2. Materials and Methods
In a retrospective search of the clinical records of our institution from between 18 May 2015, and 2 December 2019, we found 11 patients who had been diagnosed with EDS in addition to NCVS. They represented 0.8% of 1285 patients seen during that period, with diagnoses of Arnold–Chiari syndrome type I, idiopathic syringomyelia and scoliosis, platybasia, basilar impression, kinking of the brainstem, low-lying conus medullaris, and related pathologies. After excluding 1 case with incomplete imaging, we present the clinical and imaging characteristics of 10 patients with EDS and NCVS, and we compare them with the 373 patients with NCVS included in a previous study on Filum disease (FD) and NCVS [
10].
The selection of patients for initial visits in our institution and their diagnostic management have been described previously. Likewise, the collection of data for the clinical manifestations (symptoms, signs, and imaging) followed similar guidelines, though there are some appreciable differences. This time, some new symptoms, such as photophobia, sonophobia, sensations of electrical current, and involuntary movements or fasciculations, are included in the study. More clinical signs have been recorded, which were previously ruled out due to their low frequency, such as anisocoria, inverted Lasègue test, Barré test, pain upon paravertebral pressure and in the lower extremities, scoliotic attitude, gait alteration, and quadriceps paresis test.
The assessment of the MRI and X-ray images of the entire spine was conducted according to the same scales as in the aforementioned publication.
Among the 10 selected patients, 6 patients underwent SFT; 2 of these 6 patients were excluded from the study for not undergoing postoperative follow-up. Thus, we have been able to analyse the results in 4 cases at several postoperative intervals. The postoperative outcome was assessed subjectively by having the patient rate the postoperative course of every symptom, as well as provide a global percentage of improvement, if any, and objectively by having the physician evaluate the comparative findings of the clinical signs pre- and post-SFT.
For data analysis, the general data, clinical symptoms, clinical signs, and imaging features of all patients were collected, according to a table designed in a digital format database (FileMaker Pro Advanced 11.0v2, FileMaker, Inc., Santa Clara, CA, USA), from which they were transferred to a Microsoft Excel 2011 spreadsheet for Mac version 14.1.0 (Microsoft Corporation, Redmond, WA, USA) and then to a database of SPSS version 21 data (IBM Corporation, Armonk, NY, USA). The statistical significance of the differences was verified with the t test for independent samples (p < 0.05).
Patient data were anonymised in accordance with actual legislation, following the technical and organisational guarantees and measures established in the EU data protection directive 2016/679 (article 89.1). The Institut Chiari & Siringomielia & Escoliosis de Barcelona holds Research & Development (R&D) certification 1583.001.16–160920-CER-RD.001 from the Spanish Innovation Certification Agency (ACIE) [
11] and ENAC certification 33/C- PR074 [
12], Certificate IQNet and AENOR Quality Management System ISO 9001:2015 [
13], Registration Number: ES-0081/2015 for the following fields of activities: Research, Diagnosis and Treatment of the Filum Disease and Quality Management Certification according to UNE-EN ISO 9001:2008 [
14] standards.
3. Results
In the group of 10 patients with NCVS-EDS, 3 separate categories are described, and each category is represented by the patients detailed below. Patients 1, 2, and 10 are patients of more advanced age who have undergone surgery. Patients 4, 5, 6, and 7 are middle-aged, and half of them have undergone surgery. Patients 3, 8, and 9 are the youngest, all with cervical hypermobility, and none have been operated on.
3.1. Patient 1
A 60-year-old female with a history of multiple surgical procedures reported parietal and frontal headache, nausea and vomiting, blurred vision, photophobia, scotoma, sonophobia, tinnitus, dizziness, mixed dysphagia, cognitive impairment (speech, concentration, and memory disturbances), mood swings (nervousness, irritability, apathy, and depression), and general tiredness. She also reported cervical pain radiating to the shoulders and upper extremities, numbness, paraesthesia, and a sensation of loss of strength in the upper extremities, upper back pain with blockage episodes, pain in the sternal region, dyspnoea, lower back pain, pain and lack of strength in the lower extremities, and impaired gait.
The physical examination detected a scoliotic attitude, pain with pressure applied to the cervical region and a bilaterally positive quadriceps paresis test, tactile hypoaesthesia in the head, upper extremities, and foot soles, decreased deep tendon reflexes in the upper extremities and mixed in the lower extremities, slow abdominal and plantar reflexes, and a positive Romberg test.
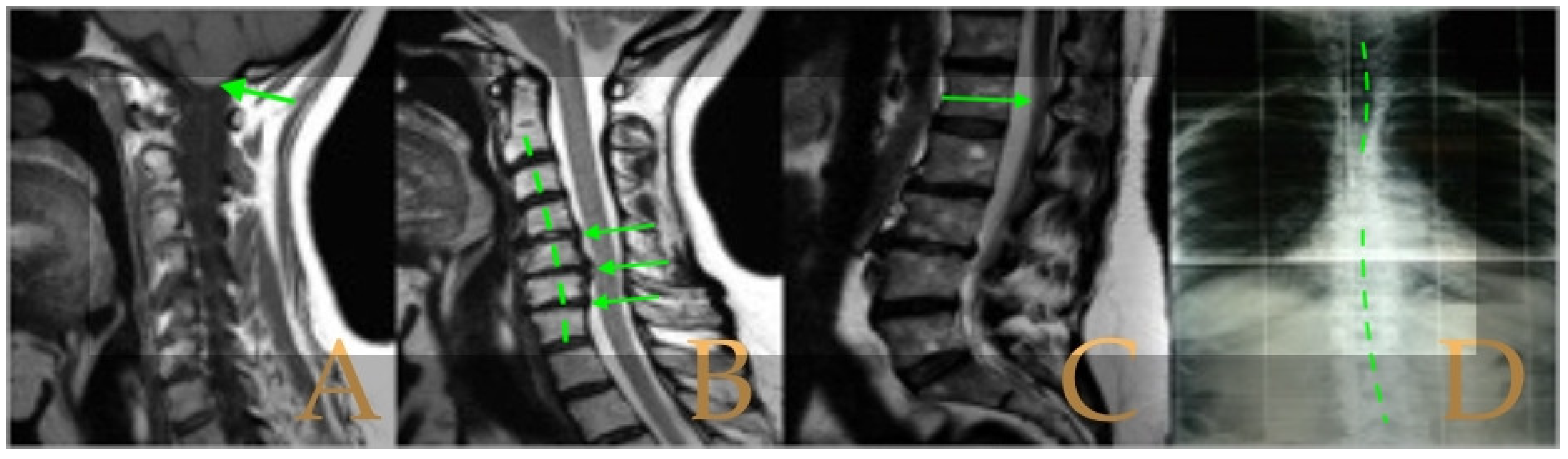
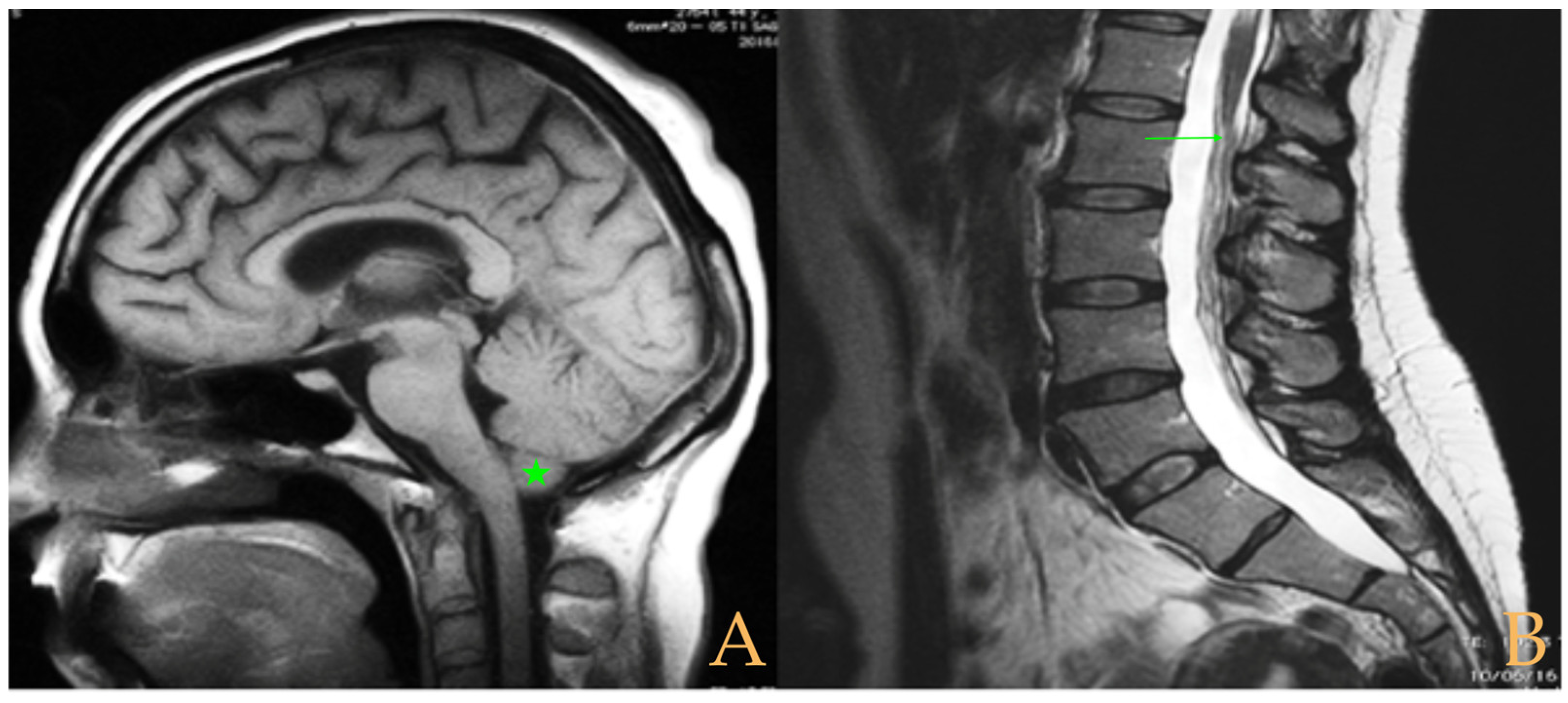
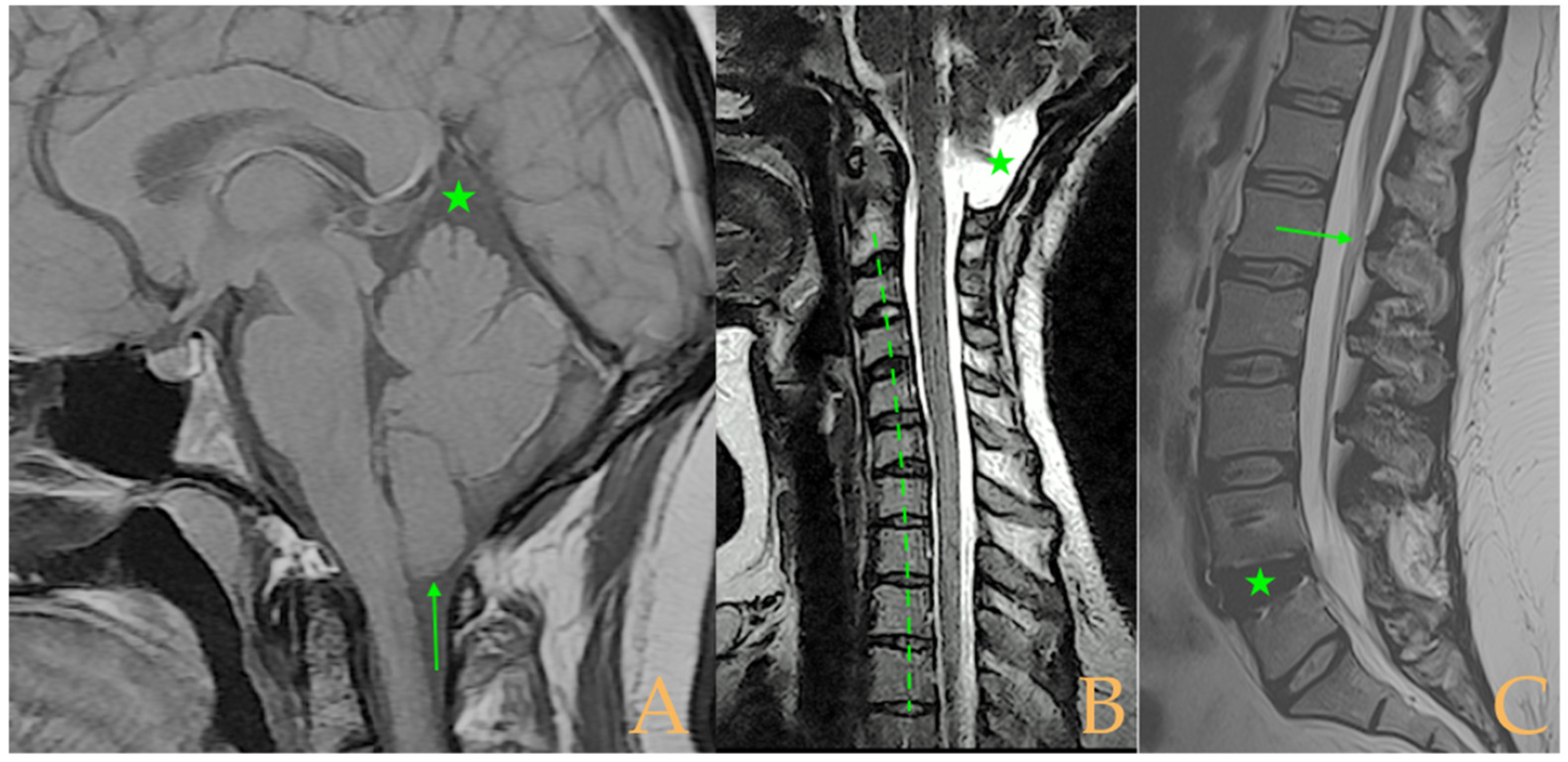
Magnetic resonance imaging showed a slight descent of the cerebellar tonsils, multiple diseased discs, and conus medullaris at the level of the middle third of the L1 vertebra. The X-ray images of the entire spine revealed a mild dextroconvex thoraco-lumbar scoliosis with 5° Cobb angle (
Figure 1).
Sectioning of the filum terminale and postoperative evaluation took place 52 days after SFT, ascertaining the improvement/disappearance of most symptoms, except the following: numbness, paraesthesias, sensation of loss of strength in the upper extremities, and lower back pain. On physical examination, cervicalgia and quadriceps paresis had disappeared, and tactile hypoaesthesia, Romberg test, and deep tendon reflexes had partially improved.
3.2. Patient 2
A 64-year-old female with a medical history of suboccipital craniectomy reported headaches, nausea, vomiting, dizziness, dysphagia, blurred and double vision, sonophobia, speech, memory and attention disturbances, depressive state, and onset of insomnia. She also reported cervicalgia radiating to the back, pain, numbness, tingling and paraesthesia in the upper extremities, dropping objects from her grip, upper and lower back pain, burning sensation, pain and paraesthesia in the lower extremities, urinary and faecal incontinence, loss of genital and anal sensation, constipation, and gait difficulties, with falls.
The physical examination revealed nystagmus, low left soft palate, and pain with pressure in the cervical and thoracic regions and lower extremities. Tactile and thermal hypoaesthesia and hyperaesthesia, altered deep tendon reflexes, absence of abdominal reflexes, flexor tendency in plantar reflexes, and gait difficulty were also identified.
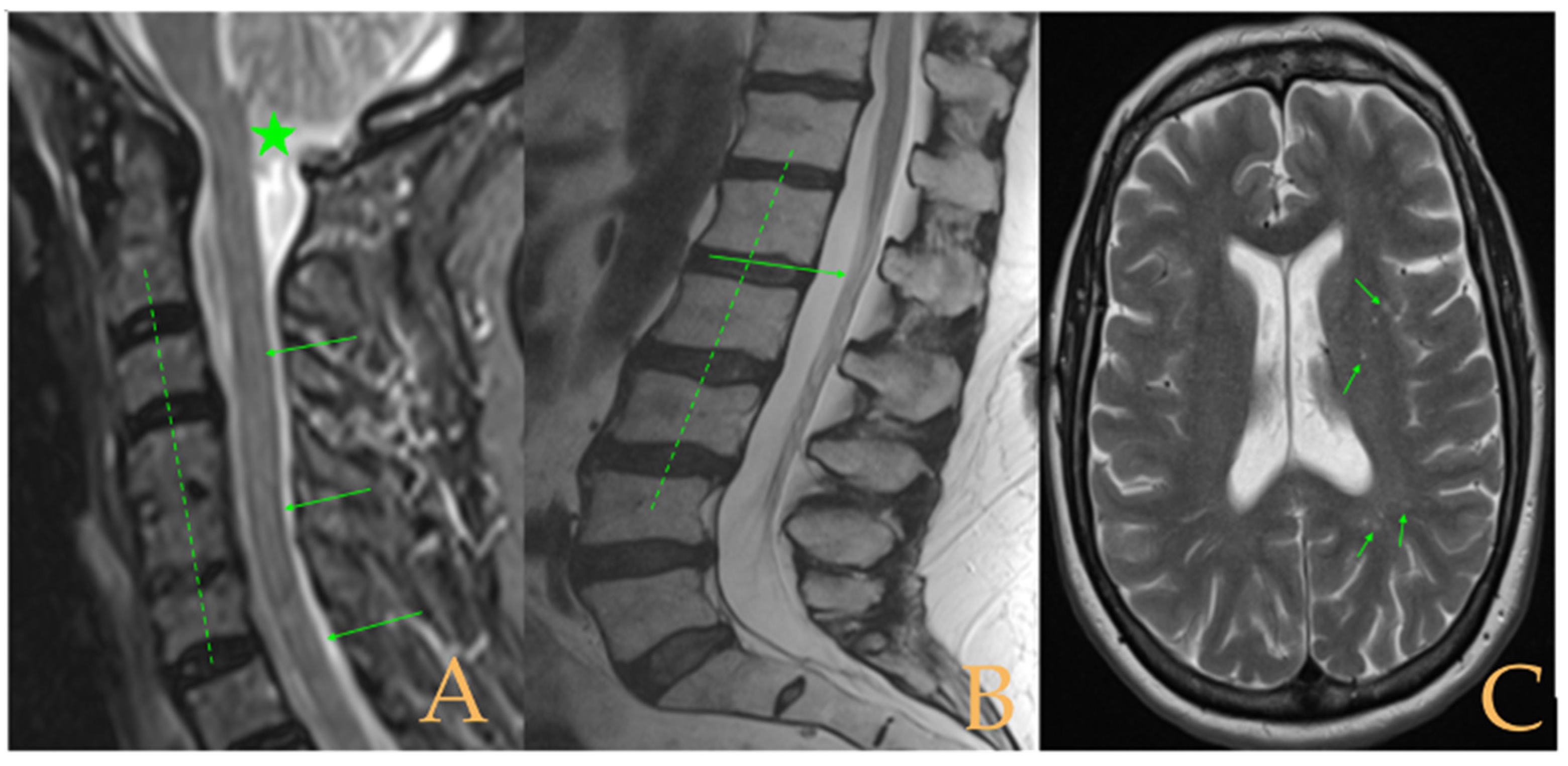
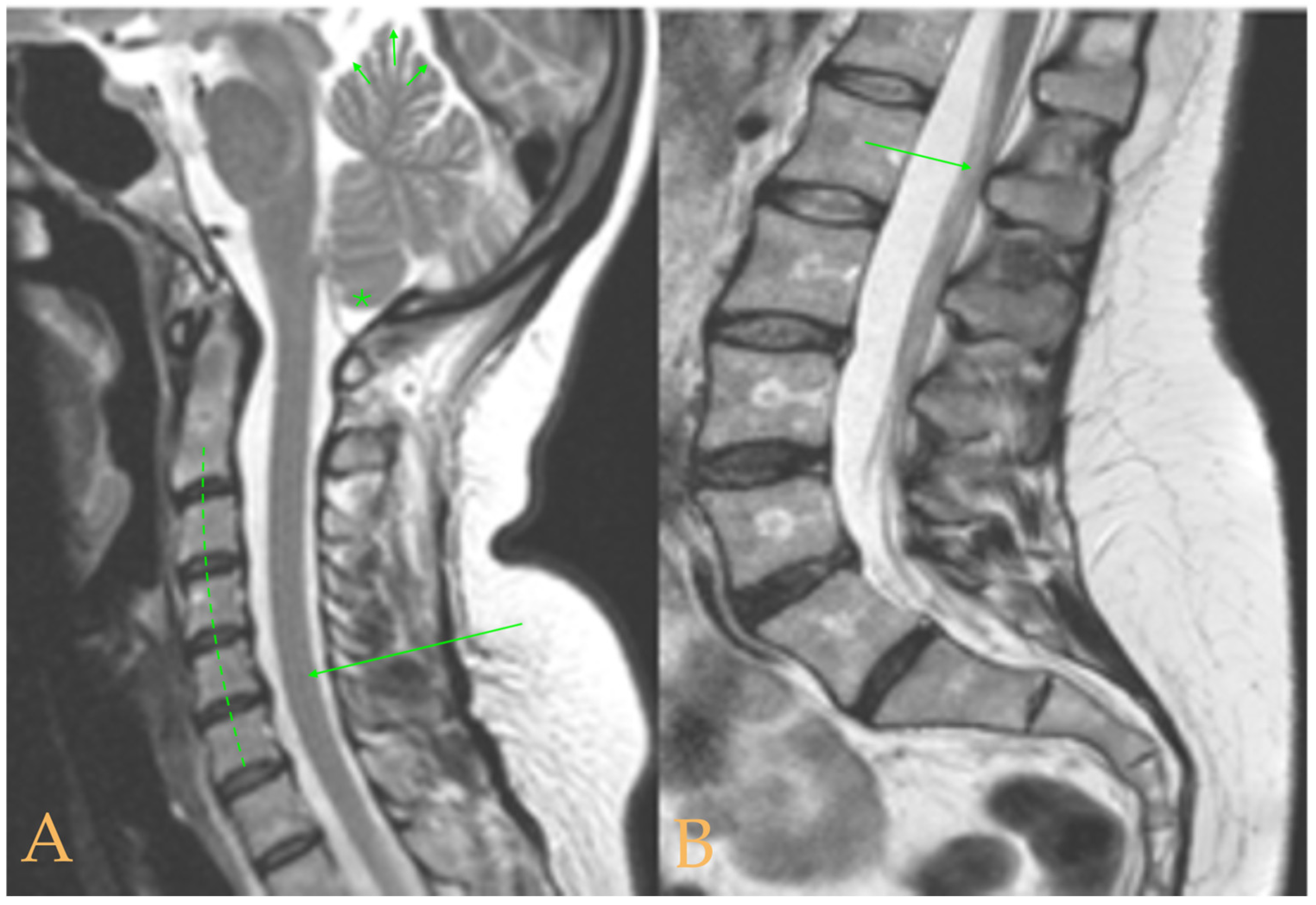
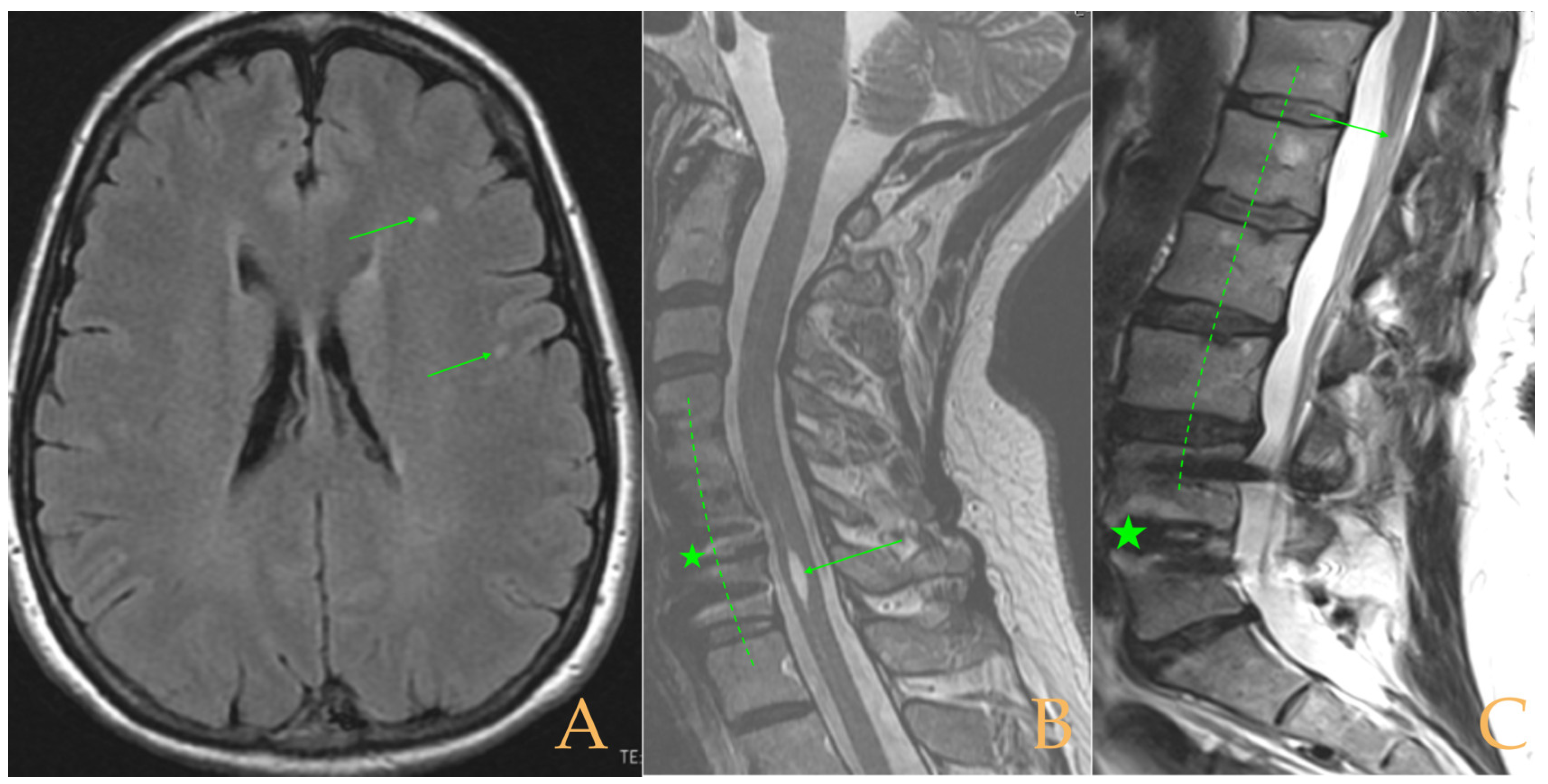
The MRI showed a descent of the cerebellar tonsils, increased supracerebral space, and micro lacunae in the bilateral white matter and the left basal ganglia. Upper cervical kyphosis and straightening in the lower levels. Surgical arthrodesis at C4-C5 and C5-C6. Cervical, thoracic and lumbar protrusions. Ischemia and oedema in the cervical and thoracic spinal cord. Slight cervical rotoscoliosis. Disc protrusions: T7-8, T11-L1, L2-L3-L4, and disc herniation L4-L5. Loss of the lumbar lordosis. Conus medullaris at the level of the middle third of the L1 vertebra (
Figure 2). The X-ray images of the entire spine showed levoconvex thoracolumbar scoliosis of 21°, dysmetria of the lower extremities with shortening of the right lower extremity by 10 mm, with respect to the level of the hip, and 99 mm at the level of the iliac crests, 16° cervical lordosis, 49° thoracic kyphosis, and 59° lumbar lordosis.
Sectioning of Filum Terminale, with Postoperative Evaluation 120 Days After Surgery
We observed improvement/disappearance of almost all mentioned symptoms, except nausea, mixed dysphagia, speech disturbance, insomnia, pain and paraesthesias in the lower extremities, urinary and faecal incontinence, loss of genital and anal sensitivity, and constipation. On physical examination, nystagmus, pain upon pressure on tender points (cervical and thoracic region, and lower legs), hypoaesthesia and hyperaesthesia to touch, and gait difficulty had disappeared. Tactile hypoaesthesia had partially improved. The other signs remained unchanged: soft palate, thermal hyperaesthesia, altered deep tendon, and abdominal and plantar reflexes.
3.3. Patient 3
A 35-year-old female presented with multiple medical-surgical antecedents, among which the diagnoses of craniocervical and atlantoaxial instability stood out, as well as fibromyalgia, chronic fatigue syndrome, and multiple chemical sensitivity. She complained of periocular and occipital headache, with relief in decubitus, paraesthesia, itching and tension in the face, absence seizures, nausea, vomiting, subjective vertigo, dysphagia, blurred vision with black dots, feeling of pressure in the ears and temporary hearing loss, tinnitus, cognitive impairment (impaired speech, memory, and attention), irritability, mixed insomnia, and possible apnoea. She also reported suffering from cervicalgia, paraesthesia and lack of strength in all extremities, lower back pain, urinary incontinence, diarrhoea, constipation, lack of ability to walk, global hyperhidrosis, cold body, and lack of sensation to temperature.
The objective examination detected descent of the left soft palate, decreased grip strength in the right hand, brisk deep tendon reflexes, absent left abdominal reflexes, slow plantar reflexes, extensive alterations in thermal and tactile sensitivity, positive Mingazzini and Barré tests, and impossible orthostatism and gait.
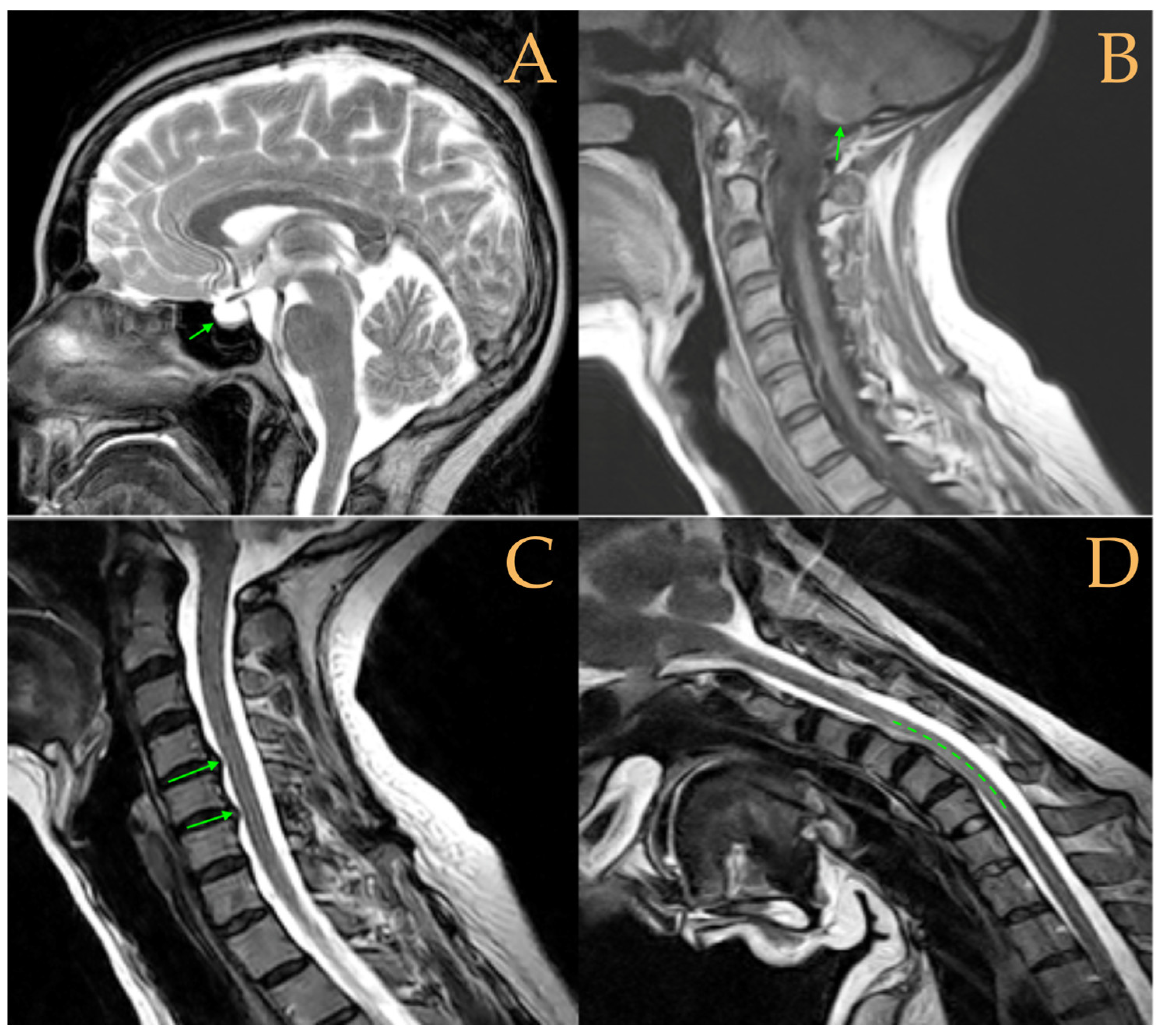
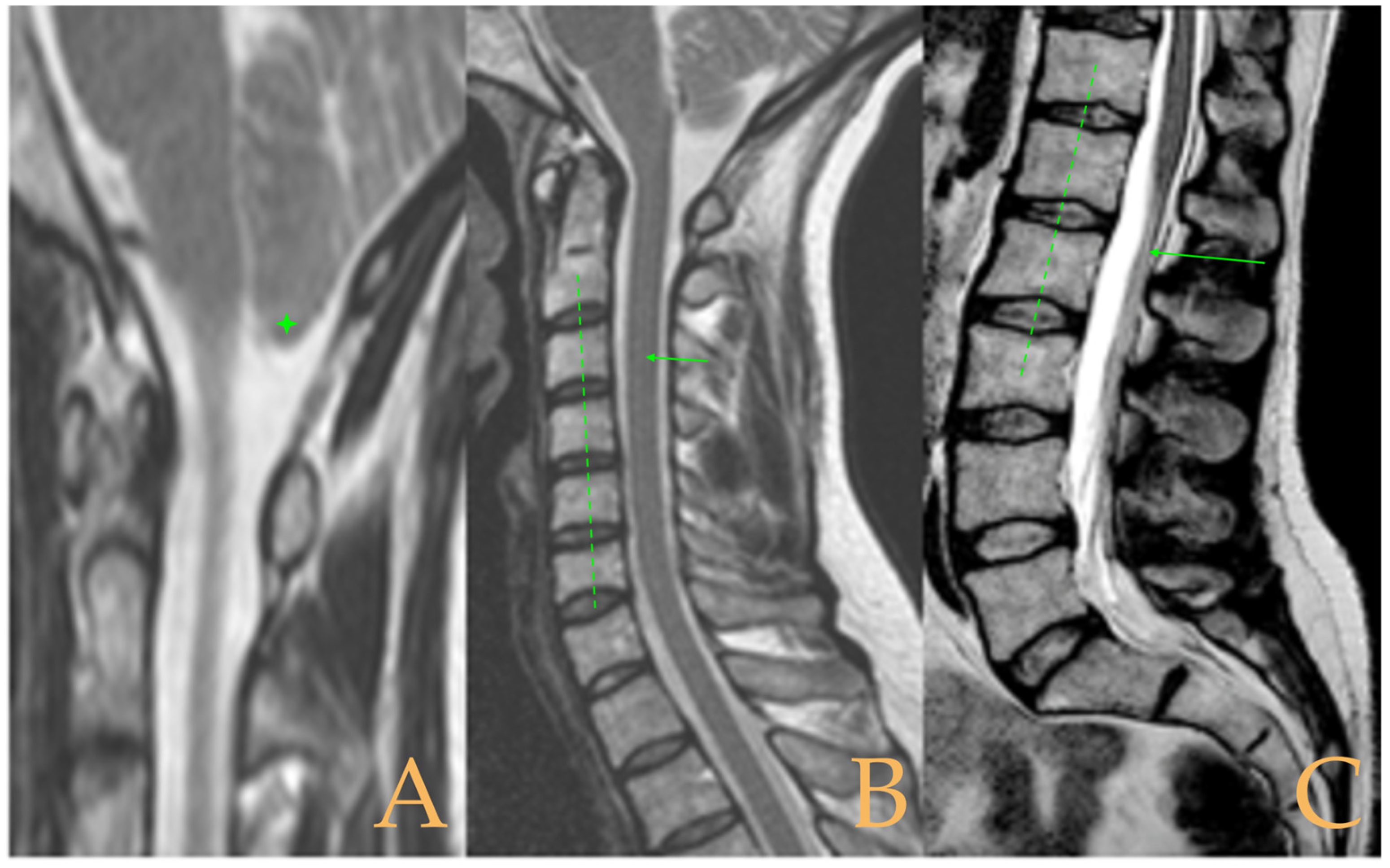
The cranio-vertebral magnetic resonance showed empty sella turcica, discrete descent of the cerebellar tonsils, C4-C5-C6 disc protrusions and cervical hypermobility with a tense medullary aspect in flexion in the incorporated dynamic test, and conus medullaris at the height of the TH12-L1 disc (
Figure 3). No scoliosis was observed in the full-spine X-rays. The patient was not operated on. Cases 8 and 9 were very similar and involved previously diagnosed cranio-cervical and atlantoaxial instability. The patient used a cervical collar, as she was afraid that she might suffer spontaneous cervical luxation.
3.4. Patient 4
A 46-year-old female with various traumatic antecedents, diagnosed with fibromyalgia and cranio-cervical and atlantoaxial instability, reported: headache, feeling of heaviness of the head, nausea, vomiting, instability, orthostatic intolerance, objective vertigo, mixed dysphagia, dysphonia, aphonia, blurred vision, photophobia, sonophobia, hearing loss, and impaired speech, memory, and attention. Maintenance insomnia, general tiredness, and muscle spasms. Cervicalgia, hypermobile shoulders, feelings of tension, pain, paresis, and paraesthesia in the upper extremities. Back pain, stiffness, palpitations in orthostatism. Lower back pain, pain and numbness in the sacral area, paraesthesia, and numbness in the lower extremities. Incontinence, urinary urgency, and constipation. Gait limited by intolerance of orthostatism.
The physical examination revealed: anisocoria, deeper waist fold on both sides, quadriceps paresis, pain upon pressure in the calf, and hyperaesthesia to temperature and hypoaesthesia to temperature and touch. Alteration of the deep tendon reflexes and abdominal reflexes were abolished, and the plantar reflexes had flexor tendency.
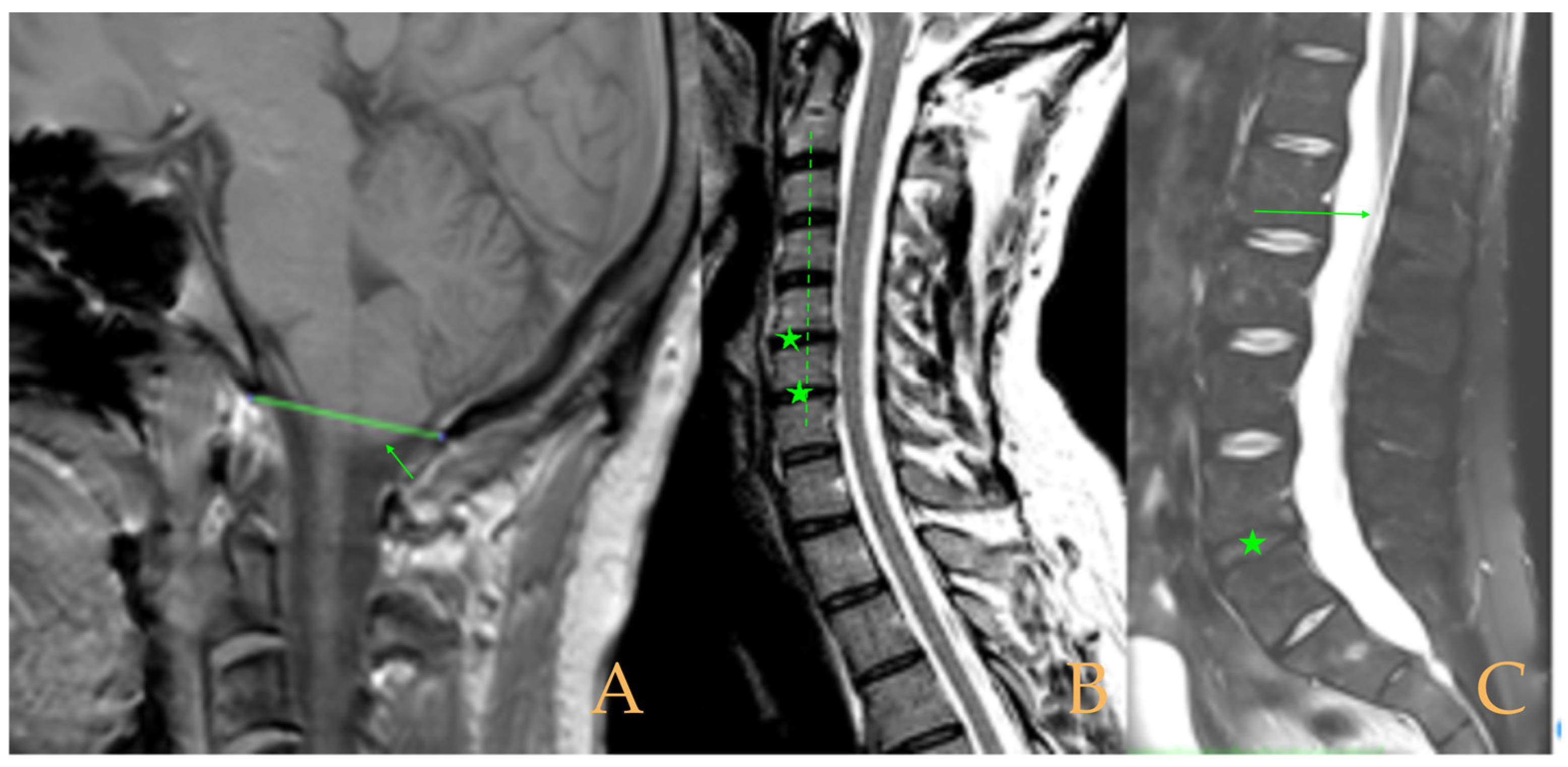
The MRI showed descent of the cerebellar tonsils, increased supracerebral space, and postsurgical repercussions of the sphenoid sinus in the right half. Empty sella turcica. Micro lacunae bilaterally in hemispheres, basal ganglia, and white matter. Straightening of the cervical spine. Cervical spinal cord with tense aspect and inferior lateral displacement, the thoracic portion appears tense with slight ischemia and oedema. Protrusions: C3-C4, C5-C6-C7, T7-T8, and L3-S1. Lumbar hyperlordosis. Sacrococcygeal hyperkyphosis. Ischemia and oedema in epiconus. Low conus medullaris at the level of the upper third of L2 vertebral body. Tarlov cyst at S2 (left) (
Figure 4). Proximal filum terminale externum visible. Levoconvex lumbar scoliosis on the spine X-ray estimated at 6°; dextroconvex cervical and thoracic scoliosis estimated at 4°.
3.5. Patient 5
A 46-year-old female with a medical history of multiple surgeries reported suffering from occipital headache radiated to retroocular and left hemicrania, nausea and vomiting, blurred vision, sonophobia, tinnitus, plugged ears, hearing loss, vertigo, dysphagia, insomnia, nervousness, cervical pain, paraesthesia and lack of strength in the upper extremities, back pain that made breathing difficult, lower back pain, fasciculations, paraesthesia, urinary urgency and incontinence, and lack of strength in the lower extremities, with falls due to loss of coordination.
The physical examination detected spontaneous nystagmus, scoliotic attitude, thermal hypoaesthesia in the lower extremities, very extensive tactile hypoaesthesia, slight alterations of the tendon reflexes, cutaneous-plantar reflexes with a tendency to extension, diminished cutaneous-abdominal reflexes, and positive Romberg test.
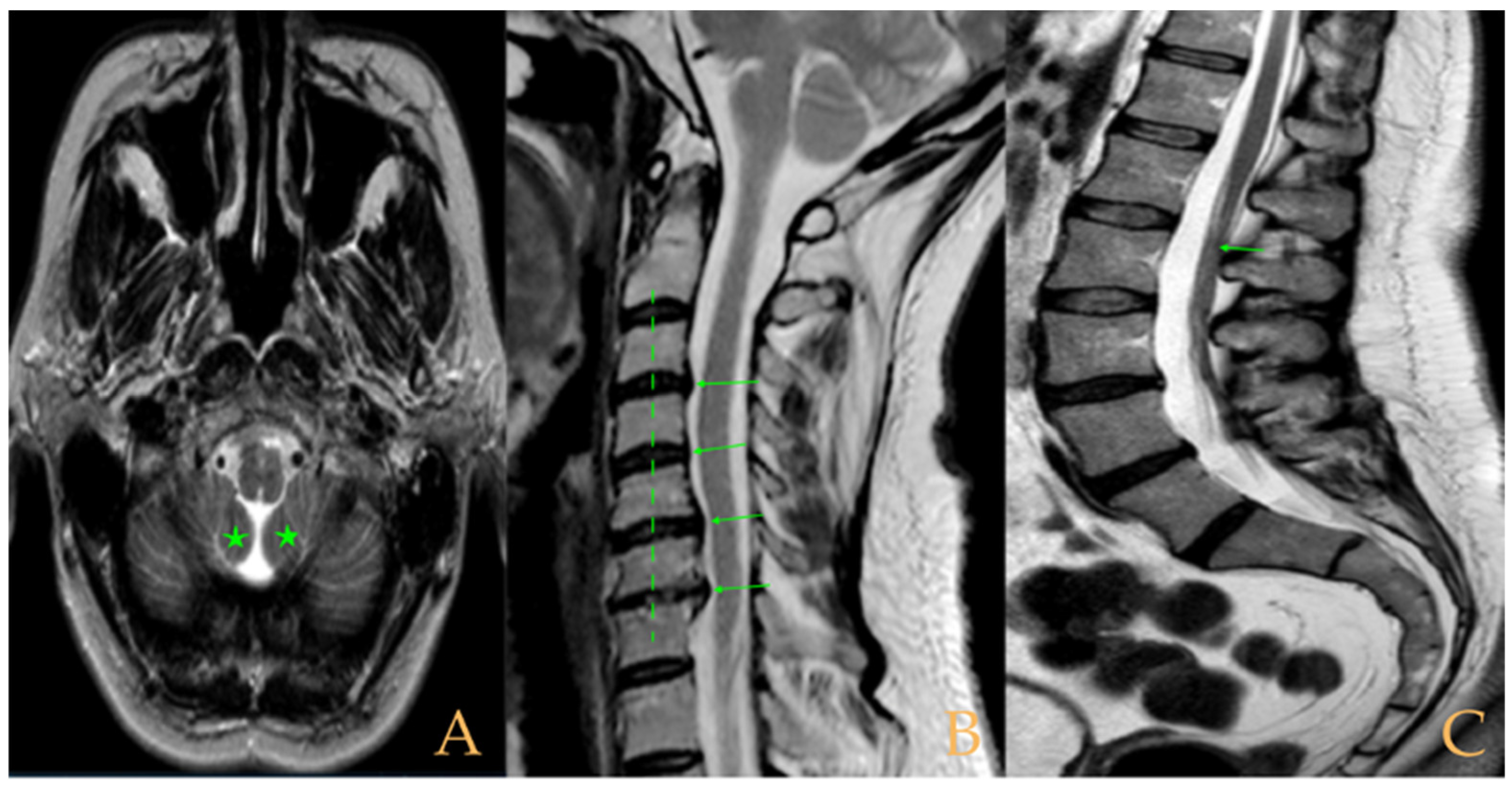
Magnetic resonance imaging showed moderate descent of the cerebellar tonsils, mild multiple disc disease, and low-lying conus medullaris at the level of the upper third of the L2 vertebra (
Figure 5). The full-spine X-ray ruled out scoliosis.
Sectioning of the Filum Terminale with Postoperative Evaluation 47 Days After SFT
We observed improvement or disappearance of most of the mentioned symptoms, except for retroocular and left hemicranial pain, nausea and vomiting, blurred vision, back pain with dyspnoea, and hearing loss. In the physical examination, almost all the signs detected in the preoperative period improved or disappeared, except for the alterations in the osteo-tendon reflexes.
3.6. Patient 6
A 44-year-old female with various traumatic antecedents and atlantoaxial instability reported: headache from the age of 2, diplopia, blurred vision, scintillating scotoma, and intense pain in the cheeks, especially when it is cold. Nausea, subjective vertigo, feeling that the throat tightens, dysphonia, photophobia and sonophobia, tinnitus, hyperosmia, impaired concentration, irritable mood, significant depression, and maintenance insomnia. Intolerance to cold, overall sensation of fatigue, cervicalgia with stiffness, pain, tingling, and a feeling of heaviness and paresis in the upper extremities. Back pain, intercostal pain and stiffness, blockage of breathing, and pain in the lumbo-sacral area and lower extremities with paresis. Very cold feet with a tingling sensation, involuntary movements of the lower extremities, pollakiuria, nocturia, constipation, and difficult gait (always accompanied). Spinal manipulations increased the pain.
The following stood out in the physical examination: uvula deviated towards the right, asymmetric soft palate, bilaterally deeper waist fold, and quadriceps paresis and pain upon pressure in the back, neck, and sacral area. Median lumbo-sacral bulge and discrete hypotrophy in the right lower leg. Hypoaesthesia and hyperaesthesia to temperature and touch. Alteration of deep tendon reflexes decreased abdominal reflexes and weak flexor tendency in the plantar reflexes.
Impaction of the cerebellar tonsils was observed in the MRI. Increased supracerebellar space, incipient descent of the cerebellar tonsils and sinuous course of the optic nerves with oedema in the sheaths. Multiple protrusions, especially C5-C6 and C7-T1, straightening of the cervical spine, and a tense aspect of the cervical spinal cord. Discrete oedema in the cervical and thoracic spinal cord. The thoracic spine tends towards straightening. Low conus medullaris at the middle third of the L1 vertebra. Lumbarisation of S1 and visible filum terminale, somewhat thickened and tight (
Figure 6). The full-spine X-rays revealed dextroconvex thoracic and lumbar scoliosis with very discrete minor compensatory curves in the upper and lower parts; straightening of the cervical spine and hyperlordosis of the lumbar spine; lumbarisation of S1; and rotoscoliosis of the lumbar spine from L5/S1 upwards (rotational subluxation).
3.7. Patient 7
A 44-year-old female diagnosed with cranio-cervical instability, fibromyalgia, chronic fatigue, postural orthostatic tachycardia syndrome, and mast cell activation syndrome reported: severe headache, burning sensation, sharp, stabbing pain in various parts of the head, sensation of retroocular pressure, nausea, instability, subjective vertigo, dysphagia with a feeling of tightening in the throat, blurred vision, photophobia and sonophobia, tinnitus, hearing loss, and plugged ears. Impaired speech, memory, and attention, brain fog, confusion, disorientation, sad and depressed mood, feelings of anxiety and nervousness, mixed insomnia, non-restorative sleep, and overall fatigue. Overall involuntary movements such as myoclonus, generalised lack of sensitivity, and lack of thermal sensitivity in the hands. Cervicalgia radiating to the lower back, pain and a feeling of heaviness in the shoulders and upper extremities, and dropping objects from her grip. Back pain and intercostal pain that block breathing and make it difficult to breathe. Sacro-coccygeal, anal, and pelvic pain; feeling of hypermobility of the coccyx and pelvis; and numbness of the genitals. Paresis, feeling of heaviness, and spasms in the lower extremities. Pollakiuria, urinary incontinence and urgency, diarrhoea, and constipation. Lack of coordination and difficult gait. The symptoms worsen in the winter. Patient presented with panic of spontaneous cervical dislocation.
The physical examination highlighted: deviated uvula, altered deep tendon reflexes, and right plantar reflexes that tended to extension, whereas the left one was indifferent. Sensitivity to temperature: generalised hypoaesthesia, anaesthesia in one hand, and hyperaesthesia in the lower back and lower extremities. Hypoaesthesia and hyperaesthesia to touch. Pain at pressure: cervical, thoracic, lumbar regions, and right lower leg. Heel walking was impossible.
The MRI showed descent of the cerebellar tonsils, 13 mm pineal cyst, and multiple disc protrusions in T4-T8 and T9-T10, with herniation T5-T6. Straightening of the thoracic and lumbar spine. Low conus medullaris at the upper third level of the L2 vertebra (
Figure 7). The full-spine X-rays showed a mild double-curved thoracic and lumbar scoliosis (<10° Cobb), and lower extremity dysmetria with shortening of the left lower extremity estimated to be 3 mm at the level of the acetabular roofs and 7 mm at the level of the iliac crests. Dextroconvex lumbar scoliosis was estimated to be 2°, and levoconvex cervicothoracic scoliosis was estimated to be 10° (both total, including all vertebrae). The estimated lateral curves, including all vertebral bodies in each region, were cervical lordosis 25°, thoracic kyphosis 34°, and lumbar lordosis 53°.
3.8. Patient 8
A 38-year-old man diagnosed with cranio-cervical and atlantoaxial instability reported: headache, nausea, instability, vertigo, darkened, blurred and double vision, halos around lights, sonophobia, tinnitus, altered speech and concentration, brain fog, depressed mood, anxiety and nervousness, mixed insomnia, non-restorative sleep, and feeling of overall fatigue. Cervicalgia radiating to the trapezius, inability to turn the head to the right, shoulder pain, and numbness and lack of strength in one hand. Intercostal pain and breathing difficulties. Lower back pain, numbness, and pain in one foot. Diarrhoea and constipation.
The physical examination yielded the following findings: deviation of the uvula, uneven shoulders, slight levoconvex scoliotic attitude, and bulging of the right hemithorax. Hyperreflexia of the deep tendon reflexes, reduction and abolition of the abdominal reflexes, and flexor plantar reflexes.
The MRI showed (
Figure 8): descent of the cerebellar tonsils and increase of the supracerebellar space, straightening of the cervical spine, multiple protrusions, and discrete central spinal cord oedema C5-C7, mild scoliosis in the thoracic spine, and somewhat tense aspect of the spinal cord. The conus medullaris was at the level of the lower third of the L2 vertebra. Disc disease L5-S1. Tarlov cyst at S2. On the full-spine X-rays, there was dysmetria of the lower extremities with shortening of the left lower extremity estimated to be 1 mm at the level of the acetabular roofs and 6 mm at the level of the iliac crests. Levoconvex lumbar scoliosis was estimated at 5°, and levoconvex cervicothoracic scoliosis was estimated at 3° (both total, including all vertebrae). The estimated lateral curvatures, including all vertebral bodies in each region, were cervical lordosis 4°, thoracic kyphosis 32°, and lumbar lordosis 40°.
Sectioning of the Filum Terminale with Postoperative Evaluation 83 Days After Surgery
Postoperative evaluation was conducted remotely with a questionnaire in relation to his symptoms. As this is incomplete information, the case is not included in the assessment of operated patients. Most of the symptoms remained unchanged, except for nausea, instability, alterations in concentration and mood (depression, anxiety, and nervousness), general feeling of fatigue, breathing difficulties, and numbness in the foot, which worsened. In addition, a new symptom appeared: dysphagia.
3.9. Patient 9
A 37-year-old female who underwent suboccipital craniectomy and had been diagnosed with fibromyalgia, postural orthostatic tachycardia syndrome, and various traumatic antecedents reported: headache, left retroocular pain, burning sensation in the head, nausea, vomiting, instability, subjective vertigo, dysphagia, blurred vision, phosphenes, diplopia, photophobia and sonophobia, tinnitus, speech, memory and concentration problems, unstable mood, mixed insomnia, general tiredness, and non-restorative sleep. Involuntary movements in the lower extremities. Cervicalgia radiating to the shoulders and interscapular region, and pain, numbness, and tingling in the upper extremities. Sensation of thoracic tightness with intercostal radiation. Lower back pain with blockages radiating to the lower extremities, and sensation of itching, burning, and weakness in the legs, with falls. Mixed urinary incontinence and urgency, frequent urination, diarrhoea, and constipation. Limited gait.
The physical examination highlighted the following: anisocoria and nystagmus, pain with pressure in the thoracic region and lower right extremity, and thermal and tactile hypoaesthesia. Scars sensitive to palpitation: extensive cervico-occipital scar, two lumbar scars, and scars in the hypochondrium. Brisk deep tendon reflexes, abolition of abdominal reflexes, and flexor plantar reflexes. Difficult gait, especially on the heels.
The MRI showed (
Figure 9): descent of the cerebellar tonsils, ischemic foci in the white matter of the cerebral hemispheres, and oedema in the optic nerve sheaths. Kyphosis of the cervical spine (especially C5-C6-C7). Tense aspect of the cervical spinal cord. Disc disease: C3-C4-C5-C6-C7-T1. Ischemia and cervical and thoracic oedema. Straightening and scoliosis of the thoracic spine. Lumbar lordosis straightening. Conus medullaris at the level of the middle third of L1. Postsurgical changes in L4-L5 (disc prosthesis). The full-spine X-rays showed cervical, thoracic, and lumbar scoliosis, with the biggest curve being dextroconvex of wide radius in the thoracic and lumbar region.
3.10. Patient 10
A 50-year-old female with a history of craniectomy and occipitocervical fusion, lumbar fusion, and hypermobile type EDS reported headaches in the occipital region, radiation of headaches, and episodes of loss of consciousness and facial paraesthesia. Nausea and vomiting, subjective vertigo or dizziness, blurred vision, diplopia, episode of amaurosis fugax, photophobia, sonophobia, tinnitus, hyposmia, hypogeusia, global dysphagia, bruxism, speech and concentration problems, mixed insomnia, and negative mood swings. Cervicalgia, with blockage episodes, that radiates into the shoulders and upper extremities. Paraesthesia, fasciculations, feeling of overall weakness, dropping objects, electric currents, and burning and pricks in the upper extremities. Thoracic back pain radiating into the intercostal region, breathing difficulty, and paraesthesia. Lower back pain, with blockage episodes, radiating into the hips, sacral region, groin, and thighs. Paraesthesia, paresis, fasciculations, and sensation of electric current and weakness in the lower extremities. Difficult gait, difficulty climbing stairs, difficulty changing position, and clumsiness when walking. Involuntary movements in all extremities when sleeping. Urinary retention and chronic constipation. Cold hands and feet, hyperhidrosis in the trunk, and loss of sensitivity to touch and temperature.
The physical examination revealed: nystagmus, uvula with mild deviation to the left, uneven shoulders, bilateral tendency of scapula alata, and deeper waist fold. Pain upon pressure in cervical, thoracic, and lumbar regions and sacral area. Hypoaesthesia to temperature and touch. Alteration of the deep tendon and abdominal reflexes. Plantar reflexes in extension (Babinski’s sign).
Magnetic resonance imaging (
Figure 10) showed macro lacunae bilaterally in the frontal white matter. Postsurgical changes from occiput to C7. Fusiform intramedullary cyst at C7, with the rest of image showing ischemia/oedema in the cervical and thoracic cord with foci of intramedullary cyst. Multiple Schmorl’s hernias. Disc disease: T2-T3, T5-T6-T7, T10-T11-T12, L3-L4, and L5-S1. Straightening of cervical lordosis and thoracic kyphosis. Conus medullaris at level T12-L1. Lumbar laminectomy with interbody fusion and transpedicular screws L4-L5. The full-spine X-rays showed slight misalignment of the spine in the anteroposterior plane and loss of the cervical lordosis and thoracic kyphosis in the sagittal plane.
Sectioning of Filum Terminale with Postoperative Evaluation 8 Days After Surgery
Improvement/disappearance of almost all the mentioned symptoms, with the exception of paresis and involuntary movements of all the extremities falling asleep, which did not change. On physical examination, the pain upon pressure (cervical, thoracic, and lumbar regions), the temperature hypoaesthesia, and the alteration of the deep tendon reflexes had disappeared, and the Babinski’s sign had partially improved. The rest of the signs remained unchanged or were not assessed.
3.11. Descriptive Analysis of NCVS–EDS
3.11.1. General Data
Among the ten selected patients, nine were females (90%) and one was male (10%). They were between 35 and 64 years old (median 45, mean 46.3, standard deviation 9.51) (
Table 1).
The time interval from the appearance of the first symptoms to the diagnosis was, in general, markedly prolonged. In the majority of cases, it exceeded ten years. With regard to the clinical course, in half of the patients, it was progressive, while in the other half, it was chronic—of long duration, but without a clear impression of worsening over time.
It is noteworthy that the ten patients presented with highly similar medical-surgical antecedents. All had a history of multiple allergies and intolerances. In five cases, various components of central sensitivity syndromes and thyroid dysfunction were recorded. Eight patients had undergone multiple surgeries and were under regular treatment with opioid or related analgesics.
3.11.2. Neurological Clinical Picture
The clinical signs and symptoms detected in the specific neurological examination of these 10 patients are presented in
Table 2 and
Table 3, respectively.
3.11.3. MRI Features
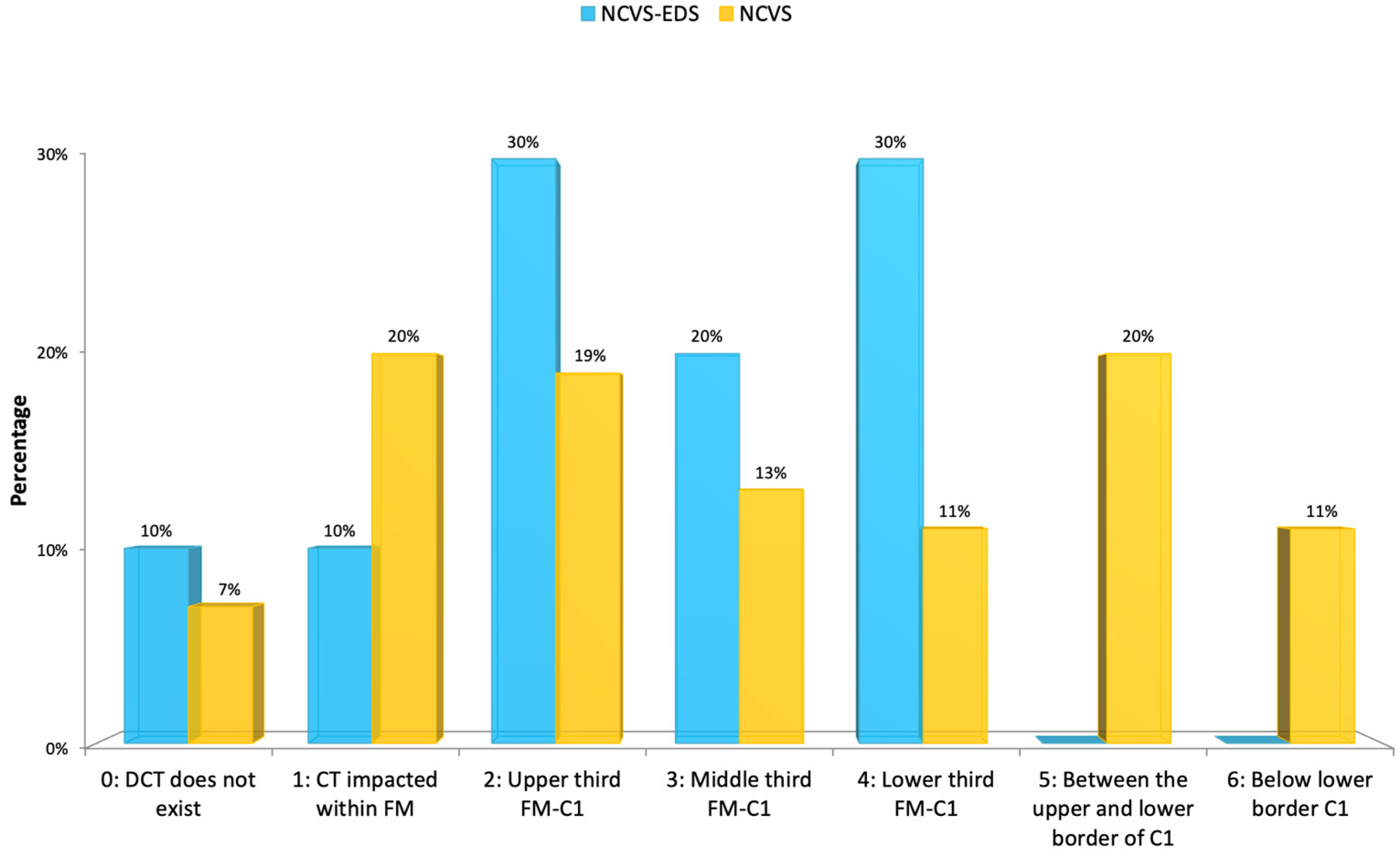
The descent of the cerebellar tonsils (Arnold–Chiari syndrome type I) was present in eight cases (80%); the majority (50%) were mild (upper/middle third of the O-C1 interval), and in one case (10%), there was only an impaction of the cerebellar tonsils, as detailed in
Table 4.
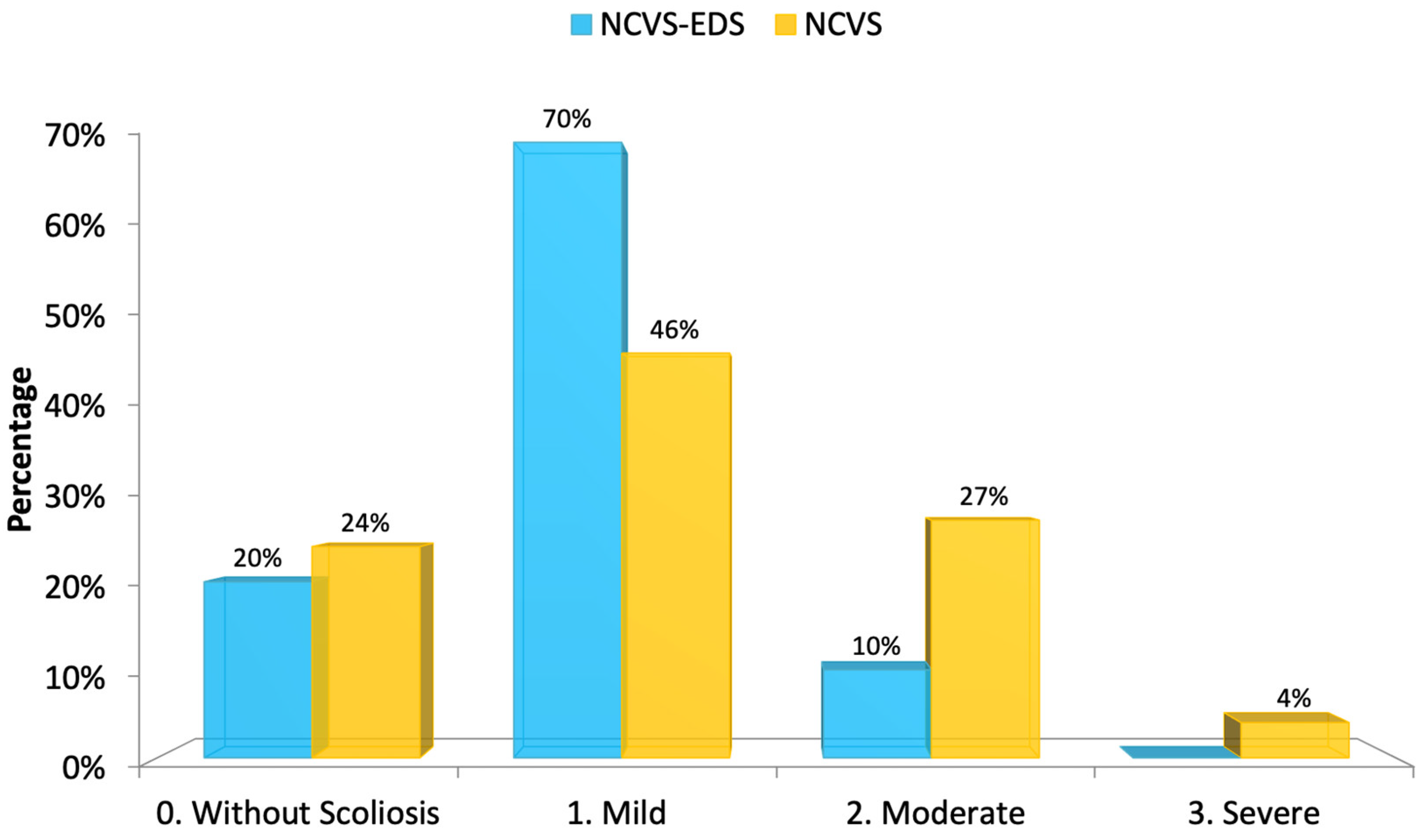
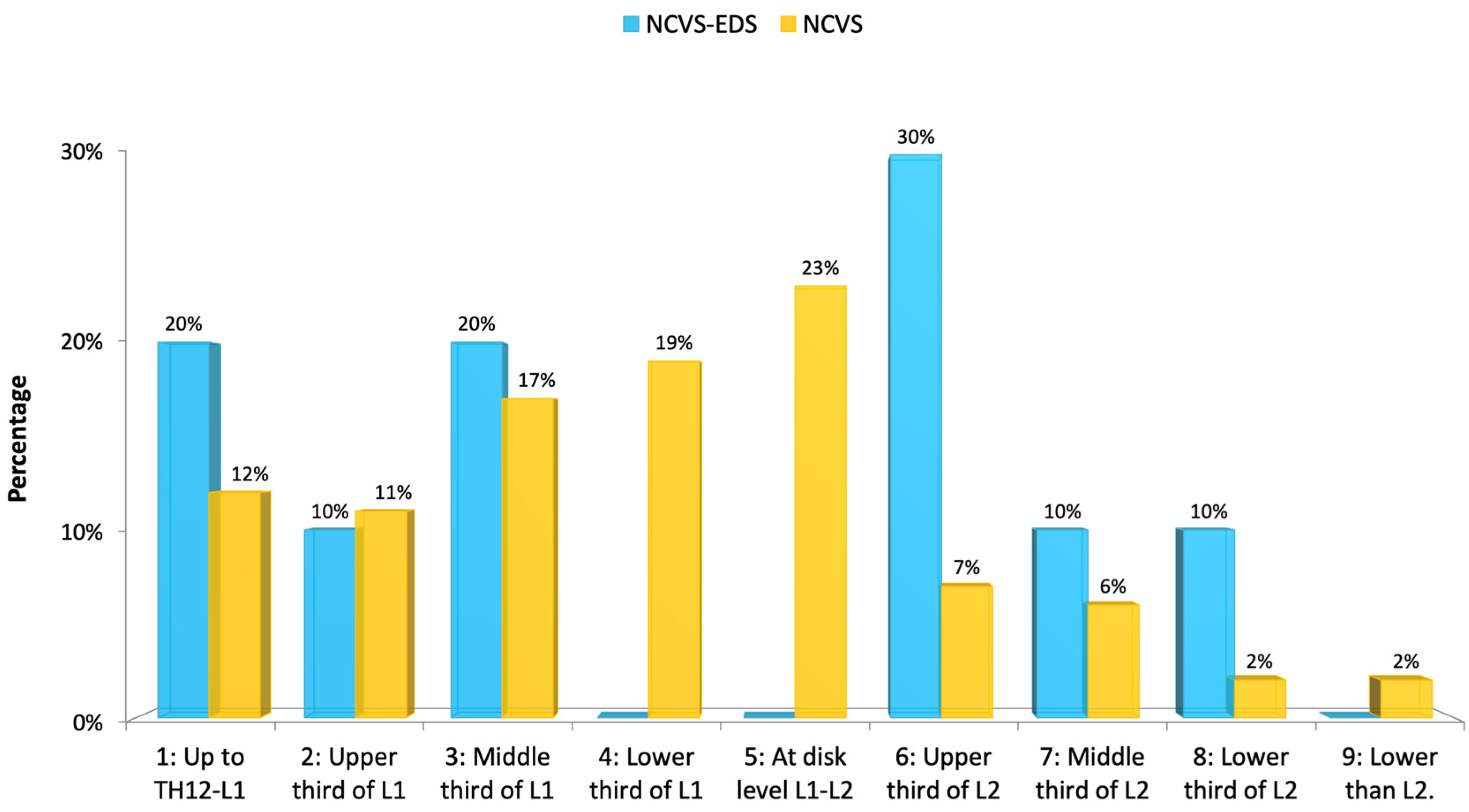
Spinal deviation consistent with idiopathic scoliosis was present in most cases, typically mild in severity. One patient exhibited a moderate curvature. An intramedullary cyst, interpreted as idiopathic syringomyelia, was only identified in case 5; however, in all other cases, areas suggestive of spinal cord ischemia–oedema were observed. The position of the conus medullaris tip varied widely, with a tendency toward lower lumbar levels—most commonly at the upper third of L2. No malformations of the occipito-cervical junction (e.g., retroflexed odontoid, basilar impression, platybasia, or brainstem kinking) were detected in this series.
3.11.4. Comparison Between the 10 Patients with NCVS–EDS and the 373 Cases with NCVS–FD
The symptomatic comparison between the 10 patients with NCVS associated with EDS and the group of 373 cases with NCVS published by Royo-Salvador et al. [
10] is represented in charts that divide the symptoms into two large categories: cranial (
Figure 11) and vertebral (
Figure 12). The comparison regarding the physical examination is represented in
Figure 13. Only the variables present in both studies have been considered when presenting this data. To compare the imaging features, we have graphically represented the descent of the cerebellar tonsils level in
Figure 14, the degree of the deviation of the spine in
Figure 15, and the conus medullaris level in
Figure 16.
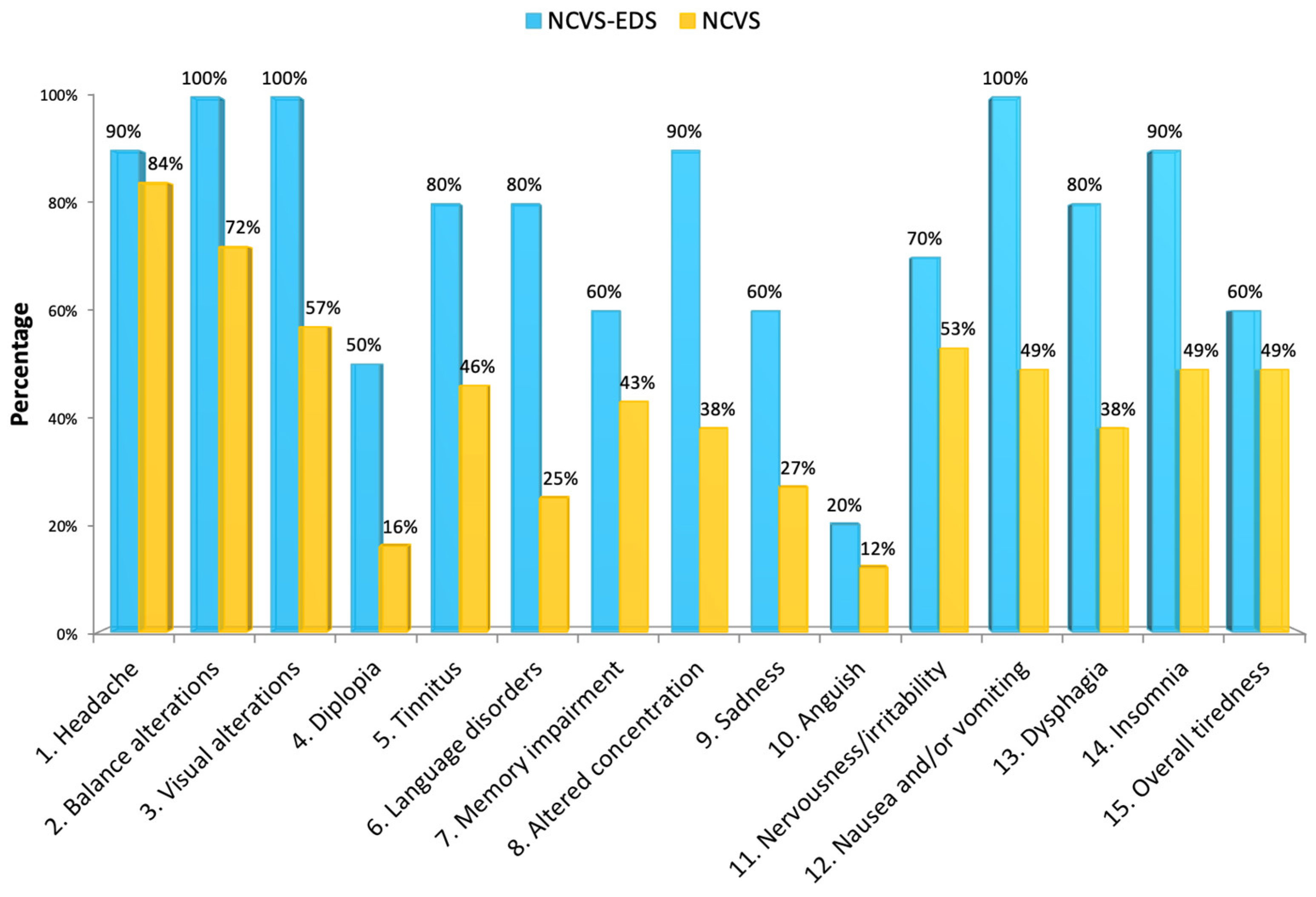
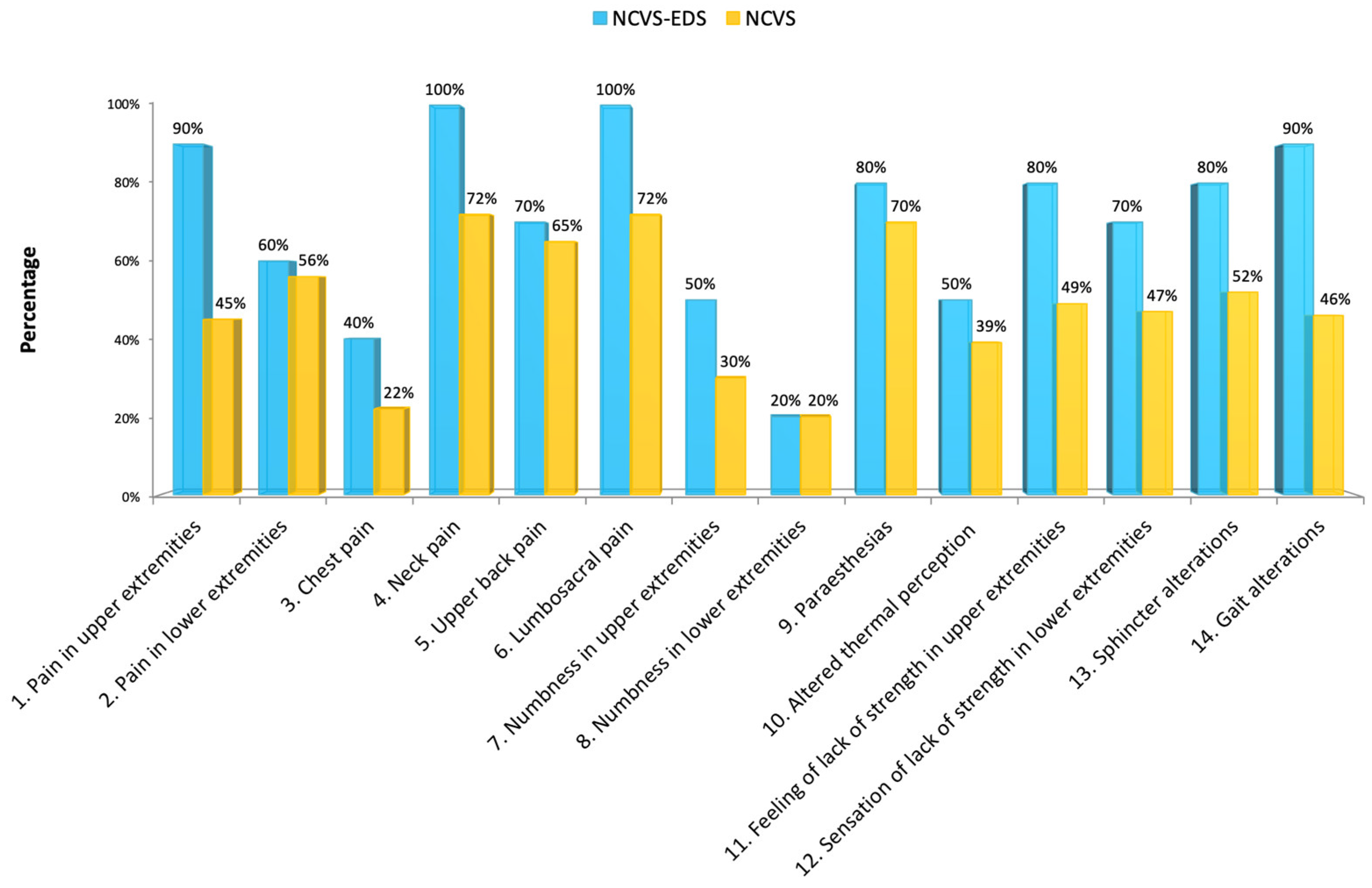
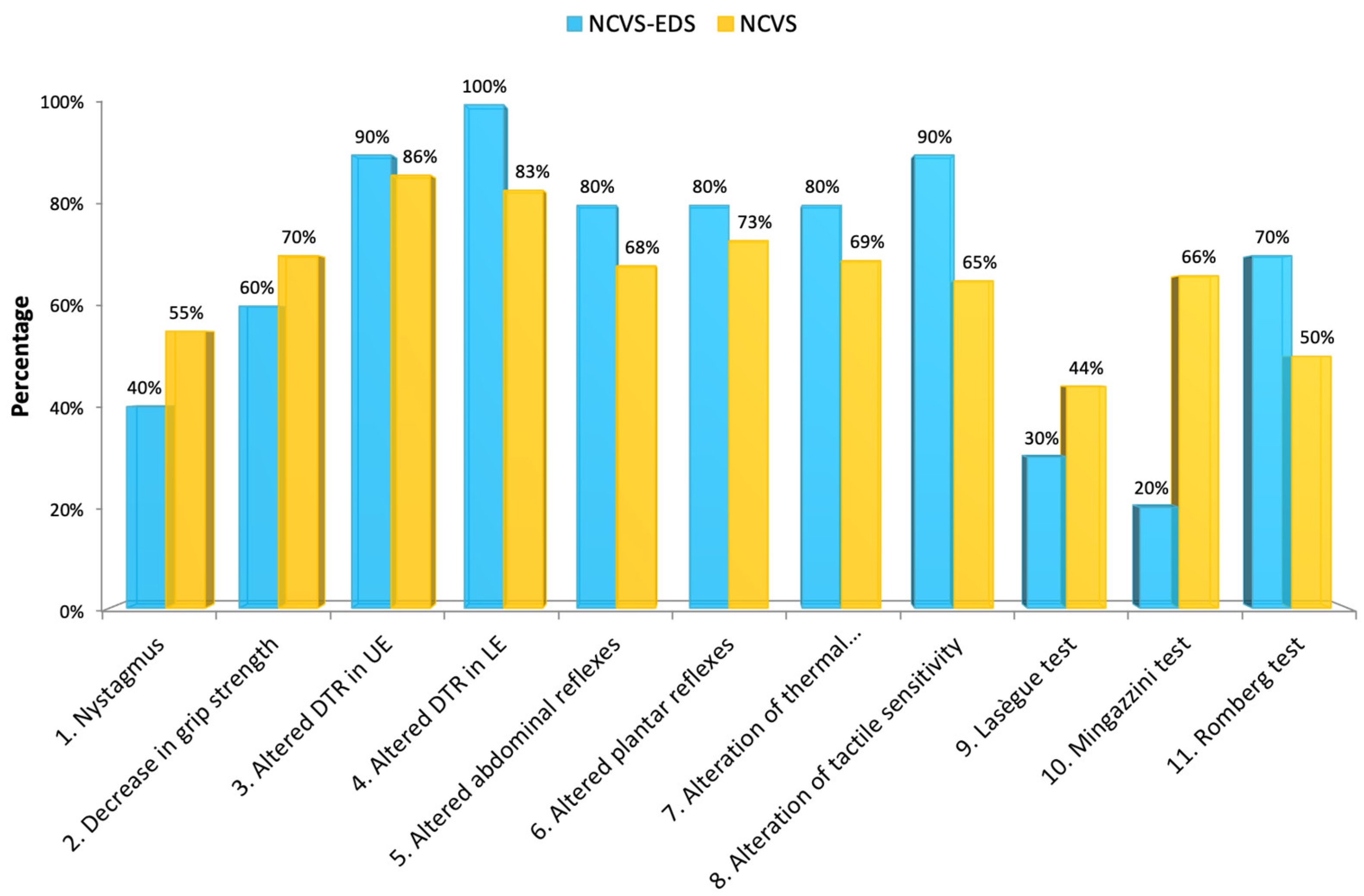
We found that patients with EDS are clearly distinguished within the group of individuals affected by NCVS, as they have more cranial (
Figure 11) and vertebral (
Figure 12) symptoms, such as: headache, visual disturbances, tinnitus, pain in the upper extremities, neck pain, lumbo-sacral pain, sensation of lack of strength in upper extremities, nausea and/or vomiting, dysphagia, insomnia, and gait disturbances.
Regarding the signs, among those that were significantly more frequent in the patients with EDS, we found: altered deep tendon reflexes in the lower extremities, altered tactile sensitivity, and a positive Romberg test. On the other hand, signs such as nystagmus, decreased grip strength, and the Lasègue and Mingazzini tests were significantly less frequent compared to the group of patients with NCVS without EDS.
There was little significant difference between the two groups regarding the MR imaging and full-spine X-rays, except for an evident lack of syringomyelia in patients with NCVS and EDS.
3.11.5. Postoperative Evolution of Four NCVS–EDS Cases
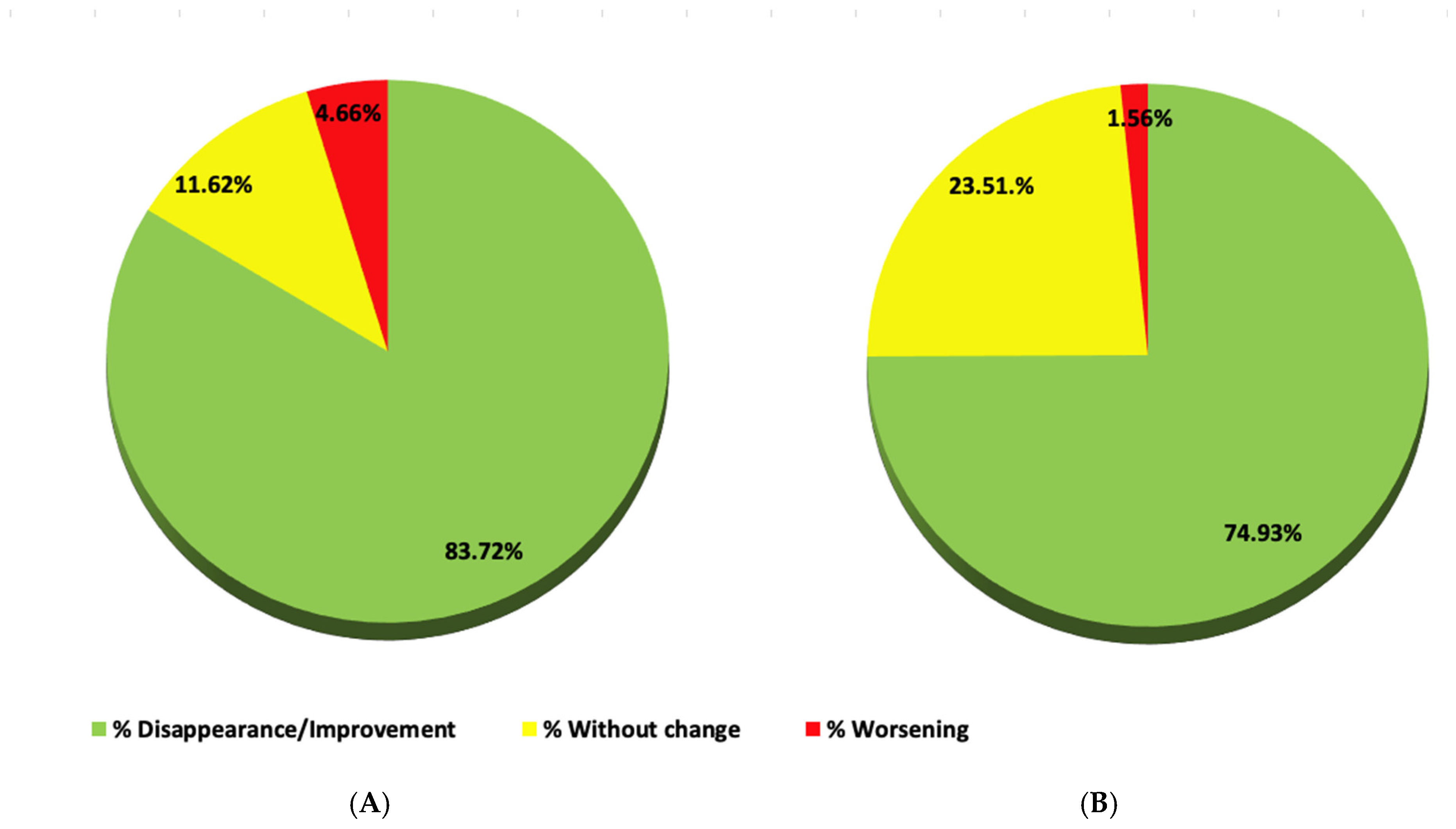
Postoperative check-up was performed 8 days, 7 weeks, 4 months, and 14 months after the SFT. On a global level, the postoperative evolution of all neurological symptoms and signs in the four patients is represented respectively in two circular charts (
Figure 17A,B). The specific evolution of each symptom and sign in each patient after the SFT is shown in
Table 5,
Table 6 and
Table 7.
Some observations can be made about the results after SFT in these four patients with NCVS and EDS:
Cranial symptoms that improved or disappeared: Chiari-type headache, diplopia, tinnitus, alterations in balance and mood, overall fatigue, memory, and concentration impairment (
Table 5);
Vertebral symptoms that improved or disappeared: pain in the upper extremities and cervical region, altered thermal perception, sensation of loss of strength in the lower extremities, and gait alterations (
Table 6);
Clinical signs that improved or disappeared: nystagmus, decreased grip strength, alteration of thermal and tactile sensitivity, Lasègue test, inverted Lasègue test, Mingazzini test, Barré test, sensitivity pressure in paravertebral region and lower extremities, positive Romberg test, altered toe/heel walking, and quadriceps paresis test (
Table 7).
4. Discussion
The series of patients presented in this paper contribute new information to the peculiarities of the neurological clinical picture in patients with EDS. At the same time, we propose an alternate explanation for EDS: an axial intramedullary tension mechanism, which replaces the increasing trend of attributing this condition to craniocervical instability.
In the medical literature, the description of cervical or atlantoaxial subluxations and dislocations in patients with EDS is scarce. Moreover, most of those that have been presented so far are associated with bone malformations such as atlas hypoplasia, os odontoideum, C1 bifid vertebra, and Klippel-Feil type vertebral fusion [
7,
15,
16]. The initiative of Halko et al. is an exception, in which 26 patients with EDS were radiologically evaluated, resulting in 2 cases (7.7%) with atlantoaxial subluxation [
17]. These findings are far from the 65% that represents the frequency of the same condition in rheumatoid arthritis [
18].
A recent study reported that Arnold–Chiari syndrome type I is a comorbid condition in hypermobile EDS and can be complicated by craniocervical instability or basilar impression [
2,
8]. While analysing the MRI of the patients with EDS and Arnold–Chiari syndrome type I, Milhorat found a greater formation of retro-odontoid pannus, compared to patients with Arnold–Chiari syndrome type I alone. In patients with EDS, the atlanto-axoid interval that defines instability remained constant when the parameter was incorporated. The parameters of the occipital–atlas–axis relationship were altered more than in patients with Arnold–Chiari syndrome type I. In another study, Eppelheimer detected a lower tonsillar position in patients with EDS and Arnold–Chiari syndrome type I, compared to patients with Arnold–Chiari syndrome type I without EDS, a result consistent with our current findings [
19].
It is interesting to observe how those that support the theory of craniocervical instability in EDS are adjusting their reasoning and moving closer to spinal traction theories [
20,
21]. They are culminating the design of an ingenious mathematical method to calculate deformative tissue stress according to movements and actions applied on the medulla oblongata and cervical spinal cord [
3]. The main problem with the theories that support instability mechanisms is that they do not ground themselves in neurological deficits, contrary to other pathologies of the nervous system. Patients are characterised by the almost constant nature of their symptoms that can greatly limit their physical and social activity. In addition, the vertebral instability theory only finds hypermobility-type anomalies at the extremes of movement, which the patient likely does not perform in daily life. The same can be reasoned about the theory of dynamic basilar impression and the consequent compression of the medulla oblongata-cervical area in patients with EDS. These arguments can also be used to make a leap toward the theory of spinal cord traction: We propose that tissue stress within the brainstem and spinal cord is abnormally high in these patients, as is the case with NCVS. This high stress is present both at rest and during head movements and changes in body position, and it is conditioned by developmental anomalies in some mechanisms that we try to explain in other works [
10,
22].
Regarding the great variety of neurological symptoms found in these patients, our data support and complement previously published information [
1,
23,
24,
25,
26,
27].
Another study highlights the strong association between EDS and spontaneous cerebrospinal fluid fistulas that may explain some neurological manifestations in these patients [
28].
Of course, the nature of neurological complications depends heavily on the exact type of EDS [
29].
Moreover, concerning occipito-cervical instability, in 2024, Gensemer et al. presented a case with spinal cord tethering in a patient with hypermobile EDS, operated with both occipito-cervical fusion and filum terminale sectioning, with very good results [
30].
Klinge contributes: “Both morphologic findings and biomechanical tests indicate limited elastic properties of the FT in hEDS (hypermobile EDS), which is no more able to dampen but still transmitting spinal movement related stretch forces. That mechanism exposes the conus medullaris to unphysiological stretch forces, causing TCS (Tethered cord syndrome), especially when considering the hypermobile spine in hEDS. This notion is supported by the observed clinical improvement following FT (filum terminale) resection in hEDS-TCS cases without a low-lying conus [
31]”. The author considers the loss of elasticity of the filum terminale responsible for the transmission of the traction force to the conus medullaris. According to the published criteria [
10], it is possible that the filum terminale in EDS, subjected to the constant traction generated by the elongation of the spine, loses elasticity, even that increased by EDS (
Figure 18). This histological finding reaffirms the assumption of the effects of EDS on the filum terminale and, in turn, the existence of the axial traction force on the CNS and spinal cord in the SNCV generated in EDS.
4.1. Comparison of the NCVS–EDS Series with the Descriptive Series of NCVS/FD
It is our objective to present a retrospective study of the clinical manifestations and imaging characteristics found in patients with NCVS associated with EDS and to compare them with a control group of 373 patients affected by NCVS, that has already been presented in another article [
10]. The patients we present have been selected from a group of conditions whose complicated neurological picture is described in detail in the mentioned article. The selected patients are characterised by having an intense and rich symptomatologic picture, with more headaches (which is usually the typical occipital headache triggered by physical exertion and Valsalva manoeuvres), visual disturbances, tinnitus, neck pain, lower back pain, pain and lack of strength in upper extremities, nausea and/or vomiting, dysphagia, insomnia, and gait disturbance.
In the objective examination, patients with NCVS–EDS show alterations comparable to the group of 373 patients with NCVS, but with significantly more alterations in the deep tendon reflexes in the lower extremities, alterations in tactile sensitivity, and positive Mingazzini test.
The images do not differ much from those found in the original group, except for a very scarce association of syringomyelia. It is tempting to attribute this to the same greater elasticity of the leptomeninge under continuous cranio-caudal tissue stress, which constitutes the main postulate of our theories. And the absence of craniocervical malformations such as basilar impression, platybasia, retroflexed odontoid, and brainstem kinking is indicative of two possible circumstances: One is the late manifestation of the pathological mechanism that generates the NCVS, and the second is the greater elasticity of the transmitting elements and spinal traction receptors.
Future studies with larger series should confirm whether the tendency of patients with EDS, compared to patients with NCVS and Filum disease, have less descent of the cerebellar tonsils, less idiopathic scoliosis, and a less low-lying conus medullaris.
4.2. Aetiopathogenic Mechanism of NCVS in EDS
Filum disease is a pathology that triggers the appearance of most of the diseases it generates, including Arnold–Chiari syndrome type I, idiopathic scoliosis and idiopathic syringomyelia, basilar impression, platybasia, retroflexed odontoid, brainstem kinking, and others. These are the consequence of a traction of the nervous system due to the conflict between asynchronous growth between the nervous system, the spine, and the filum terminale.
In the ninth week of gestation, if the spine grows more in relation to the spinal cord, it causes the spine to apply traction on the spinal cord, as they are both united at the caudal level by the filum terminale. Depending on the intensity of the conflict, neurological, cranial, and spinal injuries appear, expressing themselves in relevant cases with multiple neurological symptoms and signs.
NCVS is consequence of the same mechanism as the Filum disease, but in addition to the congenital cause, other acquired causes can add to the asymmetry between the spine and the spinal cord, as is evident in the cases presented in this paper with the existence of EDS-related anomalies.
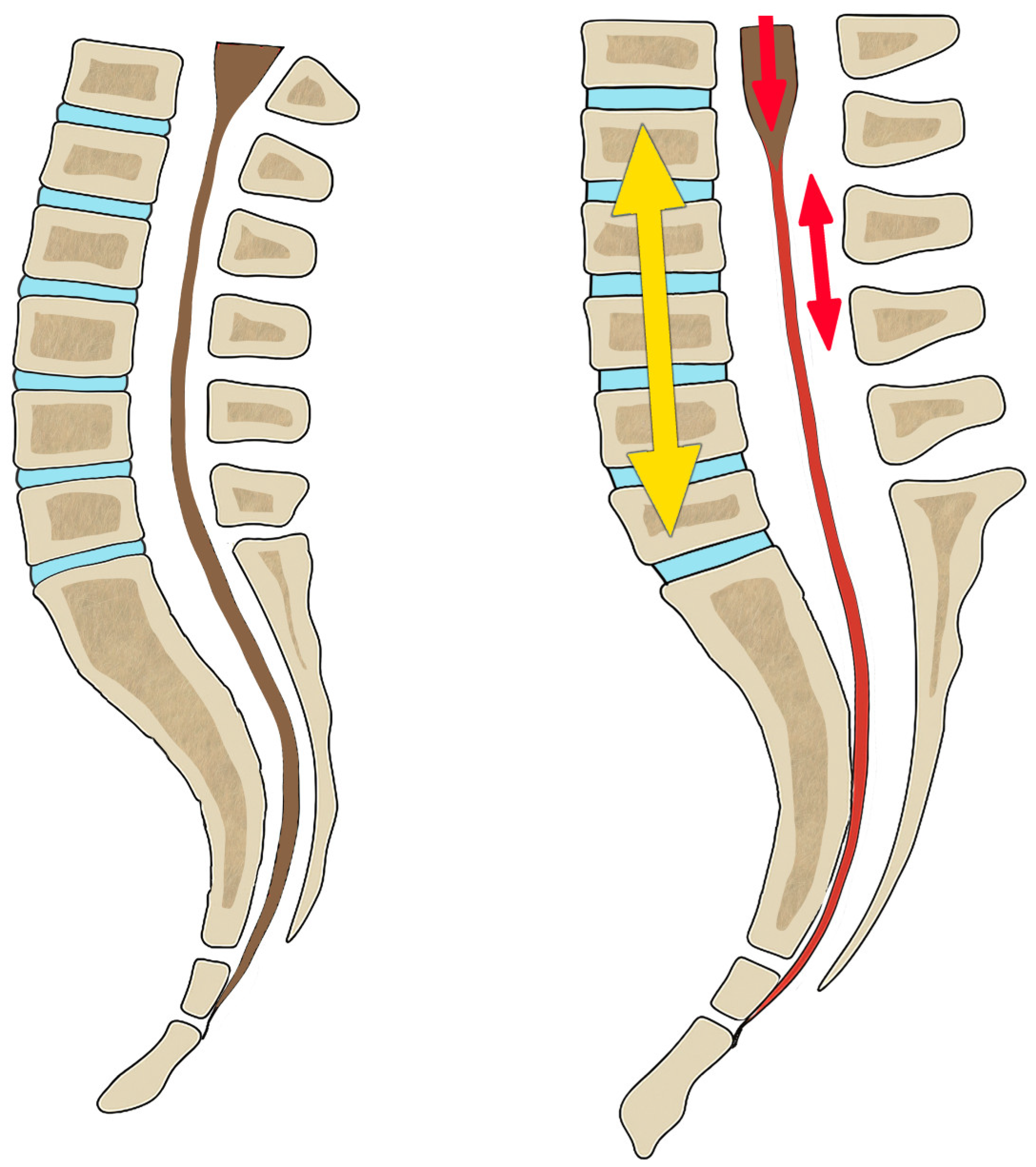
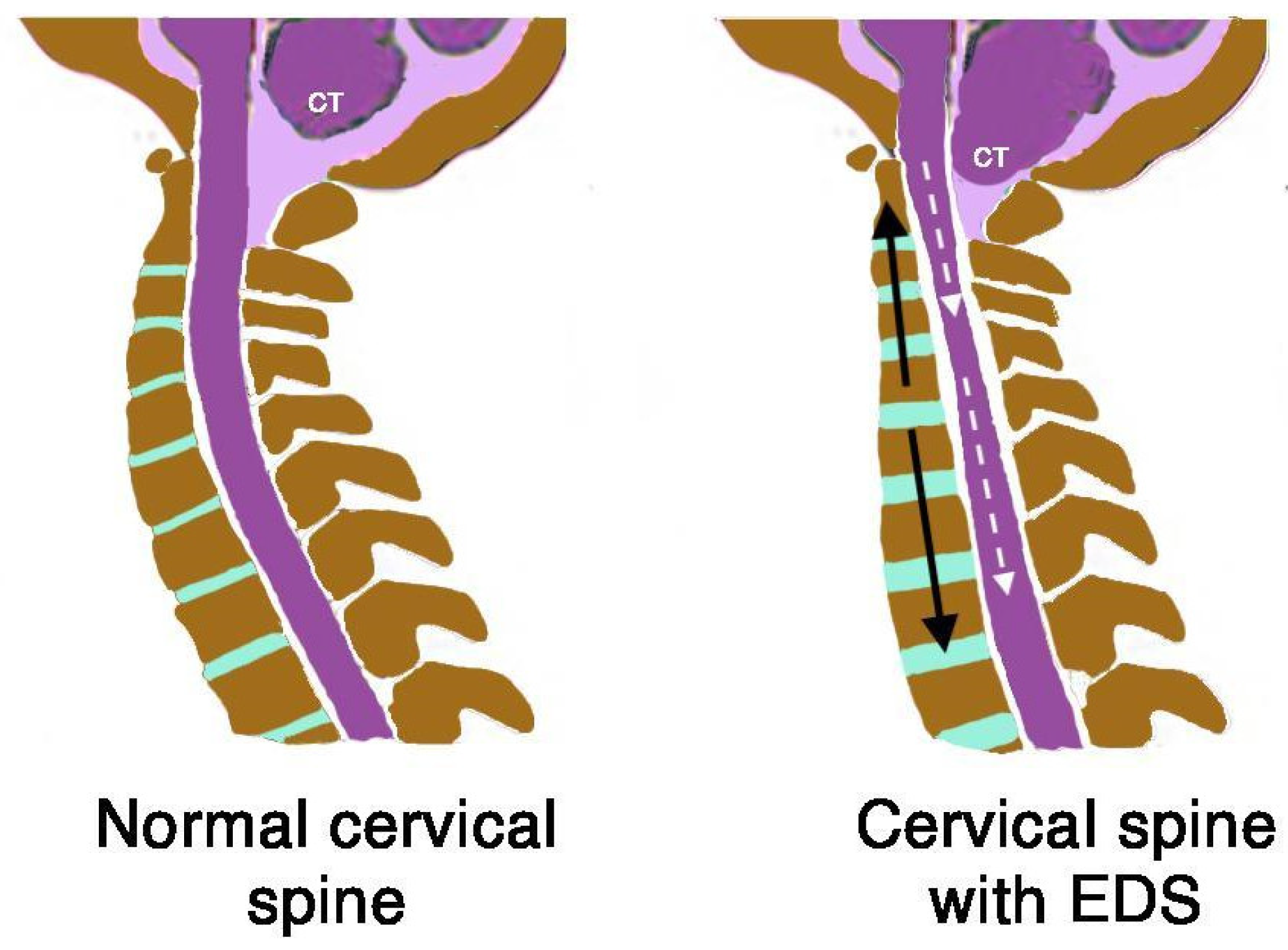
First, in the case of EDS, the generalized ligamentous laxity that affects the spine and intervertebral discs allows an elongation that does not match the spinal cord tissues. It creates a traction gradient that generates a mechanical conflict in the entire nervous system, which is impacted and anchored in the foramen magnum, and transmitted by the filum terminale (
Figure 19 and
Figure 20).
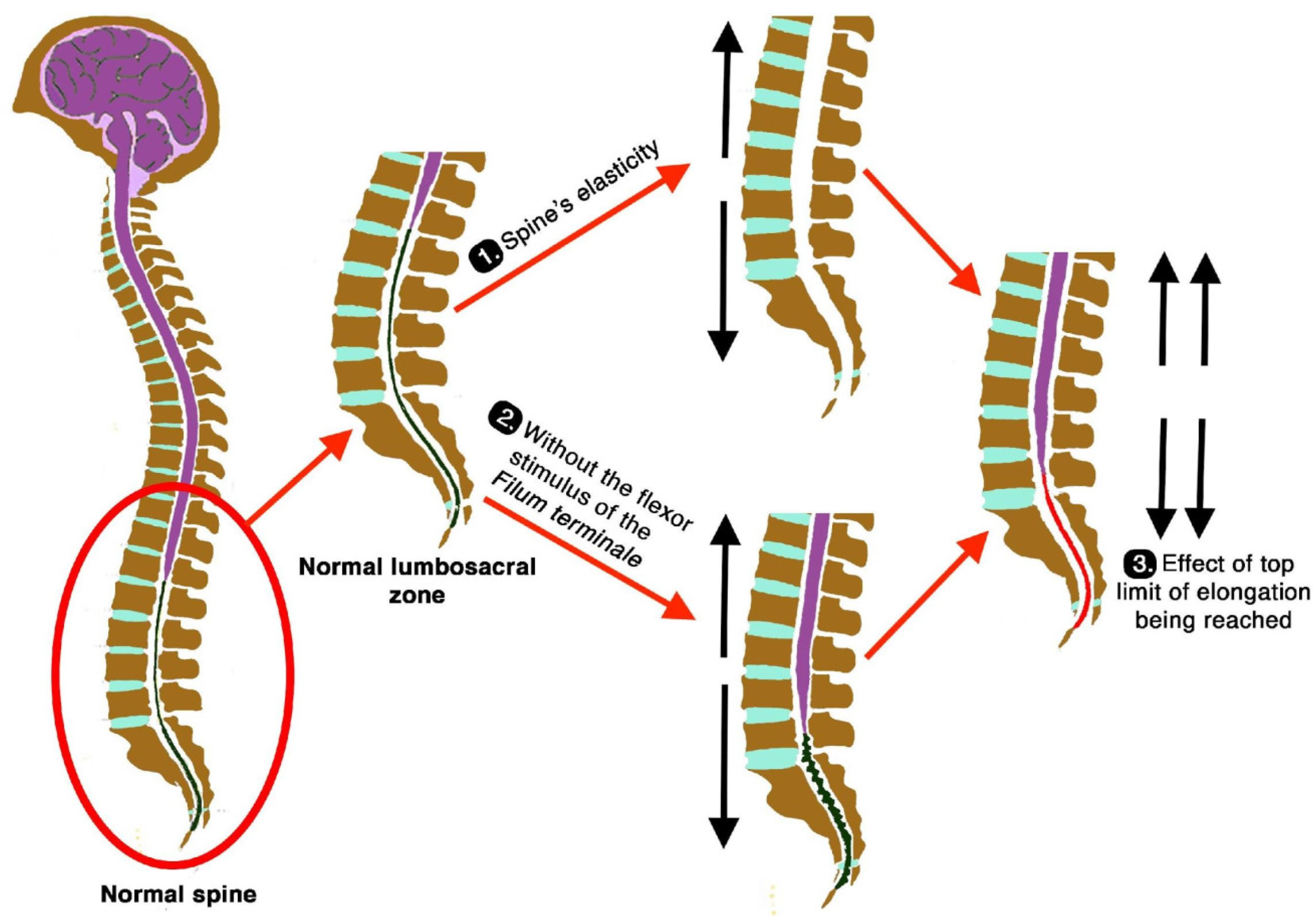
Second, in normal conditions, the filum terminale should have a flexing effect over the entire spine that contributes to the generation, together with gravity, of the physiological curves in the sagittal and coronal planes. However, these physiological curves disappear or are minimised when the filum terminale and the vertebral symphyses are loose, hence increasing the lengthening of the spine (
Figure 20).
Third, the filum terminale, despite being slacker in EDS and allowing elongation of the spine, reaches a limit during growth, where its elastic behaviour is altered and the filum terminale becomes increasingly stiff and tense. When this elongation process continues, it begins to exert a noticeable traction on the spinal cord or partially decreases the intensity of the traction of the spinal cord. As this traction is slow and of low intensity, it does not present with the bony deformations of Filum disease such as basilar impression, platybasia, and retroflexed odontoid, and idiopathic syringomyelia occurs with less frequency (
Figure 20, part 3).
A very different possible interpretation of these findings would be that patients with EDS develop a clinical picture of a central sensitivity syndrome like fibromyalgia and chronic fatigue, as they carry multiple somatic mechanical conflicts caused by abnormalities in the composition and biomechanical behaviour of the connective tissue. They develop these syndromes earlier and more intensely than other patients suffering from a Neuro-Cranio-vertebral syndrome.
4.3. Postoperative Changes in NCVS-EDS After SFT
In any case, the results of the sectioning of filum terminale surgery in patients with EDS seem to be superimposable to those of the patients with Neuro-Cranio-vertebral syndrome. However, they need prospective confirmation with more cases and more regular follow-up based on validated outcome scales.
4.4. Psychological Status of Panic of Spontaneous Cervical Dislocation
The history of the disease of some of the patients in this series shows the existence of a very marked panic of suddenly suffering a cervical dislocation due to a possible or confirmed Ehlers–Danlos syndrome. We have called this psychological syndrome “cervical dislocation panic syndrome” (CDP). It creates an obsessive state that is very difficult to mitigate. It can only be diminished with an important argument that discourages the probability of cervical dislocation or the practice of cervical arthrodeses, which are mostly unnecessary.