Real-Life Advantages and Limits of Baricitinib for the Late Treatment of Adults Hospitalized with COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- in patients who reported COVID-19 symptom onset <7 days;
- in patients with Chronic Kidney Disease (CKD) with an estimated Glomerular Filtration rate below 15 mL/min;
- in subjects who tested positive at anti-HBV screening performed at baseline;
- in those presenting with suspected or confirmed bacterial co-infections (Procalcitonin above five ng/mL).
2.2. Data Collection and Statistical Analysis
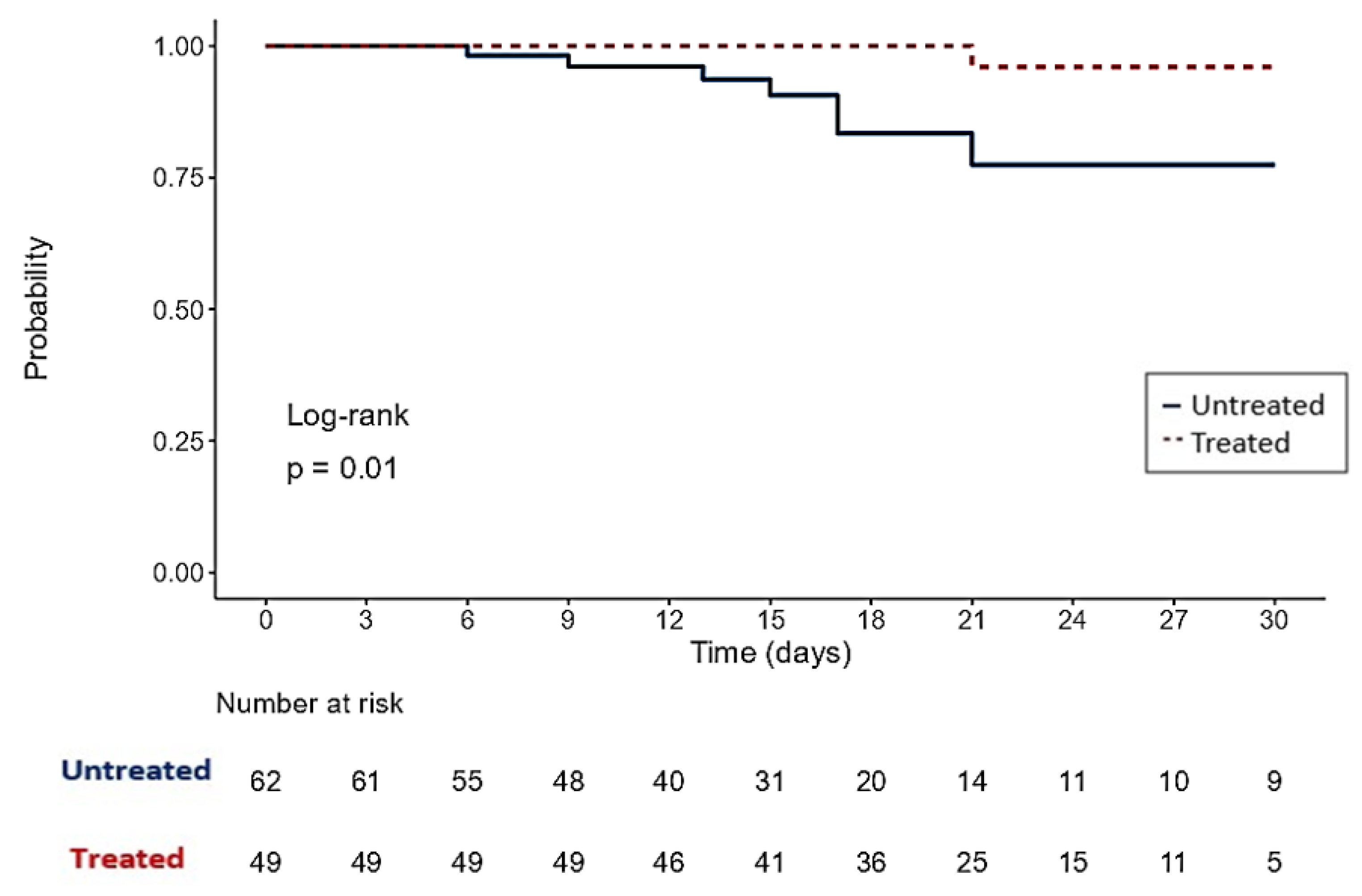
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Center for Disease Control. Available online: https://www.ecdc.europa.eu/en/covid-19/country-overviews (accessed on 8 February 2022).
- Galmiche, S.; Nguyen, L.B.L.; Tartour, E.; de Lamballerie, X.; Wittkop, L.; Loubet, P.; Launay, O. A systematic review of immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2022, 28, 163–177. [Google Scholar] [CrossRef]
- Gao, S.J.; Guo, H.; Luo, G. Omicron variant (B.1.1.529) of SARS-CoV-2, a global urgent public health alert! J. Med. Virol. 2022, 94, 1255–1256. [Google Scholar] [CrossRef] [PubMed]
- NIH COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 8 February 2022).
- Marconi, V.C.; Ramanan, A.V.; de Bono, S.; COV-BARRIER Study Group. Efficacy and safety of Baricitinibfor the treatment of hospitalized adults with COVID-19 (COV-BARRIER): A randomized, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021, 9, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; ACTT-2 Study Group. Members.Baricitinibplus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.; Tse, C.; Burry, L.; Burry, L.; Dresser, L.D. Baricitinib: A Review of Pharmacology, Safety, and Emerging Clinical Experience in COVID-19. Pharmacotherapy 2020, 40, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.; Guglielmelli, P.; Langmuir, P.; Constantinescu, S. JAK inhibitors and COVID-19. J. Immunother. Cancer 2022, 10, e002838. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Bronte, V.; Ugel, S.; Tinazzi, E.; Vella, A.; De Sanctis, F.; Canè, S.; Batani, V.; Trovato, R.; Fiore, A.; Petrova, V.; et al. Baricitinibrestrains the immune dysregulation in patients with severe COVID-19. J. Clin. Investig. 2020, 130, 6409–6416. [Google Scholar] [CrossRef] [PubMed]
- Ely, E.W.; Ramanan, A.V.; Kartman, C.E.; COV-BARRIER Study Group. Efficacy and safety of Baricitinibplus standard of care for the treatment of critically ill hospitalized adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: An exploratory, randomized, placebo-controlled trial. Lancet Respir. Med. 2022, 10, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Cantini, F.; Niccoli, L.; Nannini, C.; Matarrese, D.; Di Natale, M.E.; Lotti, P.; Aquilini, D.; Landini, G.; Cimolato, B.; Di Pietro, M.A.; et al. The beneficial impact of Baricitinibin COVID-19 moderate pneumonia; multicentre study. J. Infect. 2020, 81, 647–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005, 23, 275–306. [Google Scholar] [CrossRef]
- Pellegrini, S.; Schindler, C. Early events in signaling by interferons. Trends Biochem. Sci. 1993, 18, 338–342. [Google Scholar] [CrossRef]
- Favalli, E.G.; Biggioggero, M.; Maioli, G.; Caporali, R. Baricitinibfor COVID-19: A suitable treatment? Lancet. Infect. Dis. 2020, 20, 1012–1013. [Google Scholar] [CrossRef]
- Smolen, J.S.; Genovese, M.C.; Takeuchi, T.; Hyslop, D.L.; Macias, W.L.; Rooney, T.; Chen, L.; Dickson, C.L.; Camp, J.R.; Cardillo, T.E.; et al. Safety profile of Baricitinibin patients with active rheumatoid arthritis with over 2 years median time in treatment. J. Rheumatol. 2019, 46, 7–18. [Google Scholar] [CrossRef]
- Biggioggero, M.; Becciolini, A.; Crotti, C.; Agape, E.; Favalli, E.G. Upadacitinib and filgotinib: The role of JAK1 selective inhibition in the treatment of rheumatoid arthritis. Drugs Context 2019, 8, 212595. [Google Scholar] [CrossRef]
- Rowe, T.A.; McKoy, J.M. Sepsis in Older Adults. Infect. Dis. Clin. N. Am. 2017, 31, 731–742. [Google Scholar] [CrossRef]
- O’Toole, R.F. The interface between COVID-19 and bacterial healthcare-associated infections. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 1772–1776. [Google Scholar] [CrossRef]
- Elabbadi, A.; Turpin, M.; Gerotziafas, G.T.; Teulier, M.; Voiriot, G.; Fartoukh, M. Bacterial co-infection in critically ill COVID-19 patients with severe pneumonia. Infection 2021, 49, 559–562. [Google Scholar] [CrossRef]
- Wirth, R.; Becker, C.; Djukic, M.; Drebenstedt, C.; Heppner, H.J.; Jacobs, A.H.; Meisel, M.; Michels, G.; Nau, R.; Pantel, J.; et al. COVID-19 in old age-The geriatric perspective. Z. Gerontol. Geriatr. 2021, 54, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; KursatAzkur, A.; Azkur, D.; Gan, H.; Sun, Y.L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef] [PubMed]


| Variables | Total | Untreated | Baricitinib | Baricitinib + RDV | p-Value |
|---|---|---|---|---|---|
| (N = 111) | (N = 62) | (N = 28) | (N = 21) | ||
| Male gender, n (%) | 70 (53) | 31 (50) | 23 (82) | 16 (76) | 0.005 ^ |
| Age (median, IQR) | 73 (61–82) | 77 (70–86) | 66 (59–80) | 68 (56–73) | 0.005 § |
| Vaccinate *, n (%) | 58 (52) | 34 (54) | 12 (42) | 12 (57) | 0.51 ^ |
| Vaccinate with booster dose, n (%) | 26 (23) | 17 (26) | 7 (25) | 2 (9) | 0.24 ^^ |
| Co-existing conditions, n (%) | |||||
| Chronic Heart Disease | 70 (63) | 43 (69) | 16 (57) | 11 (52) | 0.23 ^ |
| Type II Diabetes | 31 (31) | 20 (32) | 8 (28) | 6 (28) | 0.89 ^ |
| Chronic Kidney Disease | 16 (14) | 14 (22) | 1 (3) | 1 (5) | 0.02 ^^ |
| Chronic Obstructive Pulmonary Disease | 24 (22) | 12 (19) | 7 (25) | 5 (23) | 0.83 ^ |
| Asthma | 3 (2) | 2 (3) | 0 (0) | 1 (3) | 0.24 ^^ |
| Dementia | 20 (18) | 13 (20) | 4 (14) | 3 (14) | 0.64 ^^ |
| Chronic neurological diseases | 19 (17) | 14 (22) | 5 (17) | 0 (0) | 0.05 ^^ |
| Cancer | 14 (13) | 10 (16) | 3 (11) | 1 (3) | 0.36 ^^ |
| Primitive Immunodepression | 7 (6) | 4 (6) | 1 (3) | 2 (9) | 0.69 ^^ |
| Acquired Immune depression | 12 (11) | 6 (9) | 3 (10) | 3 (14) | 0.85 ^^ |
| Charlson Co-morbidity Index, mean (±SD) | 4 (2) | 5 (5) | 4 (14) | 3 (14) | 0.01 § |
| Smoker, n (%) | 39 (40) | 20(32) | 8 (28) | 1 (3) | 0.01 ^^ |
| Laboratory test at admission | |||||
| WBC, cells × 103/mm3 ** | 8.1 (4.5) | 9.5 (4.8) | 7.3 (4.1) | 5.9 (3.1) | 0.002 § |
| Lymphocytes, cells × 103/mm3 *** | 774 (423–1070) | 689 (96–1192) | 742 (443–850) | 915 (678–11,423) | 0.13 § |
| HB, gr/dL ** | 13 (3) | 12 (2) | 14 (2) | 13 (2) | 0.07 § |
| PLT, cells × 103/mm3 ** | 238 (93) | 243 (106) | 245 (81) | 217 (74) | 0.40 § |
| D-Dimers, ng/mL *** | 1111 (722–1888) | 1351(972–2141) | 961 (709–1980) | 1063 (660–1752) | 0.35 § |
| Fibrinogen, gr/dL ** | 604 (339) | 587 (262) | 631 (259) | 592 (186) | 0.79 § |
| IL-6, pg/mL *** | 17 (8–37) | 17 (9–38) | 16 (10–36) | 17 (8–34) | 0.96 § |
| CRP, mg/L *** | 64 (26–117) | 48 (16–146) | 77 (37–109) | 54 (29–99) | 0.52 § |
| Oxygen requirement during hospitalization, n (%) | |||||
| Venturi Mask/Nasal Cannulas | 40 (36) | 34 (54) | 4 (14) | 2 (9) | |
| High Flow Nasal Cannulas | 12 (11) | 2 (3) | 5 (17) | 5 (23) | <0.001 ^^ |
| Non-Invasive Ventilation | 59 (53) | 26 (41) | 19 (67) | 14 (66) | |
| Outcome, n (%) | |||||
| Discharged | 81 (73) | 40 (64) | 22 (78) | 19 (90) | |
| Intensive Care Unit admission | 9 (8) | 8 (12) | 1 (3) | 0 (0) | |
| Dead | 21 (19) | 14 (22) | 5 (17) | 2 (9) | 0.12 ^^ |
| Hospitalization from symptoms onset, median (IQR) | 6 (1–10) | 3 (1–9) | 7 (5–11) | 8 (5–10) | 0.06 § |
| Duration of Hospital Stay, days, median (IQR) | 18 (12–23) | 14 (9–20) | 21 (18–25) | 20 (15–25) | <0.001 § |
| Variables | H.R. (95% C.I., p-Value) | aHR (95% C.I., p-Value) |
|---|---|---|
| Male sex | 0.68 (0.28–1.66, p = 0.39) | 1.10 (0.38–3.17, p = 0.86) |
| Age (×1-year increase) | 1.11 (1.06–1.17, p < 0.001) | 1.13 (1.01–1.26, p = 0.03) |
| Vaccinate * | 2.15 (0.83–5.61, p = 0.12) | 1.13 (0.36–3.52, p = 0.83) |
| Type II Diabetes | 0.43 (0.13–1.47, p = 0.177) | 1.04 (0.20–5.35, p = 0.96) |
| Chronic Kidney Disease | 2.20 (0.80–6.06, p = 0.13) | 2.26 (0.49–10.39, p = 0.29) |
| Chronic Neurological Disease | 5.15 (2.13–12.44, p < 0.001) | 3.39 (0.91–12.62, p = 0.07) |
| Treatment with Baricitinib | 0.42 (0.16–1.09, p = 0.07) | 0.52 (0.13–2.03, p = 0.35) |
| Treatment with Baricitiniband Remdesivir | 0.43 (0.10–1.88, p = 0.26) | 1.54 (0.20–11.68, p = 0.67) |
| Non-Invasive Ventilation requirement | 2.14 (0.77–5.94, p = 0.14) | 2.36 (0.72–7.72, p = 0.15) |
| Charlson Co-morbidity Index | 1.62 (1.26–2.09, p < 0.001) | 0.77 (0.40–1.51, p = 0.45) |
| Days from symptom onset to hospitalization (×1-day increase) | 1.00 (0.93–1.07, p = 0.91) | 1.05 (0.97–1.13, p = 0.23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poliseno, M.; Lacedonia, D.; Niglio, M.; De Gregorio, F.; Minafra, G.A.; Campanino, T.; Giganti, G.; Scioscia, G.; Santantonio, T.A.; Foschino Barbaro, M.P.; et al. Real-Life Advantages and Limits of Baricitinib for the Late Treatment of Adults Hospitalized with COVID-19. BioMed 2023, 3, 236-245. https://doi.org/10.3390/biomed3020021
Poliseno M, Lacedonia D, Niglio M, De Gregorio F, Minafra GA, Campanino T, Giganti G, Scioscia G, Santantonio TA, Foschino Barbaro MP, et al. Real-Life Advantages and Limits of Baricitinib for the Late Treatment of Adults Hospitalized with COVID-19. BioMed. 2023; 3(2):236-245. https://doi.org/10.3390/biomed3020021
Chicago/Turabian StylePoliseno, Mariacristina, Donato Lacedonia, Mariangela Niglio, Federica De Gregorio, Graziano Antonio Minafra, Terence Campanino, Giulio Giganti, Giulia Scioscia, Teresa Antonia Santantonio, Maria Pia Foschino Barbaro, and et al. 2023. "Real-Life Advantages and Limits of Baricitinib for the Late Treatment of Adults Hospitalized with COVID-19" BioMed 3, no. 2: 236-245. https://doi.org/10.3390/biomed3020021
APA StylePoliseno, M., Lacedonia, D., Niglio, M., De Gregorio, F., Minafra, G. A., Campanino, T., Giganti, G., Scioscia, G., Santantonio, T. A., Foschino Barbaro, M. P., & Lo Caputo, S. (2023). Real-Life Advantages and Limits of Baricitinib for the Late Treatment of Adults Hospitalized with COVID-19. BioMed, 3(2), 236-245. https://doi.org/10.3390/biomed3020021






