizziness, nausea, and vomiting, and presents at altitudes lower than those observed in HACE [3]. The symptoms of AMS peak around 24–48 h, around the time that HACE develops [1,11,12]. Due to disease time course and symptomatology, HACE is often proposed to be an end-stage presentation of AMS [4,6,11,13]. However, HACE can also present without preceding symptoms of AMS and has also been reported to present acutely within a matter of hours [1,14]. Thus, HACE may represent a distinct pathology unique from AMS and further study is warranted to clarify this matter.
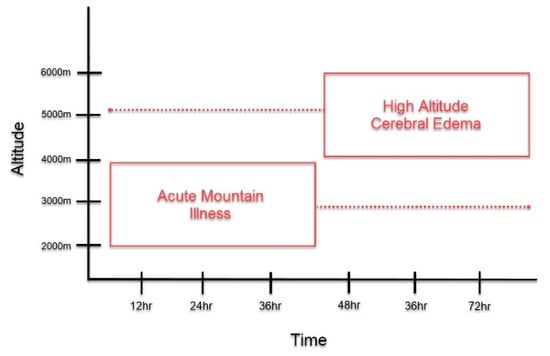
Figure 1.
Symptom Onset of Acute Mountain Illness (AMS) and High Altitude Cerebral Edema (HACE). This graph depicts the symptom onset of AMS and HACE as it relates to altitude and length of exposure to high altitudes. As compared to HACE, AMS generally presents at lower altitudes HACE and with earlier symptom onset. At the time that AMS symptoms peak, if altitude is sufficient, the onset of HACE may occur. However, as depicted in the figure, this time course is not absolute. AMS may present in a delayed fashion, while HACE could present acutely.
Ultimately, HACE is considered to be a clinical diagnosis that can be difficult to make in the context of a broad differential [3]. Although not required, workup with lumbar puncture may reveal increased opening pressure secondary to elevated intracranial pressures [4]. CT, though not as sensitive as MRI, may reveal cerebral edema [4]. Characteristic MRI findings include intense T2 signals representing cerebral edema in the white matter, most significant at the splenium of the corpus callosum [15]. New MRI technology with more advanced scanners also reveal small microbleeds with permanent imprints secondary to microvascular disruption [16]. Unfortunately, there is no apparent correlation between the degree of cerebral edema and the severity of clinical presentation and outcome [15]. One theory to explain this is based upon the Monro–Kellie doctrine. It suggests that individuals have differing baseline intracranial volumes to compensate for worsening cerebral edema [3]. Another theory suggests that symptoms are secondary to astrocytic swelling caused by the redistribution of fluid into the intracellular space [17].
As such, the pathophysiology of HACE is poorly understood. Generally, HACE is characterized by increased intracranial pressure secondary to cytotoxic and vasogenic edema [16]. A prevailing theory is that hypoxia results in severe vasodilation leading to elevated capillary pressure and leakage [10]. The neuro-hormonal response also results in the release of free radicals and reactive cytokines, further disrupting the blood–brain barrier and resulting in cerebral edema [10,18]. Another theory suggests that cerebral edema results from the suppression of Na+/K+ ATPase pumps by free radicals, produced during hypoxia [19].
Nonetheless, despite the unclear pathophysiology, the medical management of HACE continues to advance. In this paper, we review the current medical management and preventative strategies of HACE. We highlight emerging treatment options from the literature and discuss new, innovative strategies for addressing the problem of cerebral edema from high altitude exposure.
2. Current Medical Management
2.1. Prevention
Due to its associated morbidity and mortality, preventing HACE should take priority over treatment [20]. Clinically, HACE presents as a more severe form of AMS. As such, current guidelines utilize similar measures for both AMS and HACE prevention [13]. The primary focus of prevention is to improve acclimatization [21]. Inadequate acclimation is typically the result of rapid ascent, low vital capacity, or an intrinsic poor hypoxic ventilatory response [22]. Those who are unacclimated at altitudes greater than 2500 m are at greatest risk for HACE [13]. A variety of nonpharmacological and pharmacological strategies are implemented to aid in acclimatization [13,23]. The strategies chosen should depend on the anticipated altitude, prior performance at high altitudes, rate of ascent, and the availability of acclimatization days [13].
2.1.1. Nonpharmacologic Prevention
Graded Ascent
Graded ascent is an effective method of prevention for all HAI, including HACE [24]. A slow, staged ascent provides the body with an adequate amount of time to properly acclimate to greater altitudes [20,24]. It is defined by a staged increase in the altitude at which one sleeps [13]. Although randomized controlled studies are lacking, based on clinical studies, a slow, graded ascent is strongly recommended for HACE prevention [5,13,25]. In particular, above 3000 m, climbers should not increase sleeping elevation at rates greater than 500 m a day [13].
Pre-Acclimatization
Pre-acclimatization can be accomplished through multiple methods, including intermittent exposure to hypobaric hypoxia or normobaric hypoxia using hypoxic tents, chambers, or masks [26]. However, each of these devices varies in hypoxic “dose” and duration of exposure [26]. As such, only a few pre-acclimatization programs of intermittent exposure have shown decreases in AMS [13,26,27,28]. Thus, pre-acclimatization can be considered, but is not strongly recommended, in the setting of HACE prevention [13].
2.1.2. Pharmacologic Prevention
Prophylactic medication is not recommended in climbers with a low risk of HACE; however, for those with moderate-to-high risk, as defined by the Wilderness Medical Society Clinical Practice Guidelines, prophylactic treatment is crucial [13]. Despite this, it should be noted that sufficient acclimatization remains the best strategy in HACE prevention [26].
Acetazolamide
Acetazolamide is the only medication proven to aid in acclimatization and is the gold-standard for AMS and HACE prophylaxis (Table 1) [13,22]. Acetazolamide is traditionally known for its inhibitory action on carbonic anhydrase, which results in increased renal bicarbonate loss, leading to a metabolic acidosis [29]. The resulting acidosis is thought to alleviate the respiratory inhibition caused by hypoxia-induced hypocapnia, thus increasing respiratory rate, restoring oxygenation, and enhancing the acclimatization process [29]. However, alternative mechanisms have also been proposed [11,29]. Acetazolamide is the most widely used prophylactic agent with demonstrated efficacy in several clinical trials and should be strongly considered in those at moderate or high risk for AMS [13]. However, it is important to note that acetazolamide does contain a sulfa moiety and may be contraindicated in those with previous adverse reactions to sulfa-containing drugs [13].

Table 1.
Drugs Used in Prevention and Treatment of HACE (recommendations based on Wilderness Medical Society Clinical Practice Guidelines) [13].
Dexamethasone
Dexamethasone is the recommended alternative in those who cannot tolerate acetazolamide (Table 1) [13,24]. The mechanism by which dexamethasone prevents AMS and HACE is unclear; however, reduction in vascular permeability, inflammatory pathway inhibition, antioxidant balance, aquaporin-4 channel (AQP4) modulation and sympathetic blockade have all been proposed [30]. Prospective studies have shown dexamethasone to be effective in AMS prevention, which has been further confirmed in a meta-analysis [13,31,32,33].
Additional Options
Two studies have shown that ibuprofen, when compared to placebo, was effective in preventing AMS, and by extension HACE [13,34,35]. When compared to acetazolamide, one study demonstrated ibuprofen to display a similar efficacy, while a later study failed to replicate these results [13,36,37]. No studies have yet compared its efficacy to dexamethasone. Nevertheless, due to ibuprofen’s side effect profile, such as gastrointestinal bleeding, it should only be considered when one cannot tolerate both acetazolamide and dexamethasone [13].
Other potential alternatives that have been studied include budesonide, ginkgo biloba, chewed coca leaves, and acetaminophen. However, the current literature does not provide strong evidence for their efficacy and they are not recommended in the prevention of HACE at this time [13,20].
2.2. Treatment
2.2.1. Nonpharmacologic Treatment Strategies
Descent
Descent is the gold standard treatment in those who develop HACE, and the decision to descend should be made as soon as possible [13]. Descent should be at least 300 to 1000 m or continued until the patient is asymptomatic [13]. As descent is the main treatment option, other treatment options should not delay descent and should only be used in settings where descent is not possible or may be delayed [19].
Oxygen
Supplemental oxygen should be given in cases of severe HACE with a goal saturation of 90% [13]. It should only be used in combination with descent or while awaiting descent [13]. It is important to note that there are currently no controlled studies that have studied the benefit of supplemental oxygen therapy in HACE patients. Despite this, based on clinical experience, many authors suggest the use of supplemental oxygen to raise oxygen saturation and to help to resolve symptoms in HACE [13,20,26]. However, clinicians should be sure to avoid prolonged hyperoxia due to new associations with increases in mortality in critically ill patients [13].
Portable Hyperbaric Chambers
Portable hyperbaric chambers are reserved for patients with severe HACE in situations where evacuation is delayed [13]. Hyperbaric chambers can raise the ambient pressure around the patient [38]. Two psi, or 105 mmhg, is the pressure setting commonly utilized; however, chambers are capable of generating pressures up to 130 mmhg [10,39]. This increase in pressure may replicate a descent of 1800 to 2400 m, or greater, depending on starting elevation [10,39]. The chamber also contains a valve system that supplies enough ventilation to avoid carbon dioxide accumulation [26].
In order to improve symptoms, the patient must remain in the hyperbaric chamber for several hours, which can be complicated by vomiting, voiding, communication difficulties, and claustrophobia [13,20]. The symptoms of HACE may also rebound as soon the individual is removed from the chamber [13]. However, this brief improvement in symptoms may allow patients to cooperate better during the evacuation process and such an intervention should be considered when available [13].
2.2.2. Pharmacologic Treatment Strategies
Dexamethasone
Despite the emphasis on immediate descent and evacuation to a medical facility, when possible, such interventions may not always be feasible depending on altitude, terrain, and geographic location. Thus, if descent or evacuation must be delayed, dexamethasone should be administered (Table 1) [13]. Although controlled clinical studies have yet to study the efficacy of dexamethasone in the treatment of HACE, decades of clinical experience continue to support its use and it remains the mainstay of pharmaceutical intervention [13]. Of note, administration should include a loading dose of 8 mg, followed by a 4 mg dose every 6 h. It can be given intravenously, intramuscularly, or orally [13].
The mechanism by which dexamethasone exerts its therapeutic effects in the setting of HACE treatment remains unclear. Dexamethasone is traditionally known for its efficacy in treating cerebral edema of many etiologies, perhaps most widely recognized for its ability to ameliorate vasogenic edema associated with intracranial tumors [40,41]. Similarly, dexamethasone may serve to ameliorate vasogenic edema associated with HACE through the stabilization of the blood–brain barrier and the attenuation of anti-inflammatory responses [30,42,43]. Such molecular processes are similar to those proposed in the context of HACE prevention and ultimately warrant further investigation.
Acetazolamide
Although acetazolamide has been studied in the setting of AMS treatment, there is currently no evidence to support its efficacy in treating HACE [20,44]. Thus, the routine use of acetazolamide in the setting of HACE is not recommended and should not replace dexamethasone [13].
2.2.3. Re-Ascent
The safety of climbers continuing their ascent after an episode of HACE remains controversial. However, if ascent is pursued, the person must be symptom-free without medication for several days before proceeding [23]. It is also strongly recommended that those who continue ascent after HACE utilize dexamethasone prophylaxis for the remainder of ascent [23].
3. Novel Approaches
As we continue to learn more about HACE pathogenesis, more novel treatment options have also been explored. Several emerging agents with anti-inflammatory and antioxidant properties have been shown to reduce brain water content (BWC) in preclinical studies. Utilizing acute hypobaric hypoxia (AHH), an animal model used to replicate high-altitude hypoxic environments and the subsequent development of HACE, these agents have shown benefits [45,46].
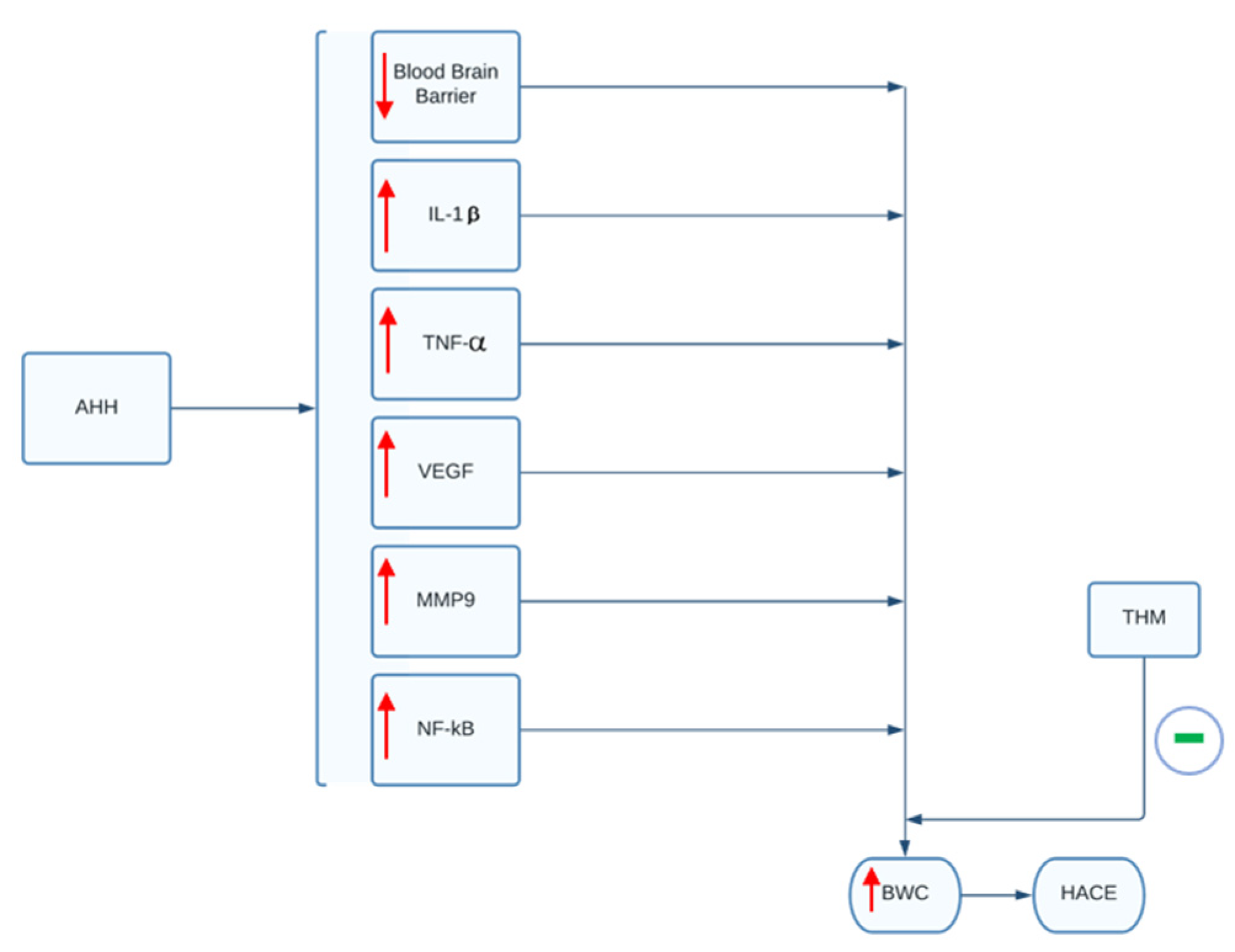
In a study by Pan and colleagues, the use of tetrahydrocurcumin (THM) in rodent models demonstrated the ability to prevent hypoxia-induced cerebral edema and inflammation [47]. THM is a powerful antioxidant derived from curcumin that is naturally found in the turmeric spice. In the study, the use of orally administered THM was shown to significantly reduce the rise in BWC (BWC: Equation (1)) following exposure to AHH, as well as prevent inflammation and blood–brain barrier damage caused by AHH (Figure 2) [47]. These effects were mediated through the inhibition of hypoxia-induced NF-κB/VEGF/MMP-9 inflammatory pathways. Based on the study, the prophylactic administration of 40 mg/kg for 3 days prior to the exposure to acute hypobaric hypoxia was able to diminish significantly the rise of BWC, interleukin-1β and tumor necrosis factors-α levels in rodents post-AHH exposure [47]. The use of THM can therefore serve as a promising therapeutic agent to treat HACE in human subjects. However, THM has poor bioavailability and would require greater than 200 mg per day orally to show similar effects as those seen in rodents. Thus, according to the authors, this quantity would likely compromise patient compliance and bioavailability must be addressed before clinical use [47].
BWC: brain water content; WB: brain weight immediately post-AHH exposer; DB: brain weight after being drained to a constant weight.
BWC = (WB − DB) ÷ WB × 100%
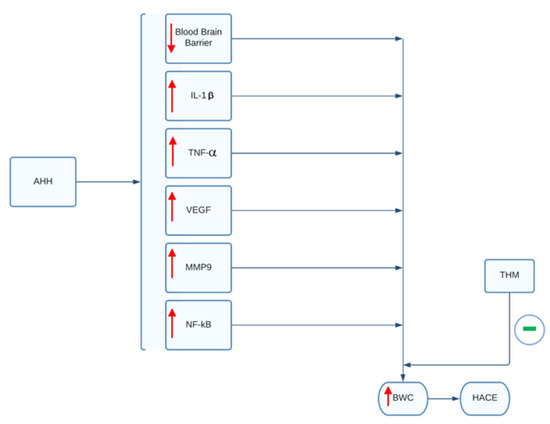
Figure 2.
THM’s effect on BWC and HACE. In animal models, AAH exposure leads to a rise in inflammatory mediators and decrease in blood-brain barrier integrity, as indicated by the red arrows. These alterations lead to a rise in BWC. As indicated by green symbol, administration of THM can attenuate the rise in inflammatory mediators and blood-brain barrier breakdown. Ultimately, this prevents increases in BWC and subsequent development of HACE. AAH, acute hypobaric hypoxia; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor; MMP9, matrix metallopeptidase; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; BWC, brain water content; THM, tetrahydrocurcumin; HACE, high-altitude cerebral edema.
The exogenous use of GM1 monosialoganglioside (GM1) has also been shown to reduce BWC following AHH [48]. GM1 has previously been demonstrated to reduce brain edema and prevent cognitive deficits and outgrowth inhibition of axons in animal models of TBI [49,50]. More recently, its therapeutic potential in models of HACE has been explored. Gong et al. demonstrated that a 40 mg/kg dosage of GM1 substantially ameliorated vascular leakage, reduced AQP4 expression, and increased Na-K-ATPase activity following AHH exposure, all of which help to minimize the increase in BWC post-AHH exposure [48]. GM1’s inhibition of the PI3K/AKT pathways also prevents inflammation and oxidative stress, further hindering the development of cerebral edema [48]. Clinical research on the exogenous use of GM1 in the treatment of HACE is warranted to establish its therapeutic efficacy.
In the manner of THM and GM1, the phenylethanoid glycosides of Phlomis younghusbandii Mukerjee (PhGC) have antioxidant and anti-inflammatory properties that may help to prevent HACE. In the case of PhGC, the production of pro-inflammatory cytokines is likely sequestered via the inhibition of the NF-κB signaling pathway [51]. The use of PhGC as a treatment option for HACE is dependent on further research to determine a more concrete mechanism for how PhGC prevents an increase in BWC [51].
4. Emerging Discovery
Although vasogenic edema and inflammatory cascades are speculated to underpin HACE pathogenesis, the true pathophysiology remains to be elucidated. Recently, attention has turned to potentially overlooked mechanisms.
The glymphatic system is an increasingly understood pathway by which the brain recycles interstitial fluid and clears metabolic waste. It is now considered to play a central role in cerebral fluid homeostasis, controlling both influx and efflux of cerebrospinal fluid (CSF) [52]. Of great interest, AQP4 is a bidirectional water channel protein present along the brain-CSF and blood–brain barrier [53]. Further, research suggests a central role of AQP4 in glymphatic flow homeostasis [54,55,56]. Several pre-clinical studies investigating various agents in models of HACE, traumatic brain injury and ischemia, implicate AQP4 modulation in both the development and resolution of edema, as summarized by Turner et al. [11]. Of interest, further research suggests that statin medications may have modulatory effects at AQP4 [57,58]. In a clinical study by Harrison and colleagues, statin use was associated with a decreased incidence of AMS among travelers flying to high altitudes [45]. Thus, statins may have therapeutic efficacy in preventing HACE, potentially through AQP4 modulation, and warrant further investigation.
Further, both acetazolamide and dexamethasone similarly display modulatory action at AQP4 and are central to HACE prevention and treatment [59,60,61,62]. The exact mechanism by which these agents prevent and treat HACE remains unclear and emerging evidence suggest it may, at least in part, be secondary to glymphatic homeostasis via APQ4 inhibition. As our understanding of glymphatics and its role in cerebral edema grows, we may be able to target this pathway better to help to restore cerebral fluid balance and to manage HACE better at high altitudes.
Another overlooked mechanism that may contribute to cerebral edema is venous outflow obstruction. The contributory role of venous congestion in the development of elevated intracranial pressures was described as early as 1935 and has been recognized to occur in mechanical ventilation secondary to increased intrathoracic pressures [63,64,65]. Venous outflow obstruction is thought to occur in both high-altitude and microgravity environments [64,66]. In microgravity environments, cerebral venous congestion is thought to occur due to cephalad fluid shifts, while at high altitudes, it is thought to be secondary to hypoxic pulmonary vasoconstriction and increased central venous pressures [64]. In the setting of increased cerebral perfusion pressures that occur in microgravity and at high altitudes, impaired venous drainage may cause severe alterations of cerebral blood and CSF homeostasis to culminate in elevated ICPs and, ultimately, cerebral edema.
To target this mechanism, nonpharmaceutical interventions, such as manipulating daytime and nighttime postures, as well as jugular venous pressure, have been suggested and warrant further investigation [11]. Recently, elevated sleeping posture has been studied at high altitudes and did not show efficacy in preventing AMS [67]. However, in light of this finding, research suggests that, instead, lateral sleeping posture may be key to increasing glymphatic and cerebral venous drainage, therefore facilitating cerebral fluid homeostasis [68,69]. Thus, such an intervention may warrant further investigation in the setting of HACE prevention.
5. Conclusions
HACE remains a serious, life-threatening form of HAI with a poorly understood pathophysiology that continues to be explored. It is proposed by some to be an end-stage presentation of AMS, reflecting acute decompensation in cerebral fluid homeostasis. However, it is also known to occur without preceding symptoms of AMS and, thus, could represent a distinct pathophysiology unique from AMS and other HAIs.
Current medical management relies heavily on both clinical experience and clinical studies. The mainstay of prevention includes graded ascent, as well as acetazolamide prophylaxis to assist with acclimatization when needed. Definitive treatment entails rapid descent, as well as the administration of dexamethasone and supportive measures, such as hyperbaric chambers and oxygen supplementation, if descent is not possible or must be delayed.
Despite the efficacy of current medical management, the incidence of HACE remains and current treatment modalities are not always feasible or absolute. Thus, further research is needed. As we continue to explore new avenues of research, several opportunities for therapeutic development emerge. Pre-clinical studies investigating novel anti-inflammatory agents have shown great promise and could soon transition to a clinical research setting. Further, the exploration of emerging concepts regarding cerebral fluid homeostasis, such as the glymphatic system and cerebral venous obstruction, have advanced our current understanding of HACE pathophysiology, enabling us to approach HACE from innovative perspectives. As new avenues of research continue to open, we can further advance medical management and the development of new strategies to address HACE from high altitude environments.
Author Contributions
Conceptualization, B.L.-W. and R.Z.; writing—original draft preparation, K.P., P.F., D.C., M.G., R.Z.; writing—review and editing, R.Z., B.L.-W.; supervision, B.L.-W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gallagher, S.A.; Hackett, P.H. High-altitude illness. Emerg. Med. Clin. N. Am. 2004, 22, 329–355. [Google Scholar] [CrossRef] [PubMed]
- Hartman-Ksycińska, A.; Kluz-Zawadzka, J.; Lewandowski, B. High altitude illness. Przegl. Epidemiol. 2016, 70, 490–499. [Google Scholar] [PubMed]
- Wilson, M.H.; Newman, S.; Imray, C.H. The cerebral effects of ascent to high altitudes. Lancet Neurol. 2009, 8, 175–191. [Google Scholar] [CrossRef]
- Hackett, P.H.; Roach, R.C. High altitude cerebral edema. High Alt. Med. Biol. 2004, 5, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Hackett, P.H.; Rennie, D.; Levine, H.D. The incidence, importance, and prophylaxis of acute mountain sickness. Lancet 1976, 2, 1149–1155. [Google Scholar] [CrossRef]
- Derby, R.; deWeber, K. The athlete and high altitude. Curr. Sports Med. Rep. 2010, 9, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Z.; Zheng, B.-H.; Wang, H.-B.; Zheng, J.-B.; Cai, Z.-X.; Long, R.-L.; Ye, G.-L.; Peng, H. The clinical characteristics of acute severe high-altitude diseases in indigenous tibetans. Chin. J. Tuberc. Respir. Dis. 2006, 29, 835–836. [Google Scholar]
- Ebert-Santos, C. High-altitude pulmonary edema in mountain community residents. High Alt. Med. Biol. 2017, 18, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Riaño López, L.; Figueredo, R.; Vásquez-Hoyos, P. Reentry high-altitude pulmonary edema in pediatric patients. Andes Pediatr. 2021, 92, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.R.; Chawla, A.; Kashyap, A.S. Acute mountain sickness, high altitude cerebral oedema, high altitude pulmonary oedema: The current concepts. Med. J. Armed Forces India 2008, 64, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.E.F.; Gatterer, H.; Falla, M.; Lawley, J.S. High-altitude cerebral edema: Its own entity or end-stage acute mountain sickness? J. Appl. Physiol. 2021, 131, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Sareban, M.; Bärtsch, P. Acute mountain sickness: Do different time courses point to different pathophysiological mechanisms? J. Appl. Physiol. 2020, 128, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Luks, A.M.; Auerbach, P.S.; Freer, L.; Grissom, C.K.; Keyes, L.E.; McIntosh, S.E.; Rodway, G.W.; Schoene, R.B.; Zafren, K.; Hackett, P.H. Wilderness medical society clinical practice guidelines for the prevention and treatment of acute altitude illness: 2019 update. Wilderness Environ. Med. 2019, 30, S3–S18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtzman, R.A.; Caruso, J.L. High-altitude illness death investigation. Acad. Forensic Pathol. 2018, 8, 83–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackett, P.H.; Yarnell, P.R.; Hill, R.; Reynard, K.; Heit, J.; McCormick, J. High-altitude cerebral edema evaluated with magnetic resonance imaging: Clinical correlation and pathophysiology. JAMA 1998, 280, 1920–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackett, P.H.; Yarnell, P.R.; Weiland, D.A.; Reynard, K.B. Acute and evolving mri of high-altitude cerebral edema: Microbleeds, edema, and pathophysiology. AJNR Am. J. Neuroradiol. 2019, 40, 464–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, D.M.; Bärtsch, P.; Knauth, M.; Baumgartner, R.W. Emerging concepts in acute mountain sickness and high-altitude cerebral edema: From the molecular to the morphological. Cell. Mol. Life Sci. CMLS 2009, 66, 3583–3594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severinghaus, J.W. Hypothetical roles of angiogenesis, osmotic swelling, and ischemia in high-altitude cerebral edema. J. Appl. Physiol. 1995, 79, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.D.; Vincent, A.L. High Altitude Cerebral Edema; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Aksel, G.; Çorbacıoğlu, Ş.K.; Özen, C. High-altitude illness: Management approach. Turk. J. Emerg. Med. 2019, 19, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Leyns, C.E.G.; Holtzman, D.M. Intercellular spread of protein aggregates in neurodegenerative disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 545–568. [Google Scholar] [CrossRef]
- Clark, S.T.; Sheraton, M. Ems High-Altitude Field Prophylaxis and Treatment; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Luks, A.M.; Swenson, E.R.; Bärtsch, P. Acute high-altitude sickness. Eur. Respir. Rev. 2017, 26, 160096. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, M.; Hefti, U.; Hefti, J.P. High-altitude illnesses: Old stories and new insights into the pathophysiology, treatment and prevention. Sports Med. Health Sci. 2021, 3, 59–69. [Google Scholar] [CrossRef]
- Beidleman, B.A.; Fulco, C.S.; Muza, S.R.; Rock, P.B.; Staab, J.E.; Forte, V.A.; Brothers, M.D.; Cymerman, A. Effect of six days of staging on physiologic adjustments and acute mountain sickness during ascent to 4300 meters. High Alt. Med. Biol. 2009, 10, 253–260. [Google Scholar] [CrossRef]
- Davis, C.; Hackett, P. Advances in the prevention and treatment of high altitude illness. Emerg. Med. Clin. N. Am. 2017, 35, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Fulco, C.S.; Beidleman, B.A.; Muza, S.R. Effectiveness of preacclimatization strategies for high-altitude exposure. Exerc. Sport Sci. Rev. 2013, 41, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Fulco, C.S.; Muza, S.R.; Beidleman, B.A.; Demes, R.; Staab, J.E.; Jones, J.E.; Cymerman, A. Effect of repeated normobaric hypoxia exposures during sleep on acute mountain sickness, exercise performance, and sleep during exposure to terrestrial altitude. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R428–R436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leaf, D.E.; Goldfarb, D.S. Mechanisms of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness. J. Appl. Physiol. 2007, 102, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Swenson, E.R. Pharmacology of acute mountain sickness: Old drugs and newer thinking. J. Appl. Physiol. 2016, 120, 204–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, E.; Chen, Y.; Luo, Y. Dexamethasone for the prevention of acute mountain sickness: Systematic review and meta-analysis. Int. J. Cardiol. 2014, 173, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, A.J.; Larson, E.B.; Strickland, D. A randomized trial of dexamethasone and acetazolamide for acute mountain sickness prophylaxis. Am. J. Med. 1987, 83, 1024–1030. [Google Scholar] [CrossRef]
- Ellsworth, A.J.; Meyer, E.F.; Larson, E.B. Acetazolamide or dexamethasone use versus placebo to prevent acute mountain sickness on mount rainier. West. J. Med. 1991, 154, 289–293. [Google Scholar] [PubMed]
- Lipman, G.S.; Kanaan, N.C.; Holck, P.S.; Constance, B.B.; Gertsch, J.H. Ibuprofen prevents altitude illness: A randomized controlled trial for prevention of altitude illness with nonsteroidal anti-inflammatories. Ann. Emerg. Med. 2012, 59, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.H.; Corbett, B.; Holck, P.S.; Mulcahy, A.; Watts, M.; Stillwagon, N.T.; Casto, A.; Abramson, C.H.; Vaughan, C.P.A.; Macguire, C.; et al. Altitude sickness in climbers and efficacy of nsaids trial (ascent): Randomized, controlled trial of ibuprofen versus placebo for prevention of altitude illness. Wilderness Environ. Med. 2012, 23, 307–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, P.; Lipman, G.S.; Warner, K.; Jurkiewicz, C.; Phillips, C.; Sanders, L.; Soto, M.; Hackett, P. Altitude sickness prevention with ibuprofen relative to acetazolamide. Am. J. Med. 2019, 132, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Lundeberg, J.; Feiner, J.R.; Schober, A.; Sall, J.W.; Eilers, H.; Bickler, P.E. Increased cytokines at high altitude: Lack of effect of ibuprofen on acute mountain sickness, physiological variables, or cytokine levels. High Alt. Med. Biol. 2018, 19, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Gaddy, J. Ems Altitude Related Conditions and Treatment; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Taber, R.L. Protocols for the use of a portable hyperbaric chamber for the treatment of high altitude disorders. J. Wilderness Med. 1990, 1, 181–192. [Google Scholar] [CrossRef]
- Jha, S.K. Cerebral edema and its management. Med. J. Armed Forces India 2003, 59, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, T.; Bittrich, P.; Noebel, C.; Kuhne, J.F.; Schroeder, J.; Schoen, G.; Fiehler, J.; Kniep, H.C.; Gellißen, S. Efficiency of dexamethasone for treatment of vasogenic edema in brain metastasis patients: A radiographic approach. Front. Oncol. 2019, 9, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietrich, J.; Rao, K.; Pastorino, S.; Kesari, S. Corticosteroids in brain cancer patients: Benefits and pitfalls. Expert Rev. Clin. Pharmacol. 2011, 4, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, B.D.; Yoshimura, K.; Kobayashi, T.; Fukushima, M.; Shibamoto, T.; Ueda, G. Dexamethasone in the treatment of acute mountain sickness. N. Engl. J. Med. 1989, 321, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Grissom, C.K.; Roach, R.C.; Sarnquist, F.H.; Hackett, P.H. Acetazolamide in the treatment of acute mountain sickness: Clinical efficacy and effect on gas exchange. Ann. Intern. Med. 1992, 116, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Luo, H.; Fan, Y.; Luo, Y.; Zhou, Q. Establishment and evaluation of an experimental animal model of high altitude cerebral edema. Neurosci. Lett. 2013, 547, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhou, Y.; Zhao, T.; Han, X.; Qiao, M.; Ding, X.; Li, D.; Wu, L.; Wu, K.; Zhu, L.-L.; et al. A method for establishing the high-altitude cerebral edema (hace) model by acute hypobaric hypoxia in adult mice. J. Neurosci. Methods 2015, 245, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, Y.; Yuan, J.; Ma, X.; Zhao, Y.; Li, Y.; Li, F.; Gong, X.; Zhao, J.; Tang, H.; et al. Tetrahydrocurcumin mitigates acute hypobaric hypoxia-induced cerebral oedema and inflammation through the nf-κb/vegf/mmp-9 pathway. Phytother. Res. 2020, 34, 2963–2977. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Yin, L.; Yuan, L.; Sui, D.; Sun, Y.; Fu, H.; Chen, L.; Wang, X. Ganglioside gm1 protects against high altitude cerebral edema in rats by suppressing the oxidative stress and inflammatory response via the pi3k/akt-nrf2 pathway. Mol. Immunol. 2018, 95, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-G.; Lu, Y.-C.; Zhu, C.; Zhang, G.-J.; Ding, X.-H.; Jiang, J.-Y. Effects of ganglioside gm1 on reduction of brain edema and amelioration of cerebral metabolism after traumatic brain injury. Chin. J. Traumatol. 2003, 6, 23–27. [Google Scholar] [PubMed]
- Rubovitch, V.; Zilberstein, Y.; Chapman, J.; Schreiber, S.; Pick, C.G. Restoring gm1 ganglioside expression ameliorates axonal outgrowth inhibition and cognitive impairments induced by blast traumatic brain injury. Sci. Rep. 2017, 7, 41269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, F.; Li, M.; Han, K.; Ma, Q.; Wang, J.; Qiu, Y.; Yu, L.; He, X.; Liu, D.; Lv, H. Phenylethanoid glycosides of phlomis younghusbandii mukerjee ameliorate acute hypobaric hypoxia-induced brain impairment in rats. Mol. Immunol. 2019, 108, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, H.; Elkin, R.; Heerdt, P.M.; Koundal, S.; Xue, Y.; Lee, H.; Wardlaw, J.; Tannenbaum, A. The glymphatic system and its role in cerebral homeostasis. J. Appl. Physiol. 2020, 129, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Tait, M.J.; Saadoun, S.; Bell, B.A.; Papadopoulos, M.C. Water movements in the brain: Role of aquaporins. Trends Neurosci. 2008, 31, 37–43. [Google Scholar] [CrossRef]
- Mestre, H.; Hablitz, L.M.; Xavier, A.L.R.; Feng, W.; Zou, W.; Pu, T.; Monai, H.; Murlidharan, G.; Rivera, R.M.C.; Simon, M.J.; et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife 2018, 7, e40070. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.M.; Kitchen, P.; Iliff, J.J.; Bill, R.M. Aquaporin 4 and glymphatic flow have central roles in brain fluid homeostasis. Nat. Rev. Neurosci. 2021, 22, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; Macdonald, J.A.; Ishida, H.; Vogel, H.J.; Almutiri, S.; Logan, A.; Kreida, S.; et al. Targeting aquaporin-4 subcellular localization to treat central nervous system edema. Cell 2020, 181, 784–799.e719. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.J.; Dai, T.M.; Shen, Y.Y.; He, J.L.; Li, J.; Tu, J.L. Atorvastatin pretreatment attenuates ischemic brain edema by suppressing aquaporin 4. J. Stroke Cerebrovasc. Dis. 2018, 27, 3247–3255. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Wu, Y.; Fan, Z.; Han, W. Simvastatin improves intracerebral hemorrhage through nf-κb-mediated apoptosis via the myd88/trif signaling pathway. Exp. Ther. Med. 2018, 15, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.T.; Zhang, H.; Xue, Y.X. Dexamethasone treatment modulates aquaporin-4 expression after intracerebral hemorrhage in rats. Neurosci. Lett. 2007, 413, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Du, K.-X.; Dong, Y.; Zhang, Y.; Hou, L.-W.; Fan, D.-X.; Luo, Y.; Zhang, X.-L.; Jia, T.-M.; Lou, J.-Y. Effects of dexamethasone on aquaporin-4 expression in brain tissue of rat with bacterial meningitis. Int. J. Clin. Exp. Pathol. 2015, 8, 3090–3096. [Google Scholar] [PubMed]
- Tanimura, Y.; Hiroaki, Y.; Fujiyoshi, Y. Acetazolamide reversibly inhibits water conduction by aquaporin-4. J. Struct. Biol. 2009, 166, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.-Q.; He, X.-Y.; Yang, X.; Xiao, Y.-C.; Duan, S.-Q.; Wang, H.; Bai, H.; Zhang, Y.; Shi, J.-Y.; Zhu, X.-L.; et al. Acetazolamide alleviate cerebral edema induced by ischemic stroke through inhibiting the expression of aqp4 mrna. Neurocrit. Care 2021, 36, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Nyquist, P.; Stevens, R.D.; Mirski, M.A. Neurologic injury and mechanical ventilation. Neurocrit. Care 2008, 9, 400–408. [Google Scholar] [CrossRef]
- Wilson, M.H.; Imray, C.H.; Hargens, A.R. The headache of high altitude and microgravity—Similarities with clinical syndromes of cerebral venous hypertension. High Alt. Med. Biol. 2011, 12, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Bedford, T.H.B. The effect of increased intracranial venous pressure on the pressure of the cerebrospinal fluid. Brain 1935, 58, 427–447. [Google Scholar] [CrossRef]
- Galdamez, L.A.; Brunstetter, T.J.; Lee, A.G.; Tarver, W.J. Origins of cerebral edema: Implications for spaceflight-associated neuro-ocular syndrome. J. Neuroophthalmol. 2020, 40, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Limper, U.; Fiala, V.; Tank, J.; Elmenhorst, E.-M.; Schaelte, G.; Hew, Y.-Y.M.; Gauger, P.; Martus, P.; Jordan, J. Sleeping with elevated upper body does not attenuate acute mountain sickness: Pragmatic randomized clinical trial. Am. J. Med. 2020, 133, e584–e588. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Xie, L.; Yu, M.; Kang, H.; Feng, T.; Deane, R.; Logan, J.; Nedergaard, M.; Benveniste, H. The effect of body posture on brain glymphatic transport. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 11034–11044. [Google Scholar] [CrossRef] [PubMed]
- Simka, M.; Czaja, J.; Kowalczyk, D. Collapsibility of the internal jugular veins in the lateral decubitus body position: A potential protective role of the cerebral venous outflow against neurodegeneration. Med. Hypotheses. 2019, 133, 109397. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).