Toxicity Studies on Essential Oil from Phoenix dactylifera (L.) Seed in Wistar Rats
Abstract
:1. Introduction
2. Results
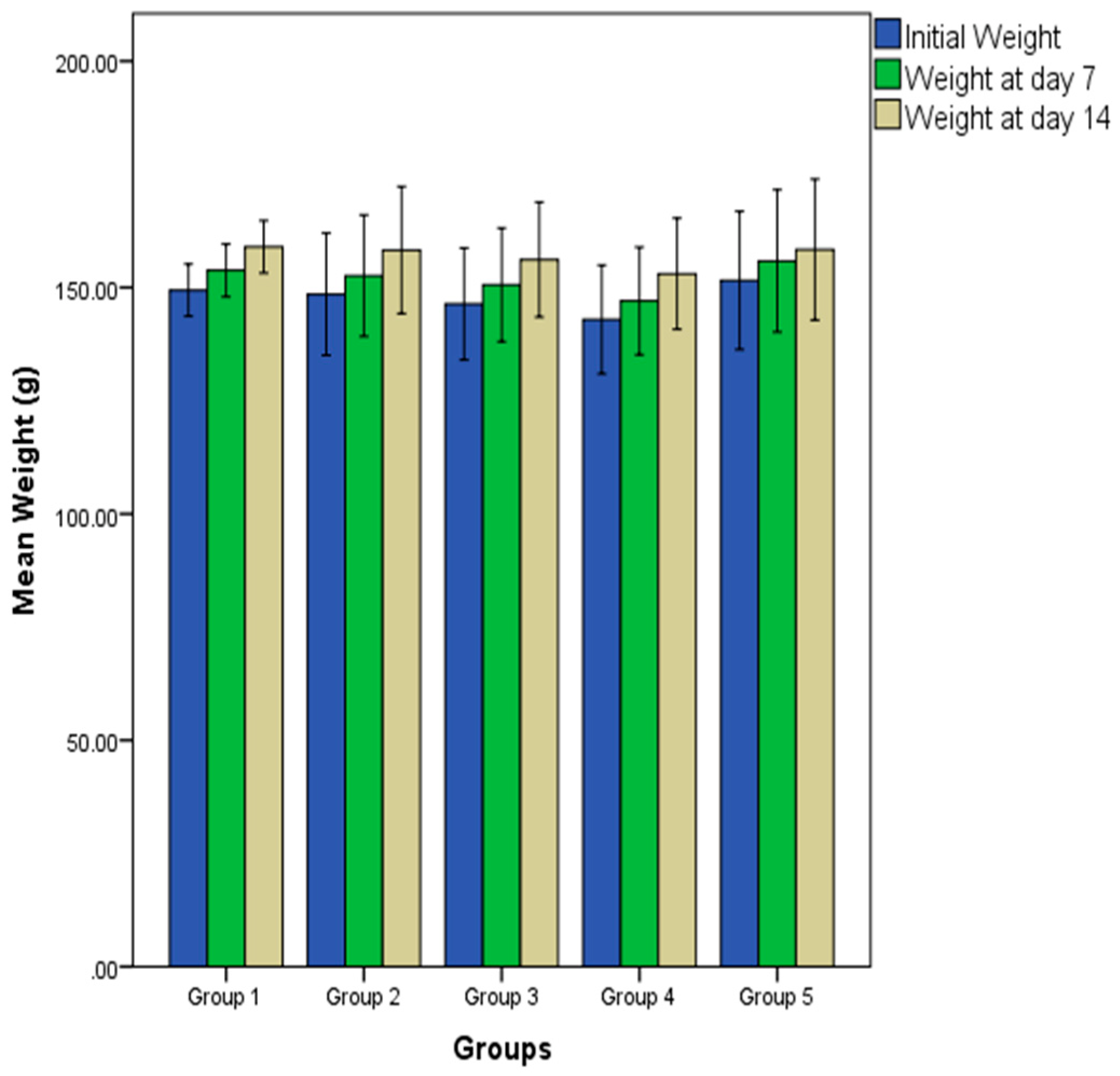
2.1. Effect of Oral Administration of Essential Oil from P. dactylifera Seed on Body Weight of Wistar Rats
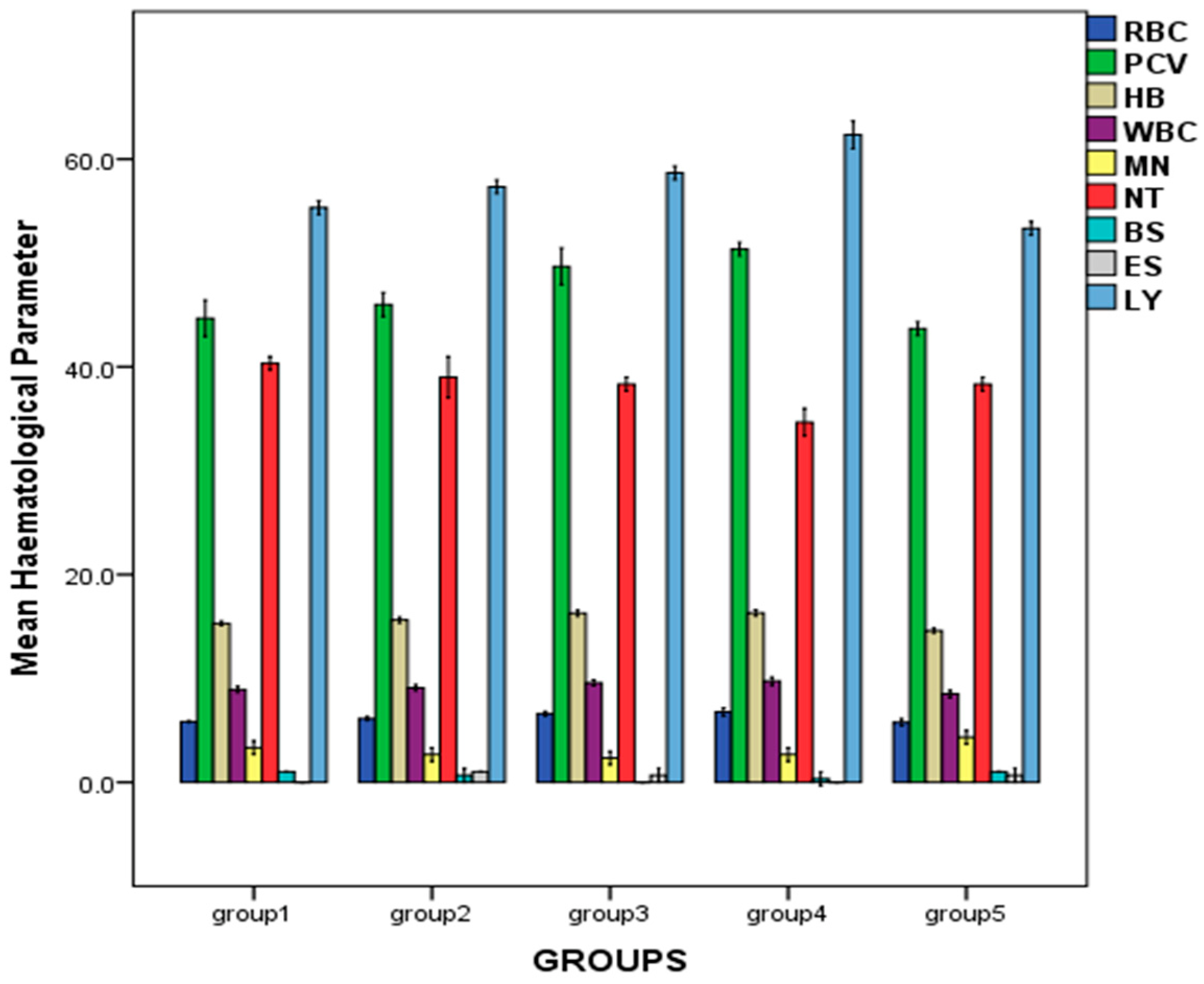
2.2. Effect of Repeated Oral Administration of Essential Oil from P. dactylifera Seed on Haematological Parameters of Wistar Rats
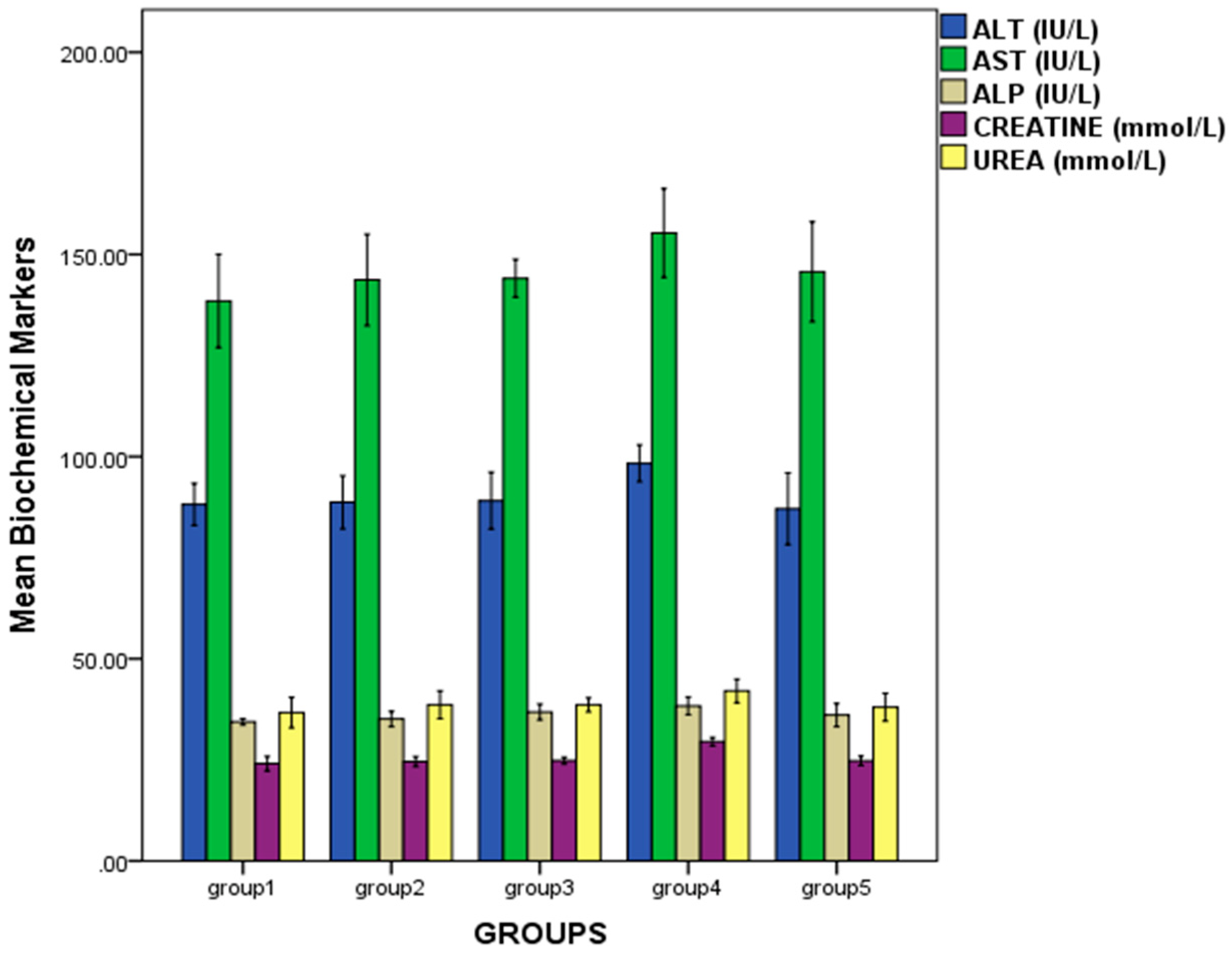
2.3. Effect of Repeated Oral Administration of Essential Oil from P. dactylifera Seed on Biochemical Parameters of Wistar Rats
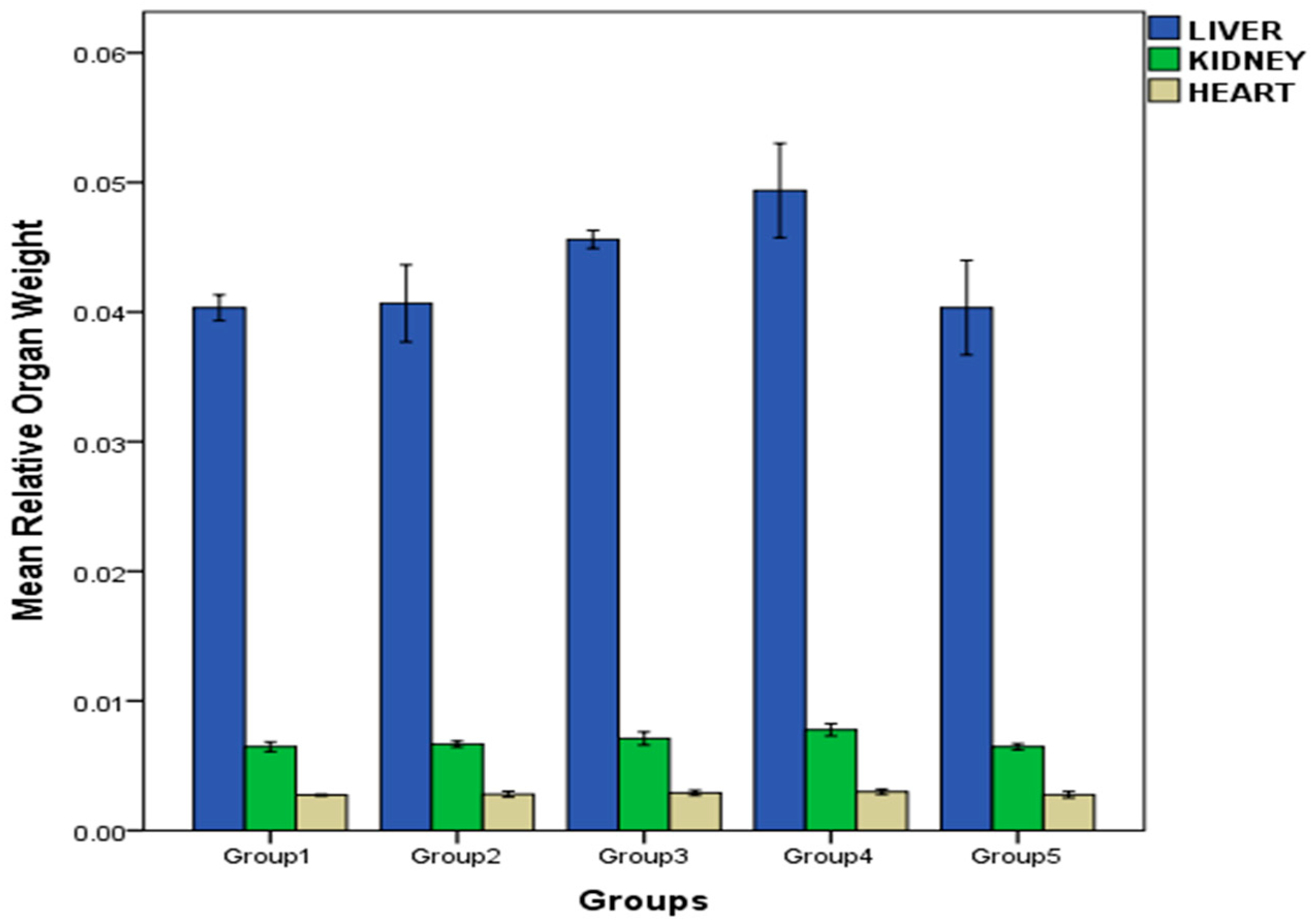
2.4. Effect of Repeated Oral Administration of Essential Oil from P. dactylifera Seed on Relative Organ Weight of Wistar Rats
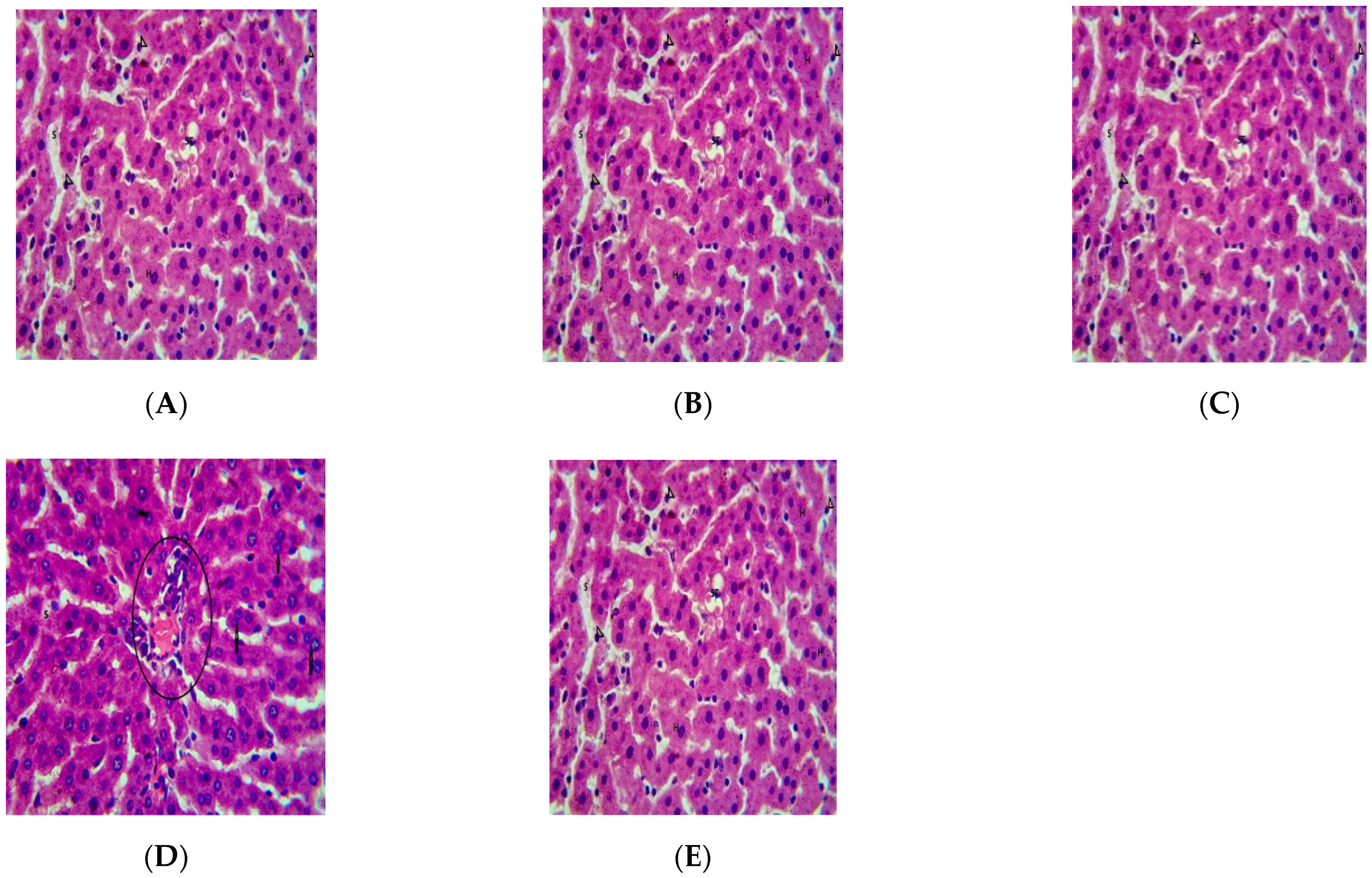
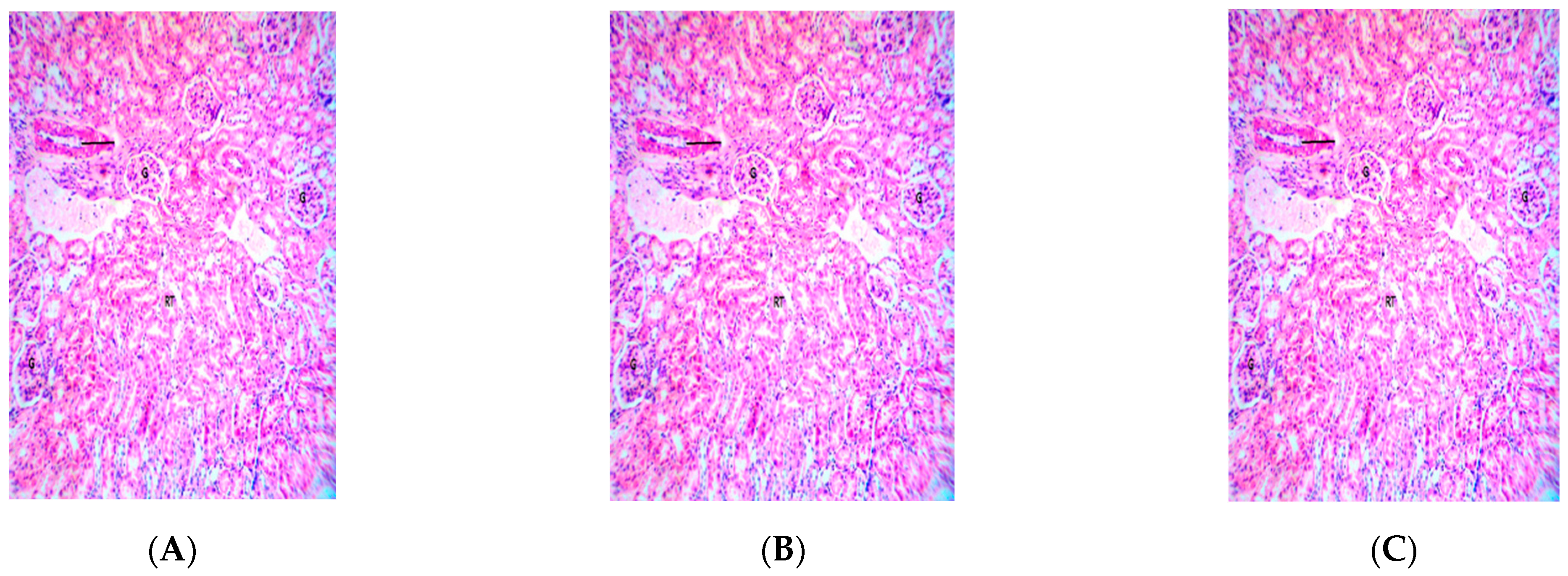
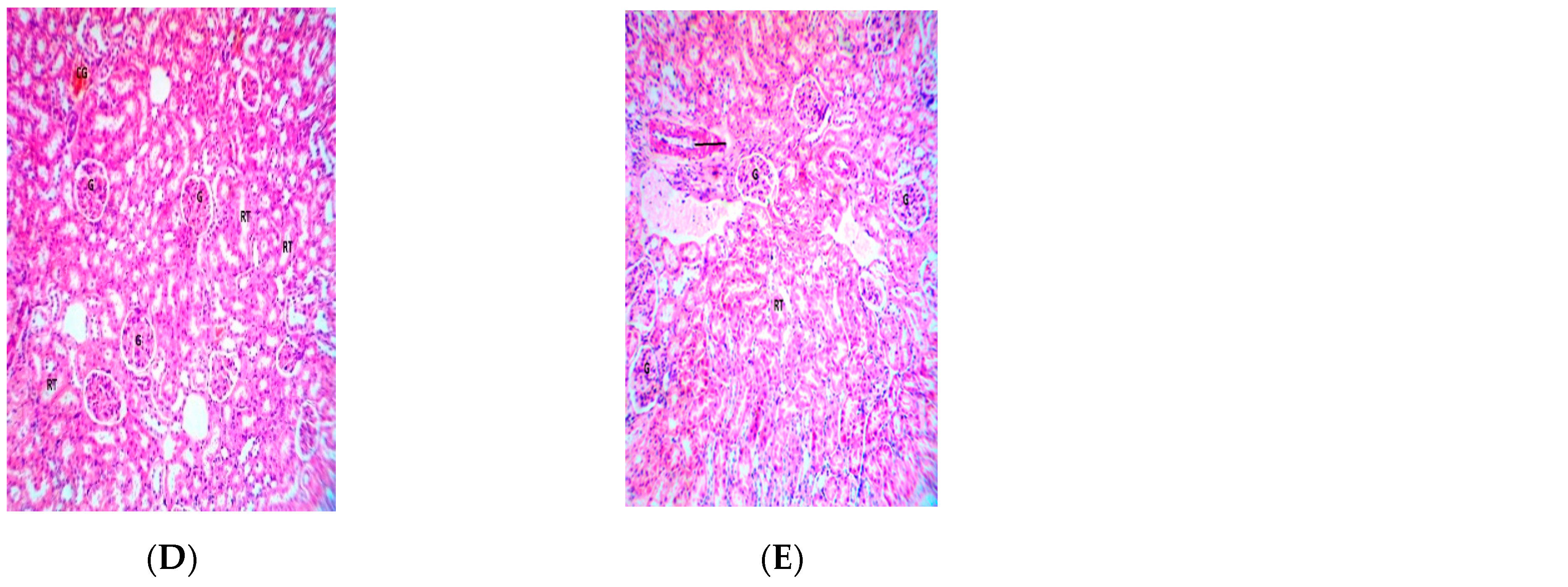
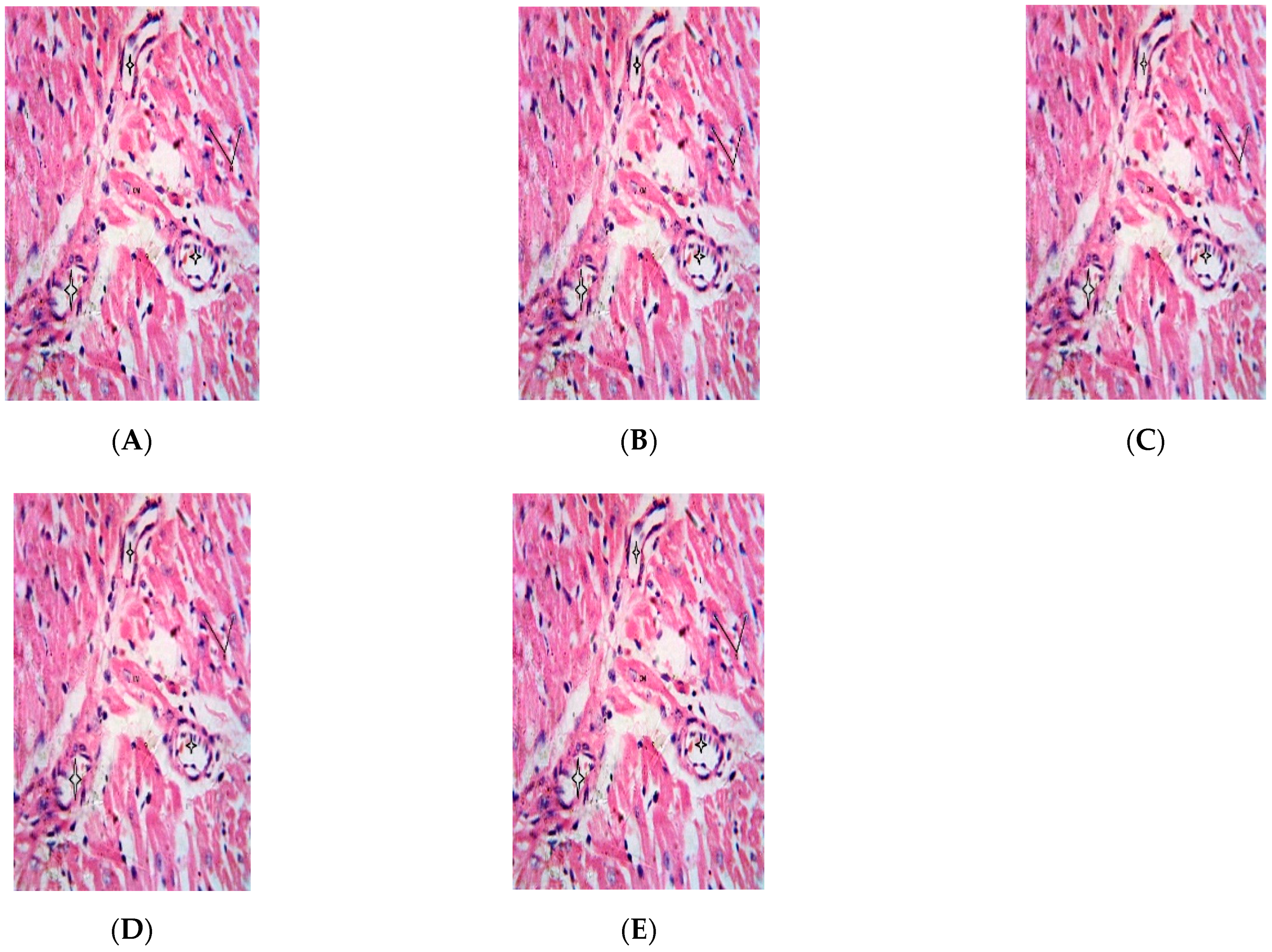
2.5. Effect of Repeated Oral Administration of Essential Oil from P. dactylifera Seed on Vital Organs of Wistar Rats
3. Discussion
4. Materials and Methods
4.1. Extraction of Essential Oil from Phoenix dactylifera Seed (PDEO)
4.2. Ethical Approval
4.3. Experimental Animals
4.4. Toxicity Studies
4.5. Haematological and Biochemical Analyses
4.6. Organ Weight
4.7. Histopathological Examination
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed. Pharm. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Ugochukwu, E.; Mazi, E.; Nuria, O.; Okoronkwo, C.O.; Agbaeze, T. Amino Acid Profile, Phytochemical Evaluation and Acute Toxicity (Ld50) Determination of Five Varieties of Date Palm (Phoenix Dactylifera) Seeds. J. Food Technol. Nutr. Sci. 2021, 3, 1–4. [Google Scholar] [CrossRef]
- Alharbi, K.L.; Raman, J.; Shin, H.-J. Date Fruit and Seed in Nutricosmetics. Cosmetics 2021, 8, 59. [Google Scholar] [CrossRef]
- Al-Farsi, M.A.; Lee, C.Y. Nutritional and Functional Properties of Dates: A Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Al-Alawi, R.A.; Al-Mashiqri, J.H.; Al-Nadabi, J.S.M.; Al-Shihi, B.I.; Baqi, Y. Date Palm Tree (Phoenix dactylifera L.): Natural Products and Therapeutic Options. Front. Plant Sci. 2017, 8, 845. [Google Scholar] [CrossRef] [Green Version]
- Uhl, N.W.; Dransfield, J. Genera Palmarum: A Classification of Palms Based on the Work of Harold E. Moore, Jr., L.H.; Baily Hortorium and the International Palm Society: Lawrence, KS, USA, 1987; pp. 214–217. [Google Scholar]
- Vyawahare, N.; Pujari, R.; Khsirsagar, A.; Ingawale, D.; Patil, M.; Kagathara, V. Phoenix dactylifera: An update of its indegenous uses, phytochemistry and pharmacology. Internet J. Pharmacol. 2009, 7, 1531–2976. [Google Scholar]
- Ghnimi, S.; Umer, S. Date fruit (Phoenix dactylifera L.): An underutilized food seeking industrial valorization. NFS J. 2017, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bouallegue, K.; Allaf, T.; Besombes, C.; Younes, R.B.; Allaf, K. Phenomenological modeling and intensification of texturing/grinding-assisted solvent oil extraction; case of date seeds (Phoenix Dactylifera L.). Arab. J. Chem. 2015, 12, 2398–2410. [Google Scholar] [CrossRef] [Green Version]
- Herman, R.A.; Ayepa, E.; Shittu, S.; Fometu, S.S.; Wang, J. Essential Oils and Their Applications—A Mini Review. Adv. Nutr. Food Sci. 2019, 4, 1–13. [Google Scholar]
- Karasawa, K.; Uzuhashi, Y.; Hirota, M.; Otani, H. A matured fruit extract of date palm tree (Phoenix dactylifera L.) stimulates the cellular immune system in mice. J. Agric. Food Chem. 2011, 59, 11287–11293. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Attia, H.A.; Al-Rasheed, N.M.; Al-Amin, M.A.; Al-Onazi, A. Aqueous Date Flesh or Pits Extract Attenuates Liver Fibrosis via Suppression of Hepatic Stellate Cell Activation and Reduction of Inflammatory Cytokines, Transforming Growth Factor-1 and Angiogenic Markers in Carbon Tetrachloride-Intoxicated Rats Extract. Evidence-Based Complement. Altern. Med. 2015, 2015, 247357. [Google Scholar]
- Khan, T.J.; Kuerban, A.; Razvi, S.S.; Mehanna, M.G.; Khan, K.A.; Almulaiky, Y.Q.; Faidallah, H.M. In vivo evaluation of hypolipidemic and antioxidative effect of ‘Ajwa’ (Phoenix dactylifera L.) date seed-extract in high-fat diet-induced hyperlipidemic rat model. Biomed. Pharmacother. 2018, 107, 675–680. [Google Scholar] [CrossRef] [PubMed]
- El Arem, A.; Zekri, M.; Thouri, A.; Saafi, E.B.; Ghrairi, F.; Ayed, A.; Zakhama, A.; Achour, L. Oxidative damage and alterations in antioxidant enzyme activities in the kidneys of rat exposed to trichloroacetic acid: Protective role of date palm fruit. J. Physiol. Biochem. 2014, 70, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Khan, T.J.; Kalamegam, G.; Pushparaj, P.N.; Chaudhary, A.; Abuzenadah, A.; Kumosani, T.; Barbour, E.; Al-Qahtani, M. Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complement. Altern. Med. 2017, 17, 418. [Google Scholar] [CrossRef] [PubMed]
- Mahdhi, A.; Bahi, A.; Mzah, D.; Bakhrouf, A. Use of mixture design to construct a consortium of date palm (Phoenix dactylifera L.) fruit extract and potentially probiotic Bacillus strain to confer protection against vibriosis in Artemia culture. J. Sci. Food Agric. 2013, 93, 3850–3855. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, K.; Otani, H. Anti-Allergic properties of a matured fruit extract of the date palm tree (Phoenix dactylifera L.) in mite-sensitized mice. J. Nutr. Sci. Vitaminol. 2012, 58, 272–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Yahya, M.; Raish, M.; AlSaid, M.S.; Ahmad, A.; Mothana, R.A.; Al-Sohaibani, M.; Al-Dosari, M.S.; Parvez, M.K.; Rafatullah, S. ‘Ajwa’ dates (Phoenix dactylifera L.) extract ameliorates isoproterenol-induced cardiomyopathy through downregulation of oxidative, inflammatory and apoptotic molecules in rodent model. Phytomedicine 2016, 23, 1240–1248. [Google Scholar] [CrossRef]
- Badeli, H.; Shahrokhi, N.; KhoshNazar, M.; Asadi-Shekaari, M.; Shabani, M.; Eftekhar Vaghefi, H.; Khaksari, M.; Basiri, M. Aqueous date fruit efficiency as preventing traumatic brain deterioration and improving pathological parameters after traumatic brain injury in male rats. Cell J. 2016, 18, 416–424. [Google Scholar]
- Ahmed, A.F.; Al-Qahtani, J.H.; Al-Yousef, H.M.; Al-Said, M.S.; Ashour, A.; Al-Sohaibani, M.; Rafatullah, S. Proanthocyanidin-Rich Date Seed Extract Protects Against Chemically Induced Hepatorenal Toxicity. J. Med. Food. 2015, 18, 280–289. [Google Scholar] [CrossRef]
- Hasan, M.; Mohieldein, A. In vivo evaluation of antidiabetic, hypolipidemic, antioxidative activities of saudi date seed extract on streptozotocin induced diabetic rats. J. Clin. Diagn. Res. 2016, 10, FF06–FF12. [Google Scholar]
- Diab, K.A.; Aboul-Ela, E.I. In vivo comparative studies on antigenotoxicity of date palm (Phoenix dactylifera L.) pits extract against DNA damage induced by n-nitroson-methylurea in mice. Toxicol. Int. 2012, 19, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehdi, I.; Omri, S.; Khalil, M.; Al-Resayes, S. Characteristics and chemical composition of date palm (Phoenix canariensis) seeds and seed oil. Ind. Crops Prod. 2010, 32, 360–365. [Google Scholar] [CrossRef]
- Ines, D.; Boudaya, S.; Ben Abdallah, F.; Turki, H.; Attia, H. Effect of Date Seed Oil on p53 Expression in Normal Human Skin. Connect. Tissue Res. 2010, 51, 55–58. [Google Scholar]
- Fatma, B.A.; Nozha, C.F.; Ines, D.; Hamadi, A.; Basma, H.; Leila, A.K. Sperm quality improvement after date seed oil in vitro supplementation in spontaneous and induced oxidative stress. Asian J. Androl. 2009, 11, 393–398. [Google Scholar]
- Lecheb, F.; Benamara, S. Feasability of a cosmetic cream added with aqueous extract and oil from date (Phoenix dactylifera L.) fruit seed using experimental design. J. Cosmet. Sci. 2015, 66, 359–370. [Google Scholar]
- Zehdi-Azouzi, S.; Cherif, E.; Moussouni, S.; Gros-Balthazard, M.; Naqvi, S.; Ludeña, B.; Castillo, K.; Chabrillange, N.; Bouguedoura, N.; Bennaceur, M.; et al. Genetic structure of the date palm (Phoenix dactylifera) in the Old World reveals a strong differentiation between eastern and western populations. Ann. Bot. 2015, 116, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Oluyele, O.; Oladunmoye, M.K. Susceptibility patterns of Staphylococcus aureus isolated from wound swabs to extracts of Vernonia amygdalina. J. Adv. Med. Pharm. Sci. 2017, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tabarraeia, J.H.; Mohammad, R.P.; Hannaneh, G.; Hossain, K.-T. Evaluation of the acute and sub-acute toxicity of the black caraway seed essential oil in Wistar rats. Toxicol. Rep. 2019, 6, 869–874. [Google Scholar] [CrossRef]
- Amaral, L.A.; Cavalcante, A.C.F.; Almeida, T.S.F.; Santos, M.M.R.; Portugal, L.C.; Santos, B.S.; Junior, F.F.B.; Troquez, T.; Nazário, C.E.D.; Cavalheiro, L.F.; et al. Acute and subacute (28 days) oral toxicity studies of tucum almond oil (Bactris Setosa Mart.) in mice. Drug Chem. Toxicol. 2021, 1–7. [Google Scholar] [CrossRef]
- Sellers, R.S.; Mortan, D.; Michael, B.; Roome, N.; Johnson, J.K.; Yano, B.L.; Perry, R.; Schafer, K. Society of toxicologic pathology position paper: Organ weight recommendations for toxicology studies. Toxicol. Pathol. 2007, 35, 751–755. [Google Scholar] [CrossRef] [Green Version]
- Gebremickael, A. Acute and Sub-Chronic Oral Toxicity Evaluation of Eucalyptus Globulus Essential Oil-Water Emulsion in Mice. J. Cytol. Histol. 2017, 8, 459. [Google Scholar] [CrossRef]
- Gryglewski, R.W.; Deptała, A.; Podolak-Dawidziak, M.; Dwilewicz-Trojaczek, J.; Walewski, J.; Jurczyszyn, A. Erythropoietin: A story of a discovery with Polish contribution. Pol. Arch. Intern. Med. 2021, 131, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Tsiftsoglou, A.S. Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine. Cells 2021, 10, 2140. [Google Scholar] [CrossRef] [PubMed]
- Cotoraci, C.; Ciceu, A.; Sasu, A.; Hermenean, A. Natural Antioxidants in Anemia Treatment. Int. J. Mol. Sci. 2021, 22, 1883. [Google Scholar] [CrossRef]
- Xanthos, T.; Vasileiou, P.V.S.; Kakavas, S.; Syggelou, A.; Iacovidou, N. The Potential Role of Erythropoietin as a Pleiotropic Agent in Post-cardiac Arrest Syndrome. Curr. Pharm. Des. 2011, 17, 1517–1529. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Rojas-Armas, J.; Arroyo-Acevedo, J.; Ortiz-Sánchez, M.; Palomino-Pacheco, M.; Castro-Luna, A.; Ramos-Cevallos, N.; Justil-Guerrero, H.; Hilario-Vargas, J.; Herrera-Calderón, O. Acute and Repeated 28-Day Oral Dose Toxicity Studies of Thymus vulgaris L. Essential Oil in Rats. Toxicol. Res. 2019, 35, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Besbes, S.; Blecker, C.; Deroanne, C.; Lognay, G.; Drira, N.; Attia, H. Heating effects on some quality characteristics of date seed oil. Food Chem. 2005, 91, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Saryono, S.; Proverawati, A. Hepatoprotective effect of date seeds works through antioxidant mechanism: A systematic review. Ann. Trop. Public Health 2019, 22, S348. [Google Scholar] [CrossRef]
- Saryono, S.; Sumeru, A.; Proverawati, A.; Efendi, F. Decreasing Carbon Tetrachloride Toxicity using Date-seed (Phoenix dactylifera L.) Steeping in Rats. Toxicol. Environ. Health Sci. 2018, 10, 139–145. [Google Scholar] [CrossRef]
- Prasad, N.; Patel, M.R. Infection-Induced Kidney Diseases. Front. Med. 2018, 5, 327. [Google Scholar] [CrossRef] [PubMed]
- Moledina, D.G.; Perazella, M.A. Differentiating Acute Interstitial nephritis from acute tubular injury: A Challenge for clinicians. Nephron 2019, 143, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Orafidiya, L.O.; Agbani, E.O.; Iwalewa, E.O.; Adelusola, K.A.; Oyedapo, O.O. Studies on the acute and sub-chronic toxicity of the essential oil of Ocimum gratissimum L. leaf. Phytomedcine 2004, 11, 71–76. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhaider, I.A.; Mohamed, M.E.; Ahmed, K.K.M.; Kumar, A.H.S. Date Palm (Phoenix dactylifera) Fruits as a Potential Cardioprotective Agent: The Role of Circulating Progenitor Cells. Front. Pharmacol. 2017, 8, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavitha, S.; John, F.; Indira, M. Amelioration of inflammation by phenolic rich methanolic extract of Ocimum sanctum Linn. leaves in isoproterenol induced myocardial infarction. Indian J. Exp. Biol. 2015, 53, 632–640. [Google Scholar] [PubMed]
- Khalil, I.; Ahmmed, I.; Ahmed, R.; Tanvir, E.M.; Afroz, R.; Paul, S.; Gan, S.H.; Alam, N. Amelioration of isoproterenol-induced oxidative damage in rat myocardium by Withania somnifera leaf extract. BioMed Res. Int. 2015, 2015, 624159. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, M.F.; Sharanabasappa, G.; Parmjyothi, S.; Seshagiri, M.; Moersel, J.-T.; Hassanien, M.F.R. Profile and levels of fatty acids and bioactive constituents in mahua butter from fruit-seeds of buttercup tree [Madhuca longifolia (Koenig)]. Eur. Food Res. Technol. 2005, 222, 710–718. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Mrabet, A.; Jiménez-Araujo, A.; Guillén-Bejarano, R.; Rodríguez-Arcos, R.; Sindic, M. Date Seeds: A Promising Source of Oil with Functional Properties. Foods 2020, 9, 787. [Google Scholar] [CrossRef] [PubMed]
- Mugao, L.G.; Gichimu, B.M.; Muturi, P.W.; Mukono, S.T. Characterization of the Volatile Components of Essential Oils of Selected Plants in Kenya. Biochem. Res. Int. 2020, 2020, 8861798. [Google Scholar] [CrossRef] [PubMed]
- NRC (National Research Council). Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- OECD (Organisation for Economic Cooperation and Development). Acute Oral Toxicity- Up-and-Down-Procedure (UDP), Guideline No. 425. OECD Guidelines for the Testing of Chemicals. Paris: Author. 3 October. 2008. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg425.pdf (accessed on 25 January 2022).
- Provan, D.; Singer, C.R.J.; Baglin, T.; Lilleyman, J. Oxford Handbook of Clinical Haematology, 2nd ed.; Oxford University Press Inc.: New York, NY, USA, 2004. [Google Scholar]
- Thammitiyagodage, M.G.; de Silva, N.R.; Rathnayake, R.; Karunakaran, C.; Kumara, W.G.; Gunatillka, M.M.; Ekanayaka, N.; Galhena, B.P.; Thabrew, M.I. Biochemical and histopathological changes in Wistar rats after consumption of boiled and un-boiled water from high and low disease prevalent areas for chronic kidney disease of unknown etiology (CKDu) in north Central Province (NCP) and its comparison with low disease prevalent Colombo, Sri Lanka. BMC Nephrol. 2020, 21, 38. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oluyele, O.; Oladunmoye, M.K.; Ogundare, A.O. Toxicity Studies on Essential Oil from Phoenix dactylifera (L.) Seed in Wistar Rats. Biologics 2022, 2, 69-80. https://doi.org/10.3390/biologics2010006
Oluyele O, Oladunmoye MK, Ogundare AO. Toxicity Studies on Essential Oil from Phoenix dactylifera (L.) Seed in Wistar Rats. Biologics. 2022; 2(1):69-80. https://doi.org/10.3390/biologics2010006
Chicago/Turabian StyleOluyele, Olumide, Muftau Kolawole Oladunmoye, and Ayodele Oluwayemisi Ogundare. 2022. "Toxicity Studies on Essential Oil from Phoenix dactylifera (L.) Seed in Wistar Rats" Biologics 2, no. 1: 69-80. https://doi.org/10.3390/biologics2010006
APA StyleOluyele, O., Oladunmoye, M. K., & Ogundare, A. O. (2022). Toxicity Studies on Essential Oil from Phoenix dactylifera (L.) Seed in Wistar Rats. Biologics, 2(1), 69-80. https://doi.org/10.3390/biologics2010006





