Characterization of Microarchitectures, Stiffness and Strength of Human Trabecular Bone Using Micro-Computed Tomography (Micro-CT) Scans
Abstract
1. Introduction
2. Investigation Approach
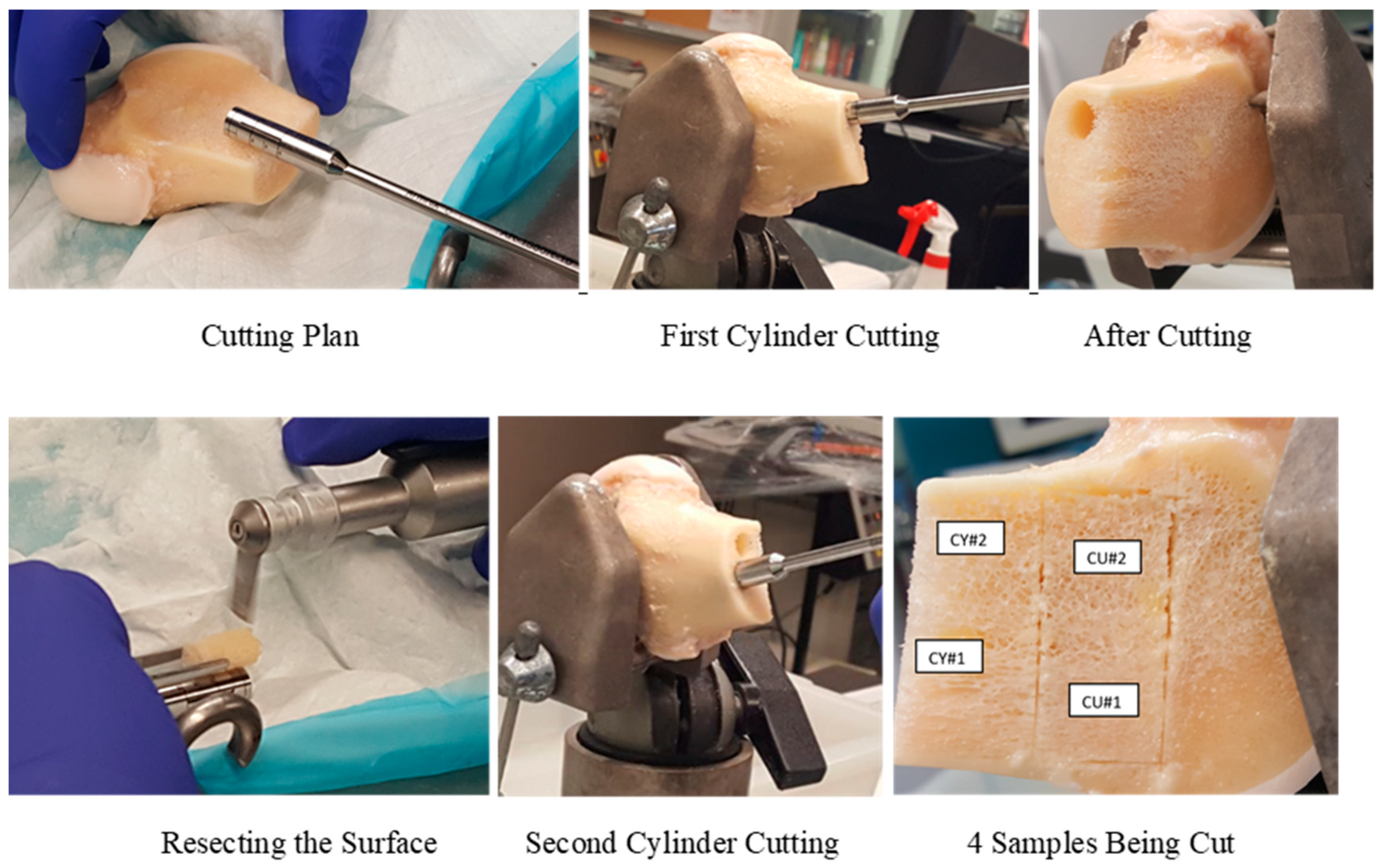
2.1. Cadaver Bone Sample Preparation
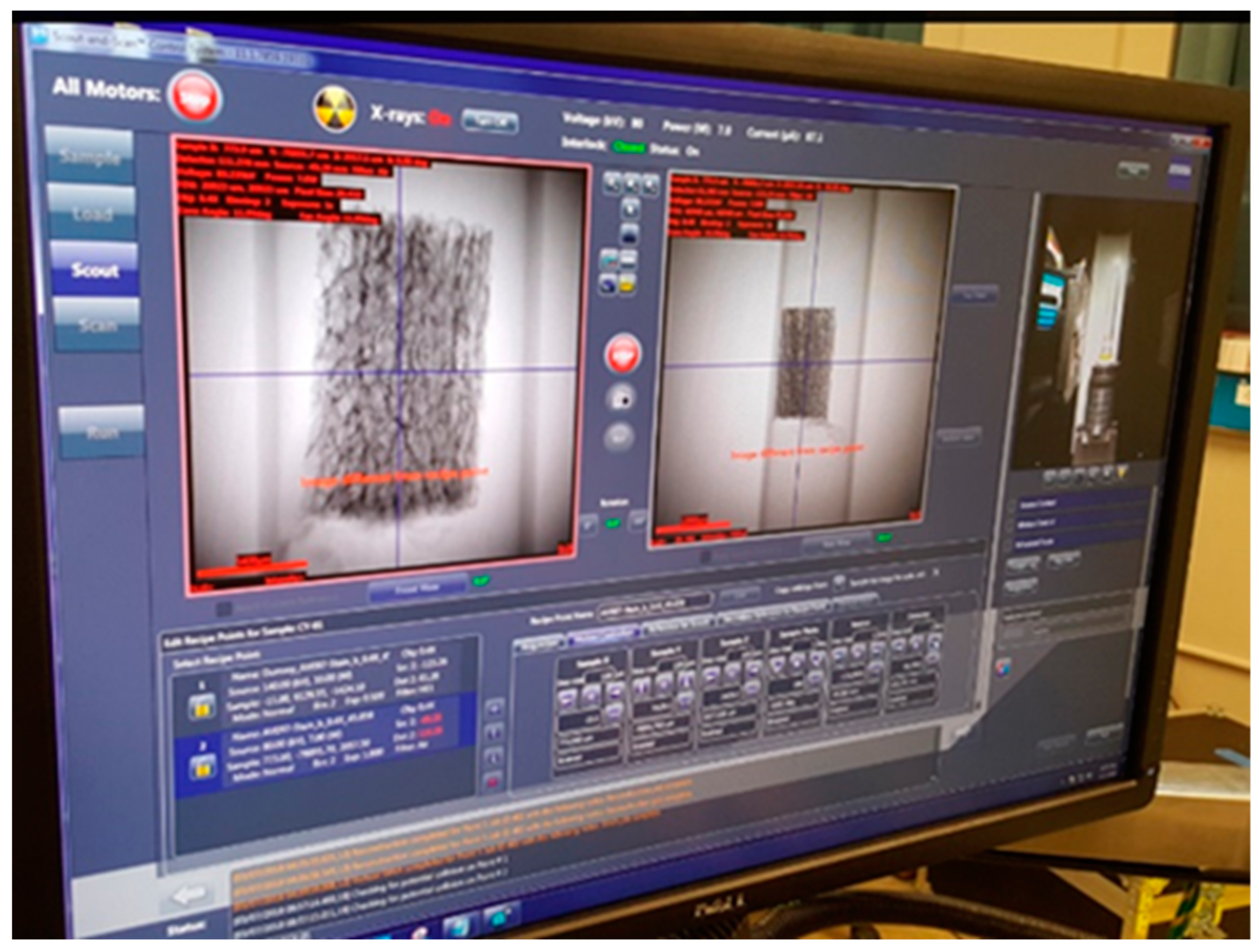
2.2. Micro-CT Imaging
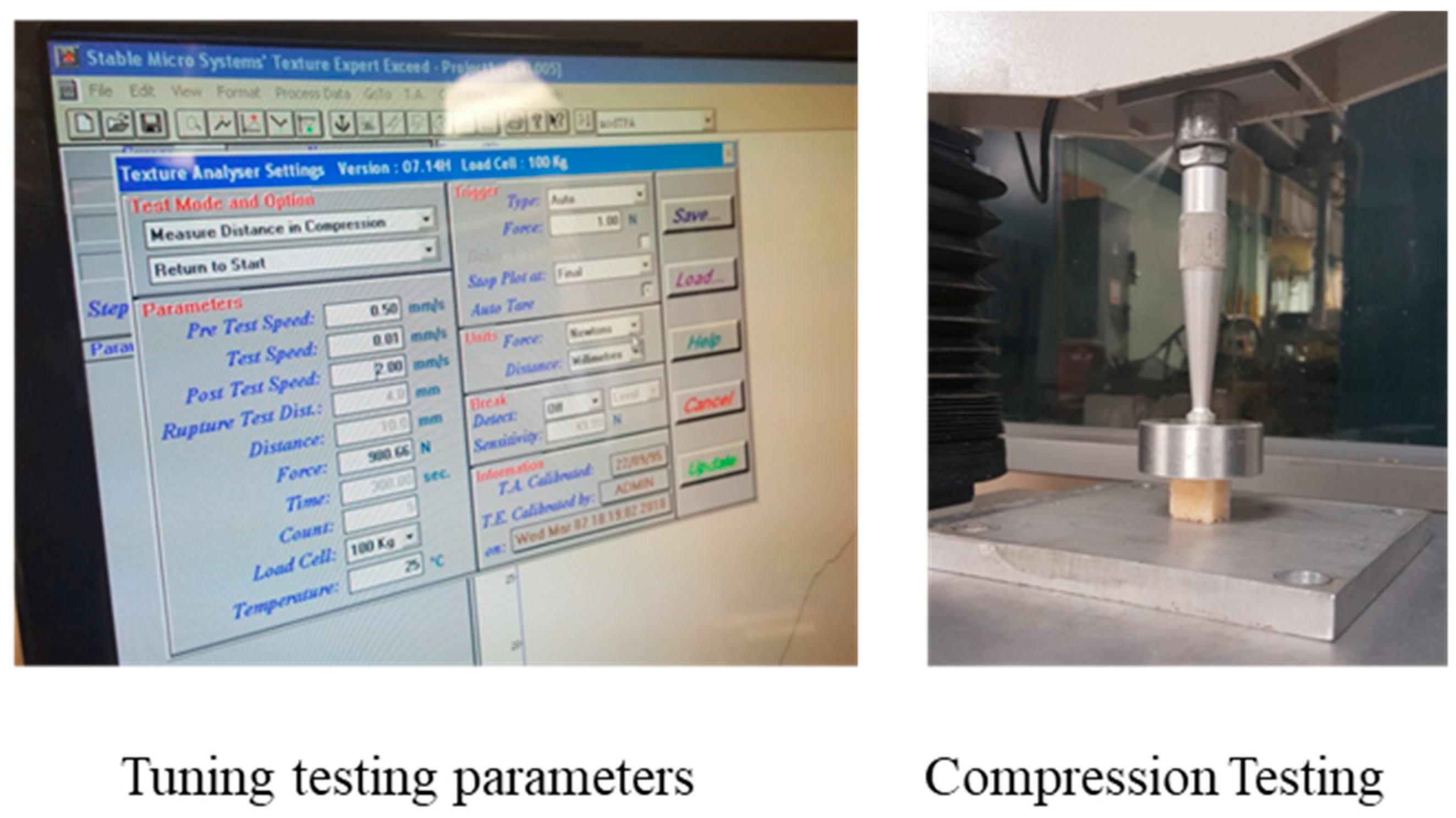
2.3. Experimental Characterization of Mechanical Properties of Bone
3. Results and Discussion
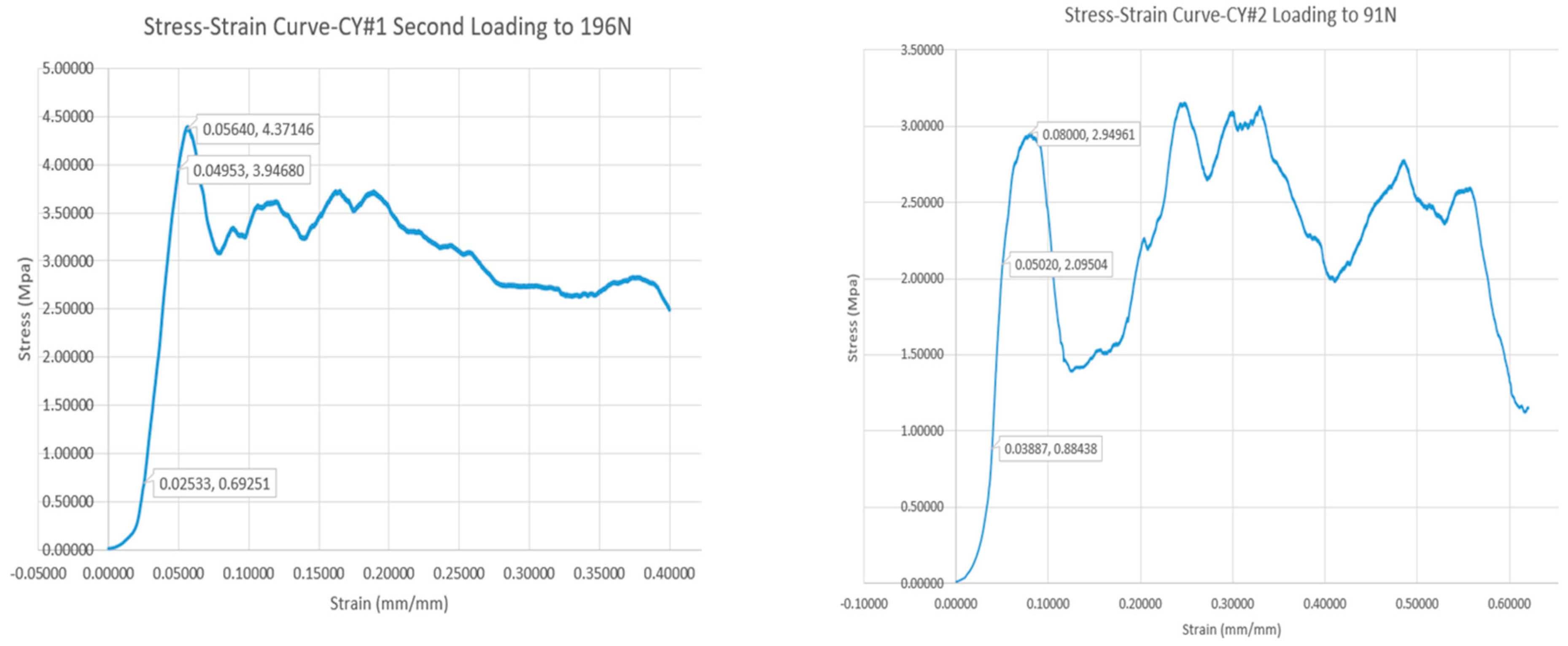
3.1. Mechanical Testing Results
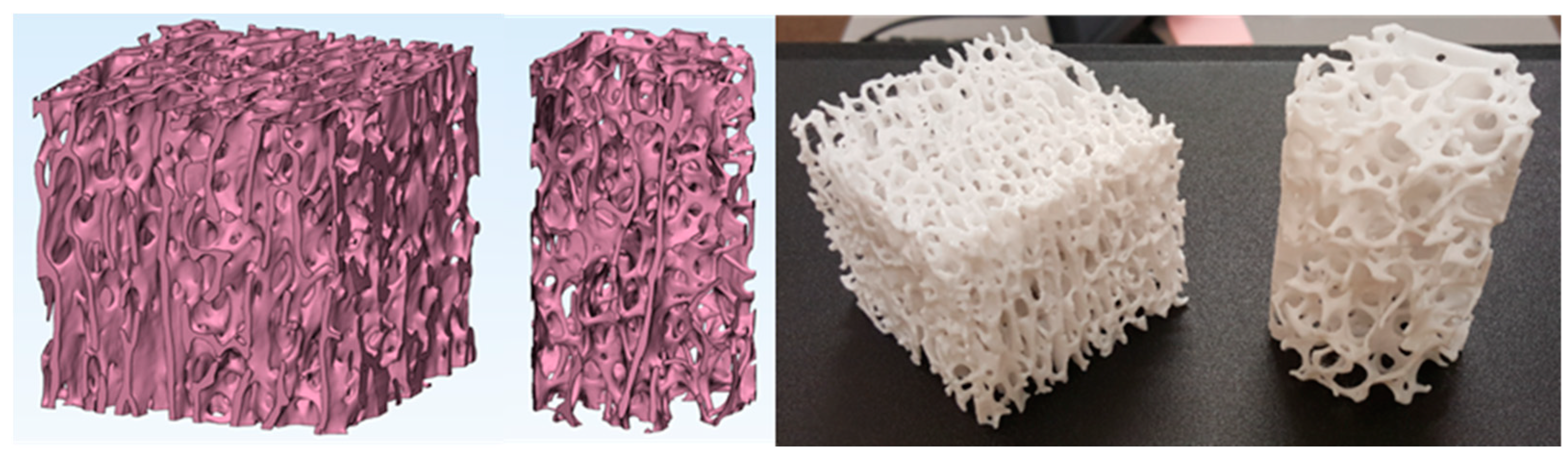
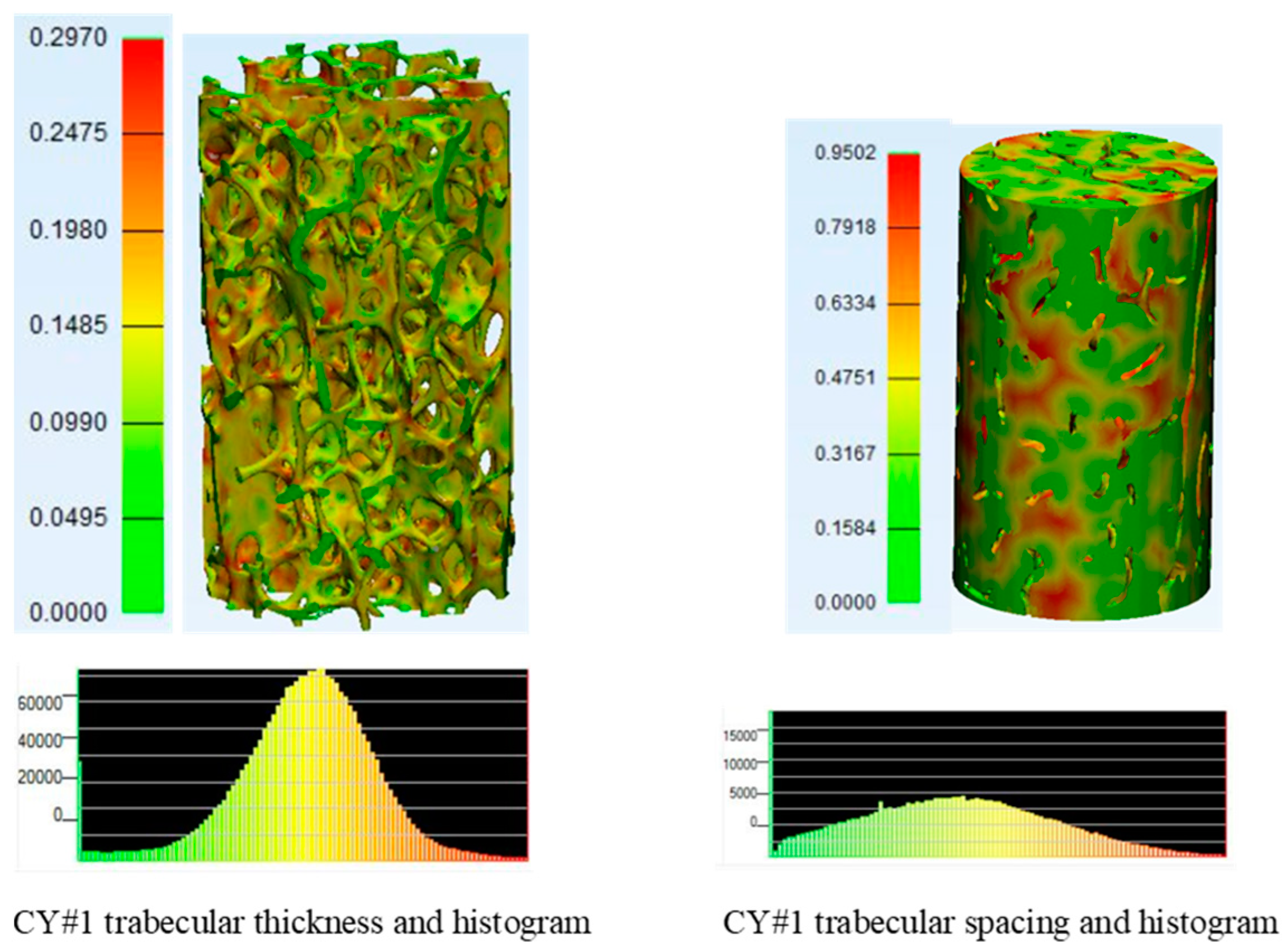
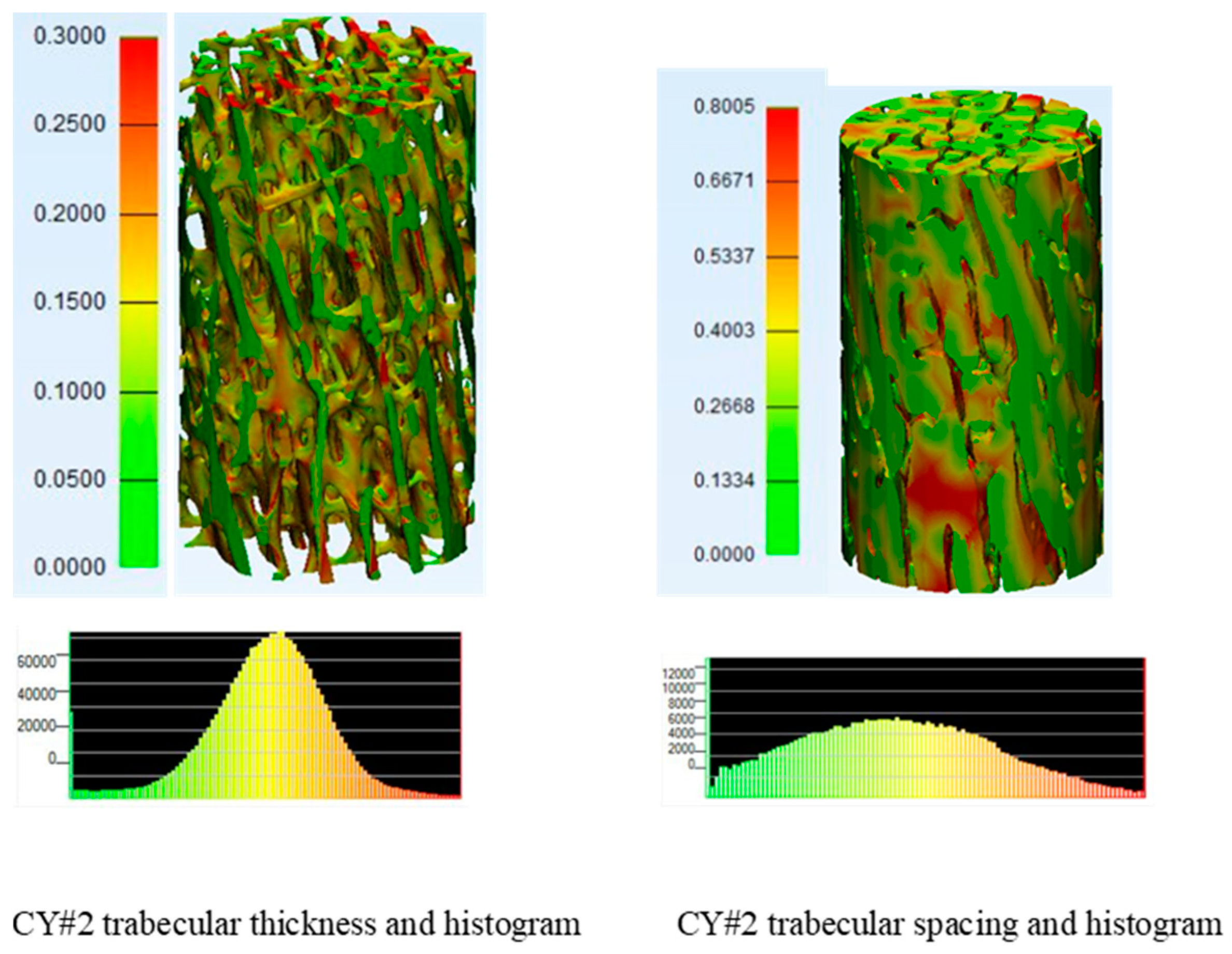
3.2. Micro-CT Scans Results and Analysis
3.2.1. Segmentation of Micro-CT Images
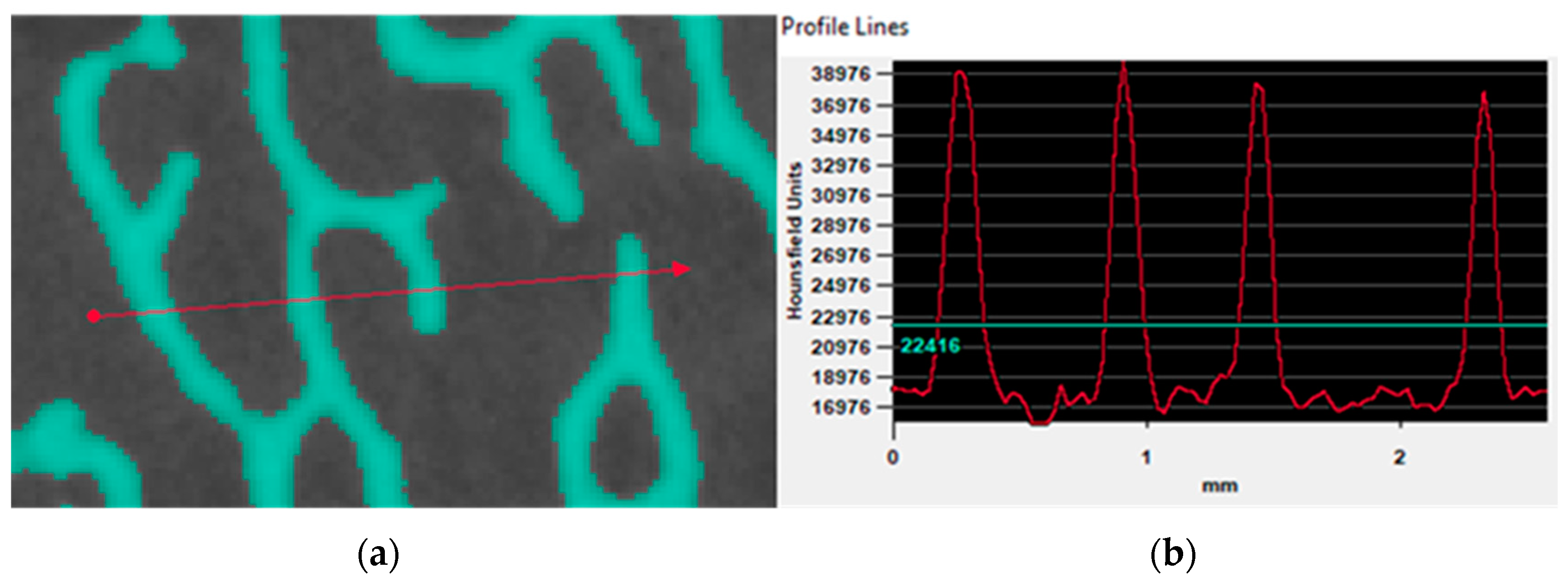
3.2.2. Gray Value Analysis on Micro-CT Data
3.2.3. Gray-Scale Value Based Bone Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciarelli, M.J.; Goldstein, S.A.; Kuhn, J.L.; Cody, D.D.; Brown, M.B. Evaluation of orthogonal mechanical-properties and density of human trabecular bone from the major metaphyseal regions with materials testing and computed-tomography. J. Orthop. Res. 1991, 9, 674–682. [Google Scholar] [CrossRef]
- Macneil, J.A.; Boyd, S.K. Bone strength at the distal radius can be estimated from high-resolution peripheral quantitative computed tomography and the finite element method. Bone 2008, 42, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Dumas, M.; Terriault, P.; Brailovski, V. Modelling and characterization of a porosity graded lattice structure for additively manufactured biomaterials. Mater. Des. 2017, 121, 383–392. [Google Scholar] [CrossRef]
- Arnone, J.; Crist, B.; Ward, C.; El-Gizawy, A.; Pashuck, T.; Della Rocca, G. Variability of human femoral geometry and its implications on nail design. Injury 2021, 52, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Adeeb, S.; Doschak, M.R. Using micro-CT derived bone microarchitecture to analyze bone stiffness—A case study on osteoporosis rat bone. Front. Endocrinol. 2015, 6, 80. Available online: www.frontiersin.org (accessed on 4 September 2022). [CrossRef] [PubMed]
- Ryan, M.; Barnet, L.; Rochester, J.; Wilkinson, J.M.; Dall’Ara, E. A new approach to comprehensively evaluate the morphological properties of the human femoral head: Example of application to osteoarthritic joint. Sci. Rep. 2020, 10, 5538. [Google Scholar] [CrossRef] [PubMed]
- Uklejewski, R.; Winiecki, M.; Patalas, A.; Rogala, P. Bone Density Micro-CT Assessment during Embedding of the Innovative Multi-Spiked Connecting Scaffold in Periarticular Bone to Elaborate a Validated Numerical Model for Designing Biomimetic Fixation of Resurfacing Endoprostheses. Materials 2021, 14, 1384. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.; Ashby, M.; Harley, B. Cellular Materials in Nature and Medicine; Cambridge University Press: Cambridge, UK, 2020; ISBN 9780521195447. [Google Scholar]
- Zhou, B.; Liu, X.S.; Wang, J.; Lu, X.L.; Fields, A.; Guo, X.E. Dependence of mechanical properties of trabecular bone on plate-rod microstructure determined by individual trabecula segmentation (ITS). J. Biomech. 2014, 47, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Chiba, K.; Okazaki, N.; Yokota, K.; Nakazoe, Y.; Kidera, K.; Yonekura, A.; Tomita, M.; Osaki, M. In vivo analysis of subchondral trabecular bone in patients with osteoarthritis of the knee using second-generation high-resolution peripheral quantitative computed tomography (HR-PQCT). Bone 2020, 132, 115155. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, N.; Chiba, K.; Motoi, M.; Osaki, M. Analysis of Subchondral Bone Microstructure by HR-PQCT: Relationship with the Severity of Knee Osteoarthritis and Alignments of Lower Extremities. Osteoarthr. Cartil. 2017, 25, S263. [Google Scholar] [CrossRef]
- Akhter, M.P.; Recker, R.R. High-resolution imaging in bone tissue research-review. Bone 2021, 143, 115620. [Google Scholar] [CrossRef] [PubMed]
- Diederichs, G.; Link, T.M.; Kentenich, M.; Schwieger, K.; Huber, M.B.; Burghardt, A.J. Assessment of trabecular bone structure of the calcaneus using multi- detector CT: Correlation with micro CT and biomechanical testing. Bone 2009, 44, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Parsa, A.; Ibrahim, N.; Hassan, H.; Van der Stelt, P.; Wismeijer, D. Bone quality evaluation at dental implant site using multi-slice CT, micro-CT, and cone beam CT. Clin. Oral Implants Res. 2015, 26, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Domander, R.; Felder, A.A.; Doube, M. BoneJ2—Refactoring Established Research Software. Available online: https://pubmed.ncbi.nlm.nih.gov/33954267 (accessed on 4 September 2022).
- Materialize. Mimics Innovation Suite. 2022. Available online: https://www.materialise.com/en/medical/mimics-innovation-suite (accessed on 4 September 2022).
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mandal, S.; Barui, S.; Vasireddi, R.; Gbureck, U.; Gelinsky, M.; Basu, B. Low temperature additive manufacturing of three-dimensional scaffolds for bone-tissue engineering applications: Processing related challenges and property assessment. Mat. Sci. Eng. R 2016, 103, 1–39. [Google Scholar] [CrossRef]


| Sample Identification | Voxel Size (Micrometer) | Kv | W | Objective | Filter | Exposure (Sec) | Projections | Time per Sample (Hours) |
|---|---|---|---|---|---|---|---|---|
| CU#1 | 27.318 | 50 | 4 | 4 | ||||
| CU#2 | 27.318 | 0.4X | LE1 | 4 | 1201 | 2 | ||
| CY#1 | 18.011 | 4.5 | ||||||
| CY#2 | 18.011 | 4.5 |
| Sample Identification | Modulus Si (MPa) | Yield Strength (MPa) | Yield Strain |
|---|---|---|---|
| CU#1 | 133 | 8.67 (Max) | 0.093 |
| CU#2 | 45 | 2.70 (Min) | 0.101 |
| CY#1 | 134 | 4.40 | 0.056 |
| CY#2 | 107 | 2.95 | 0.080 |
| AVERAGE | 105 | 4.68 | 0.082 |
| STANDARD DEVIATION | 42 | 2.76 | 0.0196 |
| Bone Sample Identification | Elastic Modulus (MPa) | Yield Strength (MPa) |
|---|---|---|
| 1 | 9.0 | 0.3 |
| 2 | 35.3 | 2.0 |
| 3 | 18.8 | 0.4 |
| 4 | 20.9 | 0.6 |
| 5 | 41.2 | 1.6 |
| 6 | 13.1 | 0.4 |
| 7 | 108.2 | 2.5 |
| 8 | 54.9 | 2.5 |
| 9 | 102.9 | 3.2 |
| 10 | 20.7 | 2.0 |
| 11 | 28.1 | 0.7 |
| 12 | 46.3 | 0.6 |
| 13 | 47.1 | 0.6 |
| 14 | 35.8 | 0.6 |
| 15 | 395.0 | 6.0 |
| 16 | 1903.4 | 8.5 |
| 17 | 33.9 | 0.6 |
| 18 | 101.6 | 2.0 |
| 19 | 241.2 | 8.7 |
| 20 | 82.4 | 2.7 |
| 21 | 134.5 | 4.4 |
| 22 | 106.8 | 3.0 |
| AVERAGE VALUE | 163.0 | 2.4 |
| STANDARD DEVIATION | 399.00 | 2.5 |
| Trabecular Boone Sample Identification | BV/TV (%) | BS/TV (MM−1) | BS/BV (MM−1) | TB.TH (MM) | TB.TH.SD (MM) | TB.SP (MM) | TB.SP.SD (MM) | DA | CONN.D (MM−3) |
|---|---|---|---|---|---|---|---|---|---|
| CU#1 | 26.876 | 3.454 | 12.851 | 0.154 | 0.037 | 0.318 | 0.151 | 0.552 | 4.523 |
| CU#2 | 18.929 | 2.685 | 14.187 | 0.148 | 0.041 | 0.363 | 0.172 | 0.270 | 3.161 |
| CY#1 | 15.876 | 2.270 | 14.296 | 0.151 | 0.048 | 0.382 | 0.206 | 0.370 | 3.032 |
| CY#2 | 22.356 | 3.061 | 13.680 | 0.152 | 0.05 | 0.341 | 0.181 | 0.556 | 2.919 |
| Average | 21.009 | 2.867 | 13.756 | 0.151 | 0.044 | 0.351 | 0.178 | 0.437 | 3.409 |
| Standard Deviation | 4.723 | 0.507 | 0.658 | 0.0025 | 0.006 | 0.028 | 0.023 | 0.141 | 0.749 |
| Grayscale Measurement Location | Calculated Stiffness (MPa) |
|---|---|
| 1 | 156 |
| 2 | 210 |
| 3 | 270 |
| 4 | 338 |
| 5 | 412 |
| 6 | 492 |
| 7 | 578 |
| 8 | 671 |
| 9 | 770 |
| 10 | 874 |
| Average (MPa) | 507 |
| Standard Deviation (MPa) | 238 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Gizawy, A.S.; Ma, X.; Pfeiffer, F.; Schiffbauer, J.D.; Selly, T. Characterization of Microarchitectures, Stiffness and Strength of Human Trabecular Bone Using Micro-Computed Tomography (Micro-CT) Scans. BioMed 2023, 3, 89-100. https://doi.org/10.3390/biomed3010007
El-Gizawy AS, Ma X, Pfeiffer F, Schiffbauer JD, Selly T. Characterization of Microarchitectures, Stiffness and Strength of Human Trabecular Bone Using Micro-Computed Tomography (Micro-CT) Scans. BioMed. 2023; 3(1):89-100. https://doi.org/10.3390/biomed3010007
Chicago/Turabian StyleEl-Gizawy, Ahmed Sherif, Xuewei Ma, Ferris Pfeiffer, James D. Schiffbauer, and Tara Selly. 2023. "Characterization of Microarchitectures, Stiffness and Strength of Human Trabecular Bone Using Micro-Computed Tomography (Micro-CT) Scans" BioMed 3, no. 1: 89-100. https://doi.org/10.3390/biomed3010007
APA StyleEl-Gizawy, A. S., Ma, X., Pfeiffer, F., Schiffbauer, J. D., & Selly, T. (2023). Characterization of Microarchitectures, Stiffness and Strength of Human Trabecular Bone Using Micro-Computed Tomography (Micro-CT) Scans. BioMed, 3(1), 89-100. https://doi.org/10.3390/biomed3010007






