Abstract
Background/Objectives: Chronic ankle instability is a common issue after lateral ankle sprain. The DMA Clinical Pilates method hypothesizes impairments in tibialis anterior and fibularis longus muscles. Methods: A total of 14 adults with chronic ankle instability, and 15 recovered and 16 healthy individuals were prospectively recruited and assessed for lower back, hip, knee, and ankle muscle activities during dominant and non-dominant sides single-leg stand on stable and unstable surfaces using wireless surface electromyography. Results: The study found consistent dysfunction in tibialis anterior muscle activity in adults with chronic ankle instability when compared with healthy adults during single-leg stand on stable and unstable surfaces, and against recovered individuals on unstable surface. As compared to healthy controls, chronic ankle instability group showed higher vastus lateralis activation during dominant side single-leg stand across surface conditions and during dominant side single-leg stand, while the higher dominant side longissimus dorsi activity on stable surface changed to higher dominant side medial gastrocnemius activity on unstable surface. As compared to recovered controls, chronic ankle instability group also showed higher gluteus medius and fibularis longus muscle activities on unstable surface. Conclusions: Tibialis anterior muscle is the main dysfunction among individuals with chronic ankle instability side.
1. Introduction
Ankle pain is the third-most-common lower limb musculoskeletal condition [1], which is commonly due to lateral ankle sprain, whereby the joint plantarflexes and inverts excessively [2]. Apart from ligamentous injuries at the lateral ankle joint [3], strains to the fibularis muscles can occur [2]. While acute symptoms such as pain and swelling resolve quickly, some individuals may experience persistent symptoms that last more than three months, thus termed chronic ankle instability [4]. In chronic ankle instability, motor activation impairment occurs not just at the ankle, but also at the hip and knee [5]. Recent studies have used the single-leg stand test to assess motor activation patterns to maintain postural stability after ankle injury [5,6]. Furthermore, for the single-leg stand test, there is no agreement on specific muscle activity dysfunction [5,7], and postural sway assessment did not yield accurate information about athletes’ ankle function [8]. Thus, a more recent study calls for further exploration of motor activation patterns among individuals with chronic ankle instability [6]. Tibialis anterior muscle activity was found to be raised among individuals with chronic ankle instability when assessed with single-leg stand on a Biodex balance system [7], but not by another group of researchers [9]. In view of the lack of consensus between studies, it is important to incorporate clinical perspectives in prospective research.
A movement science method used in clinical practice is Clinical Pilates by Dance Medicine Australia (DMA), a method that integrates physiotherapy and Pilates for the rehabilitation of athletes, dancers, and the public. Extending from managing chronic low back pain [10,11], this method has the potential to assist in the understanding of muscle impairments at peripheral joints, such as at the ankle, for individuals with chronic ankle instability. The DMA Clinical Pilates method identifies movement preferences to guide clinical diagnosis of movement impairment in chronic low back pain [12], which suggests that muscles acting opposite to the injury direction will be dysfunctional. For instance, movement of the trunk into flexion is unfavorable (discomfort), while movement into extension is favorable (comfortable); an individual will be given exercises into extension. Applying this concept to the peripheral joint with multi-directional movements, such as the ankle, an ankle inversion trauma could lead to compensatory dorsiflexion and eversion motor control plausibly due to overactivation of tibialis anterior and fibularis longus muscles (Figure 1). In addition, the Clinical Pilates method does not recommend weight-bearing exercise on the problem side because of the risk of compensatory muscle involvement during exercise.
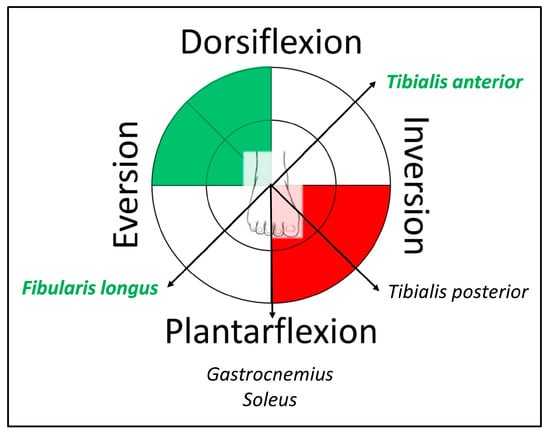
Figure 1.
Example of a right ankle sprain charted with Clinical Pilates theory of motor impairments for treatment directions to exercise (green) and avoid (red), while muscles in green font indicate predicted excitation dysfunction.
Therefore, this study was undertaken to validate the DMA Clinical Pilates method in classifying muscle impairments among individuals with chronic ankle instability through surface electromyography during the single-leg stand test. The alternative hypothesis used in this study was that individuals with chronic ankle instability would exhibit muscle activities, primarily tibialis anterior and fibularis longus muscles, that differ from healthy or recovered individuals. The findings provide clinical and research evidence to the existing literature on chronic ankle instability.
2. Materials and Methods
2.1. Study Design
A cross-sectional case–control study was carried out from April to October 2024. This study was approved by the Singapore Institute of Technology, RECAS-0267, and prospectively registered on the Australian New Zealand Clinical Trial Registry, ACTRN1262400081572. All participants provided written informed consent prior to study participation. The study procedures were carried out by the first two authors.
2.2. Sample Size
A threshold Bayes factor >3 indicates moderate evidence to support the alternative hypothesis. For the Bayes factor to be >3 at 80% desired probability, a sample size of 14 participants per group is needed. This estimate was based on the effect size of a prior study [7]. Hence, this study aimed to recruit a minimum of 14 participants per group and a maximum of 16 participants per group.
2.3. Participants
Participants were recruited from the public using electronic circulars through social media platforms such as LinkedIn and Facebook, as well as text messaging platforms such as WhatsApp and Telegram. Participants are proficient in spoken and written English and are able to perform a single-leg stand for at least 10 s. Adults who are healthy, recovered from an ankle sprain, or with chronic ankle instability were included. Healthy participants have no previous history of lower limb surgery or fracture, and no history of ankle sprain. Recovered individuals did not report any difficulty in the Foot and Ankle Ability Measure—activities of daily living questionnaire. Chronic ankle instability participants with a history of at least two episodes of lateral ankle sprain on the same leg, who experienced two giving way episodes of the ankle within the past 6 months, and reported any slight difficulty in the Foot and Ankle Ability Measure—activities of daily living questionnaire, were included [13]. Participants were excluded if they were on long-term medication that may cause dizziness, reported symptoms of vertigo, known skin allergy, had previous neurological conditions such as stroke and cerebral palsy, or had recent lower limb fractures or surgeries in the past six months.
2.4. Electromyography
To prepare the participant’s skin for electrode placement, the areas at the back and lower limb were shaved to reduce noise signals, then cleaned with alcohol swabs to reduce impedance [14]. The electrode positions of gluteus medius, longissimus dorsi, tibialis anterior, fibularis longus, medial gastrocnemius, vastus lateralis, and gluteus maximus were based on established lower limb placement recommendations [15], and longissimus dorsi placement location (Figure 2) [16]. Despite the risk of cross-talk between the gluteus medius and maximus muscles, it is important to study them as their primary actions on hip movements differ [17].
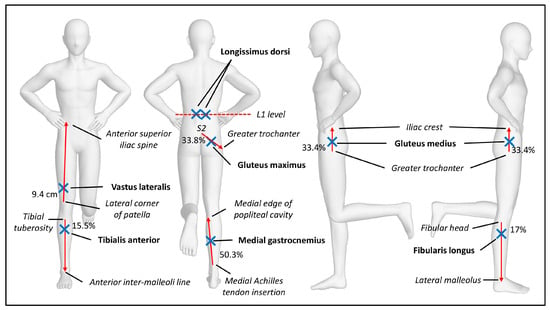
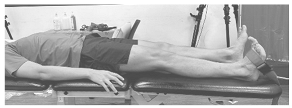
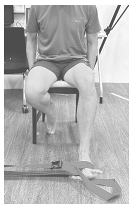
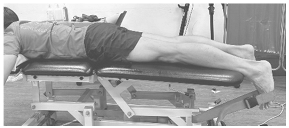
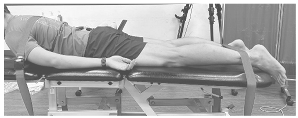
Figure 2.
Example of right single-leg stand with all electrode placements.
All electromyography (EMG) signals were recorded via the Delsys Trigno Avanti Sensor, a wireless surface EMG electrode, and EMGworks acquisition software 4.8.0 (Delsys Inc., Natick, MA, USA). EMG signals were sampled at a rate of 2148 Hz, a bandwidth of 20 to 450 Hz, and a baseline noise of 22 mV. The acquisition software was used to process raw EMG signals of maximal voluntary isometric contraction (MVIC) for single-leg stand testing.
2.5. Maximum Voluntary Isometric Contraction
MVIC of the gluteus medius, longissimus dorsi, tibialis anterior, medial gastrocnemius, fibularis longus, vastus lateralis, and gluteus maximus muscles were obtained bilaterally based on previous protocols [18]. Participants performed three trials of 5 s with a rest of 2 min between trials. To standardize the resistance applied, a mulligan belt was used during testing (Table 1). Standardized verbal encouragement, “Keep going!” was used for all participants.

Table 1.
Maximum voluntary isometric contraction testing protocol.
2.6. Experimental Procedures
Participants first performed a single-leg stand on a flat ground (stable surface) for 10 s on each leg without any shoes and socks. Participants were instructed to perform a single-leg stand with the contralateral hip in neutral and the knee flexed to 90°, measured with a handheld goniometer for standardization [6], and placed both hands on their hips to avoid any arm movements (Figure 2). Participants practiced the single-leg stand once on each side. Participants then performed all single-leg stand tests on the stable surface using the right leg before switching to the left leg. Following that, the same sequence was repeated on an Airex Foam to simulate an unstable surface. The participants sat and rested for a minute between each test.
2.7. Data Management
Normalization of EMG results with MVIC was necessary to allow for interpretation of results, as there were various factors, such as muscle fiber or diameter, which cannot be standardized between individuals [19], and recorded as %MVIC using the root mean square method with a 125 ms moving window. EMG readings from all three trials were analyzed, and the mean %MVIC of each trial was obtained, taking into account the 10 s long trial. For the reliability of data, intraclass correlation coefficients (ICCs) were used to obtain the most reliable mean %MVIC of each muscle across the different trials. It is important to establish trial-to-trial reliability to reduce the risk of type I error [20]. A 95% confidence interval ICC was used to determine the reliability of the results obtained from the different trials. In this study, we employed standardized procedures by using the two most reliable trials. We used a conservative ICC cut-off ≥0.5, which indicates moderate reliability [21].
The trial-to-trial reliability testing showed that for a stable surface, trials 1 and 2 were most reliable and so were used to calculate the mean muscle activities of healthy individuals’ dominant stance performance, while trials 2 and 3 were most reliable for non-dominant stance performance, and for unstable surface on both sides. Similarly, trials 2 and 3 were used to calculate the mean muscle activities in recovered individuals for stable surface single-leg stand performance because they were the most reliable, whereas trials 1 and 2 were most reliable for unstable surface single-leg stand performance. For participants with chronic ankle instability, the average of all trials for dominant and non-dominant stance on a stable surface was analyzed, and for dominant stance on an unstable surface, based on reliability testing, whereas for non-dominant stance and single-leg stand on an unstable surface, trials 2 and 3 were most reliable.
2.8. Statistical Analysis
All statistical analyses were performed using Jeffreys’s Amazing Statistics Program (JASP) version 0.19.3.0 for Windows. Continuous variables were presented in means and standard deviations, while categorical variables were presented in counts and percentages. To study the differences in muscle excitation between groups, the Bayesian independent t-test was used to test the alternative hypothesis [22]. In contrast to common null hypothesis testing, Bayesian analysis provides quantitative measures of evidence for both the alternative and null hypotheses. Bayes factor 10 (BF10) of 3 indicates that the data is 3 times more likely to favor the alternative hypothesis than the null hypothesis [23], which translates to a moderate level of evidence. Thus, in this study, only muscle activities that showed differences greater than or equal to a moderate level of evidence were interpreted. The 95% confidence interval (CI) was also used to interpret our study findings against the effect size of a recent study [9].
3. Results
The participants’ demographics are presented in Table 2. In the recovered group, all participants recovered naturally without seeking treatment. There was a higher proportion of female participants in the chronic ankle instability group as compared to the healthy and recovered groups. The tabulated between-group comparisons can be found in Appendix A.

Table 2.
Demographics of study participants (N = 28).
3.1. Healthy vs. Chronic Ankle Instability
On a stable surface, the chronic ankle instability group exhibited larger activation in the longissimus dorsi, vastus lateralis, and tibialis anterior muscles in dominant-leg stance (all BF10 > 3), and the most significant difference evident in the tibialis anterior in non-dominant stance (BF10 = 36.36) (Table A1). On unstable surface, the chronic ankle instability group exhibited larger activation in the vastus lateralis, tibialis anterior, and medial gastrocnemius muscles in dominant-leg stance (all BF10 > 3), and the only significant difference was evident in the tibialis anterior in non-dominant stance (BF10 = 8.22) (Table A2).
3.2. Recovered vs. Chronic Ankle Instability
On a stable surface, there was no difference between groups for muscle activation in dominant-leg stance, and the most significant difference was evident in the tibialis anterior in non-dominant stance (BF10 = 13.91) (Table A3). On an unstable surface, the chronic ankle instability group exhibited larger activation in gluteus medius, tibialis anterior, and fibularis longus muscles (all BF10 > 3), and the only significant difference was evident in the tibialis anterior in non-dominant stance (BF10 = 4.48) (Table A4).
4. Discussion
This study investigated impairments in tibialis anterior and fibularis longus muscle activities in chronic ankle instability. Among the muscle activities studied, the tibialis anterior muscle was consistently found to be hyperactive among individuals with chronic ankle instability. As compared to recovered individuals, individuals with chronic ankle instability exhibited hyperactivity in the gluteus medius and fibularis longus muscles during dominant-leg stance. Our findings partially support the hypothesis of tibialis anterior and fibularis longus muscle impairments using the DMA Clinical Pilates method.
From a biomechanics perspective, lateral ankle sprains commonly occur with excessive ankle plantarflexion and inversion, which stretches out and strains the tibialis anterior and fibularis longus muscles. The injury to these muscles could result in altered muscle activity. The increase in muscle activity in the fibularis longus muscle could be a compensatory strategy to protect against lateral ankle ligamentous instability [24], which would then antagonize the tibialis anterior muscle to increase in activation for co-contraction [25]. In our study findings, it is consistently observed that the tibialis anterior muscle activity is higher than the fibularis longus muscle among individuals with chronic ankle instability. This phenomenon was found to be related to an increase in lateral plantar pressure in patients with recurrent lateral ankle sprain [26]. Such changes in muscle activities among individuals with chronic ankle instability relate to dysfunctional proprioception in the ankle joint [27]. Our study findings on tibialis anterior hyperactivity contrasted with the study by Terada and colleagues (2022), which found diminished tibialis anterior activation because of differences in the single-leg stand protocol and EMG normalization protocol. Furthermore, their study population was predominantly female as compared to our study population. Future rehabilitation strategy for individuals with chronic ankle instability could focus on interventions that would modulate the tibialis anterior and fibularis longus muscle activity.
Higher muscle excitation is known to indicate stronger muscle contraction to generate more force. Our study findings align with a study investigating female athletes with chronic ankle instability [7], which suggests that weight-bearing exercise on the side of chronic ankle instability may be harmful and can be studied in the future. A recent small sample study suggests that individuals with chronic ankle instability showed impaired transversus abdominis contraction during single-leg pelvic bridging exercise on a stable surface [28], which could indicate issues in trunk motor control. Our finding of altered gluteus medius muscle activity supports the hip-stabilization strategies hypothesis in chronic ankle instability, as theorized by McCann and colleagues (2021). This is because lumbopelvic stability can influence peripheral movement control [29]. The trunk position of exercise for the tibialis anterior muscle matters, particularly for individuals who prefer flexion, may benefit from performing the exercise in long-sitting, while those who prefer extension will benefit from performing the exercise in supine.
There are several limitations in this study. First, there is a risk of cross-talk for the gluteus medius and gluteus maximus data collection because of their electrode placement proximity and anatomical positions. Hence, the findings for gluteus medius and maximus in the present study should be carefully interpreted. Second, we did not consider whether the participants with chronic ankle instability had received past or ongoing treatment for their ankle instability. Individuals who had received past or ongoing treatment might have a different motor recruitment pattern. Nonetheless, our findings, particularly the hyperexcitability of the tibialis anterior muscle, were aligned with the current literature. Future studies could explore how weight-bearing and non-weight-bearing exercises affect recovery among individuals with chronic ankle instability. Third, the male and female populations can differ in muscle recruitment patterns to maintain postural stability because of differences in pelvic girdle girths [30]. In our study, there were more male participants in the healthy and recovered groups, whereas there were more female participants in the chronic ankle instability group. Fourth, there might be a risk of recruitment bias as the schedule available to conduct the experiment was limited, so some participants who wanted to participate in the study could not be enrolled. Lastly, the sample size is relatively small and thus provides preliminary evidence on muscle activity dysfunction.
5. Conclusions
Individuals with chronic ankle instability exhibited a high level of tibialis anterior muscle activation during the single-leg stand as compared to healthy and recovered individuals. In contrast, fibularis longus muscle activation was only higher among individuals with chronic ankle instability when compared to recovered individuals. Therefore, tibialis anterior muscle hyperexcitation is a primary concern among individuals with chronic ankle instability.
Author Contributions
Conceptualization, All authors; methodology, All authors; software, Y.K.C. and J.R.C.A.; validation, B.C.K.; formal analysis, Y.K.C., J.R.C.A. and B.C.K.; investigation, Y.K.C., J.R.C.A. and B.C.K.; resources, Y.K.C. and J.R.C.A.; data curation, Y.K.C., J.R.C.A. and B.C.K.; writing—original draft preparation, Y.K.C. and J.R.C.A.; writing—review and editing, J.K.H.W. and B.C.K.; visualization, Y.K.C. and B.C.K.; supervision, B.C.K.; project administration, Y.K.C., J.R.C.A. and B.C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of The Singapore Institute of Technology (approval code: RECAS-0267, date of approval: 21 March 2024). The study is prospectively registered with the Australian New Zealand Clinical Trial registry (registration code: ACTRN12624000481572, date of registration: 19 April 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data from the study can be requested through the corresponding author within a reasonable request.
Acknowledgments
The authors thank Seah Jian Xing for his assistance in the use of electromyography.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DMA | Dance Medicine Australia |
| EMG | Electromyography |
| MVIC | Maximum voluntary isometric contraction |
| BF10 | Bayes factor 10 |
Appendix A

Table A1.
Bayesian t-test comparison of healthy (n = 16) against CAI (n = 14) of single-leg stance on stable surface.
Table A1.
Bayesian t-test comparison of healthy (n = 16) against CAI (n = 14) of single-leg stance on stable surface.
| Pose-Muscles | %MVIC, Mean (SD) | BF10 | Median | 95% CI | |
|---|---|---|---|---|---|
| Healthy | CAI | ||||
| Dom stance: | |||||
| Gmed | 9.55 (4.41) | 12.99 (10.65) | 0.58 | 0.32 | −0.30 to 1.00 |
| Gmed (non-dom) | 28.85 (19.73) | 30.38 (18.52) | 0.35 | 0.06 | −0.56 to 0.69 |
| LD | 10.54 (5.82) | 32.18 (26.30) | 11.86 ** | 0.98 | 0.23 to 1.79 |
| LD (non-dom) | 61.41 (67.47) | 71.52 (79.44) | 0.36 | 0.10 | −0.51 to 0.74 |
| Gluteus maximus | 29.28 (27.64) | 26.18 (14.04) | 0.39 | −0.12 | −0.79 to 0.51 |
| Vastus lateralis | 25.75 (10.88) | 36.79 (13.27) | 3.24 * | 0.73 | 0.04 to 1.50 |
| Tibialis anterior | 18.56 (15.98) | 34.72 (17.60) | 4.06 * | 0.78 | 0.08 to 1.55 |
| GasM | 42.35 (39.56) | 58.17 (46.58) | 0.51 | 0.27 | −0.34 to 0.94 |
| Fibularis longus | 46.67 (19.94) | 55.78 (30.29) | 0.50 | 0.26 | −0.35 to 0.93 |
| Non-dom stance: | |||||
| Gmed (dom) | 6.82 (5.30) | 6.64 (3.32) | 0.35 | −0.03 | −0.65 to 0.59 |
| Gmed | 30.60 (22.65) | 34.56 (24.48) | 0.37 | 0.12 | −0.49 to 0.76 |
| LD (dom) | 14.26 (41.29) | 4.92 (6.33) | 0.45 | −0.22 | −0.88 to 0.39 |
| LD | 21.03 (22.33) | 30.53 (40.06) | 0.44 | 0.22 | −0.39 to 0.88 |
| Gluteus maximus | 21.50 (31.44) | 19.51 (25.38) | 0.35 | −0.05 | −0.68 to 0.57 |
| Vastus lateralis | 34.32 (14.21) | 39.92 (16.90) | 0.50 | 0.26 | −0.35 to 0.93 |
| Tibialis anterior | 28.85 (12.22) | 58.36 (28.70) | 36.36 *** | 1.18 | 0.38 to 2.01 |
| GasM | 36.84 (25.20) | 64.15 (93.74) | 0.55 | 0.30 | −0.31 to 0.98 |
| Fibularis longus | 47.02 (25.15) | 46.08 (22.55) | 0.35 | −0.03 | −0.65 to 0.59 |
CAI: Chronic ankle instability; Dom: Dominant leg; Non-dom: Non-dominant leg; Gmed: Gluteus medius; LD: Longissimus dorsi; GasM: Medial gastrocnemius. * Moderate evidence; ** Strong evidence; *** Very strong evidence.

Table A2.
Bayesian t-test comparison of healthy (n = 16) against CAI (n = 14) of single-leg stance on unstable surface.
Table A2.
Bayesian t-test comparison of healthy (n = 16) against CAI (n = 14) of single-leg stance on unstable surface.
| Pose-Muscles | %MVIC, Mean (SD) | BF10 | Median | 95% CI | |
|---|---|---|---|---|---|
| Healthy | CAI | ||||
| Dom stance: | |||||
| Gmed | 10.63 (5.09) | 14.47 (10.78) | 0.64 | 0.35 | −0.27 to 1.04 |
| Gmed (non-dom) | 32.28 (22.57) | 32.72 (17.91) | 0.35 | 0.02 | −0.60 to 0.64 |
| LD | 18.59 (21.15) | 35.43 (36.02) | 0.88 | 0.44 | −0.19 to 1.15 |
| LD (non-dom) | 71.49 (97.28) | 83.97 (114.78) | 0.36 | 0.09 | −0.53 to 0.72 |
| Gluteus maximus | 24.43 (24.47) | 29.25 (19.20) | 0.39 | 0.16 | −0.45 to 0.80 |
| Vastus lateralis | 23.32 (12.41) | 35.51 (13.20) | 3.85 * | 0.77 | 0.07 to 1.54 |
| Tibialis anterior | 26.23 (16.18) | 61.30 (42.47) | 8.93 * | 0.93 | 0.19 to 1.73 |
| GasM | 43.64 (25.29) | 84.71 (59.36) | 3.35 * | 0.74 | 0.05 to 1.51 |
| Fibularis longus | 52.55 (24.79) | 66.01 (36.17) | 0.59 | 0.33 | −0.29 to 1.01 |
| Non-dom stance: | |||||
| Gmed (dom) | 6.83 (4.89) | 6.50 (3.55) | 0.35 | −0.06 | −0.68 to 0.56 |
| Gmed | 34.44 (23.06) | 33.67 (18.26) | 0.35 | −0.03 | −0.65 to 0.59 |
| LD (dom) | 4.53 (9.80) | 7.21 (10.41) | 0.42 | 0.19 | −0.42 to 0.85 |
| LD | 20.24 (32.51) | 27.83 (53.42) | 0.38 | 0.13 | −0.48 to 0.77 |
| Gluteus maximus | 14.30 (12.14) | 28.07 (34.77) | 0.79 | 0.41 | −0.22 to 1.11 |
| Vastus lateralis | 36.35 (16.94) | 43.14 (17.66) | 0.53 | 0.29 | −0.32 to 0.97 |
| Tibialis anterior | 37.86 (17.75) | 73.38 (43.11) | 8.22 * | 0.92 | 0.18 to 1.71 |
| GasM | 35.69 (20.00) | 70.72 (66.66) | 1.50 | 0.57 | −0.09 to 1.31 |
| Fibularis longus | 59.81 (42.36) | 56.36 (22.61) | 0.36 | −0.07 | −0.70 to 0.54 |
CAI: Chronic ankle instability; Dom: Dominant leg; Non-dom: Non-dominant leg; Gmed: Gluteus medius; LD: Longissimus dorsi; GasM: Medial gastrocnemius. * Moderate evidence.

Table A3.
Bayesian t-test comparison of recovered (n = 15) against CAI (n = 14) of single-leg stance on stable surface.
Table A3.
Bayesian t-test comparison of recovered (n = 15) against CAI (n = 14) of single-leg stance on stable surface.
| Pose-Muscles | %MVIC, Mean (SD) | BF10 | Median | 95% CI | |
|---|---|---|---|---|---|
| Recovered | CAI | ||||
| Dom stance: | |||||
| Gmed | 6.29 (3.22) | 12.99 (10.65) | 2.43 | 0.68 | −0.01 to 1.45 |
| Gmed (non-dom) | 36.47 (16.35) | 30.38 (18.52) | 0.49 | −0.25 | −0.93 to 0.36 |
| LD | 27.70 (28.17) | 32.18 (26.30) | 0.38 | 0.12 | −0.50 to 0.77 |
| LD (non-dom) | 87.85 (133.78) | 71.52 (79.44) | 0.37 | −0.11 | −0.75 to 0.51 |
| Gluteus maximus | 34.09 (21.27) | 26.18 (14.04) | 0.58 | −0.32 | −1.01 to 0.30 |
| Vastus lateralis | 41.90 (50.10) | 36.79 (13.27) | 0.37 | −0.10 | −0.74 to 0.52 |
| Tibialis anterior | 26.15 (31.30) | 34.72 (17.60) | 0.47 | 0.24 | −0.38 to 0.92 |
| GasM | 46.01 (38.10) | 58.17 (46.58) | 0.44 | 0.21 | −0.41 to 0.87 |
| Fibularis longus | 37.33 (12.37) | 55.78 (30.29) | 1.92 | 0.63 | −0.05 to 1.39 |
| Non-dom stance: | |||||
| Gmed (dom) | 8.98 (17.35) | 6.64 (3.32) | 0.38 | −0.13 | −0.78 to 0.49 |
| Gmed | 34.29 (15.47) | 34.56 (24.48) | 0.35 | 0.01 | −0.62 to 0.64 |
| LD (dom) | 2.75 (3.79) | 4.92 (6.33) | 0.56 | 0.31 | −0.32 to 1.00 |
| LD | 65.91 (118.68) | 30.53 (40.06) | 0.53 | −0.29 | −0.97 to 0.33 |
| Gluteus maximus | 21.90 (27.54) | 19.51 (25.38) | 0.36 | −0.06 | −0.70 to 0.56 |
| Vastus lateralis | 40.40 (10.97) | 39.92 (16.90) | 0.35 | −0.02 | −0.66 to 0.60 |
| Tibialis anterior | 29.11 (18.21) | 58.36 (28.70) | 13.91 * | 1.03 | 0.25 to 1.85 |
| GasM | 36.86 (21.47) | 64.15 (93.74) | 0.55 | 0.30 | −0.32 to 0.99 |
| Fibularis longus | 36.66 (22.91) | 46.08 (22.55) | 0.56 | 0.30 | −0.32 to 0.99 |
CAI: Chronic ankle instability; Dom: Dominant leg; Non-dom: Non-dominant leg; Gmed: Gluteus medius; LD: Longissimus dorsi; GasM: Medial gastrocnemius. * Strong evidence.

Table A4.
Bayesian t-test comparison of recovered (n = 15) against CAI (n = 14) of single-leg stance on unstable surface.
Table A4.
Bayesian t-test comparison of recovered (n = 15) against CAI (n = 14) of single-leg stance on unstable surface.
| Pose-Muscles | %MVIC, Mean (SD) | BF10 | Median | 95% CI | |
|---|---|---|---|---|---|
| Recovered | CAI | ||||
| Dom stance: | |||||
| Gmed | 6.40 (3.14) | 14.47 (10.78) | 5.20 * | 0.84 | 0.11 to 1.64 |
| Gmed (non-dom) | 34.82 (16.23) | 32.72 (17.91) | 0.36 | −0.09 | −0.73 to 0.53 |
| LD | 42.44 (63.22) | 35.43 (36.02) | 0.37 | −0.10 | −0.74 to 0.52 |
| LD (non-dom) | 98.27 (125.00) | 83.97 (114.78) | 0.36 | −0.09 | −0.73 to 0.54 |
| Gluteus maximus | 35.72 (18.73) | 29.25 (19.20) | 0.48 | −0.25 | −0.92 to 0.37 |
| Vastus lateralis | 26.46 (12.19) | 35.51 (13.20) | 1.34 | 0.55 | −0.11 to 1.29 |
| Tibialis anterior | 30.09 (18.59) | 61.30 (42.47) | 3.76 * | 0.77 | 0.06 to 1.56 |
| GasM | 57.99 (33.60) | 84.71 (59.36) | 0.81 | 0.42 | −0.22 to 1.13 |
| Fibularis longus | 39.97 (13.96) | 66.01 (36.17) | 3.74 * | 0.77 | 0.06 to 1.56 |
| Non-dom stance: | |||||
| Gmed (dom) | 10.66 (19.24) | 6.50 (3.55) | 0.44 | −0.21 | −0.88 to 0.40 |
| Gmed | 34.93 (15.83) | 33.67 (18.26) | 0.35 | 0.05 | −0.69 to 0.57 |
| LD (dom) | 5.47 (8.80) | 7.21 (10.41) | 0.38 | 0.13 | −0.49 to 0.78 |
| LD | 56.45 (79.20) | 27.83 (53.42) | 0.56 | −0.31 | −1.00 to 0.31 |
| Gluteus maximus | 18.95 (19.55) | 28.07 (34.77) | 0.47 | 0.24 | −0.38 to 0.91 |
| Vastus lateralis | 41.18 (9.67) | 43.14 (17.66) | 0.37 | 0.10 | −0.52 to 0.74 |
| Tibialis anterior | 37.24 (27.94) | 73.38 (43.11) | 4.48 * | 0.81 | 0.09 to 1.60 |
| GasM | 52.95 (30.48) | 70.72 (66.66) | 0.48 | 0.25 | −0.37 to 0.93 |
| Fibularis longus | 46.43 (30.65) | 56.36 (22.61) | 0.50 | 0.27 | −0.35 to 0.95 |
CAI: Chronic ankle instability; Dom: Dominant leg; Non-dom: Non-dominant leg; Gmed: Gluteus medius; LD: Longissimus dorsi; GasM: Medial gastrocnemius. * Moderate evidence.
References
- Kwok, B.C.; Chin, C.S.Y.; Wong, J.K.H.; Chan, M.A.W.K. Physiotherapy is least preferred for managing musculoskeletal pain—Findings from a pain prevalence survey. Musculoskelet. Care 2025, 23, e70090. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-M.; Needle, A.R.; Kim, J.-S.; An, Y.W.; Cruz-Díaz, D.; Taube, W. What interventions can treat arthrogenic muscle inhibition in patients with chronic ankle instability? A systematic review with meta-analysis. Disabil. Rehabil. 2024, 46, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gollhofer, A.; Lohrer, H.; Dorn-Lange, N.; Bonsignore, G.; Gehring, D. Function of ankle ligaments for subtalar and talocrural joint stability during an inversion movement—An in vitro study. J. Foot Ankle Res. 2019, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, M.; Anderson, D.D.; Wilken, J.M. Current challenges in chronic ankle instability: Review and perspective. Foot Ankle Clin. 2023, 28, 129–143. [Google Scholar] [CrossRef]
- Ghislieri, M.; Labanca, L.; Mosca, M.; Bragonzoni, L.; Knaflitz, M.; Benedetti, M.G.; Agostini, V. Balance and muscle synergies during a single-limb stance task in individuals with chronic ankle instability. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 4367–4375. [Google Scholar] [CrossRef]
- Han, S.; Oh, M.; Lee, H.; Hopkins, J.T. Emg analysis during static balance in chronic ankle instability, coper and controls. Int. J. Sports Med. 2024, 45, 48–54. [Google Scholar] [CrossRef]
- Karbalaeimahdi, M.; Alizadeh, M.H.; Minoonejad, H.; Behm, D.G.; Alizadeh, S. Higher leg and trunk muscle activation during balance control in copers versus people with chronic ankle instability and healthy female athletes. Sports 2022, 10, 111. [Google Scholar] [CrossRef]
- Toyooka, T.; Urabe, Y.; Sugiura, S.; Takata, A.; Shinozaki, M.; Takata, Y.; Ishizaki, T.; Nakamura, K.; Otsuki, K.; Oyama, T. Does the single-limb stance reflect chronic ankle instability in an athlete? Gait Posture 2018, 66, 242–246. [Google Scholar] [CrossRef]
- Terada, M.; Kosik, K.B.; McCann, R.S.; Drinkard, C.; Gribble, P.A. Corticospinal activity during a single-leg stance in people with chronic ankle instability. J. Sport. Health Sci. 2022, 11, 58–66. [Google Scholar] [CrossRef]
- Kwok, B.C.; Soh, R.E.C.; Smith, H.E.; Kong, P.W. Clinical pilates exercises for adults with chronic low back pain improves single-leg squat postural control and lumbopelvic-hip flexibility. Gait Posture 2025, 119, 127–134. [Google Scholar] [CrossRef]
- Tulloch, E.; Phillips, C.; Sole, G.; Carman, A.; Abbott, J.H. Dma clinical pilates directional-bias assessment: Reliability and predictive validity. J. Orthop. Sports Phys. Ther. 2012, 42, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Kwok, B.C.; Lim, J.X.L.; Kong, P.W. The theoretical framework of the clinical pilates exercise method in managing non-specific chronic low back pain: A narrative review. Biology 2021, 10, 1096. [Google Scholar] [CrossRef] [PubMed]
- Hoch, J.M.; Hartzell, J.; Kosik, K.B.; Cramer, R.J.; Gribble, P.A.; Hoch, M.C. Continued validation and known groups validity of the quick-faam: Inclusion of participants with chronic ankle instability and ankle sprain copers. Phys. Ther. Sport. 2020, 43, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for semg sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Rainoldi, A.; Melchiorri, G.; Caruso, I. A method for positioning electrodes during surface emg recordings in lower limb muscles. J. Neurosci. Methods 2004, 134, 37–43. [Google Scholar] [CrossRef]
- De Nooij, R.; Kallenberg, L.A.; Hermens, H.J. Evaluating the effect of electrode location on surface emg amplitude of the m. Erector spinae p. Longissimus dorsi. J. Electromyogr. Kinesiol. 2009, 19, e257–e266. [Google Scholar] [CrossRef]
- Nascimento, M.B.; Vilarinho, L.G.; Lobato, D.F.M.; Dionisio, V.C. Role of gluteus maximus and medius activation in the lower limb biomechanical control during functional single-leg tasks: A systematic review. Knee 2023, 43, 163–175. [Google Scholar] [CrossRef]
- Ekstrom, R.A.; Osborn, R.W.; Hauer, P.L. Surface electromyographic analysis of the low back muscles during rehabilitation exercises. J. Orthop. Sports Phys. Ther. 2008, 38, 736–745. [Google Scholar] [CrossRef]
- Norcross, M.F.; Blackburn, J.T.; Goerger, B.M. Reliability and interpretation of single leg stance and maximum voluntary isometric contraction methods of electromyography normalization. J. Electromyogr. Kinesiol. 2010, 20, 420–425. [Google Scholar] [CrossRef]
- Kwok, B.C.; Kong, P.W. Single-leg squat postural sway reliability: How many trials to analyze for chronic low back pain? J. Mech. Med. Biol. 2025, 25, 2450026. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Kelter, R. Analysis of bayesian posterior significance and effect size indices for the two-sample t-test to support reproducible medical research. BMC Med. Res. Methodol. 2020, 20, 88. [Google Scholar] [CrossRef]
- Van Doorn, J.; Van Den Bergh, D.; Böhm, U.; Dablander, F.; Derks, K.; Draws, T.; Etz, A.; Evans, N.J.; Gronau, Q.F.; Haaf, J.M. The jasp guidelines for conducting and reporting a bayesian analysis. Psychon. Bull. Rev. 2021, 28, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Montecinos, C.; Sanzana-Cuche, R.; Mendez-Rebolledo, G. Regional muscle fiber conduction velocity of the fibularis longus in individuals with chronic ankle instability. J. Anat. 2025, 247, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Z.; Lin, Y.-A.; Tai, W.-H.; Chen, C.-Y. Influence of landing in neuromuscular control and ground reaction force with ankle instability: A narrative review. Bioengineering 2022, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Mineta, S.; Inami, T.; Mariano, R.; Hirose, N. High lateral plantar pressure is related to an increased tibialis anterior/fibularis longus activity ratio in patients with recurrent lateral ankle sprain. Open Access J. Sports Med. 2017, 8, 123–131. [Google Scholar] [CrossRef]
- Xue, X.a.; Ma, T.; Li, Q.; Song, Y.; Hua, Y. Chronic ankle instability is associated with proprioception deficits: A systematic review and meta-analysis. J. Sport Health Sci. 2021, 10, 182–191. [Google Scholar] [CrossRef]
- McCann, R.S.; Johnson, K.; Suttmiller, A.M. Lumbopelvic stability and trunk muscle contractility of individuals with chronic ankle instability. Int. J. Sports Phys. Ther. 2021, 16, 741. [Google Scholar] [CrossRef]
- Fadaei Dehcheshmeh, P.; Gandomi, F.; Maffulli, N. Effect of lumbopelvic control on landing mechanics and lower extremity muscles’ activities in female professional athletes: Implications for injury prevention. BMC Sports Sci. Med. Rehabil. 2021, 13, 101. [Google Scholar]
- Dallinga, J.M.; van der Does, H.T.; Benjaminse, A.; Lemmink, K.A. Dynamic postural stability differences between male and female players with and without ankle sprain. Phys. Ther. Sport. 2016, 17, 69–75. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).