Clustering Diseases in Cancer and Health Organization: What Is the Gold-Standard Approach?
Abstract
1. Background
2. Methods
The Concept of Comorbidity and Frailty
3. Results
3.1. The Prevalence of Comorbidity in Cancer Patients
3.2. Mechanisms of Interaction between Neoplasia and Comorbidities
3.3. Impact of Comorbidities on the Time to Diagnosis of Neoplasms
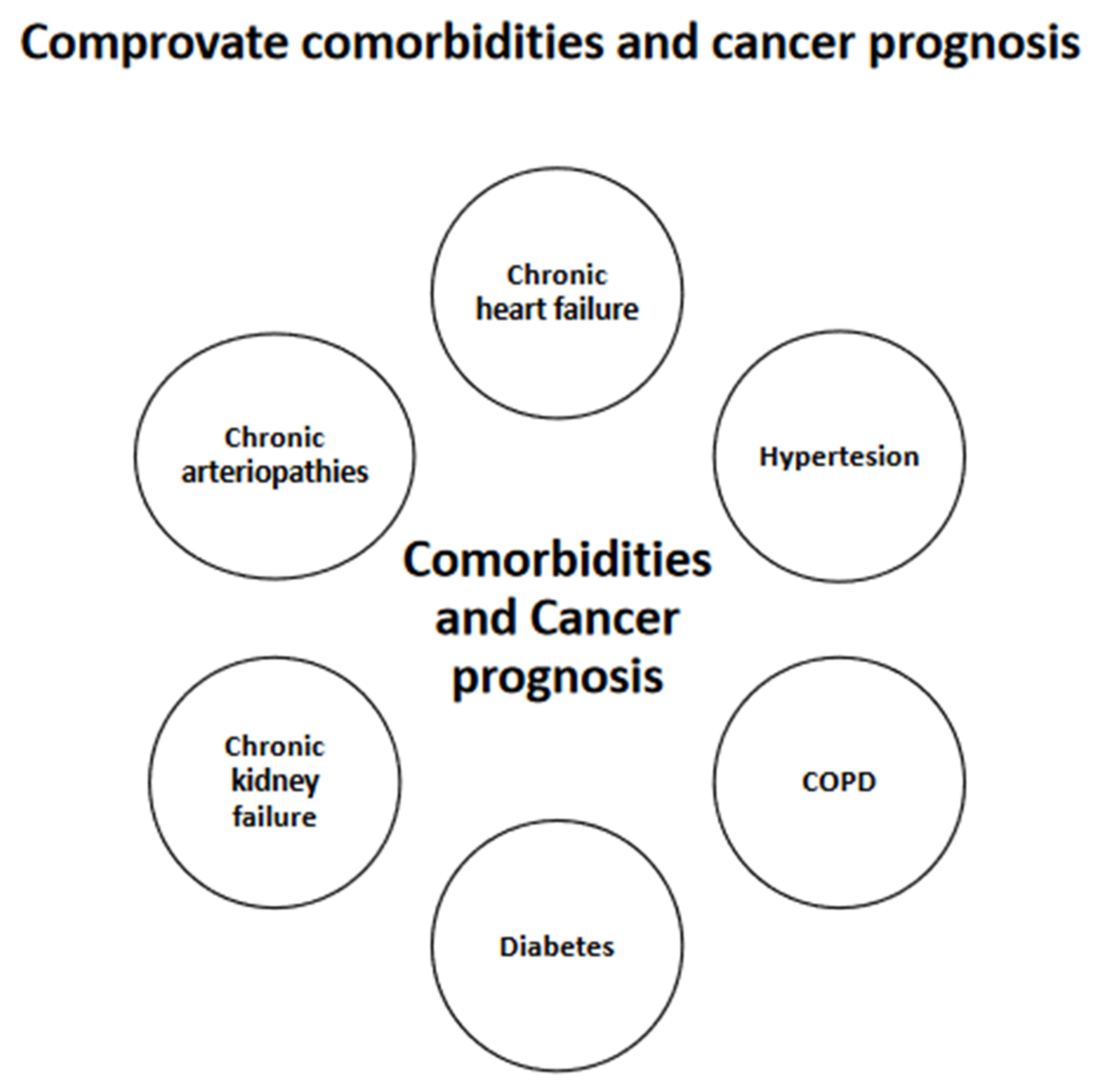
3.4. Types and Severity of Comorbidities
3.5. The Impact of the Organization of the Health Service
3.6. Impact of Comorbidity on the Choice of Treatment for Neoplasms
3.7. Impact of Comorbidities on the Outcome of Cancer Treatment
3.8. Impact on Survival
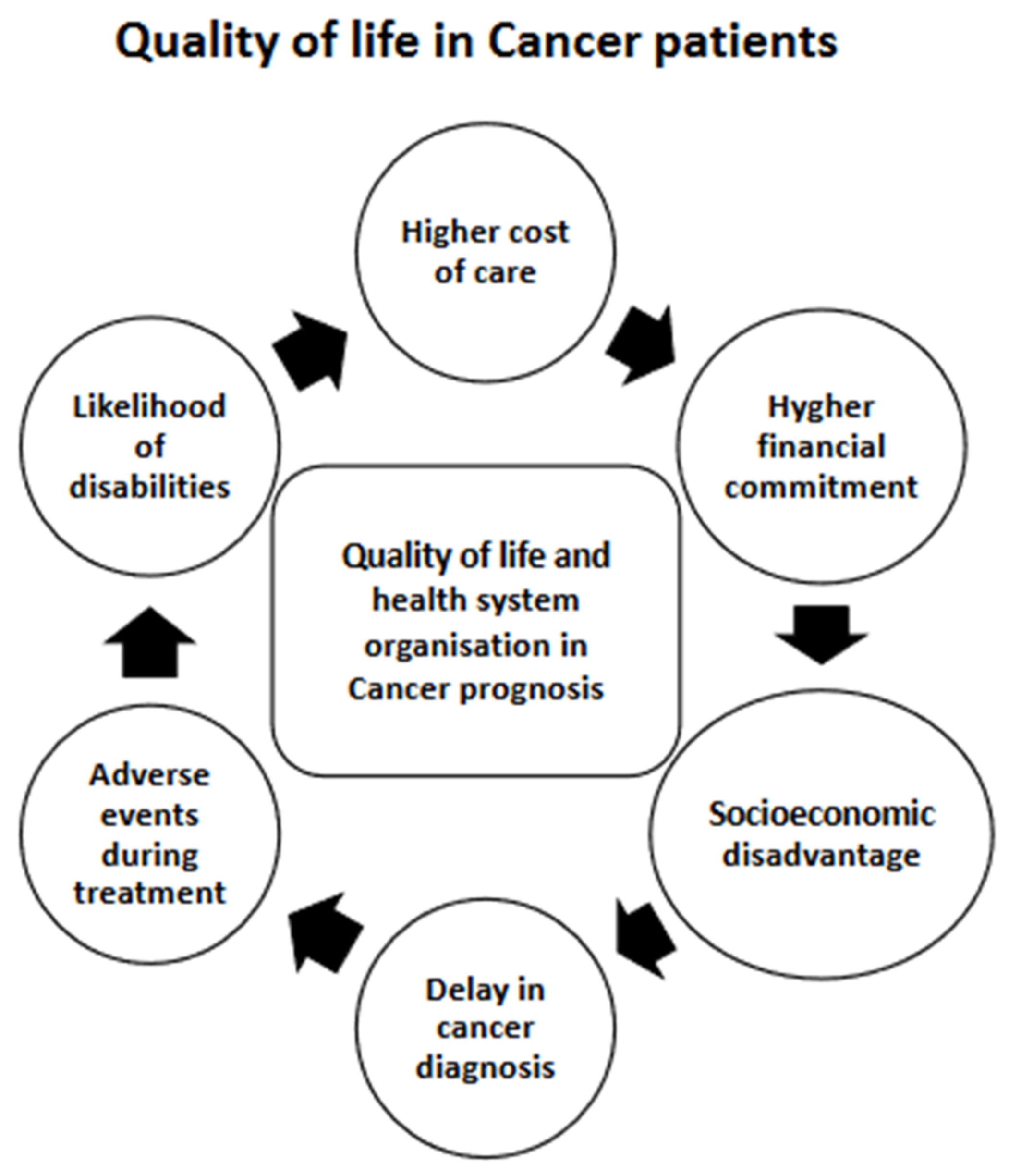
3.9. Impact on Quality of Life
3.10. Impact of Cancer Treatments on Comorbidities
3.11. COVID-19 and Management of Cancer Patients
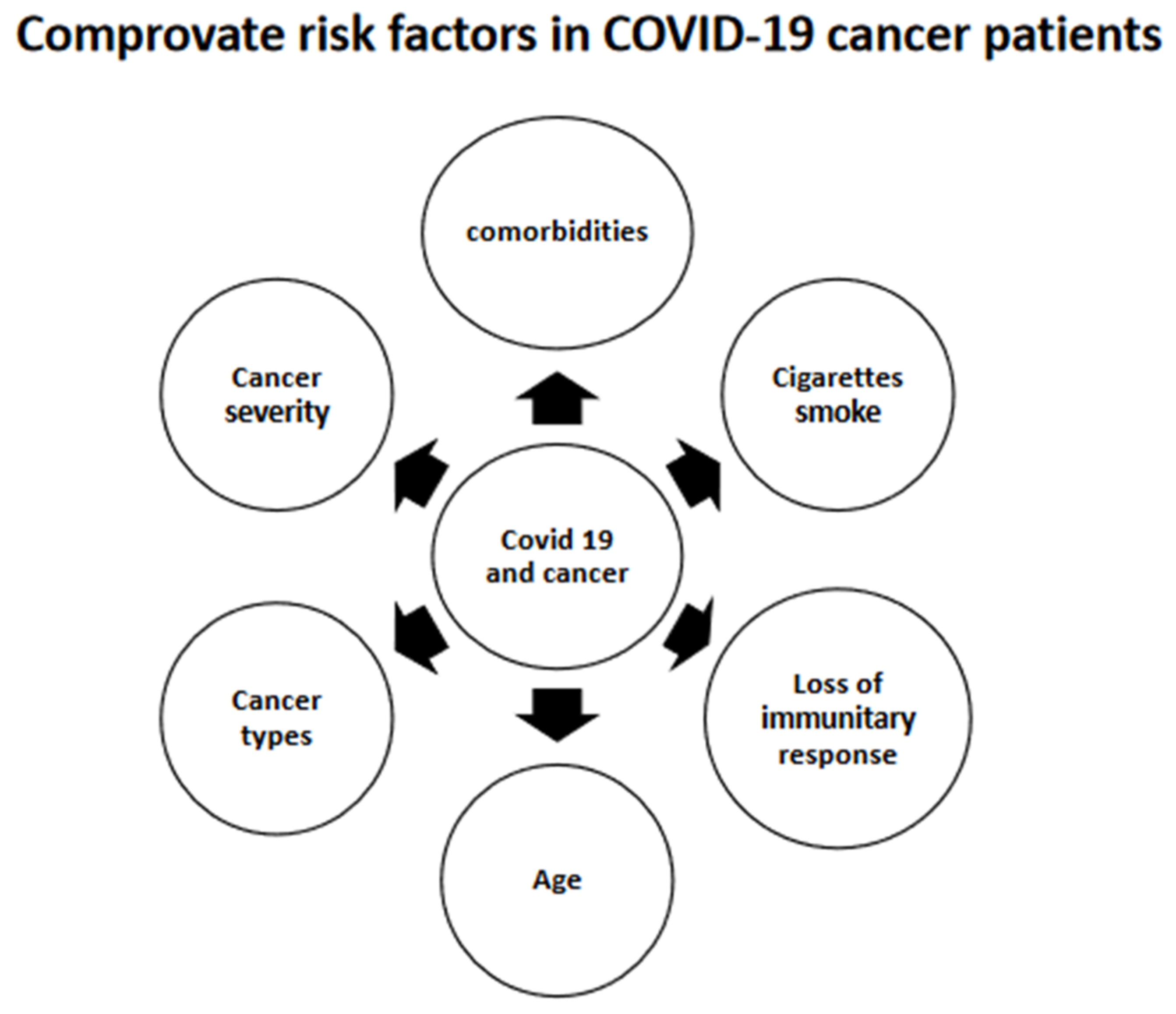
3.11.1. A. Risk Factors Associated with the Severity of COVID-19 in Cancer Patients
3.11.2. B. Health Organization and Cancer Treatment in the Pandemic Period
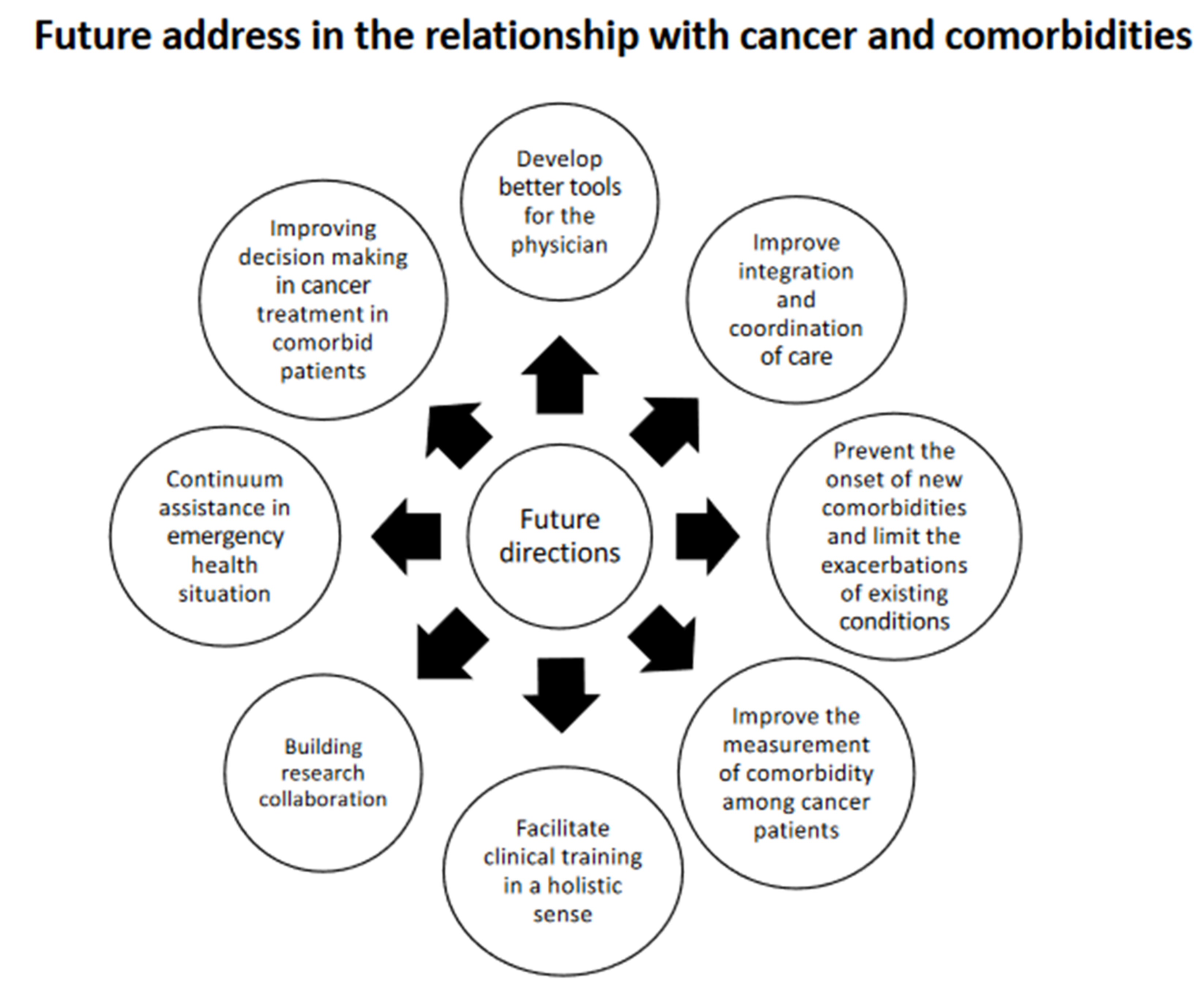
4. Future Directions
5. Preventive Measures for Clustering Disease
- Improving decision making in cancer treatment in comorbid patients
- Improve the measurement of comorbidity among cancer patients
- Improve integration and coordination of care
- Prevent the onset of new comorbidities and limit the exacerbations of existing conditions
- Develop better tools for clinicians
- Facilitate clinical training in a holistic sense
- Building research collaborations
- Continuum of assistance in emergency health situations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edwards, B.K.; Noone, M.A.; Mariotto, B.A.; Simard, P.E.; Boscoe, P.F.; Henley, J.S.; Jemal, A.; Cho, H.; Anderson, N.R.; Kohler, A.; et al. Annual report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014, 120, 1290–1314. [Google Scholar]
- Fane, M.; Weeraratna, A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Extermann, M. Measurement and impact of comorbidity in older cancer patients. Crit. Rev. Oncol. Hematol. 2000, 35, 181–200. [Google Scholar] [CrossRef]
- Extermann, M. Measuring comorbidity in older cancer patients. Eur. J. Cancer 2000, 36, 453–471. [Google Scholar] [CrossRef]
- Satariano, W.A.; Silliman, R.A. Comorbidity: Implications for research and practice in geriatric oncology. Crit. Rev. Oncol. Hematol. 2003, 48, 239–248. [Google Scholar] [CrossRef]
- Sogaard, M.; Thomsen, R.; Bossen, K.S.; Sørensen, H.T.; Nørgaard, M. The impact of comorbidity on cancer survival: A review. Clin. Epidemiol. 2013, 5, 3–29. [Google Scholar] [CrossRef]
- Institute of Medicine. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis; The National Academies Press: Washington, DC, USA, 2013. [Google Scholar]
- Feinstein, A. The pre-therapeutic classification of co-morbidity in chronic disease. J. Chronic. Dis. 1970, 23, 455–469. [Google Scholar] [CrossRef]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 255–263. [Google Scholar] [CrossRef]
- Ness, K.K.; Krull, K.R.; Jones, K.E.; Mulrooney, D.A.; Armstrong, G.T.; Green, D.M.; Chemaitilly, W.; Smith, W.A.; Wilson, C.L.; Sklar, C.A.; et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: A report from the St Jude Lifetime cohort study. J. Clin. Oncol. 2013, 31, 4496–4503. [Google Scholar] [CrossRef]
- Lee, L.; Cheung, W.Y.; Atkinson, E.; Krzyzanowska, M.K. Impact of comorbidity on chemotherapy use and out-comes in solid tumors: A systematic review. J. Clin. Oncol. 2011, 29, 106–117. [Google Scholar] [CrossRef]
- Newschaffer, C.J.; Bush, T.L.; Penberthy, L.T. Comorbidity measurement in elderly female breast cancer patients with administrative and medical records data. J. Clin. Epidemiol. 1997, 50, 725–733. [Google Scholar] [CrossRef]
- Kieszak, S.M.; Flanders, W.D.; Kosinski, A.S.; Shipp, C.C.; Karp, H. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J. Clin. Epidemiol. 1999, 52, 137–142. [Google Scholar] [CrossRef]
- Malenka, D.J.; McLerran, D.; Roos, N.; Fisher, E.S.; Wennberg, J.E. Using administrative data to describe casemix: A comparison with the medical record. J. Clin. Epidemiol. 1994, 47, 1027–1032. [Google Scholar] [CrossRef]
- Romano, P.S.; Roos, L.L.; Jollis, J.G. Further evidence concerning the use of a clinical comorbidity index with ICD-9-CM administrative data. J. Clin. Epidemiol. 1993, 46, 1085–1090. [Google Scholar] [CrossRef]
- Sarfati, D.; Hill, S.; Purdie, G.; Dennett, E.; Blakely, T. How well does routine hospitalisation data capture information on comorbidity in New Zealand? N. Z. Med. J. 2010, 123, 50–61. [Google Scholar]
- Van Doorn, C.; Bogardus, S.T.; Williams, C.S.; Concato, J.; Towle, V.R.; Inouye, S.K. Risk adjustment for older hospitalized persons: A comparison of two methods of data collection for the Charlson index. J. Clin. Epidemiol. 2001, 54, 694–701. [Google Scholar] [CrossRef]
- Sarfati, D.; Tan, L.; Blakely, T.; Pearce, N. Comorbidity among patients with colon cancer in New Zealand. N. Z. Med. J. 2011, 124, 76–88. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). CDC Health Disparities and Inequalities Report—United States. MMWR 2013, 62, 1–187. [Google Scholar]
- Valery, P.C.; Coory, M.; Stirling, J.; Green, A.C. Cancer diagnosis, treatment, and survival in indigenous and non-Indigenous Australians: A matched cohort study. Lancet 2006, 367, 1842–1848. [Google Scholar] [CrossRef]
- Schrijvers, C.T.; Coebergh, J.W.; van der Heijden, L.H.; Mackenbach, J.P. Socioeconomic variation in cancer survival in the southeastern Netherlands, 1980–1989. Cancer 1995, 75, 2946–2953. [Google Scholar] [CrossRef]
- Louwman, W.J.; Aarts, M.J.; Houterman, S.; van Lenthe, F.J.; Coebergh, J.W.W.; Janssen-Heijnen, M.L.G. A 50% higher prevalence of life-shortening chronic conditions among cancer patients with low socioeconomic status. Br. J. Cancer 2010, 103, 1742–1748. [Google Scholar] [CrossRef]
- Hill, S.; Sarfati, D.; Blakely, T.; Robson, B.; Purdie, G.; Chen, J.; Dennett, E.; Cormack, D.; Cunningham, R.; Dew, K.; et al. Survival disparities in Indigenous and non-Indigenous New Zealanders with colon cancer: The role of patient comorbidity, treatment and health service factors. J. Epidemiol. Community Health 2010, 64, 117–123. [Google Scholar] [CrossRef]
- Sheppard, A.J.; Chiarelli, A.M.; Marrett, L.D.; Nishri, E.D.; Trudeau, M.E. Stage at diagnosis and comorbidity influence breast cancer survival in First Nations women in Ontario, Canada. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2160–2167. [Google Scholar] [CrossRef]
- Martin, J.H.; Coory, M.D.; Valery, P.C.; Green, A.C. Association of diabetes with survival among cohorts of indigenous and non-indigenous Australians with cancer. Cancer Causes Control 2009, 20, 355–360. [Google Scholar] [CrossRef]
- Putt, M.; Long, J.A.; Montagnet, C.; Silber, J.H.; Chang, V.W.; Liao, K.; Schwartz, J.S.; Pollack, C.E.; Wong, Y.U.; Armstrong, K. Racial differences in the impact of comorbidities on survival among elderly men with prostate cancer. Med. Care Res. Rev. 2009, 66, 409–435. [Google Scholar] [CrossRef]
- Allard, J.E.; Maxwell, G.L. Race disparities between black and white women in the incidence, treatment, and prognosis of endometrial cancer. Cancer Control 2009, 16, 53–56. [Google Scholar] [CrossRef]
- Braithwaite, D.; Tammemagi, C.M.; Moore, D.H.; Ozanne, E.M.; Hiatt, R.A.; Belkora, J.; West, D.W.; Satariano, W.A.; Liebman, M.; Esserman, L. Hypertension is an independent predictor of survival disparity between African-American and white breast cancer patients. Int. J. Cancer 2009, 124, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Holmes, L., Jr.; Chan, W.; Jiang, Z.; Ward, D.; Essien, E.J.; Du, X.L. Impact of androgen deprivation therapy on racial/ethnic disparities in the survival of older men treated for locoregional prostate cancer. Cancer Control 2009, 16, 176–185. [Google Scholar] [CrossRef]
- Yang, R.; Cheung, M.C.; Byrne, M.M.; Huang, Y.; Nguyen, D.; Lally, B.E.; Koniaris, L.G. Do racial or socioeconomic disparities exist in lung cancer treatment? Cancer 2010, 116, 2437–2447. [Google Scholar] [CrossRef]
- Cook, L.S.; Nelson, H.E.; Cockburn, M.; Olson, S.H.; Muller, C.Y.; Wiggins, C.L. Comorbidities and endometrial cancer survival in Hispanics and non-Hispanic whites. Cancer Causes Control 2013, 24, 61–69. [Google Scholar] [CrossRef]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Tabares-Seisdedos, R.; Dumont, N.; Baudot, A.; Valderas, J.M.; Climent, J.; Valencia, A.; Crespo-Facorro, B.; Vieta, E.; Gómez-Beneyto, M.; Martínez, S.; et al. No paradox, no progress: Inverse cancer comorbidity in people with other complex diseases. Lancet Oncol. 2011, 12, 604–608. [Google Scholar] [CrossRef]
- Renehan, A.; Smith, U.; Kirkman, M.S. Linking diabetes and cancer: A consensus on complexity. Lancet 2010, 375, 2201–2202. [Google Scholar] [CrossRef]
- Onitilo, A.; Engel, J.; Glurich, I.; Stankowski, R.V.; Williams, G.M.; Doi, S.A. Diabetes and cancer II: Role of diabetes medications and influence of shared risk factors. Cancer Causes Control 2012, 23, 991–1008. [Google Scholar] [CrossRef] [PubMed]
- Din, F.V.N.; Theodoratou, E.; Farrington, S.M.; Tenesa, A.; Barnetson, R.A.; Cetnarskyj, R.; Stark, L.; Porteous, M.E.; Campbell, H.; Dunlop, M.G. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut 2010, 59, 1670–1679. [Google Scholar] [CrossRef]
- Flossmann, E.; Rothwell, P.M.; British Doctors Aspirin Trial and the UK-TIA Aspirin Trial. Effect of aspirin on long-term risk of colorectal cancer: Consistent evidence from randomised and observational studies. Lancet 2007, 369, 1603–1613. [Google Scholar] [CrossRef]
- Roe, C.M.; Fitzpatrick, A.L.; Xiong, C.; Sieh, W.; Kuller, L.; Miller, J.P.; Williams, M.M.; Kopan, R.; Behrens, M.I.; Morris, J.C. Cancer linked to Alzheimer disease but not vascular dementia. Neurology 2010, 74, 106–112. [Google Scholar] [CrossRef]
- West, A.B.; Dawson, V.L.; Dawson, T.M. To die or grow: Parkinson’s disease and cancer. Trends Neurosci. 2005, 28, 348–352. [Google Scholar] [CrossRef]
- Walter, L.C.; Bertenthal, D.; Lindquist, K.; Konety, B.R. PSA screening among elderly men with limited life expectancies. JAMA 2006, 296, 2336–2342. [Google Scholar] [CrossRef]
- Taneja, S.; Mandayam, S.; Kayani, Z.Z.; Kuo, Y.; Shahinian, V.B. Comparison of stage at diagnosis of cancer in patients who are on dialysis versus the general population. Clin. J. Am. Soc. Nephrol. 2007, 2, 1008–1013. [Google Scholar] [CrossRef]
- Fleming, S.T.; Pursley, H.G.; Newman, B.; Pavlov, D.; Chen, K. Comorbidity as a predictor of stage of illness for patients with breast cancer. Med. Care 2005, 43, 132–140. [Google Scholar] [CrossRef]
- Terret, C.; Castel-Kremer, E.; Albrand, G.; Droz, J.P. Effects of comorbidity on screening and early diagnosis of cancer in elderly people. Lancet Oncol. 2009, 10, 80–87. [Google Scholar] [CrossRef]
- Corkum, M.; Urquhart, R.; Kendell, C.; Burge, F.; Porter, G.; Johnston, G. Impact of co- morbidity and healthcare utilization on colorectal cancer stage at diagnosis: Literature review. Cancer Causes Control 2012, 23, 213–220. [Google Scholar] [CrossRef]
- Satariano, W.A.; Ragland, D.R. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann. Intern. Med. 1994, 120, 104–110. [Google Scholar] [CrossRef]
- Seok-Jun, Y.; Eun-Jung, K.; Hyun-Ju, S.; In-Hwan, O. The Association between Charlson Comorbidity Index and the Medical Care Cost of Cancer: A Retrospective Study. BioMed Res. Int. 2015, 2015, 259341. [Google Scholar] [CrossRef]
- Ng, H.S.; Roder, D.; Koczwara, B.; Vitry, A. Comorbidity, physical and mental health among cancer patients and survivors: An Australian population-based study. Asia-Pac. J. Clin. Oncol. 2018, 14, e181–e192. [Google Scholar] [CrossRef]
- Vaeth, P.A.; Satariano, W.A.; Ragland, D.R. Limiting comorbid conditions and breast cancer stage at diagnosis. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M593–M600. [Google Scholar] [CrossRef]
- Gross, C.P.; Andersen, M.S.; Krumholz, H.M.; McAvay, G.J.; Proctor, D.; Tinetti, M.E. Relation between Medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. JAMA 2006, 296, 2815–2822. [Google Scholar]
- Reid, B.C.; Warren, J.L.; Rozier, G. Comorbidity and early diagnosis of head and neck cancer in a Medicare population. Am. J. Prev. Med. 2004, 27, 373–378. [Google Scholar] [CrossRef]
- Walter, L.C.; Lindquist, K.; Nugent, S.; Schult, T.; Lee, S.J.; Casadei, M.A.; Partin, M.R. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann. Intern. Med. 2009, 150, 465–473. [Google Scholar]
- Fleming, S.T.; McDavid, K.; Pearce, K.; Pavlov, D. Comorbidities and the risk of late-stage prostate cancer. Sci. World J. 2006, 6, 2460–2470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yasmeen, S.; Chlebowski, R.T.; Xing, G.; Morris, C.R.; Romano, P.S. Severity of comorbid conditions and early-stage breast cancer therapy: Linked SEER-Medicare data from 1993 to 2005. Cancer Med. 2013, 2, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Gurney, J.; Sarfati, D.; Stanley, J. The impact of patient co- morbidity on cancer stage at diagnosis. Br. J. Cancer 2015, 113, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Spechler, S.J.; Huerta, S.; Dredar, S.; Little, B.B.; Cryer, B. Elevated HbA1c is an independent predictor of aggressive clinical behaviour in patients with colorectal cancer: A case-control study. Dig. Dis. Sci. 2008, 53, 2486–2494. [Google Scholar] [CrossRef]
- Zafar, S.Y.; Abernethy, A.P.; Abbott, D.H.; Grambow, S.C.; Marcello, J.E.; Herndon, J.E., 2nd; Rowe, K.L.; Kolimaga, J.T.; Zullig, L.L.; Patwardhan, M.B.; et al. Comorbidity, age, race and stage at diagnosis in colorectal cancer: A retrospective, parallel analysis of two health systems. BMC Cancer 2008, 8, 345. [Google Scholar] [CrossRef]
- Fisher, D.A.; Judd, L.; Sanford, N.S. Inappropriate colorectal cancer screening: Findings and implications. Am. J. Gastroenterol. 2005, 100, 2526–2530. [Google Scholar] [CrossRef]
- McBean, A.M.; Yu, X. The underuse of screening services among elderly women with diabetes. Diabetes Care 2007, 30, 1466–1472. [Google Scholar] [CrossRef]
- Kiefe, C.I.; Funkhouser, E.; Fouad, M.N.; May, D.S. Chronic disease as a barrier to breast and cervical cancer screening. J. Gen. Intern. Med. 1998, 13, 357–365. [Google Scholar] [CrossRef]
- Gross, C.P.; McAvay, G.J.; Krumholz, H.M.; Paltiel, A.D.; Bhasin, D.; Tinetti, M.E. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: Implications for screening. Ann. Intern. Med. 2006, 145, 646–653. [Google Scholar] [CrossRef]
- Schonberg, M.A.; McCarthy, E.P.; Davis, R.B.; Phillips, R.S.; Hamel, M.B. Breast cancer screening in women aged 80 and older: Results from a national survey. J. Am. Geriatr. Soc. 2004, 52, 1688–1695. [Google Scholar] [CrossRef]
- Koppie, T.M.; Serio, A.M.; Vickers, A.J.; Vora, K.; Dalbagni, G.; Donat, S.M.; Herr, H.W.; Bochner, B.H. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer 2008, 112, 2384–2392. [Google Scholar] [CrossRef]
- Blanco, J.A.; Toste, I.S.; Alvarez, R.F.; Cuadrado, G.R.; Gonzalvez, A.M.; Martín, I.J.G. Age, comorbidity, treatment decision and prognosis in lung cancer. Age Ageing 2008, 37, 715–718. [Google Scholar] [CrossRef][Green Version]
- Etzioni, D.A.; El-Khoueiry, A.B.; Beart, R.W. Rates and predictors of chemotherapy use for stage III colon cancer. Cancer 2008, 113, 3279–3289. [Google Scholar] [CrossRef]
- Hall, W.H.; Jani, A.B.; Ryu, J.K.; Narayan, S.; Vijayakumar, S. The impact of age and comorbidity on survival outcomes and treatment patterns in prostate cancer. Prostate Cancer Prostatic Dis. 2005, 8, 22–30. [Google Scholar] [CrossRef]
- Janssen-Heijnen, M.L.; Houterman, S.; Lemmens, V.E.; Louwman, M.W.J.; Maas, H.A.A.M.; Coebergh, J.W. Prognostic impact of increasing age and co-morbidity in cancer patients: A population-based approach. Crit. Rev. Oncol. Hematol. 2005, 55, 231–240. [Google Scholar] [CrossRef]
- Van der Aa, M.A.; Siesling, S.; Kruitwagen, R.F.; Lybeert, M.L.M.; Coebergh, J.W.W.; Janssen-Heijnen, M.L.G. Comorbidity and age affect treatment policy for cervical cancer: A population-based study in the south of The Netherlands, 1995–2004. Eur. J. Gynaecol. Oncol. 2008, 29, 493–498. [Google Scholar]
- Land, L.H.; Dalton, S.O.; Jensen, M.B.; Ewertz, M. Influence of comorbidity on the effect of adjuvant treatment and age in patients with early-stage breast cancer. Br. J. Cancer 2012, 107, 1901–1907. [Google Scholar] [CrossRef]
- Rodrigues, G.; Sanatani, M. Age and comorbidity considerations related to radiotherapy and chemotherapy administration. Semin. Radiat. Oncol 2012, 22, 277–283. [Google Scholar] [CrossRef]
- Sarfati, D.; Gurney, J.; Stanley, J.; Koea, J. A retrospective cohort study of patients with stomach and liver cancers: The impact of comorbidity and ethnicity on cancer care and outcomes. BMC Cancer 2014, 14, 821. [Google Scholar] [CrossRef]
- Cronin, D.P.; Harlan, L.C.; Potosky, A.L.; Clegg, L.X.; Stevens, J.L.; Mooney, M.M. Patterns of care for adjuvant therapy in a random population-based sample of patients diagnosed with colorectal cancer. Am. J. Gastroenterol. 2006, 101, 2308–2318. [Google Scholar] [CrossRef]
- Gross, C.P.; McAvay, G.J.; Guo, Z.; Tinetti, M.E. The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer 2007, 109, 2410–2419. [Google Scholar] [CrossRef]
- Lemmens, V.E.; Janssen-Heijnen, M.L.; Verheij, C.D.; Houterman, S.; van Driel, O.J.R.; Coebergh, J.W.W. Comorbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. Br. J. Surg. 2005, 92, 615–623. [Google Scholar] [CrossRef]
- Sarfati, D.; Hill, S.; Blakely, T.; Robson, B.; Purdie, G.; Dennett, E.; Cormack, D.; Dew, K. The effect of comorbidity on the use of adjuvant chemotherapy and survival from colon cancer: A retrospective cohort study. BMC Cancer 2009, 9, 116. [Google Scholar] [CrossRef]
- Khrizman, P.; Niland, J.C.; Ter Veer, A.; Milne, D.; Dunn, K.B.; William, E.C., 3rd; Engstrom, P.F.; Shibata, S.; Skibber, J.M.; Weiser, M.R.; et al. Postoperative adjuvant chemotherapy use in patients with stage II/III rectal cancer treated with neoadjuvant therapy: A National Comprehensive Cancer Network analysis. J. Clin. Oncol. 2013, 31, 30–38. [Google Scholar] [CrossRef]
- Iversen, L.H.; Norgaard, M.; Jacobsen, J.; Laurberg, S.; Sørensen, H.T. The impact of comorbidity on survival of Danish colorectal cancer patients from 1995 to 2006—A population-based cohort study. Dis. Colon Rectum 2009, 52, 71–78. [Google Scholar] [CrossRef]
- Zhang, W.; Ayanian, J.Z.; Zaslavsky, A.M. Patient characteristics and hospital quality for colorectal cancer surgery. Int. J. Qual. Health Care 2007, 19, 11–20. [Google Scholar] [CrossRef]
- Bradley, C.J.; Dahman, B.; Anscher, M. Prostate cancer treatment and survival: Evidence for men with prevalent comorbid conditions. Med. Care 2014, 52, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Keating, N.L.; Landrum, M.B.; Klabunde, C.N.; Fletcher, R.H.; Rogers, S.O.; Doucette, W.R.; Tisnado, D.; Clauser, S.; Kahn, K.L. Adjuvant chemotherapy for stage III colon cancer: Do physicians agree about the importance of patient age and comorbidity? J. Clin. Oncol. 2008, 26, 2532–2537. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowska, M.K.; Regan, M.M.; Powell, M.; Earle, C.C.; Weeks, J.C. Impact of patient age and comorbidity on surgeon versus oncologist preferences for adjuvant chemotherapy for stage III colon cancer. J. Am. Coll. Surg. 2009, 208, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Ring, A. The influences of age and co-morbidities on treatment decisions for patients with HEr2-positive early breast cancer. Crit. Rev. Oncol. Hematol. 2010, 76, 127–132. [Google Scholar] [CrossRef]
- Stairmand, J.; Signal, L.; Sarfati, D.; Jackson, C.; Batten, L.; Holdaway, M.; Cunningham, C. Consideration of comorbidity in treatment decision-making in multidisciplinary cancer team meetings: A systematic review. Ann. Oncol. 2015, 26, 1325–1332. [Google Scholar] [CrossRef]
- Schrag, D.; Cramer, L.D.; Bach, P.B.; Begg, C.B. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J. Natl. Cancer Inst. 2001, 93, 850–857. [Google Scholar] [CrossRef]
- Kutner, J.S.; Vu, K.O.; Prindiville, S.A.; Byers, T.E. Patient age and cancer treatment decisions. Patient and physician views. Cancer Pract. 2000, 8, 114–119. [Google Scholar] [CrossRef]
- Newcomb, P.A.; Carbone, P.P. Cancer treatment and age: Patient perspectives. J. Natl. Cancer Inst. 1993, 85, 1580–1584. [Google Scholar] [CrossRef]
- El Shayeb, M.; Scarfe, A.; Yasui, Y.; Winget, M. Reasons physicians do not recommend, and patients refuse adjuvant chemotherapy for stage III colon cancer: A population-based chart review. BMC Res. Notes 2012, 5, 269. [Google Scholar] [CrossRef]
- Fortin, M.; Dionne, J.; Pinho, G.; Gignac, J.; Almirall, J.; Lapointe, L. Randomized controlled trials: Do they have external validity for patients with multiple comorbidities? Ann. Fam. Med. 2006, 4, 104–108. [Google Scholar] [CrossRef]
- Lewis, J.H.; Kilgore, M.L.; Goldman, D.P.; Trimble, E.L.; Kaplan, R.; Montello, M.J.; Housman, M.G.; Escarce, J.J. Participation of patients 65 years of age or older in cancer clinical trials. J. Clin. Oncol. 2003, 21, 1383–1389. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Catalano, P.J.; Haller, D.G.; Mayer, R.J.; Macdonald, J.S.; 3rd Benson, A.B.; Fuchs, C.S. Impact of diabetes mellitus on outcomes in patients with colon cancer. J. Clin. Oncol. 2003, 21, 433–440. [Google Scholar] [CrossRef]
- Gronberg, B.H.; Sundstrom, S.; Kaasa, S.; Bremnes, R.M.; Fløtten, Ø.; Amundsen, T.; Hjelde, H.H.; von Plessen, C.; Jordhøy, M. Influence of comorbidity on survival, toxicity and health-related quality of life in patients with advanced non-small-cell lung cancer receiving platinum-doublet chemotherapy. Eur. J. Cancer 2010, 46, 2225–2234. [Google Scholar] [CrossRef]
- Lemmens, V.E.; Janssen-Heijnen, M.L.; Houterman, S.; Verheij, K.D.G.W.; Martijn, H.; van de Poll-Franse, L.; Coebergh, J.W. Which comorbid conditions predict complications after surgery for colorectal cancer? World J. Surg. 2007, 31, 192–199. [Google Scholar] [CrossRef]
- Peters, T.T.; Van der Laan, B.F.; Plaat, B.E.; Wedman, J.; Langendijk, J.A.; Halmos, G.B. The impact of comorbidity on treatment-related side effects in older patients with laryngeal cancer. Oral Oncol. 2011, 47, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Seymour, M.T.; Thompson, L.C.; Wasan, H.S.; Middleton, G.; Brewster, A.E.; Shepherd, S.F.; O’Mahony, M.S.; Maughan, T.S.; Parmar, M.; Langley, R.E. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): An open- label, randomised factorial trial. Lancet 2011, 377, 1749–1759. [Google Scholar] [CrossRef]
- Lo Conte, N.K.; Smith, M.; Alberti, D.; Bozeman, J.; Cleary, J.F.; Setala, A.N.; Wodtke, G.; Wilding, G.; Holen, K.D. Amongst eligible patients, age and comorbidity do not predict for dose-limiting toxicity from phase I chemotherapy. Cancer Chemother. Pharmacol. 2010, 65, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.W. Chemotherapy for older patients with colorectal cancer. Lancet 2011, 378, 765, author reply 765–766. [Google Scholar] [CrossRef]
- Vickers, M.M.; Powell, E.D.; Asmis, T.R.; Jonker, D.J.; Hilton, J.F.; O’Callaghan, C.J.; Tu, D.; Parulekar, W.; Jonker, M.J.M.D.J.; Hilton, J.F.; et al. Comorbidity, age and overall survival in patients with advanced pancreatic cancer—Results from NCIC CTG PA.3, a phase III trial of gemcitabine plus erlotinib or placebo. Eur. J. Cancer 2012, 48, 1434–1442. [Google Scholar] [CrossRef]
- Rieker, R.J.; Hammer, E.; Eisele, R.; Schmid, E.; Högel, J. The impact of comorbidity on the overall survival and the cause of death in patients after colorectal cancer resection. Langenbeck’s Arch. Surg. 2002, 387, 72–76. [Google Scholar] [CrossRef]
- Dehal, A.; Abbas, A.; Johna, S. Comorbidity and outcomes after surgery among women with breast cancer: Analysis of nationwide in-patient sample database. Breast Cancer Res. Treat. 2013, 139, 469–476. [Google Scholar] [CrossRef]
- Van Gestel, Y.R.; Lemmens, V.E.; de Hingh, I.H.; Steevens, J.; Rutten, H.J.T.; Nieuwenhuijzen, G.A.P.; van Dam, R.M.; Siersema, P.D. Influence of comorbidity and age on 1-, 2-and 3-month postoperative mortality rates in gastrointestinal cancer patients. Ann. Surg. Oncol. 2013, 20, 371–380. [Google Scholar] [CrossRef]
- Tomaszewski, J.J.; Uzzo, R.G.; Kutikov, A.; Hrebinko, K.; Mehrazin, R.; Corcoran, A.; Ginzburg, S.; Viterbo, R.; Chen, D.Y.T.; Greenberg, R.E.; et al. Assessing the burden of complications after surgery for clinically localized kidney cancer by age and comorbidity status. Urology 2014, 83, 843–849. [Google Scholar] [CrossRef]
- Jehn, C.F.; Boning, L.; Kroning, H.; Pezzutto, A.; Lüftner, D. Influence of co- morbidity, age and performance status on treatment efficacy and safety of cetuximab plus irinotecan in irinotecan-refractory elderly patients with metastatic colorectal cancer. Eur. J. Cancer 2014, 50, 1269–1275. [Google Scholar] [CrossRef]
- Abdollah, F.; Sun, M.; Schmitges, J.; Thuret, R.; Bianchi, M.; Shariat, S.F.; Briganti, A.; Jeldres, C.; Perrotte, P.; Montorsi, F.; et al. Survival benefit of radical prostatectomy in patients with localized prostate cancer: Estimations of the number needed to treat according to tumour and patient characteristics. J. Urol. 2012, 188, 73–83. [Google Scholar] [CrossRef]
- Earle, C.C.; Tsai, J.S.; Gelber, R.D.; Weinstein, M.C.; Neumann, P.J.; Weeks, J.C. Effectiveness of chemotherapy for advanced lung cancer in the elderly: Instrumental variable and propensity analysis. J. Clin. Oncol. 2001, 19, 1064–1070. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Rubin, D. Reducing bias in observational studies using subclassification on the propensity score. J. Am. Stat. Assoc. 1984, 79, 516–524. [Google Scholar] [CrossRef]
- Riechelmann, R.P.; Del Giglio, A. Drug interactions in oncology: How common are they? Ann. Oncol. 2009, 20, 1907–1912. [Google Scholar] [CrossRef]
- Beijnen, J.H.; Schellens, J.H. Drug interactions in oncology. Lancet Oncol. 2004, 5, 489–496. [Google Scholar] [CrossRef]
- Blower, P.; de Wit, R.; Goodin, S.; Aapro, M. Drug-drug interactions in oncology: Why are they important and can they be minimized? Crit. Rev. Oncol. Hematol. 2005, 55, 117–142. [Google Scholar] [CrossRef]
- Riechelmann, R.P.; Tannock, I.F.; Wang, L.; Saad, E.D.; Taback, N.A.; Krzyzanowska, M.K. Potential drug interactions and duplicate prescriptions among cancer patients. J. Natl. Cancer Inst. 2007, 99, 592–600. [Google Scholar] [CrossRef]
- Riechelmann, R.P.; Zimmermann, C.; Chin, S.N.; Wang, L.; O’Carroll, A.; Zarinehbaf, S.; Krzyzanowska, M.K. Potential drug interactions in cancer patients receiving supportive care exclusively. J. Pain Symptom Manag. 2008, 35, 535–543. [Google Scholar] [CrossRef]
- Miranda, V.; Fede, A.; Nobuo, M.; Ayres, V.; Giglio, A.; Miranda, M.; Riechelmann, R.P. Adverse drug re- actions and drug interactions as causes of hospital ad- mission in oncology. J. Pain Symptom Manag. 2011, 42, 342–353. [Google Scholar] [CrossRef]
- Sasaki, T.; Fujita, K.; Sunakawa, Y.; Ishida, H.; Yamashita, K.; Miwa, K.; Saji, S.; Kato, Y.; Sasaki, Y. Concomitant polypharmacy is associated with irinotecan-related ad- verse drug reactions in patients with cancer. Int. J. Clin. Oncol. 2013, 18, 735–742. [Google Scholar] [CrossRef]
- Boulos, D.L.; Groome, P.A.; Brundage, M.D.; Siemens, D.R.; Mackillop, W.J.; Heaton, J.P.W.; Schulze, K.M.; Rohland, S.L. Predictive validity of five comorbidity indices in prostate carcinoma patients treated with curative intent. Cancer 2006, 106, 1804–1814. [Google Scholar] [CrossRef]
- Kendal, W.S. Dying with cancer: The influence of age, comorbidity, and cancer site. Cancer 2008, 112, 1354–1362. [Google Scholar] [CrossRef]
- Piccirillo, J.F.; Costas, I.; Claybour, P.; Siemens, D.R.; Mackillop, W.J.; Heaton, J.P.W.; Schulze, K.M.; Siemens, S.L.R.D.R.; Mackillop, W.J.; Heaton, J.P.W.; et al. The measurement of comorbidity by cancer registries. J. Regist. Manag. 2003, 30, 8–14. [Google Scholar]
- Piccirillo, J.F.; Tierney, R.M.; Costas, I.; Grove, L.; Jr, E.L.S. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004, 291, 2441–2447. [Google Scholar] [CrossRef]
- Read, W.L.; Tierney, R.M.; Page, N.C.; Costas, I.; Govindan, R.; Spitznagel, E.L.J.; Piccirillo, J.F. Differential prognostic impact of comorbidity. J. Clin. Oncol. 2004, 22, 3099–3103. [Google Scholar] [CrossRef]
- Sarfati, D.; Blakely, T.; Pearce, N. Measuring cancer survival in populations: Relative survival versus cancer specific survival. Int. J. Epidemiol. 2010, 39, 598–610. [Google Scholar] [CrossRef]
- Kiderlen, M.; de Glas, N.A.; Bastiaannet, E.; Engels, C.C.; van de Water, W.; de Craen, A.J.M.; Portielje, J.E.A.; van de Velde, C.J.H.; Liefers, G.J. Diabetes in relation to breast cancer relapse and all-cause mortality in elderly breast cancer patients: A FOCUS study analysis. Ann. Oncol. 2013, 24, 3011–3016. [Google Scholar] [CrossRef]
- Fortin, M.; Bravo, G.; Hudon, C.; Lapointe, L.; Almirall, J.; Dubois, M.-F.; Vanass, A. Relationship between multimorbidity and health-related quality of life of patients in primary care. Qual. Life Res. 2006, 15, 83–91. [Google Scholar] [PubMed]
- Fortin, M.; Lapointe, L.; Hudon, C.; Vanasse, A.; Ntetu, A.L.; Maltais, D. Multimorbidity and quality of life in primary care: A systematic review. Health Qual. Life Outcomes 2004, 2, 51. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Ou, H.; Wang, F.; Erickson, S. A new co- morbidity index: The health-related quality of life comorbidity index. J. Clin. Epidemiol. 2011, 64, 309–319. [Google Scholar] [PubMed]
- McDaid, O.; Hanly, M.J.; Richardson, K.; Kee, F.; Kenny, R.A.; Savva, G.M. The effect of multiple chronic conditions on self-rated health, disability and quality of life among the older populations of Northern Ireland and the republic of Ireland: A comparison of two nationally representative cross-sectional surveys. BMJ Open 2013, 3, e002571. [Google Scholar] [CrossRef]
- Daskivich, T.J.; van de Poll-Franse, L.V.; Kwan, L.; Sadetsky, N.; Stein, D.M.; Litwin, M.S. From bad to worse: Comorbidity severity and quality of life after treatment for early-stage prostate cancer. Prostate Cancer Prostatic Dis. 2010, 13, 320–327. [Google Scholar] [CrossRef][Green Version]
- Litwin, M.S.; Greenfield, S.; Elkin, E.P.; Lubeck, D.P.; Broering, J.M.; Kaplan, S.H. Assessment of prognosis with the total illness burden index for prostate cancer: Aiding clinicians in treatment choice. Cancer 2007, 109, 1777–1783. [Google Scholar] [CrossRef]
- Osthus, A.A.; Aarstad, A.K.; Olofsson, J.; Aarstad, H.J. Co- morbidity is an independent predictor of health-related quality of life in a longitudinal cohort of head and neck cancer patients. Eur. Arch. Otorhinolaryngol. 2013, 270, 1721–1728. [Google Scholar] [CrossRef]
- Schubert-Fritschle, G.; Schlesinger-Raab, A.; Hein, R.; Stolz, W.; Volkenandt, M.; Hölzel, D.; Engel, J. Quality of life and comorbidity in localized malignant melanoma: Results of a German population-based cohort study. Int. J. Dermatol. 2013, 52, 693–704. [Google Scholar] [CrossRef]
- Schafer, I.; Hansen, H.; Schon, G.; Höfels, S.; Altiner, A.; Dahlhaus, A.; Gensichen, J.; Riedel-Heller, S.; Weyerer, S.; Blank, W.A. The influence of age, gender and socio-economic status on multimorbidity patterns in primary care. First results from the Multicare Cohort Study. BMC Health Serv. Res. 2012, 12, 89. [Google Scholar] [CrossRef]
- Haugnes, H.S.; Aass, N.; Fossa, S.D.; Dahl, O.; Klepp, O.; Wist, E.A.; Svartberg, J.; Wilsgaard, T.; Bremnes, R.M. Components of the metabolic syndrome in long-term survivors of testicular cancer. Ann. Oncol. 2007, 18, 241–248. [Google Scholar] [CrossRef]
- Smith, L.A.; Cornelius, V.R.; Plummer, C.J.; Levitt, G.; Verrill, M.; Canney, P.; Jones, A. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomised controlled trials. BMC Cancer 2010, 10, 337. [Google Scholar] [CrossRef]
- Carver, J.R.; Shapiro, C.L.; Ng, A.; Jacobs, L.; Schwartz, C.; Virgo, K.S.; Hagerty, K.L.; Somerfield, M.R.; Vaughn, D.J. American Society of Clinical Oncology clinical evidence review on the on- going care of adult cancer survivors: Cardiac and pulmonary late effects. J. Clin. Oncol. 2007, 25, 3991–4008. [Google Scholar] [CrossRef]
- Patnaik, J.L.; Byers, T.; Di Giuseppi, C.; Dabelea, D.; Denberg, T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res. 2011, 13, R64. [Google Scholar] [CrossRef]
- Lustberg, M.B.; Reinbolt, R.E.; Shapiro, C.L. Bone health in adult cancer survivorship. J. Clin. Oncol. 2012, 30, 3665–3674. [Google Scholar] [CrossRef]
- Wickham, R. Osteoporosis related to disease or therapy in patients with cancer. Clin. J. Oncol. Nurs. 2011, 15, E90–E104. [Google Scholar] [CrossRef]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef]
- Rogado, J.; Pangua, C.; Serrano-Montero, G.; Obispo, B.; Marino, A.M.; Perez-Perez, M.; López-Alfonso, A.; Gullón, M.; Lara, M.Á. COIVD-19 and lung cancer: A greater fatality rate? Lung Cancer 2020, 146, 19–22. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Mehta, V.; Goel, S.; Kabarriti, R.; Cole, D.; Goldfinger, M.; Acuna-Villaorduna, A.; Pradhan, K.; Thota, R.; Reissman, S.; Sparano, J.A. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020, 10, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Lara Alvarez, M.A.; Rogado Revuelta, J.; Obispo Portero, B.; Pangua Mendez, C.; Serrano Montero, G.; Lopez Alfonso, A. COVID-19 mortality in cancer patients in a Madrid hospital during the first 3 weeks of the epidemic. Med. Clin. 2020, 155, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M.; Zorzi, M.; Guzzinati, S. SARS-CoV-2 infection in the Italian Veneto region: Adverse outcomes in patients with cancer. Nat. Cancer 2020, 1, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Rizvi, H.; Preeshagul, I.R.; Egger, J.V.; Hoyos, D.; Bandlamudi, C.; McCarthy, C.G.; Falcon, C.J.; Schoenfeld, A.J.; Arbour, K.C.; et al. COVID-19 in patients with lung cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Whisenant, J.G.; Huang, L.C.; Trama, A.; Torri, V.; Agustoni, F.; Baena, J.; Banna, G.; Berardi, R.; Bettini, A.C. COVID-19 in patients with thoracic malignancies (TERAVOLT): First results of an international, registry-based, cohort study. Lancet Oncol. 2020, 21, 914–922. [Google Scholar] [CrossRef]
- Passamonti, F.; Cattaneo, C.; Arcaini, L.; Bruna, R.; Cavo, M.; Merli, F.; Angelucci, E.; Krampera, M.; Cairoli, R.; Della Porta, M.G.; et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: A retrospective, multicentre, cohort study. Lancet Haematol. 2020, 7, e737–e745. [Google Scholar] [CrossRef]
- Sanchez-Pina, J.M.; Rodriguez Rodriguez, M.; Castro Quismondo, N.; Manso, G.; Colmenares, R.; Alos, D.G.; Paciello, M.L.; Zafra, D.; Garcia-Sanchez, C.; Villegas, C. Clinical course and risk factors for mortality from COVID-19 in patients with haematological malignancies. Eur. J. Haematol. 2020, 105, 597–607. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, F.; Xie, L.; Wang, C.; Chen, R.; Guan, H.Q.; Peng, L.; Chen, Y. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 894–901. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020, 21, E181. [Google Scholar] [CrossRef]
- Ueda, M.; Martins, R.; Hendrie, P.C.; McDonnell, T.; Crews, J.R.; Wong, T.L.; McCreery, B.; Jagels, B.; Crane, A. Managing cancer care during the COVID-19 pandemic: Agility and collaboration toward a common goal. J. Natl. Compr. Cancer Netw. 2020, 18, 366–369. [Google Scholar] [CrossRef]
- Sarfati, D. review of methods to measure comorbidity in cancer populations: No gold standard exists. J. Clin. Epidemiol. 2012, 65, 924–933. [Google Scholar] [CrossRef]
- Hall, S.F. A user’s guide to selecting a comorbidity index for clinical research. J. Clin. Epidemiol. 2006, 59, 849–855. [Google Scholar] [CrossRef]
- Walsh, J.; Harrison, J.D.; Young, J.M.; Butow, P.N.; Solomon, M.J.; Masya, L. What are the current barriers to effective cancer care coordination? A qualitative study. BMC Health Serv. Res. 2010, 10, 132. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Multiple Chronic Conditions—A Strategic Framework: Optimum Health and Quality of Life for Individuals with Multiple Chronic Conditions; US Department of Health and Human Services: Washington, DC, USA, 2010.
- Institute of Medicine. Living Well with Chronic Illness: A Call for Public Health Action; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Caillet, P.; Canoui-Poitrine, F.; Vouriot, J.; Berle, M.; Reinald, N.; Krypciak, S.; Bastuji-Garin, S.; Culine, S.; Paillaud, E. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J. Clin. Oncol. 2011, 29, 3636–3642. [Google Scholar] [CrossRef]
- Extermann, M.; Hurria, A. Comprehensive geriatric assessment for older patients with cancer. J. Clin. Oncol. 2007, 25, 1824–1831. [Google Scholar] [CrossRef]
- Freyer, G.; Geay, J.F.; Touzet, S.; Provencal, J.; Weber, B.; Jacquin, J.-P.; Ganem, G.; Tubiana-Mathieu, N.; Gisserot, O.; Pujade-Lauraine, E. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: A GINECO study. Ann. Oncol. 2005, 16, 1795–1800. [Google Scholar] [CrossRef]
- Girre, V.; Falcou, M.C.; Gisselbrecht, M.; Gridel, G.; Mosseri, V.; Bouleuc, C.; Poinsot, R.; Vedrine, L.; Ollivier, L.; Garabige, V.; et al. Does a geriatric oncology consultation modify the cancer treatment plan for elderly patients? J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 724–730. [Google Scholar] [CrossRef]
- Puts, M.T.; Hardt, J.; Monette, J.; Girre, V.; Springall, E.; Alibhai, S.M.H. Use of geriatric assessment for older adults in the oncology setting: A systematic review. J. Natl. Cancer Inst. 2012, 104, 1133–1163. [Google Scholar] [CrossRef]
- Puts, M.T.; Santos, B.; Hardt, J.; Monette, J.; Girre, V.; Atenafu, E.G.; Springall, E.; Alibhai, S.M.H. An update on a systematic review of the use of geriatric assessment for older adults in oncology. Ann. Oncol. 2014, 25, 307–315. [Google Scholar] [CrossRef]
- Ramjaun, A.; Nassif, M.O.; Krotneva, S.; Huang, A.R.; Meguerditchian, A.N. Improved targeting of cancer care for older patients: A systematic review of the utility of comprehensive geriatric assessment. J. Geriatr. Oncol. 2013, 4, 271–281. [Google Scholar] [CrossRef]
- Wildiers, H.; Heeren, P.; Puts, M.; Topinkova, E.; Janssen-Heijnen, M.L.G.; Extermann, M.; Falandry, C.; Artz, A.; Brain, E.; Colloca, G. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J. Clin. Oncol. 2014, 32, 2595–2603. [Google Scholar] [CrossRef]
- McCorkle, R.; Ercolano, E.; Lazenby, M.; Schulman-Green, D.; Schilling, L.S.; Lorig, K.; Wagner, E.H. Self-management: Enabling and empowering patients living with cancer as a chronic illness. CA Cancer J. Clin. 2011, 61, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.F.; Frick, K.D.; Herbert, R.J.; Blackford, A.L.; Neville, B.A.; Wolff, A.C.; Carducci, M.A.; Earle, C.C. Quality of care for comorbid conditions during the transition to survivorship: Differences between cancer survivors and noncancer controls. J. Clin. Oncol. 2013, 31, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Earle, C.C.; Neville, B.A. Under use of necessary care among cancer survivors. Cancer 2004, 101, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Popa, M.A.; Wallace, K.J.; Brunello, A.; Extermann, M.; Balducci, L. Potential drug interactions and chemotoxicity in older patients with cancer receiving chemotherapy. J. Geriatr. Oncol. 2014, 5, 307–314. [Google Scholar] [CrossRef]
- Neefan Leeuwen, R.W.; Brundel, D.H.; Neef, C.; van Gelder, T.; Mathijssen, R.H.J.; Burger, D.M.; Jansman, F.G.A. Prevalence of potential drug-drug interactions in cancer patients treated with oral anticancer drugs. Br. J. Cancer 2013, 108, 1071–1078. [Google Scholar] [CrossRef]
- Van Leeuwen, R.W.; Swart, E.L.; Boven, E.; Boom, F.A.; Schuitenmaker, M.G.; Hugtenburg, J.G. Potential drug interactions in cancer therapy: A prevalence study using an advanced screening method. Ann. Oncol. 2011, 22, 2334–2341. [Google Scholar] [CrossRef]
- Girre, V.; Arkoub, H.; Puts, M.T.; Vantelon, C.; Blanchard, F.; Droz, J.P.; Mignot, L. Potential drug interactions in elderly cancer patients. Crit. Rev. Oncol. Hematol. 2011, 78, 220–226. [Google Scholar] [CrossRef]
- Feuer, E.J.; Lee, M.; Mariotto, A.B.; Cronin, K.A.; Scoppa, S.; Penson, D.F.; Hachey, M.; Cynkin, L.; Carter, G.A.; Campbell, D. The Cancer Survival Query System: Making survival estimates from the Surveillance, Epidemiology, and End results program timelier and more relevant for recently diagnosed patients. Cancer 2012, 118, 5652–5662. [Google Scholar] [CrossRef]
- Daskivich, T.J.; Fan, K.H.; Koyama, T.; Albertsen, P.C.; Goodman, M.; Hamilton, A.S.; Hoffman, R.M.; Stanford, J.L.; Stroup, A.M.; Litwin, M.S. Effect of age, tumour risk, and comorbidity on competing risks for survival in a US population-based cohort of men with prostate cancer. Ann. Intern. Med. 2013, 158, 709–717. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Multiple Chronic Conditions: A Framework for Education and Training; US Department of Health and Human Services: Washington, DC, USA, 2015.
| Tumors | Associate Cluster | Prevention |
|---|---|---|
| Digestive tract cancers | Helicobacter pylori infection. Being overweight or obese. Diet. Alcohol use. Previous stomach surgery, polyps, Pernicious anemia, Menetrier disease (hypertrophic gastropathy), Lynch syndrome (hereditary non-polyposis colorectal cancer, or HNPCC), Familial adenomatous polyposis (FAP), Peutz-Jeghers syndrome (PJS), familiarity. Having type A blood. | Limit alcohol, tobacco products. Avoid eating smoked and pickled foods and salted meats and fish. Diet rich in fresh fruits and vegetables and fiber. Supplementing oral vitamins. Endoscopic screening and surveillance of risk population. H. pylori eradication. Polyps screening and resection. |
| Respiratory tract cancers | Tobacco smoke. Exposure to radon, asbestos, agents in the workplace, Arsenic, radiation therapy, pollution, Chronic Obstructive Pulmonary Disease (COPD) | Avoid smoke. Test home for radon. Avoid carcinogens at work. Eat fruits and vegetables. Exercise most days of the week. Imaging and functional diagnostic strategy screening |
| Femal urogynecological tract cancers | Methylmercury, carbon monoxide, lead, ethylene oxide Cadmium, lead, mercury, chlorinated hydrocarbon solvents. Family history of breast, ovarian, uterine, or colon cancer. Obesity. Diethylstilbestrol (DES) exposure. Human papillomavirus (HPV) infection. | Pap tests, maintaining a healthy diet and lifestyle, genetic testing, and the HPV vaccine are at the forefront of gynecologic cancer prevention. Diet rich in fruit and vegetables |
| Male urogynecological tract cancers | Methylmercury, carbon monoxide, lead, ethylene oxide Cadmium, lead, mercury, chlorinated hydrocarbon solvents. Infection. Tobacco consuming. Family history. Eating habits. Agent Orange exposure. | Chemoprevention especially in prostate cancer. Dietary changes. PSA blood test. Ultrasound screening test. Urinary test. Limiting exposure to toxic agents, especially in the workplace. |
| Breast cancer | Genetic mutations. Having dense breasts. Personal history of breast cancer or certain non-cancerous breast diseases. Family history of breast or ovarian cancer. Previous treatment using radiation therapy. Exposure to the drug diethylstilbestrol (DES). Hormonal therapies. Drinking alcohol, obesity, Not breastfeeding, Menopausal hormone therapy. | Limit alcohol. Maintain a healthy weight. Be physically active. Breast-feed. Limit postmenopausal hormone therapy. Lifestyle and diet modification, vitamins, and micronutrient supplementation. Routine breast screening |
| Nervous system cancers | Home and work exposures to electromagnetic fields and ionizing radiation. Family history. Exposure to infections, viruses, and allergens. Hereditary genetic factors or conditions, including Li-Fraumeni syndrome, neurofibromatosis, nevoid basal cell carcinoma syndrome, tuberous sclerosis, Turcot syndrome, and von Hippel-Lindau disease. | Reduce the risk of developing a brain tumor by avoiding environmental hazards such as smoking and excessive radiation exposure. |
| Skin cancers | Exposure to the sun with ultraviolet rays. Dioxin, nickel, arsenic, mercury, cement (chromium), polychlorinated Biphenyls (PCBs), glues, and rubber cement. A family history of skin cancer. A personal history of skin cancer. Older age. | Seek the shade, especially between 10 AM and 4 PM. Don’t get sunburned. Avoid tanning, and never use UV tanning beds. Use a broad-spectrum (UVA/UVB) sunscreen. Adhering strategy prevention of melanoma using epiluminescence |
| Hepato-biliary tract cancers | Carbon tetrachloride, methylene chloride, vinyl chloride. Smoke, Obesity (body mass index >30 kg/m2). Heavy alcohol use, Long-standing diabetes (>5 years). Hepatitis B and C Infection, Nonalcoholic fatty liver disease (NAFLD), Hemochromatosis, male hormones or anabolic steroids, Ingestion of aflatoxin. | Maintaining a healthy weight, stopping smoking, and limiting your alcohol intake. Avoid and treat hepatitis B and C infections. Limit exposure to cancer-causing chemical agents. Treat diseases that increase liver cancer risk. |
| Hematological cancers | Arsenic, nitrates, radiation Smoking, radiation exposure, and exposure to chemicals such as benzene (a widely used industrial chemical) have all been linked to an increased risk of some types of blood cancers. Epstein-Barr virus, HIV, and human T-cell lymphoma/leukemia virus infections. Chemotherapy Drugs | Avoid exposure to radiation, chemicals such as pesticides or benzene, and smoking or tobacco in any form. Additional lifestyle behaviors, such as staying active and eating a healthy diet can help reduce your risk of developing a variety of cancers and other diseases. Periodical hematochemical controls |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciarambino, T.; Crispino, P.; Para, O.; Giordano, M. Clustering Diseases in Cancer and Health Organization: What Is the Gold-Standard Approach? BioMed 2022, 2, 282-302. https://doi.org/10.3390/biomed2030023
Ciarambino T, Crispino P, Para O, Giordano M. Clustering Diseases in Cancer and Health Organization: What Is the Gold-Standard Approach? BioMed. 2022; 2(3):282-302. https://doi.org/10.3390/biomed2030023
Chicago/Turabian StyleCiarambino, Tiziana, Pietro Crispino, Ombretta Para, and Mauro Giordano. 2022. "Clustering Diseases in Cancer and Health Organization: What Is the Gold-Standard Approach?" BioMed 2, no. 3: 282-302. https://doi.org/10.3390/biomed2030023
APA StyleCiarambino, T., Crispino, P., Para, O., & Giordano, M. (2022). Clustering Diseases in Cancer and Health Organization: What Is the Gold-Standard Approach? BioMed, 2(3), 282-302. https://doi.org/10.3390/biomed2030023






