Changes in Physical Activity and Glycemic Control before and after the Declaration of the State of Emergency Due to the COVID-19 Pandemic in Japanese Adult Females with Type 1 Diabetes: A 1-Year Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.3. Statistical Analysis
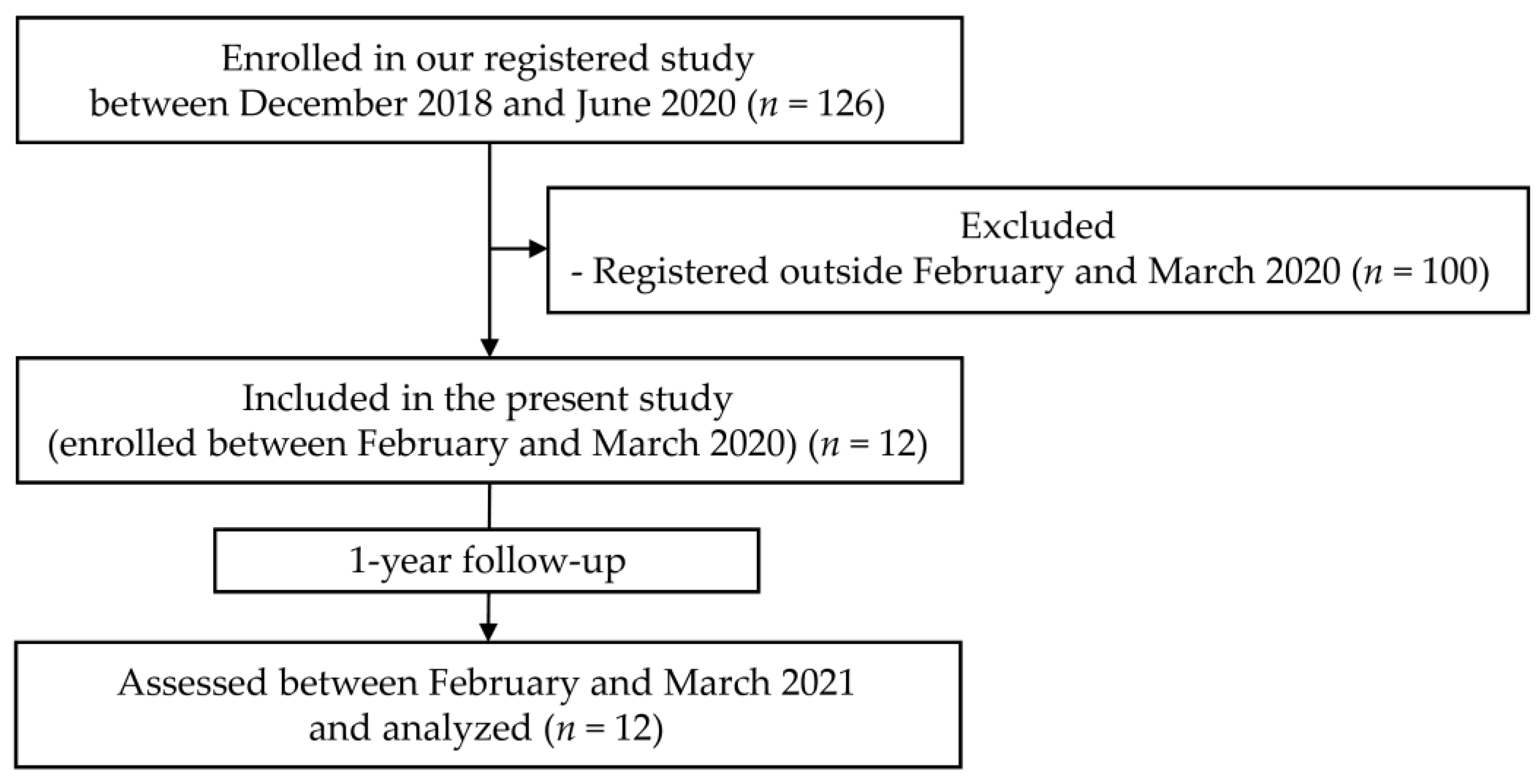
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makizako, H.; Nakai, Y.; Shiratsuchi, D.; Akanuma, T.; Yokoyama, K.; Matsuzaki-Kihara, Y.; Yoshida, H. Perceived declining physical and cognitive fitness during the COVID-19 state of emergency among community-dwelling Japanese old-old adults. Geriatr. Gerontol. Int. 2021, 21, 364–369. [Google Scholar] [CrossRef]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Otobe, Y.; Suzuki, M.; Koyama, S.; Kikuchi, T.; Kusumi, H.; Arai, H. Effect of the COVID-19 Epidemic on Physical Activity in Community-Dwelling Older Adults in Japan: A Cross-Sectional Online Survey. J. Nutr. Health Aging 2020, 24, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, M.; Ishikawa, T.; Odawara, M. Behavioral changes in patients with diabetes during the COVID-19 pandemic. Diabetol. Int. 2020, 12, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Tomonaga, O. Study on the effects of changes in lifestyle of patients with diabetes on glycaemic control before and after the declaration of the state of emergency in Japan. Diabetol. Int. 2021, 13, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, Y.; Munekawa, C.; Hashimoto, Y.; Okamura, T.; Takahashi, F.; Kawano, R.; Nakajima, H.; Majima, S.; Senmaru, T.; Nakanishi, N.; et al. The effect of COVID-19 pandemic on the lifestyle and glycemic control in patients with type 1 diabetes: A retrospective cohort study. Diabetol. Int. 2022, 13, 85–90. [Google Scholar] [CrossRef]
- Carney, T.A.; Guy, S.P.; Helliwell, C.D. Seasonal variation in HbA1c in patients with Type 2 diabetes mellitus. Diabet. Med. 2000, 17, 554–555. [Google Scholar] [CrossRef]
- Chan, C.B.; Ryan, D.A.; Tudor-Locke, C. Relationship between objective measures of physical activity and weather: A longitudinal study. Int. J. Behav. Nutr. Phys. Act. 2006, 3, 21. [Google Scholar] [CrossRef]
- Nordfeldt, S.; Ludvigsson, J. Seasonal variation of HbA1c in intensive treatment of children with type 1 diabetes. J. Pediatr. Endocrinol. Metab. 2000, 13, 529–535. [Google Scholar] [CrossRef]
- Shephard, R.J.; Aoyagi, Y. Seasonal variations in physical activity and implications for human health. Eur. J. Appl. Physiol. 2009, 107, 251–271. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee; Draznin, B.; Aroda, V.R.; Bakris, G.; Benson, G.; Brown, F.M.; Freeman, R.; Green, J.; Huang, E.; Isaacs, D.; et al. 5. Facilitating Behavior Change and Well-being to Improve Health Outcomes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S60–S82. [Google Scholar] [CrossRef]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Masse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Makizako, H.; Akaida, S.; Shono, S.; Shiiba, R.; Taniguchi, Y.; Shiratsuchi, D.; Nakai, Y. Physical Activity and Perceived Physical Fitness during the COVID-19 Epidemic: A Population of 40- to 69-Year-Olds in Japan. Int. J. Environ. Res. Public Health 2021, 18, 4832. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, B.; Annuzzi, G.; Creanza, A.; Giglio, C.; De Angelis, R.; Lupoli, R.; Masulli, M.; Riccardi, G.; Rivellese, A.A.; Bozzetto, L. Blood Glucose Control During Lockdown for COVID-19: CGM Metrics in Italian Adults with Type 1 Diabetes. Diabetes Care 2020, 43, e88–e89. [Google Scholar] [CrossRef] [PubMed]
- Maddaloni, E.; Coraggio, L.; Pieralice, S.; Carlone, A.; Pozzilli, P.; Buzzetti, R. Effects of COVID-19 Lockdown on Glucose Control: Continuous Glucose Monitoring Data From People With Diabetes on Intensive Insulin Therapy. Diabetes Care 2020, 43, e86–e87. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee; Draznin, B.; Aroda, V.R.; Bakris, G.; Benson, G.; Brown, F.M.; Freeman, R.; Green, J.; Huang, E.; Isaacs, D.; et al. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S83–S96. [Google Scholar] [CrossRef]
- Furusyo, N.; Hayashi, J. Glycated albumin and diabetes mellitus. Biochim. Biophys. Acta 2013, 1830, 5509–5514. [Google Scholar] [CrossRef]
- Hamilton, M.T.; Hamilton, D.G.; Zderic, T.W. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 2007, 56, 2655–2667. [Google Scholar] [CrossRef]
- Peddie, M.C.; Bone, J.L.; Rehrer, N.J.; Skeaff, C.M.; Gray, A.R.; Perry, T.L. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: A randomized crossover trial. Am. J. Clin. Nutr. 2013, 98, 358–366. [Google Scholar] [CrossRef]
- Smith, J.A.B.; Savikj, M.; Sethi, P.; Platt, S.; Gabriel, B.M.; Hawley, J.A.; Dunstan, D.; Krook, A.; Zierath, J.R.; Naslund, E. Three weeks of interrupting sitting lowers fasting glucose and glycemic variability, but not glucose tolerance, in free-living women and men with obesity. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E203–E216. [Google Scholar] [CrossRef]
- Garcia-Tascon, M.; Sahelices-Pinto, C.; Mendana-Cuervo, C.; Magaz-Gonzalez, A.M. The Impact of the COVID-19 Confinement on the Habits of PA Practice According to Gender (Male/Female): Spanish Case. Int. J. Environ. Res. Public Health 2020, 17, 6961. [Google Scholar] [CrossRef]
- Suga, H.; Asakura, K.; Sasaki, S.; Nojima, M.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Effect of seasonality on the estimated mean value of nutrients and ranking ability of a self-administered diet history questionnaire. Nutr. J. 2014, 13, 51. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Variables | Values |
|---|---|
| Age (years) | 48.3 (41.8, 54.9) |
| Duration of type 1 diabetes (years) | 10.2 (6.9, 13.5) |
| Insulin therapy and glucose monitoring | |
| -MDI and intermittently scanned CGM (n) (%) | 4 (33.3) |
| -CSII and intermittently scanned CGM (n) (%) | 2 (16.7) |
| -MDI and real-time CGM (n) (%) | 1 (8.3) |
| -CSII and real-time CGM (SAP) (n) (%) | 5 (41.7) |
| Oral anti-diabetic agents | |
| -Sodium-glucose cotransporter-2 inhibitor (n) (%) | 2 (16.7) |
| Diabetes complication | |
| -Neuropathy (n) (%) | 5 (41.7) |
| -Retinopathy (n) (%) | 0 (0.0) |
| -Nephropathy (n) (%) | 0 (0.0) |
| Hypertension (n) (%) | 1 (8.3) |
| Dyslipidemia (n) (%) | 5 (41.7) |
| Occupation | |
| -Homemaker (n) (%) | 2 (16.7) |
| -Sedentary worker (n) (%) | 3 (25.0) |
| -Other (n) (%) | 6 (50.0) |
| -Unemployed (n) (%) | 1 (8.3) |
| Exercise habits § (n) (%) | 3 (25.0) |
| Variables | Before | After | ES (Cohen’s d, r) | p-Value | |
|---|---|---|---|---|---|
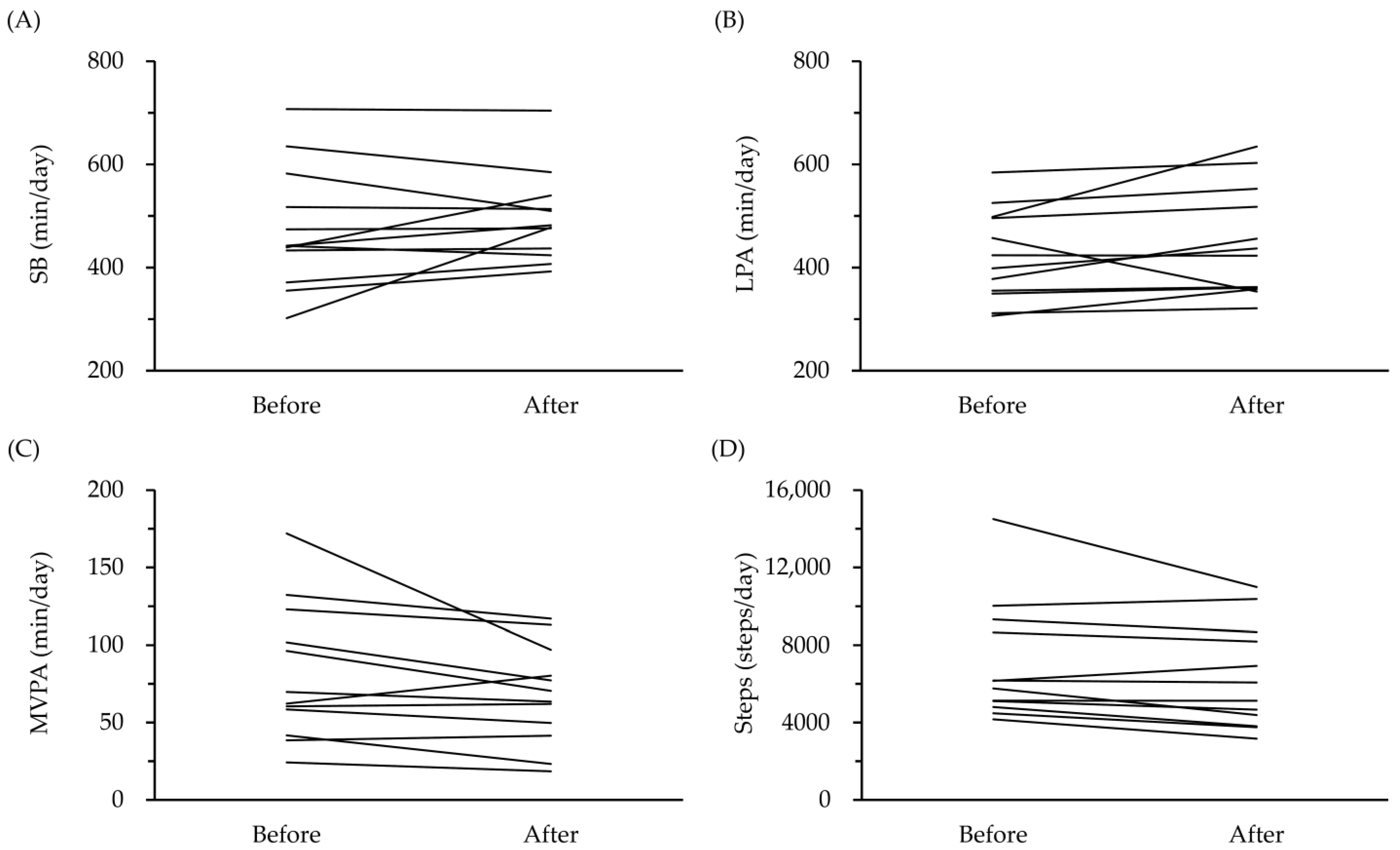
| PA | Total SB time (min/day) | 475.1 (400.1, 550.0) | 495.7 (440.8, 550.5) | 0.311 | 0.304 |
| Prolonged SB | |||||
| -Total time (min/day) † | 104.0 (64.4, 202.7) | 149.8 (111.9, 183.1) | 0.181 | 0.530 | |
| -Frequency per day (times/day) | 2.7 (1.8, 3.5) | 3.3 (2.5, 4.1) | 0.456 | 0.142 | |
| -Duration per prolonged SB (min/time) † | 46.1 (40.6, 51.8) | 44.4 (39.3, 55.0) | 0.023 | 0.937 | |
| LPA (min/day) | 423.6 (367.0, 480.2) | 448.3 (381.0, 515.6) | 0.443 | 0.153 | |
| MVPA | |||||
| -Total time (min/day) | 81.7 (53.8, 109.7) | 67.8 (47.7, 87.9) | 0.611 | 0.058 | |
| -Long bouts of MVPA (min/day) | 17.1 (8.6, 25.6) | 11.1 (5.4, 16.7) | 0.724 | 0.029 * | |
| -Short bouts of MVPA (min/day) | 64.6 (42.9, 86.3) | 56.7 (41.0, 72.4) | 0.400 | 0.193 | |
| LMVPA (min/day) | 505.4 (436.3, 574.4) | 516.1 (445.8, 586.5) | 0.166 | 0.578 | |
| Total PA volume of ≥3 METs (MET-min/day) | 421.6 (317.6, 525.7) | 400.6 (301.2, 500.0) | 0.243 | 0.417 | |
| Steps (steps/day) † | 5959.3 (4881.3, 9153.1) | 5601.5 (3952.6, 8552.7) | 0.634 | 0.028 * | |
| Energy consumption (kcal/day) | 2138.1 (1979.4, 2296.8) | 2108.0 (1960.4, 2255.7) | 0.268 | 0.373 | |
| Glycemic control | HbA1c (%) | 7.5 (7.2, 7.9) | 7.6 (7.2, 8.1) | 0.319 | 0.293 |
| GA (%) | 21.6 (19.3, 24.0) | 21.5 (19.4, 23.6) | 0.067 | 0.821 | |
| GA/HbA1c ratio | 2.9 (2.7, 3.1) | 2.8 (2.6, 3.0) | 0.229 | 0.444 | |
| Mean 24-h SG (mg/dL) | 145.8 (137.2, 154.3) | 152.4 (144.6, 160.3) | 0.417 | 0.177 | |
| TAR (%) | 23.5 (17.6, 29.5) | 29.0 (22.4, 35.6) | 0.709 | 0.032 * | |
| TIR (%) | 69.5 (62.1, 76.8) | 66.6 (59.4, 73.7) | 0.455 | 0.143 | |
| TBR (%) † | 3.0 (1.2, 12.6) | 4.3 (0.7, 7.0) | 0.308 | 0.286 | |
| Total daily insulin dose (U) † | 30.0 (22.7, 40.3) | 31.1 (23.5, 40.4) | 0.202 | 0.484 | |
| Other parameters | BMI (kg/m2) | 24.5 (21.9, 27.1) | 24.6 (21.8, 27.4) | 0.160 | 0.590 |
| Waist circumference (cm) | 80.7 (74.5, 86.9) | 80.0 (73.7, 86.4) | 0.159 | 0.593 | |
| Triglyceride (mg/dL) † | 62.5 (51.8, 120.8) | 55.5 (46.3, 84.0) | 0.374 | 0.195 | |
| LDL-cholesterol (mg/dL) | 115.1 (101.3, 128.9) | 107.9 (91.7, 124.2) | 0.324 | 0.285 | |
| HDL-cholesterol (mg/dL) | 73.8 (66.4, 81.1) | 75.8 (66.4, 85.1) | 0.240 | 0.423 | |
| Total protein (g/dL) † | 7.2 (6.8, 7.5) | 7.0 (6.7, 7.3) | 0.503 | 0.082 | |
| Albumin (g/dL) † | 4.3 (4.2, 4.4) | 4.1 (4.1, 4.3) | 0.475 | 0.100 | |
| Serum creatinine (mg/dL) | 0.61 (0.57, 0.65) | 0.61 (0.57, 0.65) | 0.064 | 0.828 | |
| eGFR (mL/min/1.73m2) | 82.4 (75.8, 88.9) | 81.5 (75.8, 87.1) | 0.233 | 0.436 |
| Variables | BMI | HbA1c | GA | GA/HbA1c Ratio | Mean 24-h SG | TAR | TIR | TBR |
|---|---|---|---|---|---|---|---|---|
| Total SB time | −0.251 (0.484) | −0.057 (0.877) | −0.189 (0.601) | −0.151 (0.677) | 0.473 (0.167) | 0.258 (0.471) | 0.427 (0.218) | −0.567 (0.087) |
| Prolonged SB | ||||||||
| -Total time | −0.005 (0.990) | −0.090 (0.804) | 0.249 (0.488) | 0.281 (0.432) | 0.703 (0.023) * | 0.485 (0.155) | 0.378 (0.281) | −0.771 (0.009) ** |
| -Frequency per day | −0.269 (0.453) | 0.029 (0.936) | −0.186 (0.606) | −0.216 (0.549) | 0.620 (0.056) | 0.428 (0.217) | 0.185 (0.610) | −0.569 (0.086) |
| -Duration per prolonged SB | 0.149 (0.680) | −0.105 (0.773) | 0.661 (0.038) * | 0.713 (0.021) * | 0.134 (0.712) | 0.087 (0.811) | 0.365 (0.299) | −0.364 (0.301) |
| LPA | −0.100 (0.783) | 0.084 (0.817) | 0.227 (0.528) | 0.169 (0.640) | −0.100 (0.782) | 0.007 (0.985) | −0.048 (0.895) | 0.013 (0.970) |
| MVPA | ||||||||
| -Total time † | −0.244 (0.498) | −0.067 (0.853) | 0.073 (0.841) | 0.155 (0.670) | −0.406 (0.244) | −0.112 (0.758) | −0.021 (0.954) | 0.537 (0.109) |
| -Long bouts of MVPA † | −0.362 (0.303) | 0.102 (0.780) | 0.259 (0.470) | 0.347 (0.326) | 0.140 (0.700) | 0.354 (0.316) | −0.287 (0.421) | 0.040 (0.913) |
| -Short bouts of MVPA | −0.175 (0.628) | −0.118 (0.745) | 0.225 (0.532) | 0.271 (0.449) | −0.345 (0.329) | −0.301 (0.398) | 0.057 (0.875) | 0.265 (0.459) |
| LMVPA | −0.162 (0.654) | 0.029 (0.937) | 0.307 (0.388) | 0.270 (0.451) | −0.167 (0.644) | −0.057 (0.877) | −0.055 (0.879) | 0.086 (0.813) |
| Total PA volume of ≥3 METs | −0.302 (0.396) | −0.368 (0.295) | 0.197 (0.585) | 0.399 (0.253) | −0.330 (0.352) | −0.295 (0.407) | 0.017 (0.962) | 0.277 (0.438) |
| Steps | −0.468 (0.172) | −0.353 (0.317) | 0.301 (0.398) | 0.487 (0.153) | 0.292 (0.413) | 0.257 (0.474) | −0.004 (0.991) | −0.269 (0.452) |
| Energy consumption | −0.120 (0.742) | −0.194 (0.591) | 0.262 (0.465) | 0.359 (0.309) | −0.289 (0.417) | −0.364 (0.301) | 0.677 (0.032) * | −0.132 (0.717) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honda, H.; Hashimoto, N.; Zenibayashi, M.; Takeda, A.; Takeuchi, T.; Yamamoto, A.; Hirota, Y. Changes in Physical Activity and Glycemic Control before and after the Declaration of the State of Emergency Due to the COVID-19 Pandemic in Japanese Adult Females with Type 1 Diabetes: A 1-Year Follow-Up Study. BioMed 2022, 2, 376-385. https://doi.org/10.3390/biomed2040029
Honda H, Hashimoto N, Zenibayashi M, Takeda A, Takeuchi T, Yamamoto A, Hirota Y. Changes in Physical Activity and Glycemic Control before and after the Declaration of the State of Emergency Due to the COVID-19 Pandemic in Japanese Adult Females with Type 1 Diabetes: A 1-Year Follow-Up Study. BioMed. 2022; 2(4):376-385. https://doi.org/10.3390/biomed2040029
Chicago/Turabian StyleHonda, Hiroto, Naoko Hashimoto, Masako Zenibayashi, Akihiko Takeda, Takehito Takeuchi, Akane Yamamoto, and Yushi Hirota. 2022. "Changes in Physical Activity and Glycemic Control before and after the Declaration of the State of Emergency Due to the COVID-19 Pandemic in Japanese Adult Females with Type 1 Diabetes: A 1-Year Follow-Up Study" BioMed 2, no. 4: 376-385. https://doi.org/10.3390/biomed2040029
APA StyleHonda, H., Hashimoto, N., Zenibayashi, M., Takeda, A., Takeuchi, T., Yamamoto, A., & Hirota, Y. (2022). Changes in Physical Activity and Glycemic Control before and after the Declaration of the State of Emergency Due to the COVID-19 Pandemic in Japanese Adult Females with Type 1 Diabetes: A 1-Year Follow-Up Study. BioMed, 2(4), 376-385. https://doi.org/10.3390/biomed2040029






