Isolation and Characterization of Bacteriophage ZCSE6 against Salmonella spp.: Phage Application in Milk
Abstract
:1. Introduction
2. Results
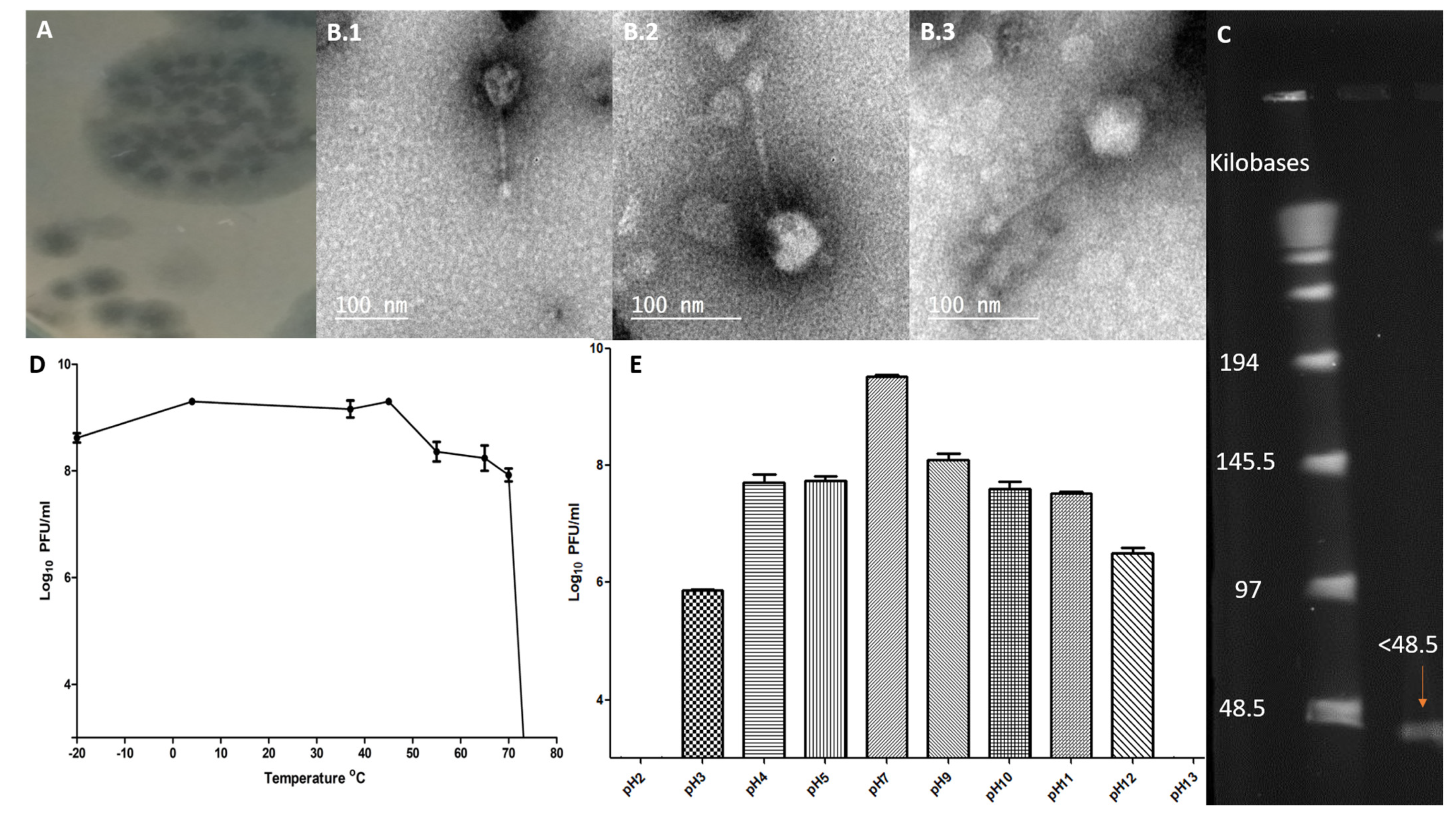
2.1. Phage Specificity, Lytic Profile, and Efficiency of Plating
2.2. Morphological Characteristics of ZCSE6 and Genome Size
2.3. Phage Stability against Temperature and pH Levels
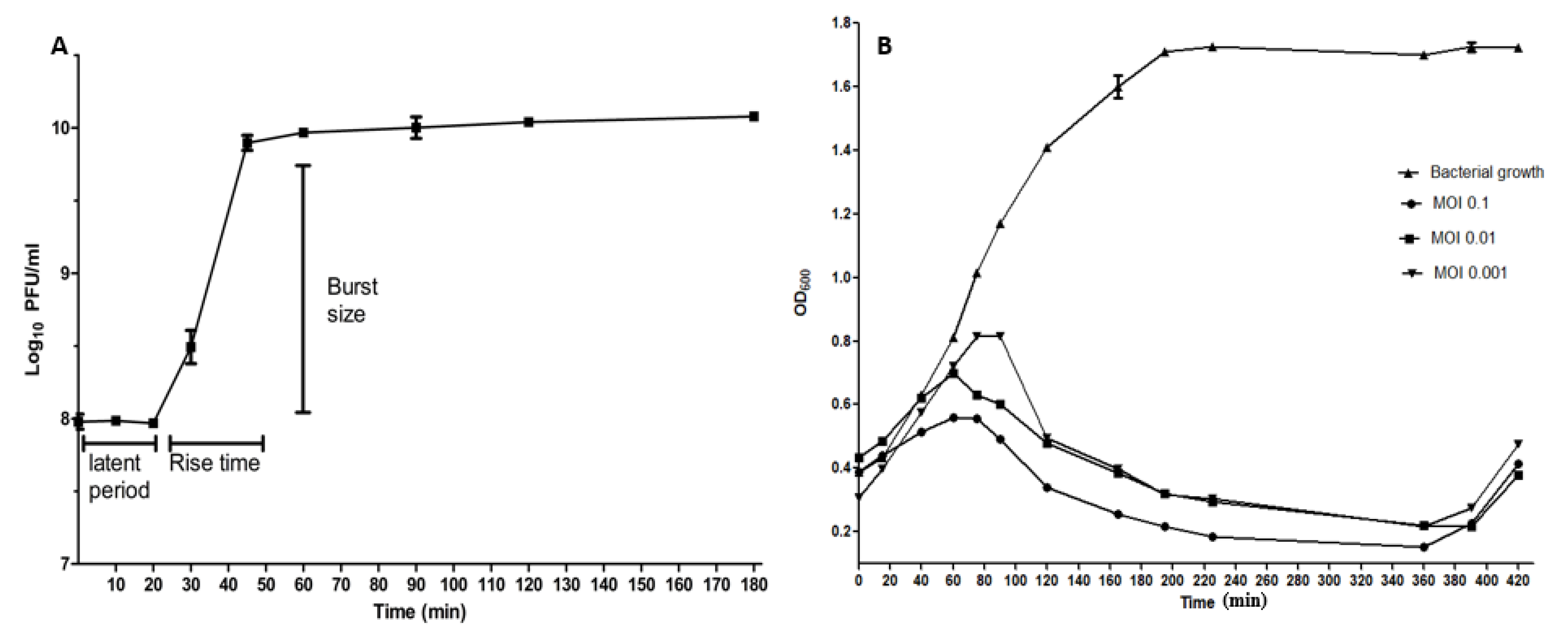
2.4. The One-Step Growth Curve
2.5. Time-Killing Curve
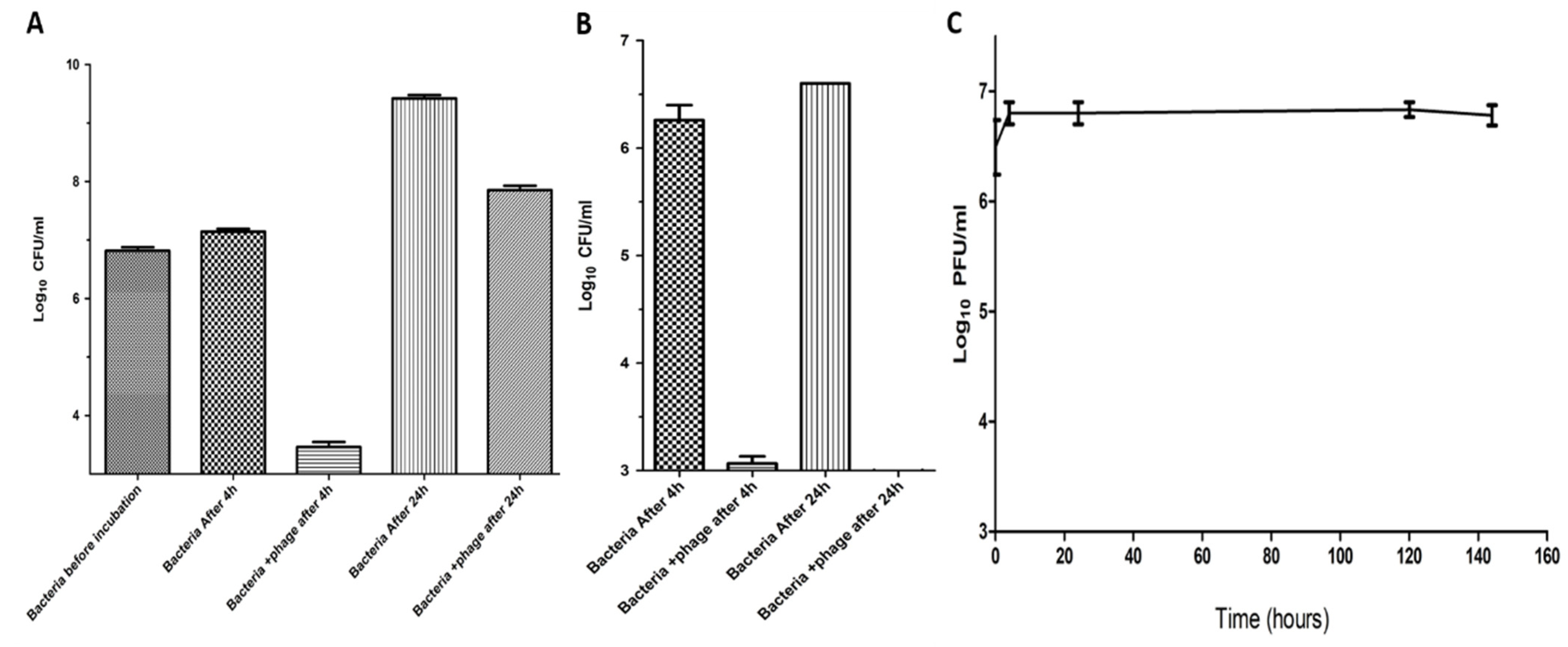
2.6. Lytic Activity at Low Temperature
2.7. Suppression of Salmonella in Milk by Using Phage ZCSE6
3. Discussion
4. Materials and Methods
4.1. Bacterial Characterization and Growth Media
4.2. Isolation, Amplification, and Purification of Phage ZCSE6 from Milk
4.3. Host Range, Lytic Profile, and EOP of Phage ZCSE6
4.4. Pulsed-Field Gel Electrophoresis (PFGE)
4.5. Morphology Investigation by Transmission Electron Microscopy (TEM)
4.6. The Effect of pH and Temperature on Phage Stability
4.7. One-Step Growth Curve of ZCSE6
4.8. Time-Killing Curves
4.9. Phage ZCSE6 Activity at Low Temperature
4.10. Determination of the Phage Stability and Lytic Activity in Milk
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faille, C.; Cunault, C.; Dubois, T.; Benezech, T. Hygienic design of food processing lines to mitigate the risk of bacterial food contamination with respect to environmental concerns. Innov. Food Sci. Emerg. Technol. 2018. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Bolton, D.; McAuliffe, O.; Coffey, A. The use of bacteriophages to control and detect pathogens in the dairy industry. Int. J. Dairy Technol. 2020, 73, 1–11. [Google Scholar] [CrossRef]
- Bao, H.; Zhang, P.; Zhang, H.; Zhou, Y.; Zhang, L.; Wang, R. Bio-control of Salmonella Enteritidis in foods using bacteriophages. Viruses 2015, 7, 4836–4853. [Google Scholar] [CrossRef]
- Afshari, A.; Baratpour, A.; Khanzade, S.; Jamshidi, A. Salmonella enteritidis and Salmonella typhimorium identification in poultry carcasses. Iran. J. Microbiol. 2018, 10, 45–50. [Google Scholar]
- Abd El-Tawab, A.; Elhalim, A.; Hegazy, M. Bacteriological and molecular studies on Salmonella species isolated from poultry farms. Benha Vet. Med J. 2019, 36, 280–293. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Tahoun, A.; El-Gohary, A.E.G.A.; El-Abasy, M.; El-Khayat, F.; Gillespie, T.; Kitade, Y.; Hafez, H.M.; Neubauer, H.; El-Adawy, H. Epidemiological, molecular characterization and antibiotic resistance of Salmonella Enterica serovars isolated from chicken farms in Egypt. Gut Pathog. 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrés Barranco, S.; Vico, J.P.; Grilló, M.J.; Mainar Jaime, R.C. Reduction of subclinical S almonella infection in fattening pigs after dietary supplementation with a ß-galactomannan oligosaccharide. J. Appl. Microbiol. 2015, 118, 284–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavallo, S.J.; Daly, E.R.; Seiferth, J.; Nadeau, A.M.; Mahoney, J.; Finnigan, J.; Wikoff, P.; Kiebler, C.A.; Simmons, L. Human outbreak of Salmonella Typhimurium associated with exposure to locally made chicken jerky pet treats, New Hampshire, 2013. Foodborne Pathog. Dis. 2015, 12, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, E.; Morales-Rueda, A.; García-Gimeno, R.M. Cross-contamination and recontamination by Salmonella in foods: A review. Food Res. Int. 2012, 45, 545–556. [Google Scholar] [CrossRef]
- El-Shibiny, A.; Dawoud, A. Bacteriophage applications for food safety. In Biocommunication of Phages; Witzany, G., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 463–484. ISBN 9783030458850. [Google Scholar]
- Soares, V.M.; Pereira, J.G.; Viana, C.; Izidoro, T.B.; dos SantosBersot, L.; de Almeida NogueiraPinto, J.P. Transfer of Salmonella Enteritidis to four types of surfaces after cleaning procedures and cross-contamination to tomatoes. Food Microbiol. 2012, 30, 453–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, M.J.; Retamales, J.; Palza, H.; Bastías, R. Encapsulation of specific Salmonella Enteritidis phage f3αSE on alginate-spheres as a method for protection and dosification. Electron. J. Biotechnol. 2018. [Google Scholar] [CrossRef]
- Sillankorva, S.M.; Oliveira, H.; Azeredo, J. Bacteriophages and their role in food safety. Int. J. Microbiol. 2012. [Google Scholar] [CrossRef] [Green Version]
- Mahony, J.; McAuliffe, O.; Ross, R.P.; van Sinderen, D. Bacteriophages as biocontrol agents of food pathogens. Curr. Opin. Biotechnol. 2011, 22, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Atterbury, R.J.; Van Bergen, M.A.P.; Ortiz, F.; Lovell, M.A.; Harris, J.A.; De Boer, A.; Wagenaar, J.A.; Allen, V.M.; Barrow, P.A. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl. Environ. Microbiol. 2007, 73, 4543–4549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo czyk-Matysiak, E.N.; Kula, D.; Owczarek, B.; Orwat, F.; Mi dzybrodzki, R.; Neuberg, J.; Bagi ska, N.; Weber-D browska, B.; Gńrski, A. Factors determining phage stability/activity: Challenges in practical phage application. Expert Rev. Anti. Infect. Ther. 2019, 17, 583–606. [Google Scholar] [CrossRef] [PubMed]
- Hungaro, H.M.; Mendonça, R.C.S.; Gouvêa, D.M.; Vanetti, M.C.D.; de OliveiraPinto, C.L. Use of bacteriophages to reduce Salmonella in chicken skin in comparison with chemical agents. Food Res. Int. 2013, 52, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Virk, S.M.; Shi, J.; Zhou, Y.; Willias, S.P.; Morsy, M.K.; Abdelnabby, H.E.; Liu, J.; Wang, X.; Li, J. Isolation, characterization, and application of bacteriophage LPSE1 against Salmonella enterica in ready to eat (RTE) foods. Front. Microbiol. 2018, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Capparelli, R.; Nocerino, N.; Iannaccone, M.; Ercolini, D.; Parlato, M.; Chiara, M.; Iannelli, D. Bacteriophage therapy of Salmonella enterica: A fresh appraisal of bacteriophage therapy. J. Infect. Dis. 2010, 201, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Dallal, M.M.S.; Nikkhahi, F.; Alimohammadi, M.; Douraghi, M.; Rajabi, Z.; Foroushani, A.R.; Azimi, A.; Fardsanei, F. Phage therapy as an approach to control Salmonella enterica serotype Enteritidis infection in mice. Rev. Soc. Bras. Med. Trop. 2019, 52. [Google Scholar] [CrossRef]
- Gao, R.; Naushad, S.; Moineau, S.; Levesque, R.; Goodridge, L.; Ogunremi, D. Comparative genomic analysis of 142 bacteriophages infecting Salmonella enterica subsp. enterica. BMC Genomics 2020, 21, 1–13. [Google Scholar] [CrossRef]
- Shin, H.; Lee, J.-H.; Kim, H.; Choi, Y.; Heu, S.; Ryu, S. Receptor diversity and host interaction of bacteriophages infecting Salmonella enterica serovar Typhimurium. PLoS ONE 2012. [Google Scholar] [CrossRef]
- Nikkhahi, F.; Dallal, M.M.S.; Alimohammadi, M.; Foroushani, A.R.; Rajabi, Z.; Fardsanei, F.; Imeni, S.M.; Bonab, P.T. Phage therapy: Assessment of the efficacy of a bacteriophage isolated in the treatment of salmonellosis induced by Salmonella enteritidis in mice. Gastroenterol. Hepatol. Bed Bench 2017, 10, 131. [Google Scholar]
- Abdelsattar, A.S.; Nofal, R.; Makky, S.; Safwat, A.; Taha, A.; El-Shibiny, A. The Synergistic Effect of Biosynthesized Silver Nanoparticles and Phage ZCSE2 as a Novel Approach to Combat Multidrug-Resistant Salmonella enterica. Antibiotics 2021, 10, 678. [Google Scholar] [CrossRef]
- Lim, T.-H.; Kim, M.-S.; Lee, D.-H.; Lee, Y.-N.; Park, J.-K.; Youn, H.-N.; Lee, H.-J.; Yang, S.-Y.; Cho, Y.-W.; Lee, J.-B. Use of bacteriophage for biological control of Salmonella Enteritidis infection in chicken. Res. Vet. Sci. 2012, 93, 1173–1178. [Google Scholar] [CrossRef]
- Phongtang, W.; Choi, G.-P.; Chukeatirote, E.; Ahn, J. Bacteriophage control of Salmonella Typhimurium in milk. Food Sci. Biotechnol. 2019, 28, 297–301. [Google Scholar] [CrossRef]
- Mobarki, N.; Almerabi, B.; Hattan, A. Antibiotic Resistance Crisis. Int. J. Med. Dev. Ctries. 2019, 3, 561–564. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.; Taha, O.; El-Sherif, H.M.; Connerton, P.L.; Hooton, S.P.T.; Bassim, N.D.; Connerton, I.F.; El-Shibiny, A. Bacteriophage ZCSE2 is a Potent Antimicrobial against Salmonella enterica Serovars: Ultrastructure, genomics and efficacy. Viruses 2020, 12, 424. [Google Scholar] [CrossRef] [Green Version]
- McCallin, S.; Sacher, J.C.; Zheng, J.; Chan, B.K. Current state of compassionate phage therapy. Viruses 2019, 11, 343. [Google Scholar] [CrossRef] [Green Version]
- Amarillas, L.; Cháidez-Quiroz, C.; Sañudo-Barajas, A.; León-Félix, J. Complete genome sequence of a polyvalent bacteriophage, phiKP26, active on Salmonella and Escherichia coli. Arch. Virol. 2013, 158, 2395–2398. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Dawoud, A.; Makky, S.; Nofal, R.; Aziz, R.K.; El-Shibiny, A. Bacteriophages: From isolation to application. Curr. Pharm. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Jurczak-Kurek, A.; Gsior, T.; Nejman-Fale czyk, B.; Bloch, S.; Dydecka, A.; Topka, G.; Necel, A.; Jakubowska-Deredas, M.; Narajczyk, M.; Richert, M. Biodiversity of bacteriophages: Morphological and biological properties of a large group of phages isolated from urban sewage. Sci. Rep. 2016, 6, 1–17. [Google Scholar] [CrossRef]
- Gwak, K.M.; Choi, I.Y.; Lee, J.; Oh, J.-H.; Park, M.-K. Isolation and characterization of a lytic and highly specific phage against Yersinia enterocolitica as a novel biocontrol agent. J. Microbiol. Biotechnol. 2018, 28, 1946–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Bai, J.; Lee, J.H.; Ryu, S. Mutation of a Staphylococcus aureus temperate bacteriophage to a virulent one and evaluation of its application. Food Microbiol. 2019. [Google Scholar] [CrossRef]
- El-Shibiny, A.; El-Sahhar, S.; Adel, M. Phage applications for improving food safety and infection control in Egypt. J. Appl. Microbiol. 2017, 123, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Abdelsattar, A.S.; Abdelrahman, F.; Dawoud, A.; Connerton, I.F.; El-Shibiny, A. Encapsulation of E. coli phage ZCEC5 in chitosan–alginate beads as a delivery system in phage therapy. AMB Express 2019, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Bochkareva, S.S.; Karaulov, A.V.; Aleshkin, A.V.; Novikova, L.I.; Kiseleva, I.A.; Rubal’skii, E.O.; Mekhtiev, E.R.; Styshnev, A.O.; Zul’karneev, E.R.; Anurova, M.N. Analysis of the Pharmacokinetics of Suppository Forms of Bacteriophages. Bull. Exp. Biol. Med. 2020, 168, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Parada, V.; Herndl, G.J.; Weinbauer, M.G. Viral burst size of heterotrophic prokaryotes in aquatic systems. J. Mar. Biol. Assoc. UK 2006, 86, 613–621. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Q.; Wang, M.; Zhao, G.; Jiang, Y.; Malin, G.; Gong, Z.; Meng, X.; Liu, Z.; Lin, T. Characterization and genome sequence of marine Alteromonas gracilis phage PB15 isolated from the Yellow Sea, China. Curr. Microbiol. 2017, 74, 821–826. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, Y.; Wang, M.; Wang, M.; Jiang, T.; Sun, J.; Gao, C.; Jiang, Y.; Guo, C.; Shao, H. Characterization and genome analysis of a novel marine Alteromonas phage P24. Curr. Microbiol. 2020, 77, 2813–2820. [Google Scholar] [CrossRef]
- Styles, K.M.; Thummeepak, R.; Leungtongkam, U.; Smith, S.E.; Christie, G.S.; Millard, A.; Moat, J.; Dowson, C.G.; Wellington, E.M.H.; Sitthisak, S. Investigating bacteriophages targeting the opportunistic pathogen Acinetobacter baumannii. Antibiotics 2020, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Park, J.-H.; Yong, D. Efficacy of bacteriophage treatment against carbapenem-resistant Acinetobacter baumannii in Galleria mellonella larvae and a mouse model of acute pneumonia. BMC Microbiol. 2019, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.; Alrashid, B.; Patel, F.; Dowah, A.S.A.; Brown, N.; Millard, A.; Clokie, M.R.J.; Galyov, E.E. vB_PaeM_MIJ3, a novel jumbo phage infecting Pseudomonas aeruginosa, possesses unusual genomic features. Front. Microbiol. 2019, 10, 2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, M.; Wahida, A.; Latz, S.; Krüttgen, A.; Häfner, H.; Buhl, E.M.; Ritter, K.; Horz, H.-P. Enhanced antibacterial effect of the novel T4-like bacteriophage KARL-1 in combination with antibiotics against multi-drug resistant Acinetobacter baumannii. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Gao, S.; Dong, Y.; Lu, C.; Liu, Y. Isolation and characterization of bacteriophages against virulent Aeromonas hydrophila. BMC Microbiol. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Tanaka, C.; Yamada, K.; Takeuchi, H.; Inokuchi, Y.; Kashiwagi, A.; Toba, T. A lytic bacteriophage for controlling Pseudomonas lactis in raw cow’s milk. Appl. Environ. Microbiol. 2018, 84, 18. [Google Scholar] [CrossRef] [Green Version]
- Grygorcewicz, B.; Chajęcka-Wierzchowska, W.; Augustyniak, A.; Wasak, A.; Stachurska, X.; Nawrotek, P.; Dołęgowska, B. In-milk inactivation of Escherichia coli O157: H7 by the environmental lytic bacteriophage ECPS-6. J. Food Saf. 2020, 40, e12747. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Dawooud, A.; Rezk, N.; Makky, S.; Safwat, A.; Richards, P.J.; El-Shibiny, A. How to Train Your Phage: The Recent Efforts in Phage Training. Biologics 2021, 1, 70–88. [Google Scholar] [CrossRef]
- Yeh, Y.; De Moura, F.H.; Van Den Broek, K.; De Mello, A.S. Effect of ultraviolet light, organic acids, and bacteriophage on Salmonella populations in ground beef. Meat Sci. 2018, 139, 44–48. [Google Scholar] [CrossRef]
- Hong, Y.; Schmidt, K.; Marks, D.; Hatter, S.; Marshall, A.; Albino, L.; Ebner, P. Treatment of Salmonella-contaminated eggs and pork with a broad-spectrum, single bacteriophage: Assessment of efficacy and resistance development. Foodborne Pathog. Dis. 2016, 13, 679–688. [Google Scholar] [CrossRef]
- Sukumaran, A.T.; Nannapaneni, R.; Kiess, A.; Sharma, C.S. Reduction of Salmonella on chicken meat and chicken skin by combined or sequential application of lytic bacteriophage with chemical antimicrobials. Int. J. Food Microbiol. 2015, 207, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, A.T.; Nannapaneni, R.; Kiess, A.; Sharma, C.S. Reduction of Salmonella on chicken breast fillets stored under aerobic or modified atmosphere packaging by the application of lytic bacteriophage preparation SalmoFreshTM. Poult. Sci. 2016, 95, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, W.A.; Azzazy, H.M.E. Phage approved in food, why not as a therapeutic? Expert Rev. Anti. Infect. Ther. 2015, 13, 91–101. [Google Scholar] [CrossRef]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol. Biol. 2009, 501, 69–76. [Google Scholar] [PubMed]
- Abdelsattar, A.; El-Shibiny, A. A Modified High-Throughput Screening Protocol to Isolate Bacteriophages from Environmental Samples. Preprints 2021. [Google Scholar] [CrossRef]
- Senczek, D.; Stephan, R.; Untermann, F. Pulsed-field gel electrophoresis (PFGE) typing of Listeria strains isolated from a meat processing plant over a 2-year period. Int. J. Food Microbiol. 2000, 62, 155–159. [Google Scholar] [CrossRef]
- Taha, O.A.; Connerton, P.L.; Connerton, I.F.; El-Shibiny, A. Bacteriophage ZCKP1: A potential treatment for Klebsiella pneumoniae isolated from diabetic foot patients. Front. Microbiol. 2018, 9, 2127. [Google Scholar] [CrossRef]

| ID | Salmonella Bacteria | Serotype | EOP | Plaque Morphology |
|---|---|---|---|---|
| CMPZCSB1 | Salmonella enterica | enterica serotype Typhimurium | ≥1 | plaques with a halo |
| CMPZCSB2 | Salmonella enterica | enterica serotype Blegdam | <0.5 | plaques with a halo |
| CMPZCSB3 | Salmonella enterica | enterica serotype Kentucky | ≥1 | turbid plaque |
| CMPZCSB4 | Salmonella enterica | enterica serotype Blegdam | <1 ≥0.5 | plaques with a halo |
| CMPZCSB5 | Salmonella enterica | enterica serotype Typhimurium | ≥1 | turbid plaque |
| CMPZCSB6 | Salmonella enterica | enterica serotype Gallinarum | <1 ≥0.5 | plaques with a halo |
| CMPZCSB7 | Salmonella enterica | enterica serotype Enteritidis | <0.5 | plaques with a halo |
| CMPZCSB8 | Salmonella enterica | Salmonella enterica spp. | <1 ≥0.5 | plaques with a halo |
| CMPZCSB9 | Salmonella enterica | enterica serotype Enteritidis | ≥1 | plaques with a halo |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelsattar, A.S.; Safwat, A.; Nofal, R.; Elsayed, A.; Makky, S.; El-Shibiny, A. Isolation and Characterization of Bacteriophage ZCSE6 against Salmonella spp.: Phage Application in Milk. Biologics 2021, 1, 164-176. https://doi.org/10.3390/biologics1020010
Abdelsattar AS, Safwat A, Nofal R, Elsayed A, Makky S, El-Shibiny A. Isolation and Characterization of Bacteriophage ZCSE6 against Salmonella spp.: Phage Application in Milk. Biologics. 2021; 1(2):164-176. https://doi.org/10.3390/biologics1020010
Chicago/Turabian StyleAbdelsattar, Abdallah S., Anan Safwat, Rana Nofal, Amera Elsayed, Salsabil Makky, and Ayman El-Shibiny. 2021. "Isolation and Characterization of Bacteriophage ZCSE6 against Salmonella spp.: Phage Application in Milk" Biologics 1, no. 2: 164-176. https://doi.org/10.3390/biologics1020010
APA StyleAbdelsattar, A. S., Safwat, A., Nofal, R., Elsayed, A., Makky, S., & El-Shibiny, A. (2021). Isolation and Characterization of Bacteriophage ZCSE6 against Salmonella spp.: Phage Application in Milk. Biologics, 1(2), 164-176. https://doi.org/10.3390/biologics1020010








