Abstract
As inflammatory lifestyle factors become more prevalent and as the population ages, the management of inflammation will become increasingly relevant. Plant polyphenols are powerful antioxidants that are known to have beneficial effects in a number of diseases with an inflammatory or oxidative component, such as malignancy, cardiovascular disease and arthritis. Polyphenol-rich sugarcane extract (PRSE) is a novel preparation with high concentrations of polyphenolic antioxidants, with some evidence to show benefits in health, but there is limited research investigating its effects on immunomodulation. This study determined the effects of PRSE on human monocyte cells in vitro. We show that PRSE has an immunomodulatory effect in U937 human monocyte cells, altering the expression of cellular surface markers, with an increased expression of CD16 and CD11b, as well as small changes in CD40, CD80, CD80, CD206 and MHCI. It also modulates the profile of secreted cytokines, increasing IL-1β, TNFα, IL-6, IL-8, IL-4 and IL-10. These changes are consistent with the advanced differentiation of the monocyte, as well as the switch from the M1 to M2 phenotype in macrophages. We also demonstrate that this effect is likely to be independent of the NF-κB signalling pathway, suggesting that other mechanisms drive this effect. PRSE exerts an immunomodulatory effect on U937 monocytes in vitro, potentially facilitating the conversion from inflammation to healing. Future studies should identify specific mechanisms underlying the changes and evaluate their effectiveness in animal models of disease.
1. Introduction
Inflammation is a key component of a wide range of pathological states and is a consequence of an increasingly large number of lifestyle factors in the modern world [1,2]. Conditions such as cardiovascular disease, malignancies, metabolic syndrome and mental health disorders all have an underlying inflammatory process, either as a causative factor or as a related consequence of the pathology [3,4]. Despite both its widespread incidence and significant health impacts, the treatment of chronic inflammation is currently limited to harsh medication with significant side effects that limit their long term use [5,6]. In order to combat its growing burden, novel treatments that are safe and effective are needed for use in the long term. In recent times, the focus has turned to lifestyle modifications, such as exercise [7,8,9,10], diet [11] and natural products [12,13], as an answer to the growing burden of chronic inflammation [14].
Diets rich in plant products have long been known to reduce systemic inflammation [15]. One of the key mediators of this process is the powerful antioxidant plant polyphenols [16]. Polyphenols are organic chemicals characterized by the presence of at least one phenolic hydroxyl group, typically found in large quantities in fruits, vegetables and cereals, as well as in preserved beverages, such as tea and coffee [17]. Polyphenols have been shown to have a number of beneficial effects in vitro, with antioxidant, anti-angiogenic and anti-proliferative effects in models of cancer and inflammatory disease [18,19]. These findings have yet to be translated into human studies, largely due to both a lack of known biomarkers and in vitro studies using high concentrations that make them impractical for biological use. While a number of individual polyphenols have been studied, a number of plant sources of these chemicals have yet to be scientifically evaluated.
Sugarcane (Saccharum officinarum L.) is known to be a rich source of polyphenols and has been shown to have a number of biological effects. Preparations of the liquid extracts of sugarcane have been shown to be both antimicrobial [20] and antioxidant [21], as well as being able to modulate insulin responses to food intake [22,23]. Sugarcane is an abundant crop, with high value as both a source of nutrition and as an economic vehicle in countries around the world. This makes it an attractive target for further utilization in the development of “nutraceuticals”, as it is readily available, cost-effective and safely edible. A novel preparation named “polyphenol-rich sugarcane extract” (PRSE) has been shown to have a large concentration of both polyphenols and other antioxidant compounds, and has been shown to have some beneficial effects on energy uptake and metabolism in cancer cells, as well as being able to impact the actions of insulin-secreting pancreatic β-cells [24]. It is also able to impart benefits in diabetes, has anti-bacterial effects and improves the cosmetic effects of aging in the skin [25]. PRSE has also been shown to modulate the immune system, with effects on inflammation, cancer and neurological disorders [26,27].
We extended these findings to evaluate the effect of PRSE on human immune monocyte cells, hypothesizing that they may have an immunomodulatory effect at biologically relevant concentrations. For this research, the U937 human pro-monocytic cell line was used to test the effect of PRSE on the secretion of cytokines and the differentiation and maturation of monocytes in vitro. It is hypothesized that PRSE may have a direct anti-inflammatory mechanism of action on monocytes independent from its in vivo antioxidant effect.
2. Results
2.1. PRSE Modulates the Expression of Cell Surface Markers in U937 Monocyte Cells
U937 cells expressed CD11b, CD14, CD86 and MHC-I upon differentiation with vitamin D3, and expression levels were not further enhanced by LPS. Differentially regulated cell surface markers after 24 h and 48 h incubation are shown in Figure 1 and Figure 2, respectively. After 24 h, the monocytes that were differentiated from U937 cells exhibited a classical monocyte phenotype expressing CD14, but not CD16 (CD14+CD16−). At 24 h, PRSE did not alter the expression of CD14, although a small percentage of cells became non-classical monocytes (CD14+CD16+) in the presence of PRSE. Interestingly, PRSE, but not ibuprofen, increased the expression of CD11b from 42.3% to 56.9% (14.6% increase), which is suggestive of initiating an inflammatory response. In addition, PRSE, but not ibuprofen, weakly upregulated the expression of CD40, CD80, CD83 and MHC-II; CD86 was weakly upregulated in the presence of ibuprofen. As expected, CD206 was not expressed by monocytes but was weakly increased in the presence of PRSE or ibuprofen. MHC-I was expressed by monocytes and was weakly decreased in the presence of PRSE or ibuprofen, from 62.5% to 56.7% and 56.3%, respectively.
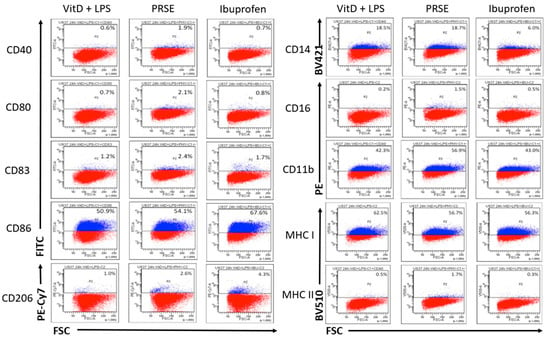
Figure 1.
Expression of cell surface markers after 24 h of incubation by flow cytometry: PE, phycoerythrin; AF, Alexa Fluor; PE-Cy7, phycoerythrin-cyanine 7; BV, Brilliant Violet.
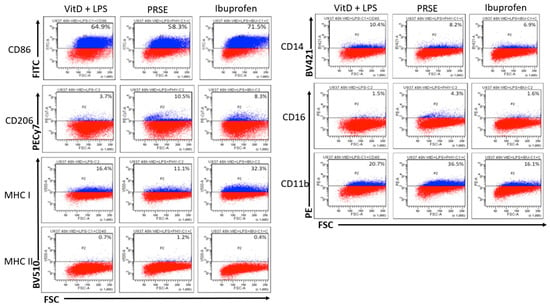
Figure 2.
Expression of cell surface markers after 48 h of incubation by flow cytometry: PE, phycoerythrin; AF, Alexa Fluor; PE-Cy7, phycoerythrin-cyanine 7; BV, Brilliant Violet.
Following incubation with PRSE for 48 h, there was no significant change in the expression of CD14, although a small percentage of cells remained as CD14+CD16+ non-classical monocytes in the presence of PRSE, which was similar to that noted at 24 h. Again, PRSE, but not ibuprofen, increased the expression of CD11b from 20.7% to 36.5% (43.3% increase), which was suggestive of initiating an inflammatory response. No significant changes to the expression of CD40, CD80 and CD83 in the presence of PRSE or ibuprofen were noted; CD86 was weakly upregulated in the presence of ibuprofen. As expected, CD206 was not expressed by monocytes but was significantly increased in the presence of PRSE (64.8% increase) or ibuprofen (55.4% increase). MHC-I was weakly expressed by monocytes, which decreased in the presence of PRSE; MHC-II was marginally increased, similarly to the 24 h time point.
2.2. PRSE Alters the Production of Immunomodulatory Cytokines
Cytokine expression after 24 h is shown in Figure 3. PRSE significantly increased the secretion of IL-1β (p < 0.005), IL-6 (p < 0.005), IL-8 (p < 0.05), IL-4 (p < 0.05), IL-10 (p < 0.05) and TNFα (p < 0.05). There was an indication of an increase in IL-2, IFNγ and GM-CSF; however, changes were small and not significant.
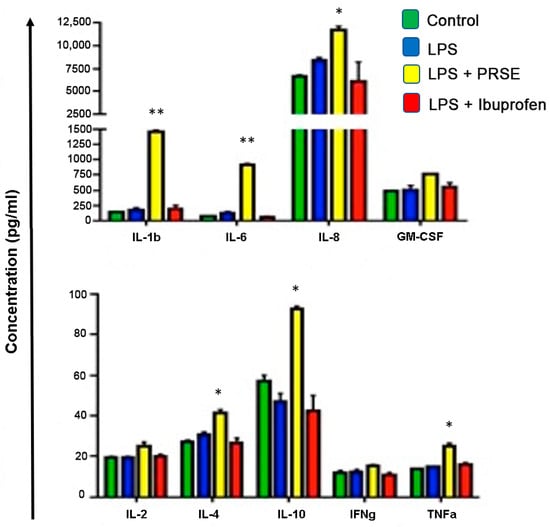
Figure 3.
Cytokine secretion after 24 h of treatment, * p < 0.05, ** p < 0.01.
2.3. Immunomodulatory Actions of PRSE Are Not Mediated by NF-κB
The stimulation of an inflammatory phenotype with LPS increased NF-κB expression, as expected. This was partially inhibited by the presence of ibuprofen, but PRSE exerted no detectable effect (Figure 4).
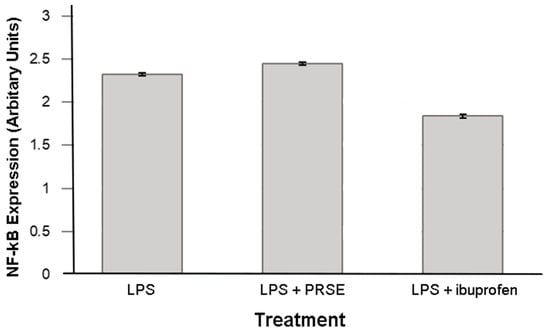
Figure 4.
Expression of NF-κB after 24 h treatment.
3. Discussion
These results show that PRSE has the capacity to modulate the immune function of monocytes. These changes were shown at low, potentially clinically relevant concentrations, providing support for the dietary supplementation of PRSE. While some studies have shown that polyphenols exert an anti-inflammatory effect, the results of this study instead demonstrate the modulation of the innate immune system, which is key to the acute phase reaction and pathogen detection, as well as tissue healing and repair.
It appears that PRSE has the capacity to affect the differentiation of monocytes in a number of ways. Firstly, PRSE causes the differentiation of a portion of U937 monocytes into the CD14+CD16+ non-classical, or resident, monocytes. These cells are involved in the maintenance of the vascular endothelial microenvironment and are believed to be anti-inflammatory in nature [28]. Their anti-inflammatory action is thought to be due to vascular protection and wound healing; however, in chronic settings, they correlate with the disease burden, though this is likely a response to ongoing vascular damage [29]. PRSE also causes an increase in CD11b, a marker of monocyte differentiation into macrophages [30]. Macrophages are an important mediator of the innate immune response, acting to clear debris and pathogens and modulate the transition from the acute phase reaction into tissue repair. These two changes together are suggestive of PRSE modulating an acute response, allowing it to transition into a state of tissue repair and subsequent healing.
These effects are also supported by the cytokine expression profile of the treated cells. The interleukin family of cytokines has a key role in regulating the immune response. While some of the interleukins have a strong inflammatory role, IL-4, IL-6 and IL-10 are known to have an overall dampening effect on immune responses. The capacity for PRSE to increase the expression of these cytokines supports its effect as being one of modulating the transition from acute phase inflammation to a steady state of tissue repair and healing. IL-4 aids in the modulation of T-cell responses and antibody production, and, importantly, stimulates macrophage differentiation into an M2 phenotype, while inhibiting the production of M1 macrophages [31,32]. M2 macrophages are involved with the discontinuation of an immune response and the ultimate healing of the affected tissue, whereas M1 cells drive inflammation and leukocyte infiltration [33]. The M2 macrophage transition is also associated with IL-10 production, which is classically an anti-inflammatory mediator, suppressing the production of T-helper cell cytokines, blocking the NF-κB pathway and decreasing the expression of pro-inflammatory cytokines, such as TNFα and IFNγ, by myeloid lineage cells [34,35]. While these two cytokines are classically associated with anti-inflammatory effects, IL-6 has a more complex model of regulatory action. IL-6 has pleiotropic effects on the immune function, with both pro-and anti-inflammatory effects [36]. IL-6 is involved in the synthesis of acute phase proteins, such as C-reactive protein (CRP) from the liver, accelerating the inflammatory response. However, it also has a complex role of mediating the transition from the acute, innate immune response, to the acquired, through the regulation of naïve T-cells and B-cells. It also inhibits some pro-inflammatory cytokines, such as TNFα, and upregulates IL-10 secretion, further enhancing this transition [37].
While the increase in these cytokines provides support for an anti-inflammatory, immunomodulatory role, the accelerated production of IL-1β, IL-8 and TNFα is harder to place in this model. IL-8 is a potent mediator of neutrophil chemotaxis to an injury site, as well as increasing the activity of phagocytes more generally, whereas IL-1β is a key pyrogen, being the primary mediator of the fever associated with acute infection and inflammation [38,39]. TNFα is both a key element of macrophage biology and the master regulator of the cytokine cascade. Its production is one of the early signs of acute inflammation, triggering macrophage secretion of pro-inflammatory cytokines [40]. While the overexpression of TNFα suggests an inflammatory response, it also plays a role in the cessation of inflammation, as it drives efferocytosis by neutrophils, causing them to clear the area of injury once pathogens and debris are cleared [40]. In summary, the increase in IL-1β, IL-8 and TNFα in monocyte/macrophage cells is likely to both speed the acute phase response and possibly cause a rapid transition from the inflammatory cycle into a transitional phase of tissue repair and healing.
While NF-κB is a likely mechanism underpinning immunomodulatory changes, it appears that it is not the mechanism by which PRSE exerts an effect. Other possible mechanisms may involve PI3K/Akt signalling, which is known to aid in the transition away from acute inflammation and stimulate the production of IL-10 by macrophages or mTOR signalling, known to be strongly involved in their activation [41]. Akt signalling is also critical in the M2 polarization of macrophages, which is central to the hypothesis posed by this research [42]. While these mechanisms are possible candidates for the findings of this research, further investigation is required to explore this further. Other potential candidates include GAB1 and GAB2, as well as PPARγ signalling, both of which are associated with M2 polarization [43].
While novel, this study has some limitations. Firstly, it examines only the immortalized U937 cell line, which, while an excellent model for monocyte/macrophage behaviour, is known to have some flaws in in vivo translation. Secondly, as PRSE is a composite product with a large number of individual polyphenol compounds within it, it is unclear as to the exact identity of the active molecule/s. It is possible that the effects here are attributable to only one specific polyphenol in PRSE, or different effects due to different compounds. Thorough assessment and testing of the individual components of PRSE will allow for a fuller understanding, as well as an optimization of its production.
4. Materials and Methods
4.1. Materials
PRSE was prepared as described previously [24]. PRSE was obtained from The Product Makers (Melbourne, Australia) as lyophilized powder and reconstituted at the appropriate concentration in phosphate-buffered saline (PBS, cat no. 10010023, Gibco, New York, NY, USA) before treatment. The chemical composition of PRSE has been described previously [26] and is prepared by the processing of sugarcane with ethanol extraction of the hydrophobic polyphenols, as described here [44]. Briefly, the PRSE used in this study is made using a patented proprietary process from the juice of the sugarcane plant Saccharum officinarum. By-products from the post-harvest processing of the juice are fractionated by a hydrophobic resin, FPX-66 (Dow, Midland, MI, USA) with the material binding to the resin membrane and subsequently concentrated by vacuum evaporation and spray dried in order to produce a free-flowing shelf-stable powder. This powder contains a unique mixture of phenolic acids, glycone and aglycone flavonoids, including chlorogenic acid, syringic acid, diosmin, diosmetin, etc, some of which are chelated with divalent anions (Mg, Ca, Zn) [45]. Ibuprofen (α-Methyl-4-(isobutyl) phenylacetic acid, (±)-2-(4-Isobutylphenyl) propanoic acid), (cat no. 15687-27-1, Sigma-Aldrich, St. Louis, MO, USA) as a powder, which was reconstituted in ethanol at the appropriate concentration as per manufacturer instruction.
4.2. U937 Cell Culture
U937 cells (ATCC®®CRL-1593.2) are an immortalized promonocytic cancer cell line. The U937 line was chosen as they are well characterized and are one of a limited pool of human monocyte lineage cells. They have been used widely in immunological research to test monocyte responses [46,47]. U937 cells were cultured in RPMI 1640 media supplemented with 2 mM L-glutamine (Sigma-Aldrich, St. Louis, MO, USA), 100 U/mL penicillin, 100 µg/mL streptomycin (Sigma-Aldrich) and 10% heat-inactivated foetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA) at 37 °C and 5% CO2. Culture media was changed every 2–3 days and cells were passaged to maintain sub-confluence by centrifugation at 100× g for 5 min, and gentle resuspension. Once the cells had reached 80–90% confluency, they were used in experiments.
4.3. U937 Cell Differentiation and Proinflammatory Activation
U937 cells are pre-monocytes and differentiate into mature monocytes when exposed to vitamin D3 (VitD3) (Sigma-Aldrich). After being seeded for experiments, cells were incubated with 100 nM VitD3 for 72 h. In order to induce an inflammatory state, monocytes were exposed to 1 µg/mL of lipopolysaccharide (LPS) derived from E. coli (Sigma-Aldrich).
4.4. Treatment Groups
After activation with VitD3 and LPS, cells were cultured in the following conditions: (a) 400 µg/mL PRSE, (b) 100 µg/mL ibuprofen and (c) untreated control. Ibuprofen was used as a positive, anti-inflammatory control. Cells were processed for downstream analysis after 24 h in culture. PRSE at 400 µg/mL was chosen based on previous in vitro experiments on modulation and anti-inflammatory effects of cancer cell lines [27]. All analyses were performed on three technical replicates, and with sample means presented. Independent t-tests were used to identify significant differences between means, with an alpha value of 0.05. All statistics were performed using the GraphPad prism software (GraphPad Software, San Diego, CA, USA).
4.5. Cell Surface Marker Analysis
At 24 h, cells were harvested and washed by centrifugation at 100× g for 5 min and resuspended in FACS buffer (BD Biosciences, VIC Australia) for immunofluorescent staining. Cells were first incubated with FcR blocking reagent (BD Biosciences, VIC Australia) for 10 min, before a 30-min incubation with the conjugated antibodies described in Table 1. Antibody concentrations were titrated before the experiments. Flow cytometry was performed on a three-color BD FACSCanto II, with violet (405 nm), blue (488 nm) and red (633 nm) lasers. Analysis was performed with the BD FACSDiva software (v8.0.1). All analyses were performed with single-color samples, so compensation was not required. Dead cells were excluded based on standard forward and side-scatter geometry gating. Unstained cells and isotype controls (Figure 5) were used to identify background and non-specific staining. These controls were used to define the gating strategy, with negative cut-offs set with the unstained cells and verified with matching isotypes. Indicative dot plots of marker expression are presented.

Table 1.
Antibody targets, conjugations and working concentrations.
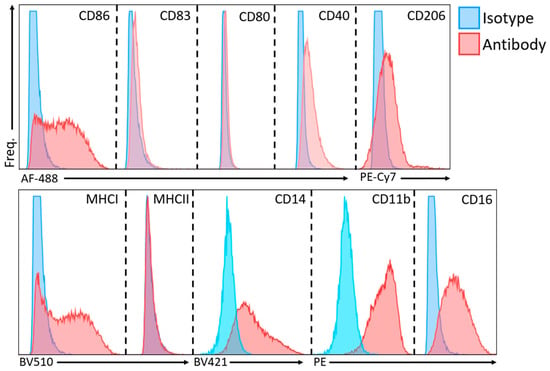
Figure 5.
Overlay histograms of isotype controls and sample of interest for flow cytometry. PE, phycoerythrin; AF, Alexa Fluor; PE-Cy7, phycoerythrin-cyanine 7; BV, Brilliant Violet.
4.6. Cytokine Analysis
Following treatment for 24 h, supernatants were isolated by centrifugation at 1000× g for 15 min at 4 °C and analyzed with a 9-analyte bioplex cytokine bead array kit (Bio-Rad, Melbourne, VIC, Australia), as per the manufacturer’s instructions. Briefly, 50 μL of supernatant was added to a 96-well plate along with the conjugated magnetic beads and incubated on a shaker for 30 min at room temperature. The plates were washed and detection antibodies were added at the manufacturer recommended concentration, before 10 min incubation on a shaker at room temperature. The plates were again washed, and a phycoerythrin-streptavidin (PE-SD) conjugate was added to the sample wells. These were mixed and data were collected on a Bio-Rad Bio-Plex 200 system, with the Bio-Plex Manager software (v6.1) (Bio-Rad, Hercules, CA, USA). Culture media and blank controls were used for calibration.
4.7. NF-κB ELISA
Levels of NF-κB were assessed via enzyme-linked immunosorbent assay (ELISA) after treatment for 24 h. Cells were collected, counted and 1 × 105 cells from each sample lysed in 500 µL lysis buffer for ELISA and stored at −80 °C. An NF-κB ELISA kit (Abcam, Melbourne, VIC, Australia) was performed using 50 µL lysate per well, as per manufacturer’s instructions. A Bio-Rad 680 microplate reader was used to measure light absorbance at 450 nm, and blank and lysis buffer controls were used to calibrate the measurements.
4.8. Statistical Analysis
All statistical analyses were performed using GraphPad Prism (version 9.1.1). Alpha level of significance was set at 0.05, with p-values below this being considered statistically significant. Analysis of Variance (ANOVA) models were used to identify mean differences between groups, with posthoc Tukey testing used to identify specific differences.
5. Conclusions
This research has demonstrated that PRSE can cause immunomodulatory changes in human monocytes that are consistent with both M1 to M2 macrophage transformation and the acceleration of the transition through acute inflammation into a state of tissue healing and repair. Future studies should identify its effects in primary human cells and attempt to identify the underlying mechanisms of the effects at a mechanistic signalling level. This will allow for a deeper understanding of the beneficial effects of polyphenols (PRSE) on disease and health, as well as assisting in the optimal use of PRSE as a method of their delivery to the public.
Author Contributions
Conceptualization, V.A.; formal analysis, M.D.P., J.F.; investigation, J.F.; writing—original draft preparation, J.F.; writing—review and editing, J.F., M.D.P., L.S., M.R.F., B.K. and V.A.; visualization, J.F.; supervision, V.A. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by an Innovation Connections Grant, Department of Industry, Innovation and Science (RC51666), VIC Australia. In addition, the study received funding and resources from The Product Makers (Melbourne, Australia) Pty Ltd., in order to evaluate the effects of their product; they had no input into the design, execution, analysis or publication of the research.
Data Availability Statement
All data underpinning the findings of this paper are available from the authors on request.
Acknowledgments
The authors would like to thank the support from the Institute for Health and Sport and the Immunology and Translational Group within the Mechanisms and Interventions in Health and Disease Program, Victoria University, Melbourne Australia. J.F. was supported by a University of Melbourne postgraduate scholarship.
Conflicts of Interest
M.R.F. and B.K. are employed by The Product Makers (Australia) Pty Ltd., in the bioactives division. They provided and advised on the use of PRSE, but had no role in the execution of the research, analysis of the data or preparation of the manuscript. They provided comments to the paper. All other authors have no conflicts to declare.
References
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.P.; Nicholls, A.J.; Wong, C.H. Partners in crime: Neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018, 371, 551–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, inflammation, and cancer. Ann. Rev. Path. 2016, 11, 421–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Candales, A.; Burgos, P.M.H.; Hernandez-Suarez, D.F.; Harris, D. Linking chronic inflammation with cardiovascular disease: From normal aging to the metabolic syndrome. J. Nat. Sci. 2017, 3, e341. [Google Scholar]
- Tabas, I.; Glass, C.K. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Headland, S.E.; Norling, L.V. The resolution of inflammation: Principles and challenges. Semin. Immunol. 2015, 27, 149–160. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Borkoles, E.; Polman, R.; Stojanovska, L. Physical and immunological aspects of exercise in chronic diseases. Immunotherapy 2014, 6, 1145–1157. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Polenakovic, M.; Bosevski, M.; Apostolopoulos, V. Exercise and mental health. Maturitas 2017, 106, 48–56. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Prakash, M.; Apostolopoulos, V. The effects of vitamin B on the immune/cytokine network and their involvement in depression. Maturitas 2017, 96, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Stojanovska, L.; Apostolopoulos, V.; Polman, R.; Borkoles, E. To exercise, or, not to exercise, during menopause and beyond. Maturitas 2014, 77, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Apostolopoulos, V.; de Courten, M.P.; Stojanovska, L.; Blatch, G.L.; Tangalakis, K.; de Courten, B. The complex immunological and inflammatory network of adipose tissue in obesity. Mol. Nutr. Food Res. 2016, 60, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Dargahi, N.; Johnson, J.; Apostolopoulos, V. Streptococcus thermophilus alters the expression of genes associated with innate and adaptive immunity in human peripheral blood mononuclear cells. PLoS ONE 2020, 15, e0228531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dargahi, N.; Johnson, J.; Donkor, O.; Vasiljevic, T.; Apostolopoulos, V. Immunomodulatory effects of probiotics: Can they be used to treat allergies and autoimmune diseases? Maturitas 2019, 119, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Sears, B. Anti-inflammatory diets. J. Am. Coll. Nutr. 2015, 34, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E. Diet components can suppress inflammation and reduce cancer risk. Nutr. Res. Pract. 2014, 8, 233–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostyuk, V.A.; Potapovich, A.I.; Suhan, T.O.; de Luca, C.; Korkina, L.G. Antioxidant and signal modulation properties of plant polyphenols in controlling vascular inflammation. Eur. J. Pharmacol. 2011, 658, 248–256. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Araujo, J.R.; Goncalves, P.; Martel, F. Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutr. Res. 2011, 31, 77–87. [Google Scholar] [CrossRef]
- Bucio-Noble, D.; Kautto, L.; Krisp, C.; Ball, M.S.; Molloy, M.P. Polyphenol extracts from dried sugarcane inhibit inflammatory mediators in an in vitro colon cancer model. J. Proteom. 2018, 177, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ahtesh, F.B.; Stojanovska, L.; Feehan, J.; de Courten, M.P.; Flavel, M.; Kitchen, B.; Apostolopoulos, V. Polyphenol Rich Sugar Cane Extract Inhibits Bacterial Growth. Prilozi 2020, 41, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.E.; El Gedaily, R.A.; Mocan, A.; Farag, M.A.; El-Seedi, H.R. Profiling Metabolites and Biological Activities of Sugarcane (Saccharum officinarum Linn.) Juice and its Product Molasses via a Multiplex Metabolomics Approach. Molecules 2019, 24, 934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, T.P.; Wright, A.G.; Clifton, P.M.; Ilag, L.L. Postprandial insulin and glucose levels are reduced in healthy subjects when a standardised breakfast meal is supplemented with a filtered sugarcane molasses concentrate. Eur. J. Nutr. 2016, 55, 2365–2376. [Google Scholar] [CrossRef]
- Wright, A.G.; Ellis, T.P.; Ilag, L.L. Filtered molasses concentrate from sugar cane: Natural functional ingredient effective in lowering the glycaemic index and insulin response of high carbohydrate foods. Plant Foods Hum. Nutr. 2014, 69, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yang, X.; Flavel, M.; Shields, Z.P.; Kitchen, B. Antioxidant and Anti-Diabetic Functions of a Polyphenol-Rich Sugarcane Extract. J. Am. Coll. Nutr. 2019, 38, 670–680. [Google Scholar] [CrossRef]
- Ji, J.; Yang, X.; Flavel, M.; Shields, Z.P.; Neoh, J.; Bowen, M.-L.; Kitchen, B. Age-Deterring and Skin Care Function of a Polyphenol Rich Sugarcane Concentrate. Cosmetics 2020, 7, 30. [Google Scholar] [CrossRef]
- Ji, J.; Flavel, M.; Yang, X.; Chen, O.C.; Downey, L.; Stough, C.; Kitchen, B. A polyphenol rich sugarcane extract as a modulator for inflammation and neurological disorders. PharmaNutrition 2020, 12, 100187. [Google Scholar] [CrossRef]
- Prakash, M.D.; Stojanovska, L.; Feehan, J.; Nurgali, K.; Donald, E.L.; Plebanski, M.; Flavel, M.; Kitchen, B.; Apostolopoulos, V. Anti-cancer effects of polyphenol-rich sugarcane extract. PLoS ONE 2021, 16, e0247492. [Google Scholar] [CrossRef]
- Narasimhan, P.B.; Marcovecchio, P.; Hamers, A.A.J.; Hedrick, C.C. Nonclassical Monocytes in Health and Disease. Annu. Rev. Immunol. 2019, 37, 439–456. [Google Scholar] [CrossRef]
- Zawada, A.M.; Rogacev, K.S.; Schirmer, S.H.; Sester, M.; Böhm, M.; Fliser, D.; Heine, G.H. Monocyte heterogeneity in human cardiovascular disease. Immunobiology 2012, 217, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Solovjov, D.A.; Pluskota, E.; Plow, E.F. Distinct roles for the α and β subunits in the functions of integrin αMβ2. J. Biol. Chem. 2005, 280, 1336–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casella, G.; Garzetti, L.; Gatta, A.T.; Finardi, A.; Maiorino, C.; Ruffini, F.; Martino, G.; Muzio, L.; Furlan, R. IL4 induces IL6-producing M2 macrophages associated to inhibition of neuroinflammation in vitro and in vivo. J. Neuroinflamm. 2016, 13, 139. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Zhou, J.; Liu, N.; Wang, L.; Gao, Q.; Wu, Y.; Zhao, Q.; Liu, P.; Wang, S.; Liu, Y. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J. Mol. Cell. Cardiol. 2015, 85, 131–139. [Google Scholar] [CrossRef]
- Mills, C. M1 and M2 macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef] [Green Version]
- Aste-Amezaga, M.; Ma, X.; Sartori, A.; Trinchieri, G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J. Immunol. 1998, 160, 5936–5944. [Google Scholar] [PubMed]
- Opp, M.; Smith, E.; Hughes, T., Jr. Interleukin-10 (cytokine synthesis inhibitory factor) acts in the central nervous system of rats to reduce sleep. J. Neuroimmunol. 1995, 60, 165–168. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro-and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Dinarello, C.A. Blocking IL-1 in systemic inflammation. J. Exp. Med. 2005, 201, 1355–1359. [Google Scholar] [CrossRef]
- Dinarello, C.A. The biological properties of interleukin-1. Eur. Cytokine Netw. 1994, 5, 517–531. [Google Scholar] [PubMed]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Aksoylar, H.I.; Horng, T. Control of macrophage metabolism and activation by mTOR and Akt signaling. Semin. Immunol. 2015, 27, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS–) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Ilag, L.L.; Smythe, J.; Ellis, P.T.; Weisinger, R.S. Sugar Extracts. U.S. Patent 9572852B2, 21 February 2017. [Google Scholar]
- Deseo, M.A.; Elkins, A.; Rochfort, S.; Kitchen, B. Antioxidant activity and polyphenol composition of sugarcane molasses extract. Food Chem. 2020, 314, 126180. [Google Scholar] [CrossRef]
- Chanput, W.; Peters, V.; Wichers, H. THP-1 and U937 Cells. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 147–159. [Google Scholar] [CrossRef] [Green Version]
- Baek, Y.-S.; Haas, S.; Hackstein, H.; Bein, G.; Hernandez-Santana, M.; Lehrach, H.; Sauer, S.; Seitz, H. Identification of novel transcriptional regulators involved in macrophage differentiation and activation in U937 cells. BMC Immunol. 2009, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).