Latent Class Analysis of Aeroallergen Sensitization Profiles: Correlations with Sex, Age, and Seasonal Variation in Serum-Specific IgE—Cross-Sectional Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Collection
2.5. Data Analysis
2.6. Latent Class Analysis
2.7. Ethical Aspects
3. Results
3.1. Characteristics of the Studied Population
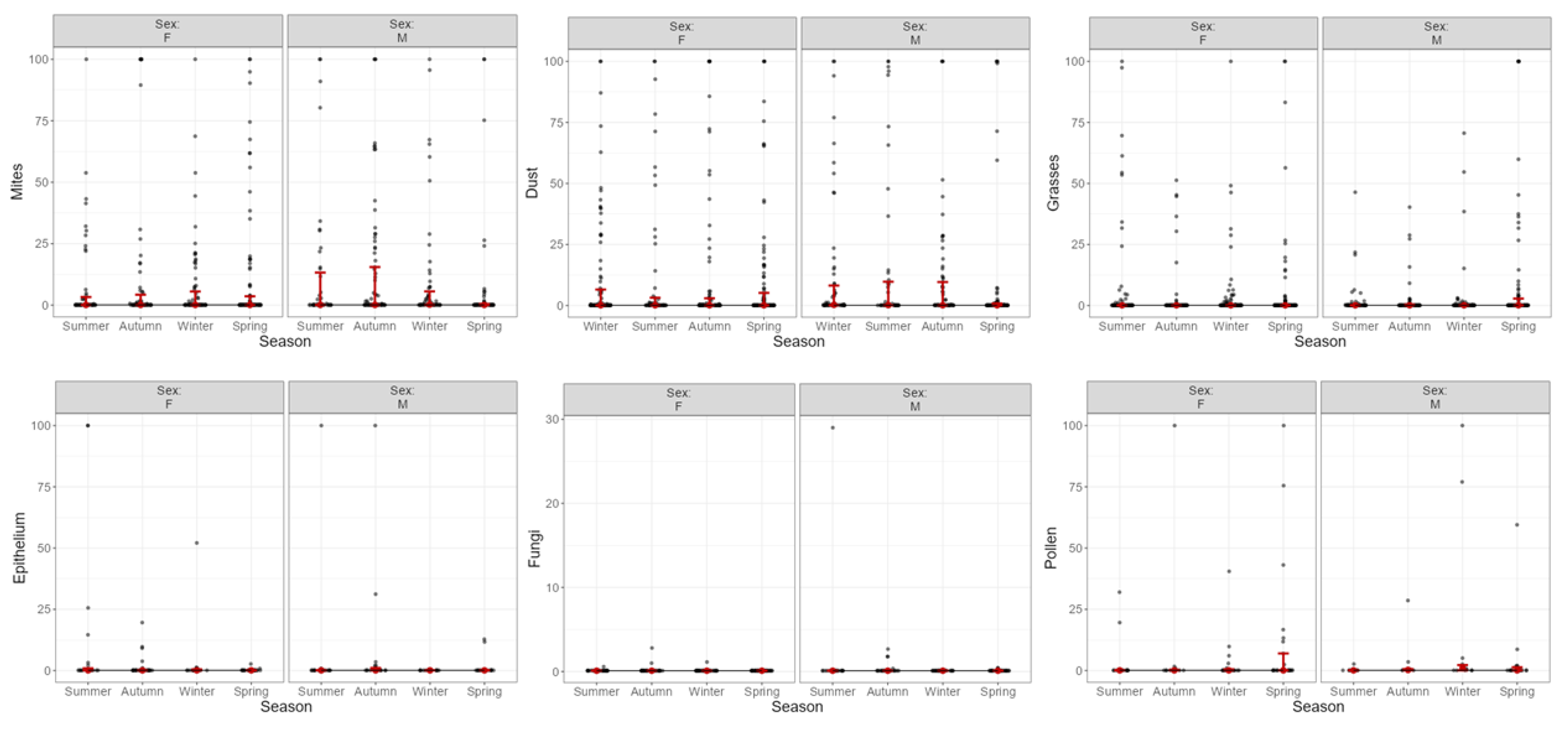
3.2. Sensitization and Aeroallergens: Variation According to IgE Levels
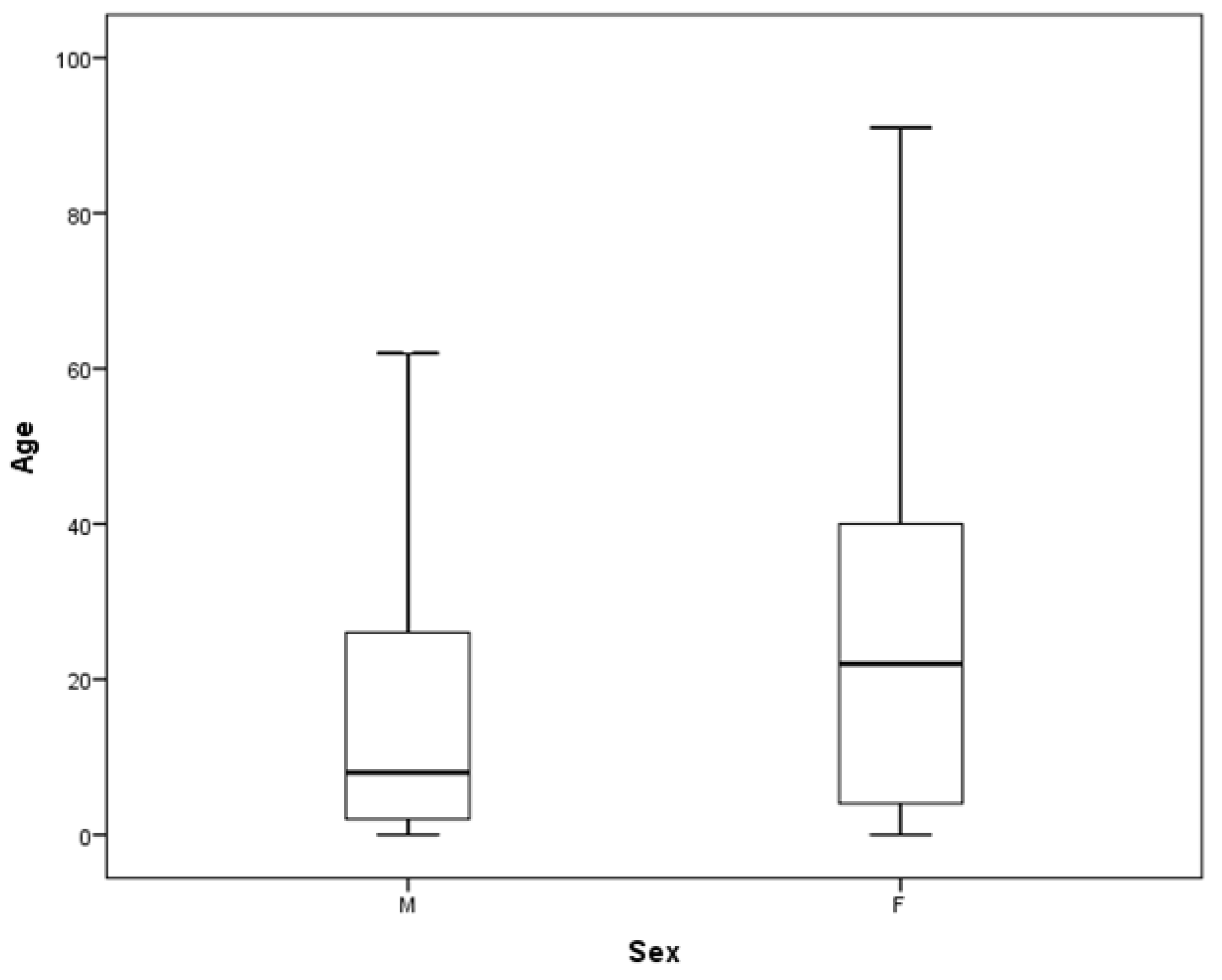
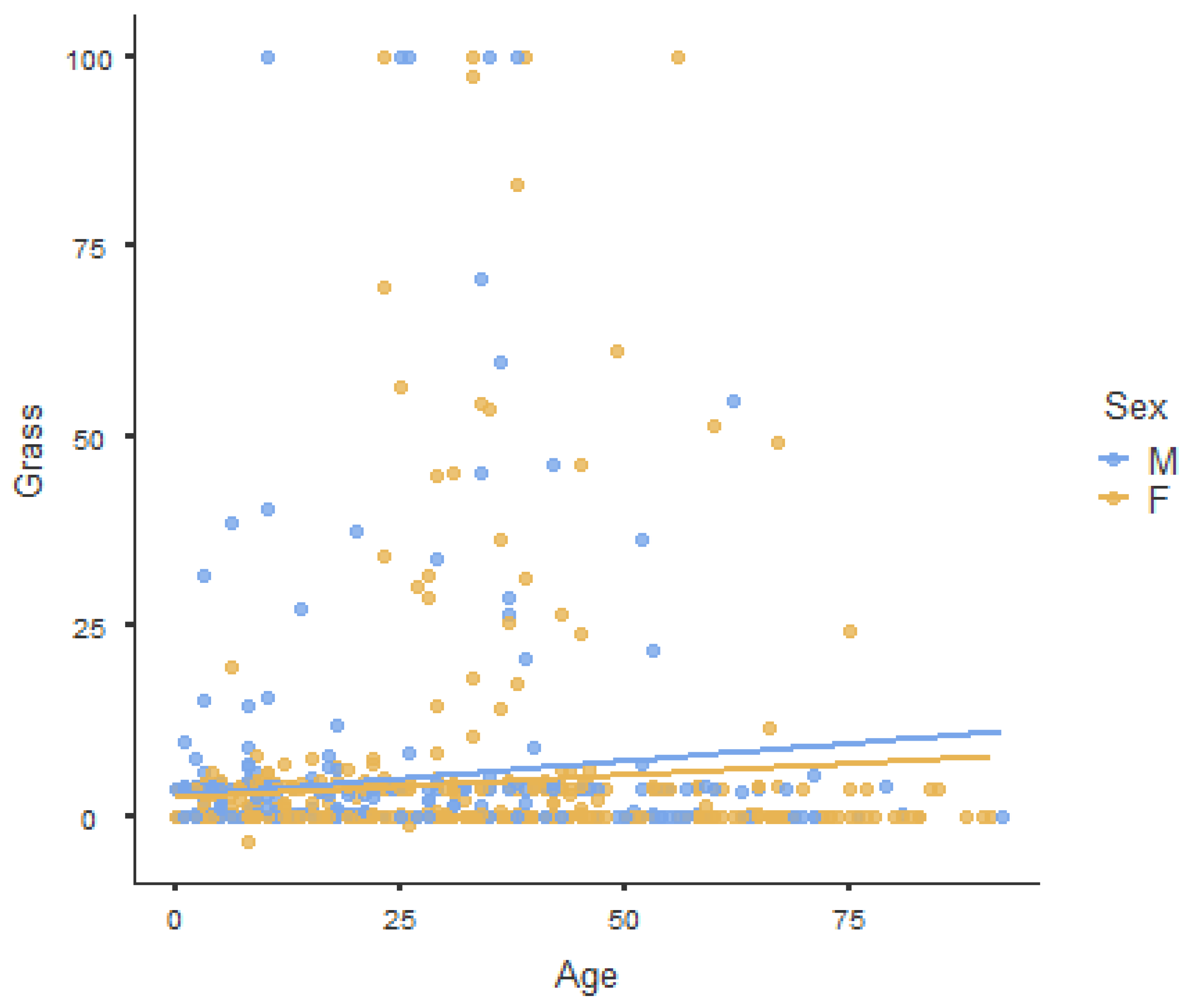
3.3. Sensitization: Exploiting the Age–Sex Pyramid
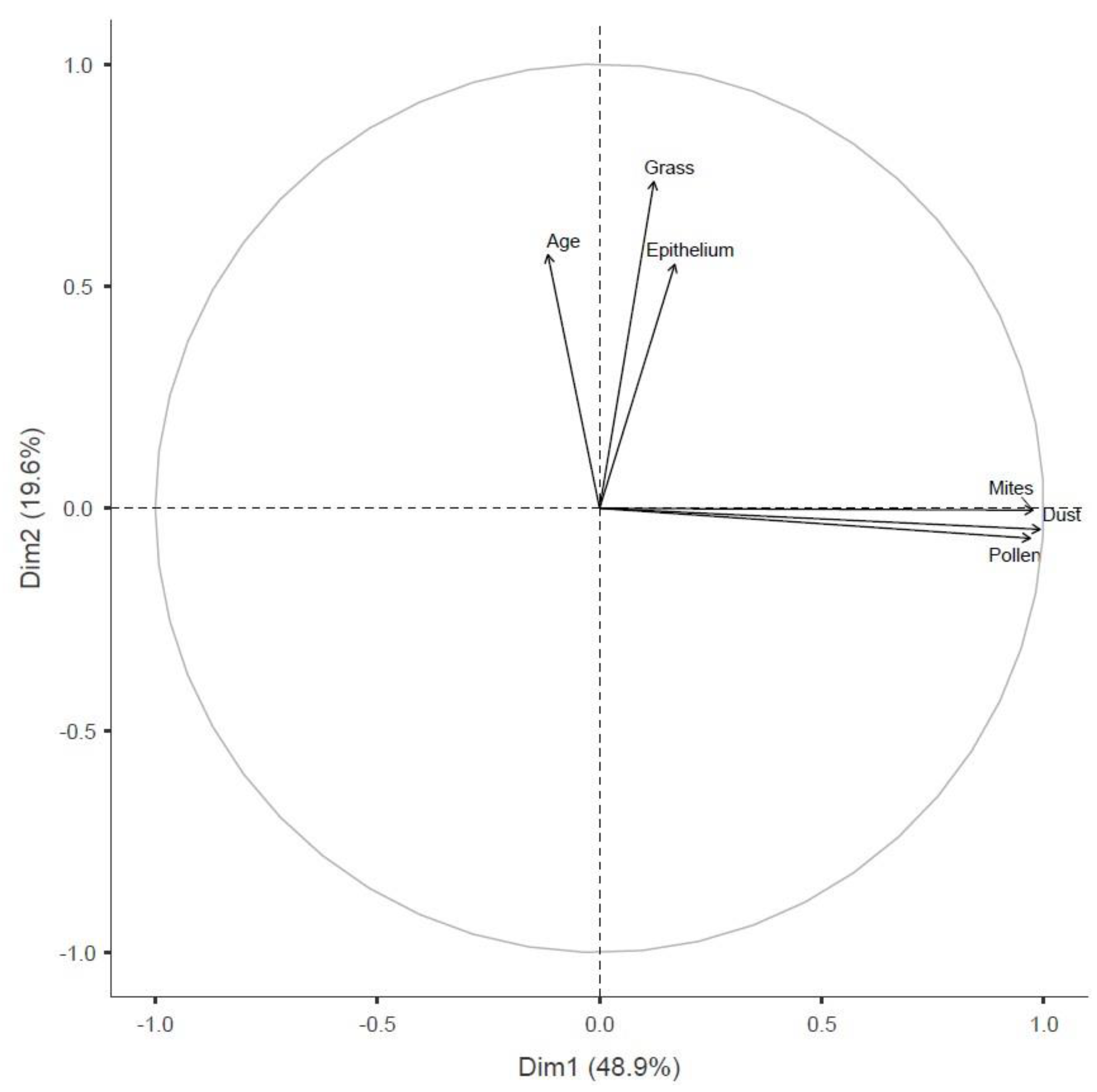
3.4. Principal Component Analysis
3.5. Model Fit Indices for LCA
3.6. Latent Class Definitions for Specific IgE Sensitization
4. Discussion
5. Limitations and Future Research
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pawankar, R.; Canonica, G.W.; Holgate, S.T.; Lockey, R.F.; Blaiss, M. The WAO White Book on Allergy (Update 2013); WAO: Mindanao, Philippines, 2013. [Google Scholar]
- Bousquet, J.; Schunemann, H.J.; Togias, A.; Bachert, C.; Erhola, M.; Hellings, P.W.; Klimek, L.; Pfaar, O.; Wallace, D.; Ansotegui, I.; et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J. Allergy Clin. Immunol. 2019, 145, 70–80.e3. [Google Scholar] [CrossRef]
- Sztandera-Tymoczek, M.; Szuster-Ciesielska, A. Fungal Aeroallergens—The Impact of Climate Change. J. Fungi 2023, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Del Moral, M.G.; Martínez-Naves, E. The Role of Lipids in Development of Allergic Responses. Immune Netw. 2017, 17, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Justiz-Vaillant, A.A.; Vashisht, R.; Zito, P.M. Immediate Hypersensitivity Reactions. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Xu, G.; Liu, B.; Yang, W.; Snetselaar, L.G.; Chen, M.; Bao, W.; Strathearn, L. Association of Food Allergy, Respiratory Allergy, and Skin Allergy with Attention Deficit/Hyperactivity Disorder among Children. Nutrients 2022, 14, 474. [Google Scholar] [CrossRef] [PubMed]
- Katoch, C.D.S.; Kumar, K.; Marwah, V.; Bhatti, G. Pattern of skin sensitivity to various aeroallergens by skin prick test in patients of allergic airway disease in South Western Maharashtra. Med. J. Armed Forces India 2022, 78, 400–404. [Google Scholar] [CrossRef]
- Soares, F.A.A.S.; Segundo, G.R.S.; Alves, R.; Ynoue, L.H.; Resende, R.O.; Sopelete, M.C.; Silva, D.A.O.; Sung, S.J.; Taketomi, E.A. Perfil de sensibilização a alérgenos domiciliares em pacientes ambulatoriais. Rev. Assoc. Med. Bras. 2007, 53, 25–28. [Google Scholar] [CrossRef]
- Bakonyi, S.M.C.; Oliveira, I.M.D.; Martins, L.C.; Braga, A.L. Poluição atmosférica e doenças respiratórias em crianças na cidade de Curitiba, PR. Rev. Saúde Pública 2004, 38, 695–700. [Google Scholar] [CrossRef]
- Aldakheel, F.M. Allergic Diseases: A Comprehensive Review on Risk Factors, Immunological Mechanisms, Link with COVID-19, Potential Treatments, and Role of Allergen Bioinformatics. Int. J. Environ. Res. Public Health 2021, 18, 12105. [Google Scholar] [CrossRef]
- Sakano, E.; Sarinho, E.S.C.; Cruz, A.A.; Pastorino, A.C.; Tamashiro, E.; Kuschnir, F.; Castro, F.F.M.; Romano, F.R.; Wandalsen, G.F.; Chong-Neto, H.J.; et al. IV Brazilian Consensus on Rhinitis—An update on allergic rhinitis. Braz. J. Otorhinolaryngol. 2018, 84, 3–14. [Google Scholar] [CrossRef]
- BRASIL. Protocolo Clínico e Diretrizes Terapêuticas da Asma; Ministério da Saúde: Brasília, Brazil, 2021. [Google Scholar]
- Demoly, P.; Liu, A.H.; Rodriguez Del Rio, P.; Pedersen, S.; Casale, T.B.; Price, D. A Pragmatic Primary Practice Approach to Using Specific IgE in Allergy Testing in Asthma Diagnosis, Management, and Referral. J. Asthma Allergy 2022, 15, 1069–1080. [Google Scholar] [CrossRef]
- Eiringhaus, K.; Renz, H.; Matricardi, P.; Skevaki, C. Component-Resolved Diagnosis in Allergic Rhinitis and Asthma. J. Appl. Lab. Med. 2019, 3, 883–898. [Google Scholar] [CrossRef]
- Sonntag, H.J.; Filippi, S.; Pipis, S.; Custovic, A. Blood Biomarkers of Sensitization and Asthma. Front. Pediatr. 2019, 7, 251. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.G. Clinical laboratory assessment of immediate-type hypersensitivity. J. Allergy Clin. Immunol. 2010, 125, S284–S296. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.G.; Adkinson, N.F. In vitro assays for the diagnosis of IgE-mediateddisorders. J. Allergy Clin. Immunol. 2004, 114, 213–225. [Google Scholar] [CrossRef] [PubMed]
- JAMOVI. Version 2.3; The Jamovi Project. 2022. Available online: https://www.jamovi.org (accessed on 1 December 2022).
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.1; R Packages Retrieved from MRAN Snapshot 2022-01-01; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://cran.r-project.org (accessed on 1 December 2022).
- SPSS Inc. IBM SPSS Statistics Base 20; SPSS Inc.: Chicago, IL, USA, 2011. [Google Scholar]
- Yan, T.; Zheng, R.; Li, Y.; Sun, S.; Zeng, X.; Yue, Z.; Liao, Y.; Hu, Q.; Xu, Y.; Li, Q. Epidemiological Insights into the Omicron Outbreak via MeltArray-Assisted Real-Time Tracking of SARS-CoV-2 Variants. Viruses 2023, 15, 2397. [Google Scholar] [CrossRef]
- Ying, X.; Qi, X.; Yin, Y.; Wang, H.; Zhang, H.; Jiang, H.; Yang, L.; Wu, J. Allergens sensitization among children with allergic diseases in Shanghai, China: Age and sex difference. Respir. Res. 2022, 23, 95. [Google Scholar] [CrossRef]
- Shikha, G.; Swami, H. Trends of sensitization pattern to aeroallergens among the patients with allergic rhinitis and/or bronchial asthma in Bangalore: A cross sectional study. Med. J. Armed Forces India 2022, 78, 430–436. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L.; Wei, G.; Zhang, H.; Yang, B. Prevalence of allergen-specific IgE in southern China: A multicenter research. Aging 2021, 13, 18894–18911. [Google Scholar] [CrossRef]
- Ahmed, H.; Ospina, M.B.; Sideri, K.; Vliagoftis, H. Retrospective analysis of aeroallergen’s sensitization patterns in Edmonton, Canada. Allergy Asthma Clin. Immunol. 2019, 15, 6. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Wickman, M.; Keil, T.; Valenta, R.; Haahtela, T.; Lodrup Carlsen, K.; van Hage, M.; Akdis, C.; Bachert, C.; et al. Are allergic multimorbidities and IgE polysensitization associated with the persistence or re-occurrence of foetal type 2 signalling? The MeDALL hypothesis. Allergy 2015, 70, 1062–1078. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, Y.; Zhu, H.; Lin, Y.; Su, H.; Hu, J.; Zhang, M.; Bao, W. Age, Sex, and Symptom-Dependent Variations in Total IgE and Eosinophils in Atopic Patients: A Five-Year Retrospective Study. J. Asthma Allergy 2025, 18, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.; Belgrave, D.; Papastamoulis, P.; Simpson, A.; Rattray, M.; Custovic, A. Evolution of IgE responses to multiple allergen components throughout childhood. J. Allergy Clin. Immunol. 2018, 142, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Liu, J.; Zhao, Q.; Li, M.; Zhou, Y.; Chen, Z.; Zhang, Y. Investigation of Allergic Sensitizations in Children with Allergic Rhinitis and/or Asthma. Front. Pediatr. 2022, 10, 842293. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Anto, J.M.; Akdis, M.; Auffray, C.; Keil, T.; Momas, I.; Postma, D.S.; Valenta, R.; Wickman, M.; Cambon-Thomsen, A.; et al. Paving the way of systems biology and precision medicine in allergic diseases: The MeDALL success story: Mechanisms of the Development of ALLergy; EU FP7-CP-IP; Project No: 261357; 2010–2015. Allergy 2016, 71, 1513–1525. [Google Scholar] [CrossRef]
- Yong Rodríguez, A.; Macías Weinmann, A.; Palma Gomez, S.; Arias Cruz, A.; Pérez Vanzzini, R.; Gutiérrez Mujica, J.J.; González Díaz, S.N. Perfil de sensibilización a alergenos en niños con dermatitis atópica atendidos en el Servicio de Alergología del Hospital Universitario de la Universidad Autónoma de Nuevo León, México. Rev. Alerg. México 2015, 62, 98–106. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Y.; An, X.; Gao, N.; Li, Z.; Zhang, S.; Liang, Y.; Ruan, W.; Bu, Y.; Xin, J.; et al. Effects of airborne pollen on allergic rhinitis and asthma across different age groups in Beijing, China. Sci. Total Environ. 2024, 912, 169215. [Google Scholar] [CrossRef]
- Karadoğan, D.; Telatar, T.G.; Dönmez, H.; Dursun, A.B. Relationship between aeroallergen sensitization pattern and clinical features in adult asthmatics. Heliyon 2023, 9, e15708. [Google Scholar] [CrossRef]
- Souza, T.M.O.; Fernandes, J.S.; Santana, C.V.N.; Lessa, M.M.; Cruz, Á.A. Aeroallergen sensitization patterns among patients with chronic rhinitis with or without concomitant asthma. Braz. J. Otorhinolaryngol. 2023, 90, 101351. [Google Scholar] [CrossRef]
- Rodinkova, V.; Grewling, Ł.; Ribeiro, H.; Antunes, C.; Apangu, G.P.; Çelenk, S.; Costa, A.; Eguiluz-Gracia, I.; Galveias, A.; Gonzalez Roldan, N.; et al. Outdoor airborne allergens: Characterization, behavior and monitoring in Europe. Sci. Total Environ. 2023, 905, 167042. [Google Scholar] [CrossRef]
- Andersson, K.; Lidholm, J. Characteristics and immunobiology of grass pollen allergens. Int. Arch. Allergy Immunol. 2003, 130, 87–107. [Google Scholar] [CrossRef]
- Kleine-Tebbe, J.; Davies, J. Grass pollen allergens. In Global Atlas of Allergy; Akdis, C.A., Agache, I., Eds.; European Academy of Allergy and Clinical Immunology: Zurich, Switzerland, 2014; pp. 22–26. [Google Scholar]
- Hemmer, W.; Schauer, U.; Trinca, A.M.; Neumann, C. Endbericht 2009 zur Studie: Prävalenz der Ragweedpollen-Allergie in Ostösterreich; Amt der NÖ Landesregierung, Landesamtsdirektion, Abteilung Gebäudeverwaltung, Amtsdruckerei: St. Pölten, Austria, 2010. [Google Scholar]
- Johansen, N.; Weber, R.W.; Ipsen, H.; Barber, D.; Broge, L.; Hejl, C. Extensive IgE cross-reactivity towards the Pooideae grasses substantiated for a large number of grass-pollen-sensitized subjects. Int. Arch. Allergy Immunol. 2009, 150, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Bullimore, A.; Batten, T.; Hewings, S.; Fischer von Weikersthal-Drachenberg, K.J.; Skinner, M. Cross-reactivity in Grasses: Biochemical Attributes Define Exemplar Relevance. World Allergy Organ. J. 2012, 5, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Susanto, N.H.; Schoos, A.-M.M.; Standl, M.; Lowe, A.J.; Dharmage, S.C.; Svanes, C.; Salim, A.; von Berg, A.; Lehmann, I.; Rasmussen, M.A.; et al. Environmental grass pollen levels in utero and at birth and cord blood IgE: Analysis of three birth cohorts. Environ. Int. 2018, 119, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Minn, D.; Kim, S.; Kim, J.K.; Cho, J.H. Aeroallergen Sensitization Status in South Korea from 2018 to 2021. Clin. Exp. Otorhinolaryngol. 2022, 15, 254–263. [Google Scholar] [CrossRef]
- Khasawneh, R.; Al-Hiary, M.; Al-Abadi, B.; Bani-Salameh, A.; Al-Momani, S. Total and Specific Immunoglobulin E for Detection of Most Prevalent Aeroallergens in a Jordanian Cohort. Med. Arch. 2019, 73, 272–275. [Google Scholar] [CrossRef]
- Levetin, E. Aeroallergens and Climate Change in Tulsa, Oklahoma: Long-Term Trends in the South Central United States. Front. Allergy 2021, 2, 726445. [Google Scholar] [CrossRef]
- Oh, J.W. Pollen Allergy in a Changing Planetary Environment. Allergy Asthma Immunol. Res. 2022, 14, 168–181. [Google Scholar] [CrossRef]
- Meher, B.K.; Pradhan, D.D.; Mahar, J.; Sahu, S.K. Prevalence of Allergic Sensitization in Childhood Asthma. Cureus 2021, 13, e15311. [Google Scholar] [CrossRef]
- Gleeson, P.K.; Morales, K.H.; Buckey, T.M.; Fadugba, O.O.; Apter, A.J.; Christie, J.D.; Himes, B.E. Factors associated with aeroallergen testing among adults with asthma in a large health system. J. Allergy Clin. Immunol. Glob. 2023, 2, 100167. [Google Scholar] [CrossRef]
- Olivier, C.E.; Pinto, D.G.; Teixeira, A.P.M.; Santana, J.L.S.; Santos, R.A.P.G.S.; Lima, R.P.S.; Monteiro, E.S. Evaluating Non-IgE-Mediated Allergens’ Immunoreactivity in Patients Formerly Classified as “Intrinsic” Asthmatics with Help of the Leukocyte Adherence Inhibition Test. Eur. J. Clin. Med. 2023, 4, 1–7. [Google Scholar] [CrossRef]
- Olivier, C.E.; Pinto, D.G.; Teixeira, A.P.M.; Santana, J.L.S.; Santos, R.A.P.G.S.; Lima, R.P.S.; Monteiro, E.S. Evaluating Non-IgE-mediated Allergens’ Immunoreactivity in Patients with “Intrinsic” Persistent Rhinitis with Help of the Leukocyte Adherence Inhibition Test. Eur. J. Med. Health Sci. 2023, 5, 17–22. [Google Scholar] [CrossRef]
- Bouman, A.; Heineman, M.J.; Faas, M.M. Sex hormones and the immune response in humans. Hum. Reprod. Update 2005, 11, 411–423. [Google Scholar] [CrossRef]
- Hachmeriyan, A.; Toneva, A.; Marinova-Achkar, M.; Pancheva, R. Early-Life Environmental Determinants of Allergic Conditions in Children with Atopic Heredity: A Single Center Cross-Sectional Study from Bulgaria. Med. Sci. 2025, 13, 198. [Google Scholar] [CrossRef]
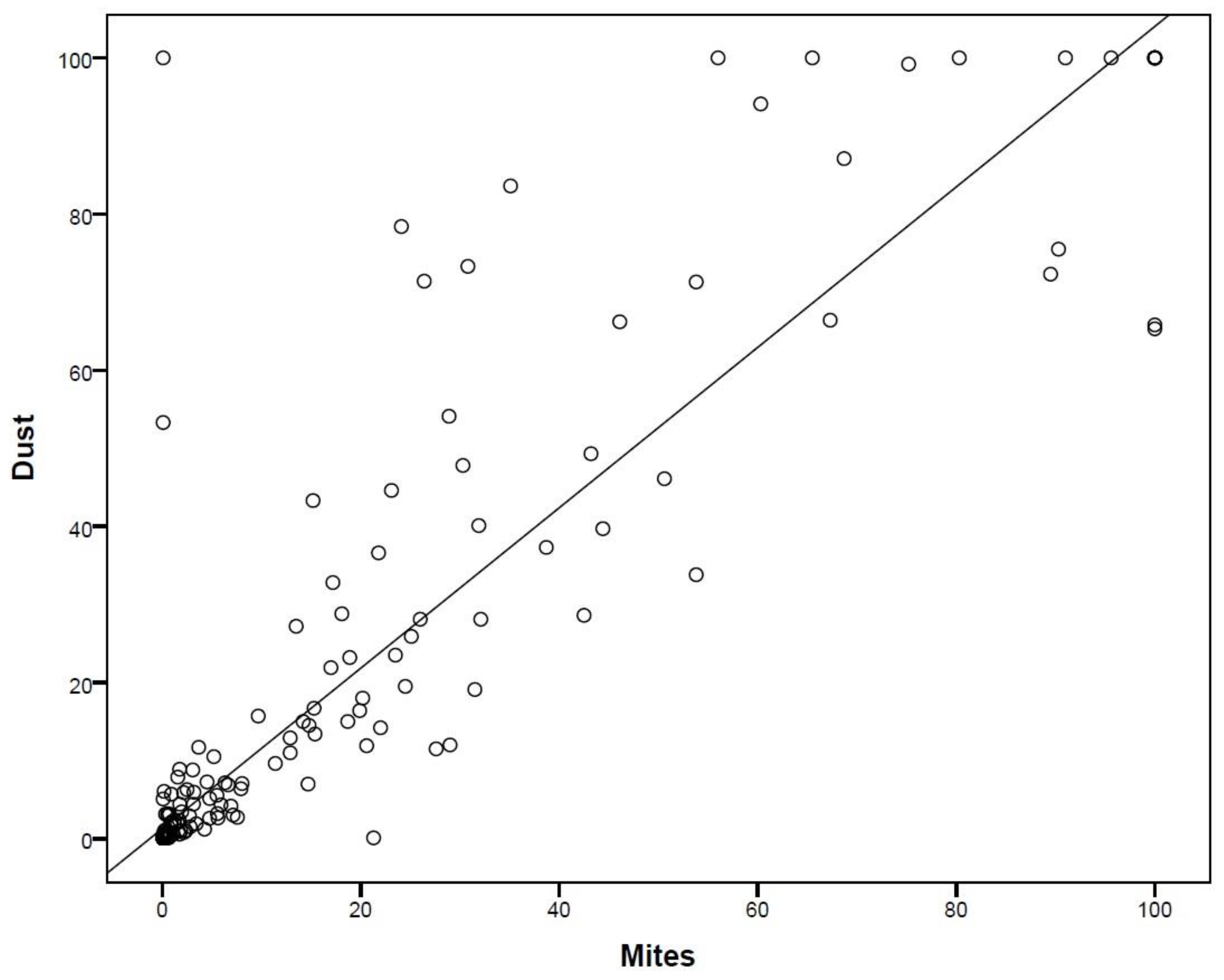
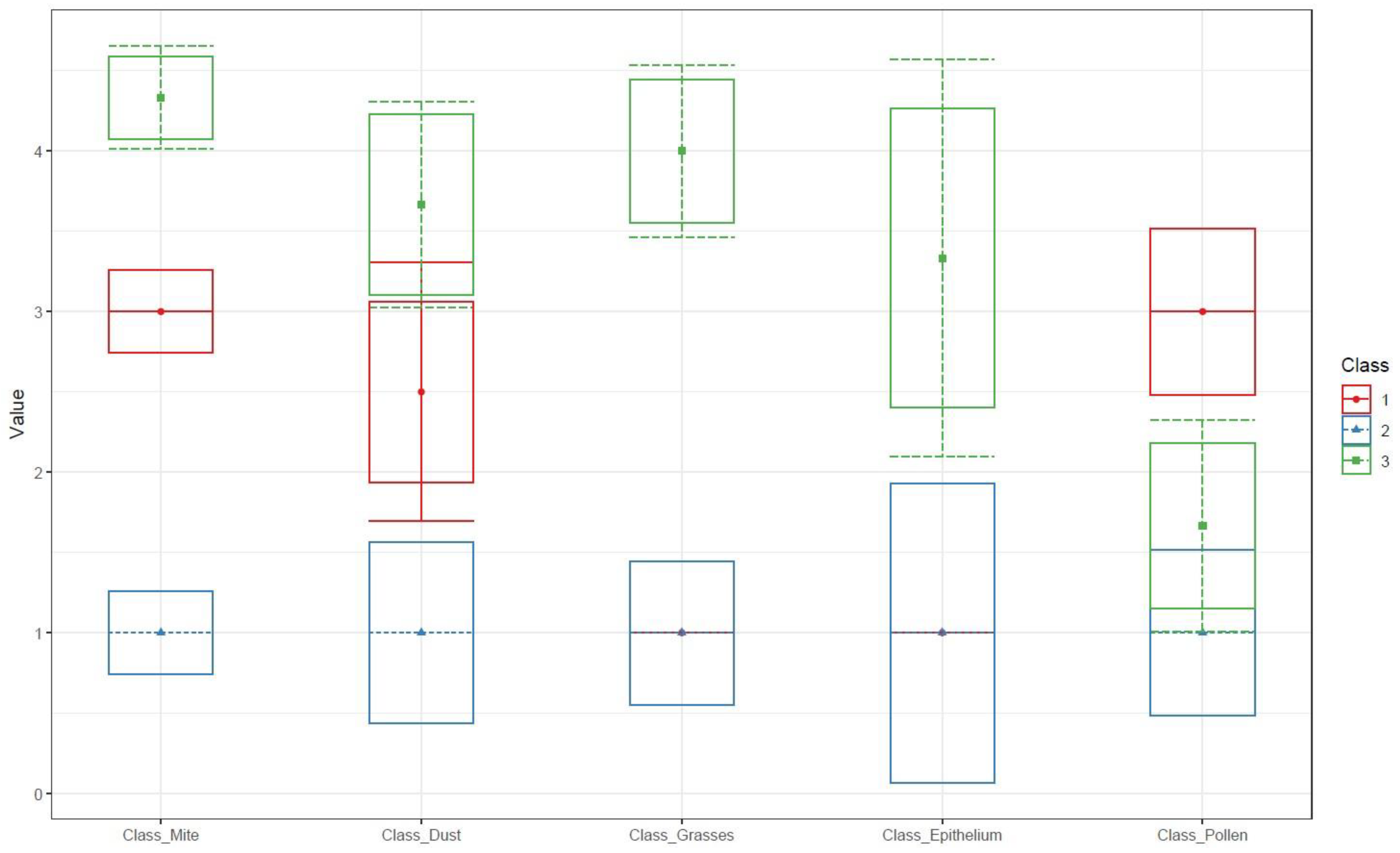
| Categories | Undetectable (<0.35 kU/L) |
Low
(≥0.35 kU/L–<0.7 kU/L) |
Moderate
(≥0.7 kU/L–<3.5 kU/L) |
High
(≥3.5 kU/L–<17.5 kU/L) |
Very High
(≥17.5 kU/L) |
|---|---|---|---|---|---|
| Mites | 334 (62.2) * | 17 (3.2) | 40 (7.4) | 57 (10.6) | 89 (16.6) |
| Dust | 368 (62.3) | 15 (2.5) | 46 (7.8) | 64 (10.8) | 98 (16.6) |
| Grass | 587 (79.2) | 21 (2.8) | 51 (6.9) | 32 (4.3) | 50 (6.7) |
| Epithelial cells | 143 (79.9) | 9 (5) | 12 (6.7) | 7 (3.9) | 8 (4.5) |
| Fungi | 266 (96) | 4 (1.4) | 6 (2.2) | 0 (0) | 1 (0.4) |
| Pollen | 85 (68.5) | 5 (4) | 14 (11.3) | 9 (7.3) | 11 (8.9) |
| Class | AIC | BIC | CAIC | Entropy | p-Value |
|---|---|---|---|---|---|
| 2 | 3907 | 4147 | 4196 | 0.77 | - |
| 3 | 3733 | 4101 | 4176 | 0.82 | <0.001 |
| OR | Coefficient | Standard Error | t | p-Value | |
|---|---|---|---|---|---|
| Class1/3. (Intercept) | 8.11 | 2.09 | 0.49 | 4.19 | <0.001 |
| Class1/3.Sex | 1.37 | 0.31 | 0.28 | 1.12 | 0.26 |
| Class1/3.Age | 0.63 | −0.44 | 0.06 | −6.53 | <0.001 |
| Class2/3. (Intercept) | 0.04 | −3.07 | 0.59 | −5.19 | <0.001 |
| Class2/3.Sex | 1.48 | 0.39 | 0.26 | 1.49 | 0.13 |
| Class2/3.Age | 1.08 | 0.07 | 0.01 | 7.06 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szekut, M.S.; Jung, T.; da Cruz, Á.K.; Ohlweiler, L.M.; Pedralli, L.; Witz, R.W.; Majolo, F.; da Silva, G.L. Latent Class Analysis of Aeroallergen Sensitization Profiles: Correlations with Sex, Age, and Seasonal Variation in Serum-Specific IgE—Cross-Sectional Study. BioMed 2025, 5, 24. https://doi.org/10.3390/biomed5040024
Szekut MS, Jung T, da Cruz ÁK, Ohlweiler LM, Pedralli L, Witz RW, Majolo F, da Silva GL. Latent Class Analysis of Aeroallergen Sensitization Profiles: Correlations with Sex, Age, and Seasonal Variation in Serum-Specific IgE—Cross-Sectional Study. BioMed. 2025; 5(4):24. https://doi.org/10.3390/biomed5040024
Chicago/Turabian StyleSzekut, Michelle Silva, Tatiana Jung, Ágatha Kniphoff da Cruz, Laura Marina Ohlweiler, Luiza Pedralli, Rafaela Wickert Witz, Fernanda Majolo, and Guilherme Liberato da Silva. 2025. "Latent Class Analysis of Aeroallergen Sensitization Profiles: Correlations with Sex, Age, and Seasonal Variation in Serum-Specific IgE—Cross-Sectional Study" BioMed 5, no. 4: 24. https://doi.org/10.3390/biomed5040024
APA StyleSzekut, M. S., Jung, T., da Cruz, Á. K., Ohlweiler, L. M., Pedralli, L., Witz, R. W., Majolo, F., & da Silva, G. L. (2025). Latent Class Analysis of Aeroallergen Sensitization Profiles: Correlations with Sex, Age, and Seasonal Variation in Serum-Specific IgE—Cross-Sectional Study. BioMed, 5(4), 24. https://doi.org/10.3390/biomed5040024







