Abstract
This study evaluated the toxicological effect of oral administration of Phoenix dactylifera seed essential oil (PDEO) in Wistar rats. PDEO was extracted through a steam-distillation technique. Acute toxicity study evaluated administration of a single dose of the oil in a group (n = 5) of rats followed by 24 h observation, for sub-acute toxicity evaluation, the animals were randomly divided into five groups (n = 3). Group 1 to 4 rats, respectively, received 62.5, 125, 250, and 500 mg/kg bw of PDEO for fourteen days, while the fifth group served as control. At the termination of the study, blood samples were obtained for biochemical and hematological analyses, while vital organs were histopathologically examined. Results from this study revealed no mortality or abnormal behavioral changes in the animals. A dose-related increase in bodyweight and hematological parameters was observed across the treated groups (p < 0.05). At a dosage of 500 mg/kg bw, PDEO caused slight elevation in biochemical marker levels and mild changes in histological architecture of liver and kidney of the test rats. This study revealed that PDEO exhibited significant hematopoietic attributes with no adverse effect on the experimental rats’ vital organs at concentrations below 500 mg/kg bw.
1. Introduction
Natural products extracted from plants host a vast repertoire of bioactive molecules representing opulent prospects for drug development. Medicinal plants have been an integral part of human life all through civilization history [1]. Recognized as one of the oldest fruits globally, the date palm (Phoenix dactylifera) has beneficial nutritional and medicinal values [2]. It plays a vital role in the social and economic life of the people in date producing countries. Asia and Africa are the major producers of date palm with a total production share of 57% and 42.2%, respectively [3]. The date palm (Phoenix dactylifera L.), is an angiosperm, a monocotyledonous plant belonging to the family Arecaceae [4]. The species name dactylifera meaning “finger-bearing” refers to the fruit clusters produced by the plant [5]. It is characterized by pinnated leaves and conduplicate leaflets with acute tips [6]. Flowers of the date palm tree are small and yellow-colored, attached directly to spikelets, which develop as fruits [5]. The fruits that are known as dates are oval-cylindrical, having a single seed [7].
Date seeds, also called kernels or pits, which are usually discarded after consuming date fruits, constitute 10–15% of the fruit weight [8]. Annually, date seeds are generated in large quantities from the date processing industries as waste products [9]. This by-product could represent an excellent source of food ingredients, dietary fiber and phytomedicine [3]. Over the last few decades, essential oils have evoked immense global attention. These natural products have broad-spectrum pharmacological potentials and biotechnological applications [10].
Several previous studies have reported the diverse bioactivities of extracts from date palm fruit and seed. In experimental animal models, Phoenix dactylifera fruits have been shown to display immunomodulatory [11], hepatoprotective [12,13], nephroprotective [14], anticancer [15], antimicrobial [16], anti-allergic [17], cardioprotective [18] and neuroprotective [19] activities. Hydroacetone extract from Phoenix dactylifera seeds protects against chemically induced hepatorenal toxicity [20]. In hyperlipidemic mice given atorvastatin, alanine transferase (ALT), and aspartate transferase (AST) levels were lower with Phoenix dactylifera aqueous seed extract consumption compared to those not given, showing the hepatoprotective ability of the seed [13]. The boiled water seed extract showed an antidiabetic property in streptozotocin induced diabetic rats [21]. Antigenotoxicity of the distilled water seed extract against DNA damage induced by n-nitroson-methylurea in mice has been established [22]. The date seed oil can protect against UV-B and UV-A radiation [23,24], likewise, it has a protective effect on sperm parameters [25] and potential application in formulation cosmetic creams [26].
Despite extensive bioactivity studies on the date plant and its constituents, studies on the pharmacological activities of the date seed oil are infrequent. Relevant data on the toxicological profile of the essential oil from the seed is lacking in the literature, thus underscoring the necessity for this study which evaluated the toxicological effect of oral administration of essential oil from the seed in albino Wistar rats.
2. Results
2.1. Effect of Oral Administration of Essential Oil from P. dactylifera Seed on Body Weight of Wistar Rats
Figure 1 shows the effect of oral administration of different doses of PDEO on the bodyweight of Wistar rats. There was a gradual and sustained increase in the bodyweight of rats in all the experimental groups all through the duration of the animal bioassay. Analysis of the result shows that the gain (percentages) in bodyweight after 14 days were 6.41% (group 1), 6.58% (group 2), 6.70% (group 3), 7.07% (group 4) and 4.49% (group 5).
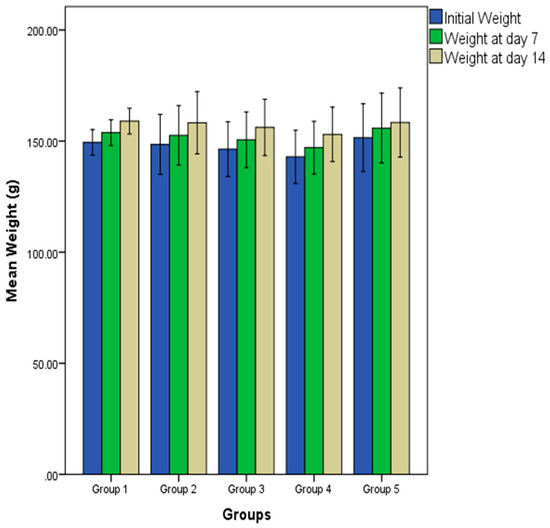
Figure 1.
Effect of repeated oral administration of P. dactylifera seed essential oil on weight of Wistar rats.
2.2. Effect of Repeated Oral Administration of Essential Oil from P. dactylifera Seed on Haematological Parameters of Wistar Rats
As presented in Figure 2, a dose-related increase in hematological parameters such as red blood cell count (RBC), hemoglobin (HB), packed cell volume (PCV), and white blood cell count (WBC) was observed across the treated groups, with the highest values obtained in the group treated with 500 mg/kg bw of the oil: RBC (6.77 ± 0.20 × 106/mm3), PCV (51.33 ± 0.33%), HB (16.30 ± 0.15 g/dL), WBC (9.73 ± 0.20 × 103/mm3). There were significant differences in the hematological parameters of the rats (p < 0.05).
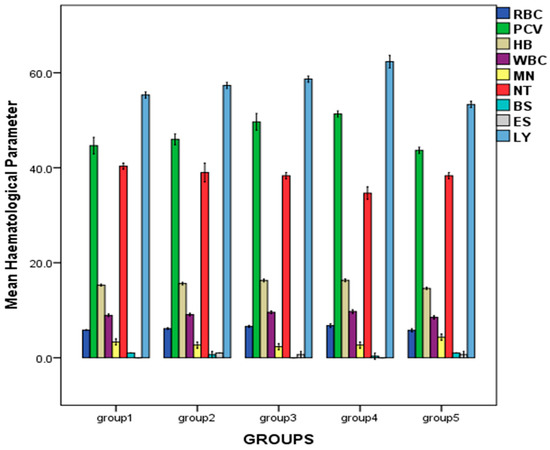
Figure 2.
Effect of repeated oral administration of P. dactylifera seed essential oil on hematological parameters of Wistar rats. Legend: Red Blood Cell count = RBC (×106 mm−3), Packed Cell Volume = PCV (%), Hemoglobin = HB (g/dL), White Blood Cell count = WBC (×103 mm−3), Monocyte = MN (%), Neutrophil = NT (%), Basophil = BS (%) (%), Eosinophil = ES (%), Lymphocyte = LY (%).
2.3. Effect of Repeated Oral Administration of Essential Oil from P. dactylifera Seed on Biochemical Parameters of Wistar Rats
Administration of the oil did not cause any major variation in the evaluated biochemical parameters such as alanine transferase (ALT), aspartate transferase (AST), alkaline phosphatase (ASP), creatinine and urea in the treated groups when compared to the control. However, rats in group 4 showed elevation in these biomarker levels: ALT (98.33 ± 2.27 IU/L), AST (155.27 ± 5.49 IU/L), ALP (38.33 ± 1.10 IU/L), creatinine (29.50 ± 0.52 mmol/L) and urea (42.03 ± 1.47 mmol/L). There was no statistical significance in the biomarker levels within all the groups, except for creatinine (p < 0.05). The results are presented in Figure 3.
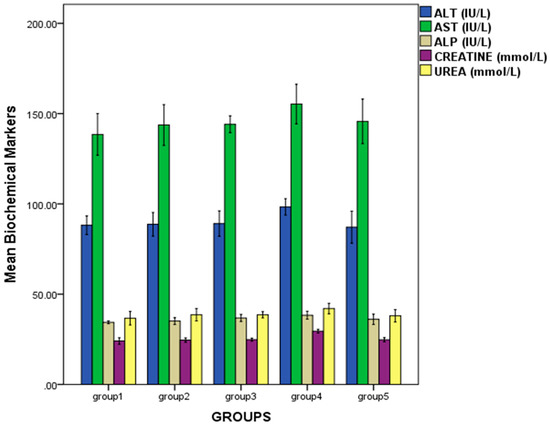
Figure 3.
Effect of repeated oral administration of P. dactylifera seed essential oil on biochemical parameters of Wistar rats.
2.4. Effect of Repeated Oral Administration of Essential Oil from P. dactylifera Seed on Relative Organ Weight of Wistar Rats
As shown in Figure 4, the mean relative organ weight of the treated rats in all groups did not show any significant changes relative to the control group, except for the liver of rats in group 3 and 4 and kidney of rats in group 4, which showed appreciable weight increase.
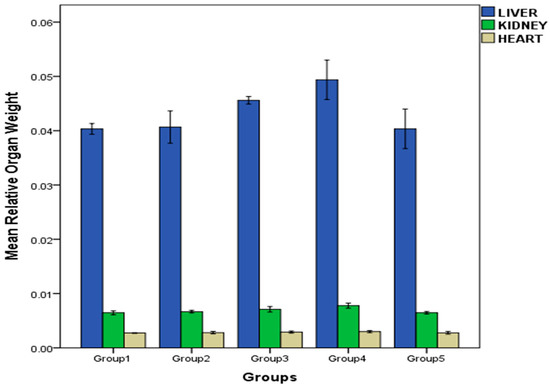
Figure 4.
Effect of repeated oral administration of P. dactylifera seed essential oil on relative organ weight of Wistar rats.
2.5. Effect of Repeated Oral Administration of Essential Oil from P. dactylifera Seed on Vital Organs of Wistar Rats
As presented in Figure 5, Figure 6 and Figure 7, histological examination of the rats’ organs revealed the following: no pathological changes in the architecture of the heart in all the groups; no pathological changes in the histological sections of the liver of rats in all the treated groups except the group administered with 500 mg/kg bw of PDEO (slightly enlarged hepatocytes were observed); and no pathological changes in the kidney of rats in all treated groups except for the group administered with 500 mg/kg bw of PDEO (mild congestion was observed).
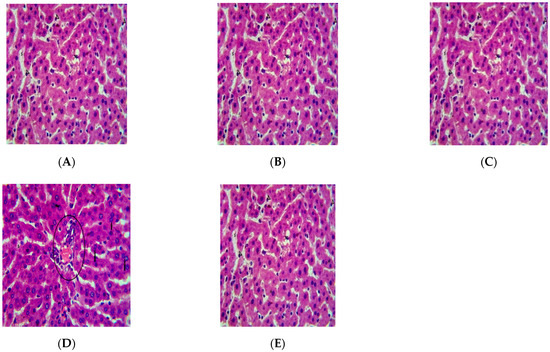
Figure 5.
Photomicrograph of liver tissue section of Wistar rats repeatedly dosed with (A) 62.5 mg/kg bw, (B) 125 mg/kg bw, (C) 250 mg/kg bw of P. dactylifera essential oil and (E) control showing steatosis (ST), well arranged hepatocytes (H) separated by the sinusoids (S) (×400 H and E Staining); (D) 500 mg/kg bw P. dactylifera essential oil showing portal triad with mild periportal hepatitis, hepatocytes showed bi-nucleation (arrow) (×400 H and E Staining).
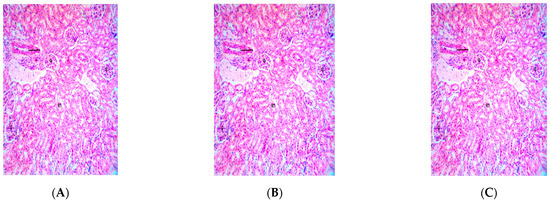
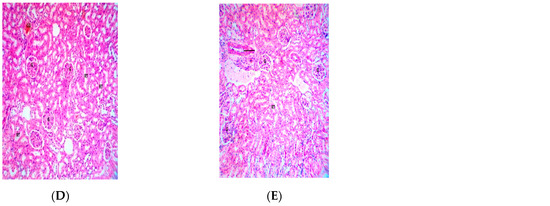
Figure 6.
Photomicrograph of kidney section of Wistar rats repeatedly dosed with (A) 62.5 mg/kg bw, (B) 125 mg/kg bw, (C) 250 mg/kg bw of P. dactylifera essential oil and (E) control showing intact glomerulus (G), other renal features appear normal and unremarkable (×400 H and E Staining); (D) 500 mg/kg bw of P. dactylifera essential oil showing mild congestion (CG).
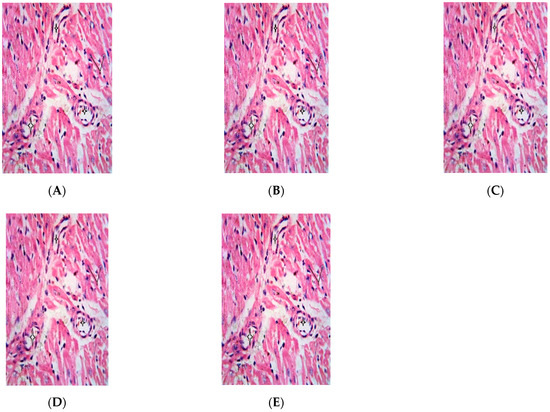
Figure 7.
Photomicrograph shows the heart of Wistar rats repeatedly dosed with (A) 62.5 mg/kg bw, (B) 125 mg/kg bw, (C) 250 mg/kg bw, (D) 500 µg/kg bw P. dactylifera essential oil and (E) control. Cardiac muscle (CM) arranged in bundle, the nucleus (N) and branches of the coronary vessel (star) are normal (×400 H and E Staining).
3. Discussion
Phoenix dactylifera L., called the ‘Tree of Life’ by the Arabs, is considered one of the oldest cultivated fruit trees, which has accompanied the development of early human societies. They are highly symbolic for Muslim, Christian and Jewish religions, and are reputed to have several medicinal benefits [27]. It is believed that consumption of date fruits, specifically in the morning before any meal, can reverse the actions of any toxic substance a subject may have been exposed to [7]. Different parts of this plant are traditionally employed for the treatment of a broad spectrum of ailments including memory disturbances, cardiac disorders, fever, loss of consciousness and nervous disorders [5,7].
Plant extracts can serve as sources of lead molecules for drug development [28]. However, toxicological evaluations are necessary to determine the safety of their use, thus, studies are presently being intensified in this direction. Although information on various extracts from P. dactylifera fruit and seed is ample in the literature, information on bioactivity of the seed oil is sparse, and no data is available on the toxicological profile of essential oil from the seed, sponsoring the impetus for this study. Changes in the hematological and serum biochemical parameters; and organ architecture in animals have been considered as veritable indices for predicting toxicity [29,30].
Oral administration of PDEO induced no mortality in (both for acute and the sub-acute studies) any of the test groups at the end of the treatment period, indicating the LD50 is greater than 2000 mg/kg bw. Additionally, no significant change in skin, fur, stool, behavioral and locomotive activities was noticed in all the test groups.
There was a steady increase in weight gain of the rats as the administered concentration of PDEO increased, however no significant difference was observed within the groups. The weight gain is an indication of infection free state, which is opposed to the debilitating effects of emaciation in morbid animals. This may be a pointer to the health sustaining benefit of PDEO in keeping the rats free from infection and stabilizing their vital hematological and immunological parameters for healthy living.
There was no considerable alteration in relative organ (heart, kidney and liver) weight of the treated animals in virtually all the groups (except the liver of group 3 and 4, and kidney of group 4 animals) relative to the control. No statistical significance was observed in the results obtained. It is conceivable that PDEO did not seem to induce any harmful effect on the examined organs whose relative weight remained fairly constant during the test period. Atrophy of organs during drug administration is symbolic of the toxic effect of the tested compound [31].
The degree of toxic assault of drugs can be determined via assessment of hematological parameters that supply crucial information on the response of the body to stress, injury and deprivation of nutrient [29,32]. Hematological parameters such as RBC, HB, PCV and WBC of experimental animals in groups 3 and 4 showed significant differences in comparison with the control group (p < 0.05). The general dose-dependent increase in levels of PCV, RBC and HB in the test groups could be as a result of PDEO having hematopoietic potential to stimulate erythropoietin release from the kidneys, which is the humoral regulator of RBC production [33,34]. Plant extracts that exert this property could serve as good blood supplements and may be used to cure anemia [35]. Erythropoietin release also has beneficial effect on non-hematopoietic tissues due to its anti-apoptotic, anti-inflammatory, and anti-oxidant properties which confers neuro and cardioprotection [36].
Xenobiotics trigger free radicals that can induce oxidative stress, and activate hepatic stellate cells, leading to liver inflammation. Pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1-beta (IL-1β) can escalate the liver damage [18]. AST, ALT and ALP are biomarkers released in the liver and an elevation in their plasma levels signifies hepatic dysfunction or hepatocellular injury. These enzymes participate in amino acid metabolism and are applicable in monitoring the functional integrity of the liver. ALP is also considered as a marker of cholestasis [37]. The kidney is a vital organ for clearance and excretion of metabolic wastes and xenobiotics, including drugs and drug products from the body. Renal function indices such as urea and creatinine can be used for evaluation of the functional capacity of the nephrons and are regarded as viable indices of kidney function and integrity.
The normal levels of serum biomarkers at dosages below 500 mg/kg bw indicate that PDEO did not adversely interfere with hepatic and renal functions at these dosages. This agrees with the conclusions from the toxicological evaluation of essential oil from Thymus vulgaris (L) at similar concentrations, which showed no detrimental effect on hematological and biochemical parameters of Wistar rats [38]. For all the animal groups in this study, there was no statistical significance in all the biomarker levels except creatine (p ≤ 0.05). Polyphenols and phytosterols, previously reported in date seed oil [23,39], are endowed with anti-inflammatory potentials [37] and may shield the liver from toxic assault. Phenolic compounds act as anti-inflammatory agents by inhibiting leukocyte migration and reducing serum lysozyme, nitric oxide, prostaglandin-E2 (PGE-2), and malondialdehyde levels [40,41]. Downregulation of cyclooxygenase-1 (COX-1), COX-2, PGE-2, interleukin-1-beta (IL-1β) and interleukin-12 (IL-12) have also been reported as another mechanism through which date seed extracts exerts anti-inflammatory action [41]. The slightly elevated levels of biochemical markers of group 4 rats could be as a result of the amplified stimulating effect of the essential oil at this concentration on immune cells such as Kupffer and mesangial cells in the absence of any active infection which putatively led to inflammatory reaction in the liver and kidney, respectively, consequently an elevation in levels of the enzyme biomarkers in the sera of the rats.
Histopathological examination of tissues and organs is best for evaluating treatment related pathological changes, which may serve to further verify alterations in their cellular structure [42]. Mild periportal hepatitis was observed in the histological sections of the rat group given 500 mg/kg of the essential oil which might be due to its accumulation over the protracted duration of administration without prior infection which led to the stimulation of immune response from the liver. The binucleation of hepatocytes observed denotes recovery is steadily being achieved by the regenerative ability of the liver, as these binucleated cells can preferentially undergo the cell cycle and divide afterwards into new hepatocytes with separate nuclei to replace the damaged hepatocytes. Meanwhile, the histological slides of the liver samples of the control rats and the rats given lower doses of the essential oil showed relatively better appearances of well-arranged hepatocytes adequately separated with sinusoids, and little accumulation of fat was observed in the liver tissues, which could be used up subsequently. Buttressing this observation, one study noted that administration of date seed extract reduced inflammatory markers (IL-6, TNF-α, IL-1β, and C-reactive protein) and improved lymphocyte count in rats exposed to a toxic agent [41].
The mild congestion observed in the histological sections of kidneys harvested from rats treated with 500 mg/kg bw of PDEO is an indication of acute interstitial nephritis, which could result from acute kidney injury, interstitial inflammation, oedema or tubulitis. This condition can be induced by drugs, infectious agents or autoimmune response [43,44]. Inflammation in organs and tissues may advance from acute to chronic when the acute inflammatory response cannot be resolved due to interference in the normal resolution process, prolonged exposure to the potentially toxic agent or persistence of the injurious agent [45]. The photomicrograph of the kidney sections of the control rats and rats given lower doses of the essential oil appeared relatively normal. In support of the foregoing, histopathological examination and measurements of hepatorenal serum indices from a different study demonstrated that hydroacetone extract from date seed elicited substantial protective potential against carbon-tetrachloride (CCl4)-induced liver and kidney injury in Wistar rats [20].
The histological sections of the harvested heart from the treated and the control rats showed cardiac cells well arranged in bundles, the nuclei and the coronary vessels were intact. PDEO does not seem to exert damage on the heart despite the dosage and the duration of treatment administration, the architecture of the heart was not observed to be disrupted. Phenolic compounds and flavonoids which are richly found in date fruit [46] and seed oil [23], have been noted for their ameliorating effect on myocardial infarction induced damage in experimental models [47,48]. In addition, phytosterols, tocopherols and unsaturated fatty acids, which are also present in date oil have cardio-protective and anti-inflammatory functions [49,50,51,52].
In a related studies on date fruit extract, administration of the extract mobilized endogenous circulating progenitor cells (CD34 and CD133 positive cells) towards the site of myocardial injury resulting in tissue repair and improvement of the state of myocardial infarction in rats [46]. In another study, extract of date fruits showed cardioprotective, anti-inflammatory, hypolipidemic, and anti-apoptotic potential against myocardial damage in Wistar rats by downregulating the expressions of pro-inflammatory cytokines (IL-6, IL-10, and TNFα) and apoptotic markers (caspase-3 and Bax), and upregulating the anti-apoptotic protein Bcl2 [15]. Histological data from same study also revealed that the extract reduced myonecrosis, edema, and infiltration of inflammatory cells and restored cardiomyocytes architecture [18].
4. Materials and Methods
4.1. Extraction of Essential Oil from Phoenix dactylifera Seed (PDEO)
Phoenix dactylifera fruits were purchased from a local market in Jigawa State, Nigeria. The fruit flesh were manually separated from the endocarp (seed), followed by milling of the seed to obtain a powdery form. Essential oil from the seed was extracted using the Clevenger steam-distillation method [53]. The milled seed powder was dispensed into the biomass chamber connected to a 2000 mL round bottomed flask containing sterile distilled water. The distillation unit was switched on for the extraction process, which was run for the time until no further oil was extracted (about 4 h). The essential oil obtained was dried using anhydrous sodium sulphate and stored at −18 °C until further use. The yield of the oil was 0.26%.
4.2. Ethical Approval
The Committee for Ethical Use of Research Animals of the Federal University of Technology Akure, Nigeria, approved the methodology for all animal experiments carried out in this study. Processes involving experimental animals were carried out in accordance with institutional protocols and the guideline for care and use of laboratory animals [54].
4.3. Experimental Animals
The experiment was performed with 8–12 weeks old Wistar rats weighing 131–162 g. The rats were acquired from the Animal House of the Faculty of Pharmacy, Obafemi Awolowo University Ile-Ife, Nigeria. The rats were acclimatized for a week before commencement of the experiment. The animals’ housing was maintained under regulated environmental conditions of temperature (25–27 °C), humidity (40–60%) and light (12-h light/dark cycle). They were fed ad libitum with commercial rat feed and drinking water.
4.4. Toxicity Studies
All the animals were fasted overnight before commencing the experiment. Acute toxicity study was carried out by oral gavage administration of a single dose of 2000 mg/kg bw of PDEO to a group of Wistar rats (n = 5) in accordance with the limit test procedure prescribed by Organization for Economic Cooperation and Development [55], followed by observation for 24 h for signs and symptoms of toxicity and mortality, while; for sub-acute toxicity evaluation, the animals were randomly divided into five groups (n = 3). Four different groups of rats, respectively, received 62.5, 125, 250, and 500 mg/kg bw of PDEO for fourteen consecutive days, while the fifth group served as control. The weight and behavioral changes of the animals were monitored. At termination of the experiment, the rats were denied access to food overnight and anesthetized by ethyl ether inhalation.
4.5. Haematological and Biochemical Analyses
After 24 h from the last oral administration of PDEO, blood samples were collected by cardiac puncture. A portion of the blood samples obtained from each animal was collected in separate Ethylene Diamine Tetra-acetic Acid (EDTA) sample bottle, and the other portion in plain sample bottle [56]. Blood samples from EDTA bottles were processed immediately to determine hematological parameters that included: RBC, HB, PCV, WBC and white blood cell differential count. Blood samples in the plain bottles were allowed to stand for about 2 h to achieve complete clotting, followed by centrifuging at 2500 rpm for 15 min to obtain the sera. The sera were biochemically analyzed to determine the concentrations of ALT, AST, ALP, creatinine and urea.
4.6. Organ Weight
The organs of rats from the various groups were surgically excised immediately after blood collection. The organs were then trimmed of fat and connective tissues, and placed in a Petri dish containing 10% normal saline, after which they were gently blotted dry with filter paper and weighed immediately (paired organs were weighed together).
4.7. Histopathological Examination
Samples from the organs (liver, kidney and heart) were fixed in 10% neutral buffered formalin for 24 h and processed via routine tissue processing technique. The samples were dehydrated in a graded series of ethanol, cleared in xylene, embedded in paraffin wax, sectioned at 4–7 µm and stained with hematoxylin and eosin (H&E) [57]. The stained sections were carefully examined under binocular compound light microscope. After which, photomicrograph of sections from selected samples were taken under a magnification ×400 using automated built-in digital photo camera.
4.8. Statistical Analysis
All data obtained were analyzed using Statistical Package for Social Sciences (SPSS) version 22.0 (IBM Corp. Armonk, NY, USA); data were subjected to one way analysis of variance (ANOVA) and differences between means were determined by Duncan’s New Multiple Range Test at (p ≤ 0.05).
5. Conclusions
This study showed that essential oil from P. dactylifera seed exhibited significant hematopoietic attributes in a dose-related manner in the test murine model. Administration of the oil caused no mortality, and no major variation in the evaluated biochemical parameters in the treated rat groups, except for slight elevation observed in rats given 500 mg/kg bw of the oil. Histological examination of the rats’ organs revealed no pathological changes in the architecture of the heart, liver and kidney in all the groups, except mild changes noticed in the liver and kidney of the group administered with 500 mg/kg bw of the oil. Preservation of the integrity of the experimental rats’ vital organs at concentrations below 500 mg/kg bw putatively presents PDEO as biologically safe for use at these concentrations. These findings showcase PDEO as a non-toxic agent, which may be used for the clinical development of a good blood supplement, this will be of great value in anemic conditions. Further studies are needed to determine its exact mechanism of action for the observed activity.
Author Contributions
Conceptualization, O.O., M.K.O. and A.O.O.; methodology, O.O. and M.K.O.; software, O.O.; validation, M.K.O. and A.O.O.; formal analysis, O.O.; investigation, O.O. and M.K.O.; resources, O.O., M.K.O. and A.O.O.; data curation, O.O., M.K.O. and A.O.O.; writing—original draft preparation, O.O.; writing—review and editing, O.O., M.K.O. and A.O.O.; supervision, M.K.O. and A.O.O.; project administration, O.O.; funding acquisition, O.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Committee for Ethical Use of Research Animals of the Federal University of Technology Akure, with approval code: (FUTA/ETH/20/28).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed. Pharm. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Ugochukwu, E.; Mazi, E.; Nuria, O.; Okoronkwo, C.O.; Agbaeze, T. Amino Acid Profile, Phytochemical Evaluation and Acute Toxicity (Ld50) Determination of Five Varieties of Date Palm (Phoenix Dactylifera) Seeds. J. Food Technol. Nutr. Sci. 2021, 3, 1–4. [Google Scholar] [CrossRef]
- Alharbi, K.L.; Raman, J.; Shin, H.-J. Date Fruit and Seed in Nutricosmetics. Cosmetics 2021, 8, 59. [Google Scholar] [CrossRef]
- Al-Farsi, M.A.; Lee, C.Y. Nutritional and Functional Properties of Dates: A Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Al-Alawi, R.A.; Al-Mashiqri, J.H.; Al-Nadabi, J.S.M.; Al-Shihi, B.I.; Baqi, Y. Date Palm Tree (Phoenix dactylifera L.): Natural Products and Therapeutic Options. Front. Plant Sci. 2017, 8, 845. [Google Scholar] [CrossRef] [Green Version]
- Uhl, N.W.; Dransfield, J. Genera Palmarum: A Classification of Palms Based on the Work of Harold E. Moore, Jr., L.H.; Baily Hortorium and the International Palm Society: Lawrence, KS, USA, 1987; pp. 214–217. [Google Scholar]
- Vyawahare, N.; Pujari, R.; Khsirsagar, A.; Ingawale, D.; Patil, M.; Kagathara, V. Phoenix dactylifera: An update of its indegenous uses, phytochemistry and pharmacology. Internet J. Pharmacol. 2009, 7, 1531–2976. [Google Scholar]
- Ghnimi, S.; Umer, S. Date fruit (Phoenix dactylifera L.): An underutilized food seeking industrial valorization. NFS J. 2017, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bouallegue, K.; Allaf, T.; Besombes, C.; Younes, R.B.; Allaf, K. Phenomenological modeling and intensification of texturing/grinding-assisted solvent oil extraction; case of date seeds (Phoenix Dactylifera L.). Arab. J. Chem. 2015, 12, 2398–2410. [Google Scholar] [CrossRef] [Green Version]
- Herman, R.A.; Ayepa, E.; Shittu, S.; Fometu, S.S.; Wang, J. Essential Oils and Their Applications—A Mini Review. Adv. Nutr. Food Sci. 2019, 4, 1–13. [Google Scholar]
- Karasawa, K.; Uzuhashi, Y.; Hirota, M.; Otani, H. A matured fruit extract of date palm tree (Phoenix dactylifera L.) stimulates the cellular immune system in mice. J. Agric. Food Chem. 2011, 59, 11287–11293. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Attia, H.A.; Al-Rasheed, N.M.; Al-Amin, M.A.; Al-Onazi, A. Aqueous Date Flesh or Pits Extract Attenuates Liver Fibrosis via Suppression of Hepatic Stellate Cell Activation and Reduction of Inflammatory Cytokines, Transforming Growth Factor-1 and Angiogenic Markers in Carbon Tetrachloride-Intoxicated Rats Extract. Evidence-Based Complement. Altern. Med. 2015, 2015, 247357. [Google Scholar]
- Khan, T.J.; Kuerban, A.; Razvi, S.S.; Mehanna, M.G.; Khan, K.A.; Almulaiky, Y.Q.; Faidallah, H.M. In vivo evaluation of hypolipidemic and antioxidative effect of ‘Ajwa’ (Phoenix dactylifera L.) date seed-extract in high-fat diet-induced hyperlipidemic rat model. Biomed. Pharmacother. 2018, 107, 675–680. [Google Scholar] [CrossRef] [PubMed]
- El Arem, A.; Zekri, M.; Thouri, A.; Saafi, E.B.; Ghrairi, F.; Ayed, A.; Zakhama, A.; Achour, L. Oxidative damage and alterations in antioxidant enzyme activities in the kidneys of rat exposed to trichloroacetic acid: Protective role of date palm fruit. J. Physiol. Biochem. 2014, 70, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Khan, T.J.; Kalamegam, G.; Pushparaj, P.N.; Chaudhary, A.; Abuzenadah, A.; Kumosani, T.; Barbour, E.; Al-Qahtani, M. Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complement. Altern. Med. 2017, 17, 418. [Google Scholar] [CrossRef] [PubMed]
- Mahdhi, A.; Bahi, A.; Mzah, D.; Bakhrouf, A. Use of mixture design to construct a consortium of date palm (Phoenix dactylifera L.) fruit extract and potentially probiotic Bacillus strain to confer protection against vibriosis in Artemia culture. J. Sci. Food Agric. 2013, 93, 3850–3855. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, K.; Otani, H. Anti-Allergic properties of a matured fruit extract of the date palm tree (Phoenix dactylifera L.) in mite-sensitized mice. J. Nutr. Sci. Vitaminol. 2012, 58, 272–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Yahya, M.; Raish, M.; AlSaid, M.S.; Ahmad, A.; Mothana, R.A.; Al-Sohaibani, M.; Al-Dosari, M.S.; Parvez, M.K.; Rafatullah, S. ‘Ajwa’ dates (Phoenix dactylifera L.) extract ameliorates isoproterenol-induced cardiomyopathy through downregulation of oxidative, inflammatory and apoptotic molecules in rodent model. Phytomedicine 2016, 23, 1240–1248. [Google Scholar] [CrossRef]
- Badeli, H.; Shahrokhi, N.; KhoshNazar, M.; Asadi-Shekaari, M.; Shabani, M.; Eftekhar Vaghefi, H.; Khaksari, M.; Basiri, M. Aqueous date fruit efficiency as preventing traumatic brain deterioration and improving pathological parameters after traumatic brain injury in male rats. Cell J. 2016, 18, 416–424. [Google Scholar]
- Ahmed, A.F.; Al-Qahtani, J.H.; Al-Yousef, H.M.; Al-Said, M.S.; Ashour, A.; Al-Sohaibani, M.; Rafatullah, S. Proanthocyanidin-Rich Date Seed Extract Protects Against Chemically Induced Hepatorenal Toxicity. J. Med. Food. 2015, 18, 280–289. [Google Scholar] [CrossRef]
- Hasan, M.; Mohieldein, A. In vivo evaluation of antidiabetic, hypolipidemic, antioxidative activities of saudi date seed extract on streptozotocin induced diabetic rats. J. Clin. Diagn. Res. 2016, 10, FF06–FF12. [Google Scholar]
- Diab, K.A.; Aboul-Ela, E.I. In vivo comparative studies on antigenotoxicity of date palm (Phoenix dactylifera L.) pits extract against DNA damage induced by n-nitroson-methylurea in mice. Toxicol. Int. 2012, 19, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehdi, I.; Omri, S.; Khalil, M.; Al-Resayes, S. Characteristics and chemical composition of date palm (Phoenix canariensis) seeds and seed oil. Ind. Crops Prod. 2010, 32, 360–365. [Google Scholar] [CrossRef]
- Ines, D.; Boudaya, S.; Ben Abdallah, F.; Turki, H.; Attia, H. Effect of Date Seed Oil on p53 Expression in Normal Human Skin. Connect. Tissue Res. 2010, 51, 55–58. [Google Scholar]
- Fatma, B.A.; Nozha, C.F.; Ines, D.; Hamadi, A.; Basma, H.; Leila, A.K. Sperm quality improvement after date seed oil in vitro supplementation in spontaneous and induced oxidative stress. Asian J. Androl. 2009, 11, 393–398. [Google Scholar]
- Lecheb, F.; Benamara, S. Feasability of a cosmetic cream added with aqueous extract and oil from date (Phoenix dactylifera L.) fruit seed using experimental design. J. Cosmet. Sci. 2015, 66, 359–370. [Google Scholar]
- Zehdi-Azouzi, S.; Cherif, E.; Moussouni, S.; Gros-Balthazard, M.; Naqvi, S.; Ludeña, B.; Castillo, K.; Chabrillange, N.; Bouguedoura, N.; Bennaceur, M.; et al. Genetic structure of the date palm (Phoenix dactylifera) in the Old World reveals a strong differentiation between eastern and western populations. Ann. Bot. 2015, 116, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Oluyele, O.; Oladunmoye, M.K. Susceptibility patterns of Staphylococcus aureus isolated from wound swabs to extracts of Vernonia amygdalina. J. Adv. Med. Pharm. Sci. 2017, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tabarraeia, J.H.; Mohammad, R.P.; Hannaneh, G.; Hossain, K.-T. Evaluation of the acute and sub-acute toxicity of the black caraway seed essential oil in Wistar rats. Toxicol. Rep. 2019, 6, 869–874. [Google Scholar] [CrossRef]
- Amaral, L.A.; Cavalcante, A.C.F.; Almeida, T.S.F.; Santos, M.M.R.; Portugal, L.C.; Santos, B.S.; Junior, F.F.B.; Troquez, T.; Nazário, C.E.D.; Cavalheiro, L.F.; et al. Acute and subacute (28 days) oral toxicity studies of tucum almond oil (Bactris Setosa Mart.) in mice. Drug Chem. Toxicol. 2021, 1–7. [Google Scholar] [CrossRef]
- Sellers, R.S.; Mortan, D.; Michael, B.; Roome, N.; Johnson, J.K.; Yano, B.L.; Perry, R.; Schafer, K. Society of toxicologic pathology position paper: Organ weight recommendations for toxicology studies. Toxicol. Pathol. 2007, 35, 751–755. [Google Scholar] [CrossRef] [Green Version]
- Gebremickael, A. Acute and Sub-Chronic Oral Toxicity Evaluation of Eucalyptus Globulus Essential Oil-Water Emulsion in Mice. J. Cytol. Histol. 2017, 8, 459. [Google Scholar] [CrossRef]
- Gryglewski, R.W.; Deptała, A.; Podolak-Dawidziak, M.; Dwilewicz-Trojaczek, J.; Walewski, J.; Jurczyszyn, A. Erythropoietin: A story of a discovery with Polish contribution. Pol. Arch. Intern. Med. 2021, 131, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Tsiftsoglou, A.S. Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine. Cells 2021, 10, 2140. [Google Scholar] [CrossRef] [PubMed]
- Cotoraci, C.; Ciceu, A.; Sasu, A.; Hermenean, A. Natural Antioxidants in Anemia Treatment. Int. J. Mol. Sci. 2021, 22, 1883. [Google Scholar] [CrossRef]
- Xanthos, T.; Vasileiou, P.V.S.; Kakavas, S.; Syggelou, A.; Iacovidou, N. The Potential Role of Erythropoietin as a Pleiotropic Agent in Post-cardiac Arrest Syndrome. Curr. Pharm. Des. 2011, 17, 1517–1529. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Rojas-Armas, J.; Arroyo-Acevedo, J.; Ortiz-Sánchez, M.; Palomino-Pacheco, M.; Castro-Luna, A.; Ramos-Cevallos, N.; Justil-Guerrero, H.; Hilario-Vargas, J.; Herrera-Calderón, O. Acute and Repeated 28-Day Oral Dose Toxicity Studies of Thymus vulgaris L. Essential Oil in Rats. Toxicol. Res. 2019, 35, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Besbes, S.; Blecker, C.; Deroanne, C.; Lognay, G.; Drira, N.; Attia, H. Heating effects on some quality characteristics of date seed oil. Food Chem. 2005, 91, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Saryono, S.; Proverawati, A. Hepatoprotective effect of date seeds works through antioxidant mechanism: A systematic review. Ann. Trop. Public Health 2019, 22, S348. [Google Scholar] [CrossRef]
- Saryono, S.; Sumeru, A.; Proverawati, A.; Efendi, F. Decreasing Carbon Tetrachloride Toxicity using Date-seed (Phoenix dactylifera L.) Steeping in Rats. Toxicol. Environ. Health Sci. 2018, 10, 139–145. [Google Scholar] [CrossRef]
- Prasad, N.; Patel, M.R. Infection-Induced Kidney Diseases. Front. Med. 2018, 5, 327. [Google Scholar] [CrossRef] [PubMed]
- Moledina, D.G.; Perazella, M.A. Differentiating Acute Interstitial nephritis from acute tubular injury: A Challenge for clinicians. Nephron 2019, 143, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Orafidiya, L.O.; Agbani, E.O.; Iwalewa, E.O.; Adelusola, K.A.; Oyedapo, O.O. Studies on the acute and sub-chronic toxicity of the essential oil of Ocimum gratissimum L. leaf. Phytomedcine 2004, 11, 71–76. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhaider, I.A.; Mohamed, M.E.; Ahmed, K.K.M.; Kumar, A.H.S. Date Palm (Phoenix dactylifera) Fruits as a Potential Cardioprotective Agent: The Role of Circulating Progenitor Cells. Front. Pharmacol. 2017, 8, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavitha, S.; John, F.; Indira, M. Amelioration of inflammation by phenolic rich methanolic extract of Ocimum sanctum Linn. leaves in isoproterenol induced myocardial infarction. Indian J. Exp. Biol. 2015, 53, 632–640. [Google Scholar] [PubMed]
- Khalil, I.; Ahmmed, I.; Ahmed, R.; Tanvir, E.M.; Afroz, R.; Paul, S.; Gan, S.H.; Alam, N. Amelioration of isoproterenol-induced oxidative damage in rat myocardium by Withania somnifera leaf extract. BioMed Res. Int. 2015, 2015, 624159. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, M.F.; Sharanabasappa, G.; Parmjyothi, S.; Seshagiri, M.; Moersel, J.-T.; Hassanien, M.F.R. Profile and levels of fatty acids and bioactive constituents in mahua butter from fruit-seeds of buttercup tree [Madhuca longifolia (Koenig)]. Eur. Food Res. Technol. 2005, 222, 710–718. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Mrabet, A.; Jiménez-Araujo, A.; Guillén-Bejarano, R.; Rodríguez-Arcos, R.; Sindic, M. Date Seeds: A Promising Source of Oil with Functional Properties. Foods 2020, 9, 787. [Google Scholar] [CrossRef] [PubMed]
- Mugao, L.G.; Gichimu, B.M.; Muturi, P.W.; Mukono, S.T. Characterization of the Volatile Components of Essential Oils of Selected Plants in Kenya. Biochem. Res. Int. 2020, 2020, 8861798. [Google Scholar] [CrossRef] [PubMed]
- NRC (National Research Council). Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- OECD (Organisation for Economic Cooperation and Development). Acute Oral Toxicity- Up-and-Down-Procedure (UDP), Guideline No. 425. OECD Guidelines for the Testing of Chemicals. Paris: Author. 3 October. 2008. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg425.pdf (accessed on 25 January 2022).
- Provan, D.; Singer, C.R.J.; Baglin, T.; Lilleyman, J. Oxford Handbook of Clinical Haematology, 2nd ed.; Oxford University Press Inc.: New York, NY, USA, 2004. [Google Scholar]
- Thammitiyagodage, M.G.; de Silva, N.R.; Rathnayake, R.; Karunakaran, C.; Kumara, W.G.; Gunatillka, M.M.; Ekanayaka, N.; Galhena, B.P.; Thabrew, M.I. Biochemical and histopathological changes in Wistar rats after consumption of boiled and un-boiled water from high and low disease prevalent areas for chronic kidney disease of unknown etiology (CKDu) in north Central Province (NCP) and its comparison with low disease prevalent Colombo, Sri Lanka. BMC Nephrol. 2020, 21, 38. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).