1. Introduction
LBP is the most common medical condition for those living with a disability; in most countries, it is also the top medical condition requiring rehabilitation [
1]. A classical medical approach is disease-oriented and directs all management efforts of LBP towards identifying underlying causes of pain in the low back, namely, structural and/or functional impairments or sensory impairments associated with chronic pain, and treating these impairments to alleviate the pain and improve a person’s health state. Rehabilitative and preventive medicine conceive pain in the low back as a medical condition associated with a person’s impaired functioning and health state. Its efforts are primarily directed towards interventions that minimize the person’s disability and optimize his/her functioning level associated with LBP. Disability is defined as an individual’s perceived impaired functioning and health state, i.e., the perceived impaired functions/structures and limited activities/participation modulated by personal and contextual factors. As both aspects are subject to the management of patients with LBP, we aim to discuss the advantages and disadvantages of disease versus a health approach if a person with low back pain seeks help in a medical office.
Before this article’s question can be answered, several basic terms must be defined. These terms include, among others, definitions of “pain”, “health”, “disease”, “pain”, “medicine”, and “health care”. This terminology is also the foundation for the following discussion on whether low back pain should be understood as a disease or condition of impaired functional health. Finally, definition-inherent consequences for the comprehensive care of back pain patients are discussed.
2. Terminology: Health
The term “health” is elusive in its definition. Health terms, such as “disease” are understood as a construct to capture an important condition of living organisms. The definition of human health has an enormous impact on the practice of medicine and intervention options to improve the health condition of individuals. Defining health is both crucial and difficult. The WHO definition of “a state of complete physical, mental and social wellbeing, and not merely the absence of disease or infirmity” is not perfect but points in the right direction. One problem with this definition is the attempt to define complete health. Keeping that aspect of absolute health counteracts that important statement with the idea that health is “not merely the absence of disease or infirmity.” The International Classification of Functioning, Disability and Health (ICF) [
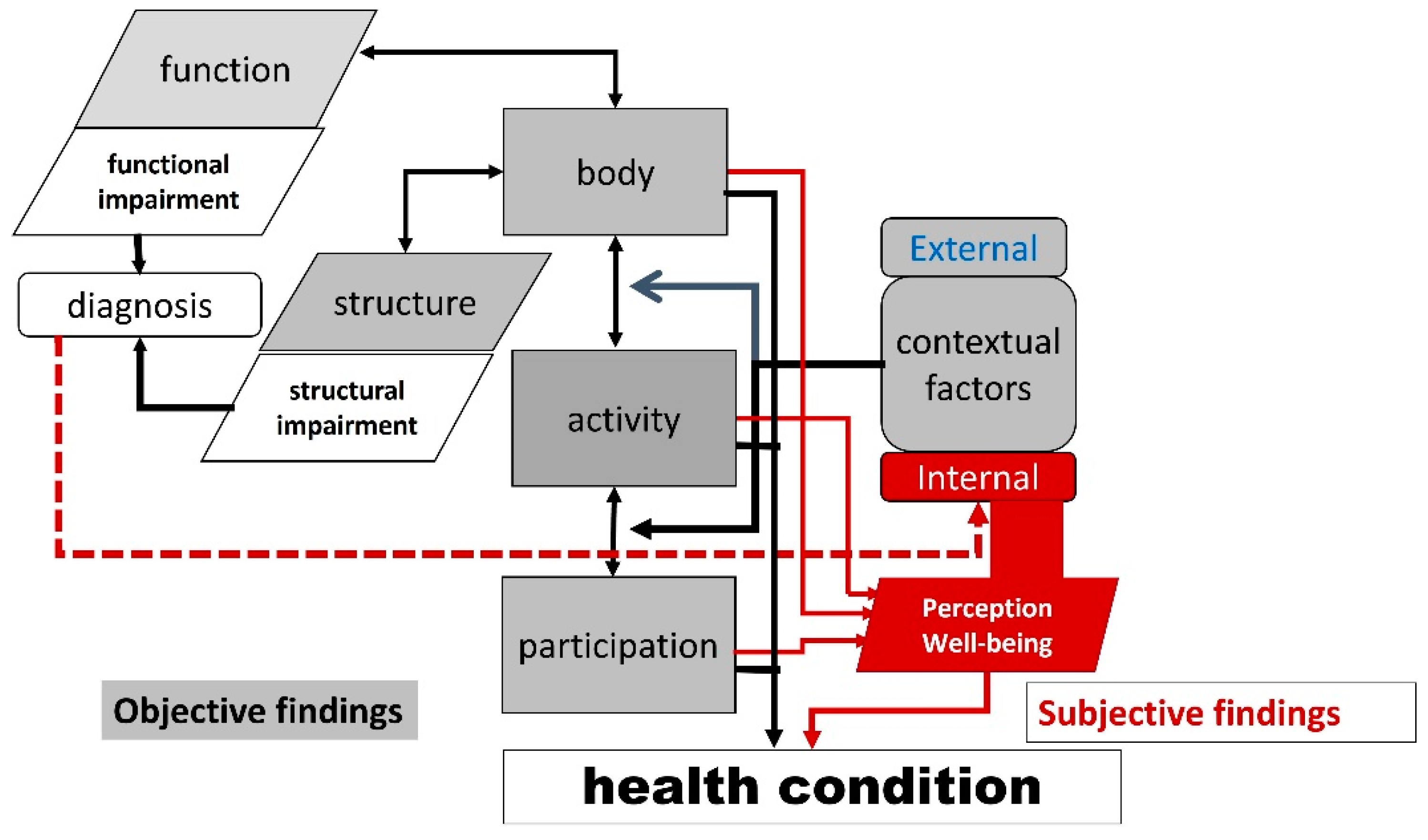
2] clarified health as the summary of physical, mental, and social functioning in relation to diseases. ICF-based classifications provide a catalog encompassing graded physical function, personal activity/participation, and an individual’s physical and social environment. This catalog is associated with a diagnosed disease in the original ICF model, as represented in the International Classification of Diseases (ICD). We recently proposed a modification to the original ICF model [
3]. In this modification, the diagnosis is linked to impaired bodily functions/structure and perception of the diagnostic process; the result is understood as an important aspect of internal context factors. Internal context factors include the perception of bodily functioning, activity/participation, external context factors, and the level of subjective health conditions and well-being. In the modified ICF model (
Figure 1), health is not an absolute state but rather a graded condition determined by objective assessment results, subjective perception and the experience of being alive. The term “condition” is used in a descriptive sense as “the state of something regarding its appearance, quality, or working order”. The relationship between conditions and diseases is different; the general health condition integrates objective and subjective evaluations of ICF components. Conditions are associated with health, but in a medical sense, the disease determines a person’s health state.
3. Terminology: Disease
Disease may be defined as a disorder of structure or function in a human, animal, or plant, especially one that produces specific symptoms or affects a specific location that is not simply a direct result of physical injury [
4]. Thus, the term “disease” refers to a pathological process identified as a deviation from a biological norm. There is objectivity about disease that physicians can see, touch, smell, and measure. This approach is predominant in science-based medicine and textbooks describing disease entities for which patients are the manifestation field. This definition has served as a basic term in a debate on whether chronic non-malignant pain (CP) should be regarded as a condition or disease entity [
5]. The term “disease” differentiates from other descriptions of unhealth [
6], which are illness and sickness.
Illness refers to a feeling or experience of unhealth in a person’s interior that is entirely personal. Disease and illness are related, but signs of illness are difficult and almost impossible to measure even when psychological assessment tools objectivate patients’ personal experiences. On the other hand, subjective health experience relates to the definition of unhealth for persons afflicted with a disease. Thus, for anyone, it is real, true, and as valid as any external evaluation of this subjective experience.
The third mode of unhealth is
sickness. Sickness is a social role, status, negotiated position in the world, a bargain struck between the person henceforward called “sick” and the society prepared to recognize and sustain them. Confirmation of being sick often relies on a confirmed disease and is accepted on different levels for various diseases and their affected patients. The whiplash epidemic in Australia declined because health authorities massively reduced sickness certifications [
7].
Illness and sickness would be overlooked if unhealth was reduced to the disease mode stipulated by a mandatory WHO ICD classification, thus creating the impression that diseases are strange creatures or small biological agents that attack and modify individuals’ health. Fighting against these “disease personalities” is mandatory to eradicate those “demons” forever. “Disease” is defined as a model that describes and explains an experienced impairment of a person’s state of health or the risk of future health impairments based on structural and functional conditions [
3]. In other words, the community recognizes the personal experience of being unwell or ill as being sick and medically and causally explained by disease. The quasi-causal position of pathology may lead to the oversimplification of causal chains stemming from the disease-defining pathological lesion.
In order to define a disease, a diagnosis must be established. The diagnosis distinguishes between pathological lesions and is defined as:
The art or act of identifying a disease, illness, or problem by examining someone or something;
A statement or conclusion that describes the reason for a disease, illness, or problem;
The art or act of identifying a disease from its signs and symptoms;
The decision reached by the diagnosis [
8].
From a quality assurance perspective, diagnosis is understood as a process rather than a classification label necessary for public health statistics. The core of the diagnostic process is gathering, integrating, and interpreting information based on a patient’s experiences of a health problem derived from clinical history, physical examination, referrals, consultation, and diagnostic tests. The established diagnosis is then communicated to the patient and serves as the basis for therapeutic interventions. The outcome of treatment serves as feedback to the core process of diagnostic information processing [
9].
Undoubtedly, a person reporting pain in the low back area can be examined, but the underlying causes of this pain (the “problem”) in the low back are typically complex and may relate to trauma, a tumor, inflammation, or malformation. The “act of identifying” causes of low back pain shows that a leading perception impairment, the “symptom pain”, cannot be classified as a “disease” at the same time. The “decision reached by diagnosis” would, according to the definitions, be based on diagnostic findings of the disease.
Therefore, one may reasonably question whether “low back pain” represents a diagnosis and thus be classified as a disease. In the new International Classification of Diseases (ICD 11), low back pain is classified under “Symptoms, signs or clinical findings of the musculoskeletal system” Chapter 21—ME84.2, along with other relevant numbers to be excluded (MG30.02; MG30.3; MG30.5; FA80-FA8.Z)
The detection and treatment of diseases are the core tasks of medicine, whereas health care expands its aims beyond disease management and comprehensively includes all determinants of health. Physical activity of various intensities also plays a central role in health maintenance [
10].
4. Terminology: Infirmity/Frailty/Vulnerability
The WHO also defines another antonym to health, i.e., infirmity. This archaic term is a precursor to the more modern term frailty. In 1949, infirmity was a condition understood differently than a disease, although with features of personally experienced illness and/or socially recognized sickness. The medical definition of frailty shares similar features to disease, including thresholds for normal physical structures and functions, such as body mass, muscle strength, walking speed, and physical activity [
11]. The term frailty was criticized due to inherent pejorative and exclusionary tendencies [
12]. The FRAILOMIC Initiative promotes the following definition of frailty as “an age-associated syndrome characterized by a decrease of biological reserve and resistance to stressors due to functional decline of several physiological systems and placing the individual at enhanced risk of disability, hospitalization, and death”. In other words, frailty refers to a decline in both robustness and recovery (resilience). According to the International Classification of Impairments, Disabilities and Handicaps-ICIDH (WHO, 1980), disability is defined as “any restriction or lack (resulting from an impairment) of ability to perform an activity in the manner or within the range considered normal for a human being” ([
13]. Thus, the term frailty is associated with vulnerability, particularly at advanced ages and/or consuming diseases.
5. Terminology: Pain and Definition of LBP
The current IASP definition of pain is “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” [
14]. One of their explanatory notes states that “Pain and nociception are different phenomena as pain cannot be inferred solely from activity in sensory neurons” but this depends on an individual’s associations with pain and related automatic and voluntary reactions/responses to pain. This statement is confusing at first glance since the perception of a noxious stimulus is painful by definition. The second sentence in the note removes the confusion of nociception being defined as detecting potentially or injurious stimuli followed by a reflex withdrawal [
15].
The person In pain is the only source of explaining and describing the pain experience. Thus, the pain has features of both illness (“it hurts”) and sickness (“a person in pain”) but presents few indirect characteristics that are easily captured by medical examination, as expected in the case of a disease. From a descriptive view, pain is a condition or health state associated with actual or potential tissue damage. A disease resulting in tissue damage may cause symptoms of pain. However, the classification of pain as a disease requires functional and/or structural findings and not just the perception of pain that arises from actual or threatened damage to non-neural tissue and the activation of nociceptors. Similarly, “neuropathic pain” is also not a disease and is defined as pain caused by a lesion or disease of the somatosensory nervous system. The recently promoted term “nociplastic pain” does not describe a structural and/or functional pathology required for disease classification. “Nociplastic pain” is defined as pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage from the activation of peripheral nociceptors, which are free nerve endings that transduce harmful information via A-δ and C fibers to the spinal cord, where this information is relayed to higher centers of the CNS. Most importantly, the intensity of perceiving the noxious pain stimulus depends on the context/environmental factors under which the pain was perceived [
16]. This finding provides evidence for diseases or lesions within the tissues causing pain [
17]. Nociplastic pain may contribute to the pain experience of common musculoskeletal pain syndromes, such as low back pain or fibromyalgia.
A variety of techniques and aids for documenting pain experiences transformed this subjective phenomenon into objective estimates of this unpleasant experience. Simple visual analog scales (VAS) became the standard to measure the intensity of spontaneous, recalled, or provoked pain [
18]. Sensory testing helps detect allodynia; an acquired misinterpretation of non-painful stimuli presented to the skin [
19]. Multidimensional questionnaires capture pain characteristics other than intensity [
20] and detect relationships to emotions, expectations, and pain triggering circumstances [
21].
Low back pain is a health condition associated with a higher risk for years of life living with disability (YLDs) frequently reported worldwide [
1]. Low back pain (LBP) is defined as localized pain and discomfort below the costal margin and above the inferior gluteal folds, with or without leg pain. Nonspecific (common) low back pain is defined as pain not attributed to recognizable, known, or specific pathology (e.g., infection, tumor, osteoporosis, ankylosing spondylitis, fracture, inflammatory process, radicular syndrome, or cauda equina syndrome) [
22].
An important aspect of pain experience is the duration of this unpleasant sensation. Manifestations lasting up to 6 weeks are commonly classified as “acute”, “subacute” for manifestations between 6 and 12 weeks, and “chronic” for conditions/diseases lasting longer than 12 weeks.
5.1. Health: A Condition or Absence of Disease/Infirmity
Functional health is primarily a condition representing the complex interaction of the physical, mental, and social components in an individual’s life. The basic assumption of medicine is that all deviations from normal functioning are caused by objectively detected deficits in physiological functions and/or body structures. This finding leads to a binary classification of people into “healthy” or “diseased” categories. Therefore, in medicine, an individual’s health is classified as a disease based on external assessments, evaluation processes, and the impact on lifetime prevalence. In other words, the older a population, the smaller the percentage of healthy persons. This model’s subjective perspective of diseased persons is unfavorable since health is both a rare state and dependent on external judgment.
Considering health and its impairments as a condition has several advantages. Understanding health as the summary of functioning transforms the categorical classification of the antonyms “health” and “disease or infirmity, respectively”, into a continuum of experiences living on different levels of functioning. Functional assessments, personal activity/participation, and the external environment come first, and investigations into the physical causes of the observed condition come second. In the condition’s evaluation process, subjective experience and objective findings equally contribute to the final level of an individual’s health state. Since an individual’s health state results from subjective and objective inputs on their functioning, the individual’s perspective on their health may improve or be maintained at a low level of dysfunction. The condition’s description also helps compare the health states of different diseases. However, for deciding interventions, labeling the health condition with a single or collection of ICF codes creates similar problems to representing disease by a single or a collection of diagnostic codes provided in the International Classification of Diseases (ICD).
5.2. Non-Specific Low Back Pain as Condition
If LBP is defined as a person’s pain sensations in the low back region of the body, then this definition should fulfill the requirements of a “condition”. The definition of low back pain notes that various underlying diseases may cause the pain. The first step in a diagnostic workup is to recognize the physical condition of any health impairment. In the case of low back pain, clinicians are advised to check for red flags (or alarm signals) while recording the patient’s medical history and conducting a physical examination to evaluate severe underlying pathology [
23]. Despite the wide variety of red flags presented in guidelines for low back pain, there is a lack of consensus on which red flags to endorse. Such a condition-oriented approach allows the separation of patients with specific low back pain from those with nonspecific low back pain. Currently, the duration of the back pain episode is used to assign the most effective treatment for patients with nonspecific low back pain since several studies have raised evidence that patients with acute, subacute, or chronic nonspecific low back pain respond differently to the same type of therapeutic intervention [
24].
Similar to a diagnosis, a one-dimensional description of a physical condition cannot capture the complex components associated with pain experienced in the low back region. Core outcome sets were proposed for back pain trials at the end of the 20th century for back pain sufferers in acute health care [
25]. The three outcome domains in the outcome set include (1) physical functioning, (2) pain intensity, and (3) health-related quality of life (HRQoL). The Oswestry Disability Index version 2.1a (ODI 2.1a) or the 24-item Roland Morris Disability Questionnaire (RMDQ-24) are recommended as assessment tools for physical functioning. Pain intensity should be recorded using a Numeric Rating Scale (NRS). For HRQoL, the recommended questionnaires are either the Short Form Health Survey 12 (SF12) or the 10-item PROMIS Global Health (PROMIS-GH-10) ([
25].
Patients with acute conditions may develop ongoing pain for more than 12 weeks, and treatment results may be biased when derived from populations with back pain present ≤6 weeks. The transmission from acute to chronic low back pain may be associated with psychosocial distress [
26,
27]. Chronicity can also be triggered by the patient’s emotional experience with pain and their beliefs and expectations about the course of the pain [
28]. Several questionnaires for chronicity screening in patients with acute back pain have been developed [
29,
30]. The STarT Back Screening Tool [
31] or Orebro Screening Tool [
32] primarily screen for psychosocial risk factors. They were established for their good discriminative abilities. Matching treatment programs with risk classes detected by the STarT tool results in greater health benefits for back pain patients stratified to risk-matched treatment, than patients receiving physical therapy based on the physiotherapist’s evaluation [
33].
5.3. Non-Specific Low Back Pain as a Disease
As previously mentioned, classifying a health condition as a disease requires that the confirmed deviation from normal function and/or body structure relates to tissue lesions, inclusive of nerves and inflammation, due to any cause which establishes a causal relationship with objectively detected signs of the perceived health impairment and explains corresponding symptoms. Thus, removing the cause of the illness by eradicating infectious agents, correction, or compensation for physiological functions and damaged structure repair appears to be an appropriate way of re-establishing health. However, establishing causality is more difficult than it appears at first glance. Physiological systems do not function exclusively in a monocausal relationship, such as stimulus and response. The physiological system of living beings is a complex network of functions connected by necessary and/or sufficient and/or contributory causes via multiple pathways. A typical contributory cause is described as INUS, “an insufficient but necessary part of a condition which is itself unnecessary but sufficient for the result” [
34]. Lesions generated by external causes, such as physical forces in the case of injuries or biological agents causing infections are better understood than lesions arising from derangement of structures and/or specific functions. For the latter lesion, a multifactorial genesis seems to be predominant.
Several common alterations in the structure and motion of the low back region have been proposed as an underlying disease of non-specific low back pain. However, there is no evidence that these alterations are the single cause of back pain, which would otherwise be labeled as specific back pain. Due to gaps in understanding the causal chains initiated by dysfunction of the lumbar spine vertebral motion segment, this central pathology of manual/osteopathic/chiropractic medicine is currently not accepted by scientific/evidence-based medicine as a cause of back pain.
This explanation appears widely endorsed by national and international guidelines on non-specific back pain that identify vertebral fractures, infections, radiculopathies/neuropathies, tumors and axial spondylarthritis as the leading causes of specific back pain. However, they admit that more subtle contributors to LBP cannot be identified in clinical practice due to the lack of accurate and reliable differential diagnostic methods for assessing myofascial and joint-related structures’ contribution to back pain syndrome.
Thus, the German guideline on nine structural entities and two functional entities as specific causes for back pain, which proposes that all these entities possess an ICD-code and can be ruled out by objective imaging techniques, is arguable [
35]. There is a striking mismatch between the 2016 English and German ICD codes. The first structural entity is “the lumbar facet syndrome/spondylarthrosis”, and the
Supplementary Table S1 shows the German terminology and English ICD codes side by side. The ICD codes M45– M49 classify spondylopathies, structural changes of the spinal joints, and vertebral bodies as “degeneration”, appearing under code M47 as “spondylosis, including arthrosis or osteoarthritis of spine and degeneration of facet joints”. Code M47.1 combines spondylosis with myelopathy, whereas code M47.2 is reserved for spondylosis causing radiculopathy. Code M47.8 describes spondylosis without myelopathy or radiculopathy, and code M47.9 is used for unspecified spondylosis. The differentiation between codes M47.8 and M47.9 remains unclear, but spondylosis without associated clinical symptoms may be the correct entity for M47.9 (
Supplementary Materials Table S1).
The German guideline combines code M47 spondylarthrosis with facet syndrome. “
Syndrome” is defined as a group of symptoms that consistently occur together or a condition characterized by associated symptoms. The clinical description of a facet syndrome does not fulfill the classification requirements for a syndrome. In addition, the adjective scale of acute and chronic pain describes the duration of a disease/condition, not its signs/symptoms; therefore, it should not be coded as being “associated with radiculopathy”. Another unclear term is “facet irritation—Facettenreizung”, coded by M54.5. The word “strain” in “low back strain” may have been the reason to code “acute lumbago with facet irritation” in M54.4. However, if the ligamentous joint structures were damaged by force, this condition is typically called a “sprain”, whereas the term “strain” is used for muscle injuries and ligamentous/tendinous attachments to bone. The assumption that acute or unspecified low back pain is regularly associated with irritated facet joints should be excluded from codes intended for the objective classification of diseases. However, Chapter 2 of the structural entities “Discogenic lumbar syndrome/vertebral osteochondrosis” relates to adult osteochondrosis of the spine, M43.1, and chronic degenerative low back pain, falsely coded as M47.26 despite the absence of radiculopathy. M43.1 also relates to M54.4. with the descriptions “acute „lumbar syndrome”, “chronic-recurrent lumbar syndrome”, “chronic lumbar syndrome”, “chronic lumbar syndrome with pseudo-radicular referred pain”, and “lumbar syndrome with pseudo-radicular referred pain” (
Supplementary Materials Table S1).
These definitions of specific LBP widely ignore findings typically observed in clinical imaging or manual medical examinations that may or may not relate to the leading symptoms of back pain with or without irradiation. Such a view would be strongly supported by X-ray or MRI findings indicating disk herniation, degenerative joints with or without signs of inflammation in the facet joints, or spinal stenosis presenting in a relatively high proportion of pain-free persons [
36]; this is particularly the case in middle-aged and older persons.
5.4. “Joint Blockage”
The “hypomobile segmental dysfunction of the lumbar spine” plays a central role in Manual Medicine’s pathophysiological back pain models. Minute movements between the intervertebral joints are evaluated by manual palpation, and restricted motion towards one direction is considered reversible hypomobile dysfunction. Specific passive movement interventions are applied to reinstall the normal, limited “joint play” range. Diagnostic and therapeutic interventions are based on scientific neurophysiological and biomechanical principles [
37]. Due to a lack of epidemiological data on back pain associated with “vertebral blockages” and fair–moderate results in both the repeatability and reproducibility studies of palpation [
38], “vertebral blockage” is not yet accepted as a specific cause for LBP.
5.5. Structural and Functional Alterations within the Myofascial System
Objective examinations of musculoskeletal structures are highly limited to imaging studies by MRI/CT or diagnostic ultrasound. Muscle atrophy and increasing fat content within the paravertebral muscles are related to LBP. However, these findings have been either weakly/inconsistently related or unrelated to pain intensity or muscle weakness and daily activities [
39,
40,
41]. In addition, manual medical examinations attempt to differentiate between normal and increased muscle tone or muscle spasms even if the pain widely depends on the examiner’s palpatory skills and perception. Therefore, a reliable diagnostic examination to objectify impaired muscle tone function is unlikely. Measurements of muscle function, such as strength, power, and endurance also depend on the cooperation of the patient. Palpatory findings from myofascial structures are one of the basic diagnostic procedures of any manual medical examination. Identifying trigger points, tender points, myofascial gliding, and muscular hypertonus/spasms are important indicators of myofascial pain syndromes. However, both trigger points and myofascial tender points also present themselves in back pain-free individuals. However, they are considered causes of low back pain when observed in patients with LBP. Trigger points, which are defined as structural–morphological alterations within a muscle and identified by referring to pain on palpation [
42,
43], indicate lesions and malfunction within the neuromuscular structures. These must be differentiated from “tender points”, which are painful, hyperaesthetic spots on palpation in typical locations but without structural changes or referred to as pain on palpation. The presence of tender points may be interpreted as a decreased pain defense control and/or neuropathic pain (released by provocation) because it presents where sensory nerves perforate fascial layers.
When tender points are present together with trigger points, indicating a decline in the central pain defense mechanisms, they are considered morphological manifestations of neurophysiological and neurovegetative changes within myofascial structures resulting from back pain and are not the source of pain itself. It is worth mentioning that these alterations within the myofascial system, which may also be related to inflammatory tissue processes and reduced blood flow, maintain and thus contribute to processes within the CNS associated with pain chronification [
44].
Likewise, muscle functional changes with weakness, hypertonus, and changes in the fascial system (densification/fibrosis) impair the neuromuscular output necessary to induce movement and stability for the spine. Altered neuromuscular control and changes within the myofascial system change the biomechanic output of muscles contributing to spine stability, movement, and posture. Depending on yellow and blue flags, i.e., the psychosocial expectancy and demands of a person in life, the activity’s behavior will likely change. If the pain is not behaving appropriately, relative overuse can result in myofascial overload or disuse in atrophy and deconditioning [
45]. Both disuse and overuse fuel a vicious cycle that contributes to the maintenance of pain through stimulating peripheral and central sensitization processes relevant to enhanced pain perception, pain chronicity, and disablement [
46]. Although myofascial structural and functional changes contribute to the maintenance of pain and explain flair-ups in LBP, they rarely represent a specific cause for LBP.
5.6. Treatment of a Disease Versus Comprehensive Care of a Health Condition
Understanding LBP as a disease or a condition has important consequences for the management of LBP patients. Classifying a disease as deficits in either structure and/or function does not consider pain sensation. In the case of nociceptive pain, the underlying cause is the noxious stimulus, as neuropathy is the underlying disease of neuropathic pain. Likewise, inflammatory low back pain disease is an inflammatory process in the lumbar region, and neuropathic back pain is caused by various affections of the neural structures located in the spine. Although the separation of back pain cases by the severity of health impairment is a well-established procedure (“red flags”), reducing the treatment of specific back pain to the underlying disease may overlook the illness and sickness components of the patient’s pain condition.
Focusing on specific conditions causing back pain overlooks other important health components. Gender and age influence the assessment and classification of LBP [
47,
48], and context factors of age and gender were implemented into new clinical practice guidelines was proposed [
49].
Epidemiological data, such as prevalence, incidence, prognosis and responsiveness to therapeutic interventions for LBP are other sources for decision making in the management of LBP patients. Many people occasionally report LBP [
50,
51]. As noticed in daily practice, many people recover without medical intervention [
52]. The lifetime prevalence of nonspecific LBP is reported to be 84%, and the prevalence of chronic LBP is about 23%, with 11–12% of the population experiencing disability [
53]. LBP incidence in athletes is between 1 and 30%, and about 90% improve without medical intervention [
54]. Therefore, diagnostic procedures must primarily focus on excluding red and yellow flags, and it may be reasonable to “wait and see” as part of therapeutic procedures.
Therapeutic procedures should be based on a multidisciplinary biopsychosocial approach [
55,
56] because this procedure shows greater cost effectiveness, particularly in Germany [
57].
Key findings in the review article by van Tulder et al. [
24] were that bed rest and exercise therapy is ineffective for patients with acute back pain. Furthermore, manipulation is not superior to the effects of physiotherapeutic applications, such as massage, shortwave diathermy, or exercise. Analgesics, non-steroidal anti-inflammatory drugs (NSAIDs) and, to a lesser degree, muscle relaxants are effective remedies for acute nonspecific low back pain. The effectiveness of NSAIDs for acute low back pain was recently revised: NSAIDs are slightly more effective than placebos for short-term pain reduction (moderate certainty), disability (high certainty), and global improvement (low certainty); however, the magnitude of these effects is minor and not clinically relevant [
58]. A systematic review published in 2017 on the effects of spinal manipulation reported modest improvements in pain and function with transient minor musculoskeletal harms. The evidence was moderate for beneficial effects but greater for transient unwanted effects. These results align with the outcomes of the 1997 study by van Tulder et al. [
24].
Analgesics, NSAIDs and muscle relaxants are less effective for chronic low back pain than in acute low back pain. Exercise therapy is an effective treatment for chronic low back pain. At least moderate evidence exists for the benefit of manipulation. A recent review article evaluated the effectiveness of commonly used interventions in primary care for chronic radicular or non-radicular low back pain. The authors reported a moderate certainty of evidence that exercise, oral NSAIDs, and duloxetine, a serotonin-norepinephrine reuptake inhibitor, provide clinically meaningful benefits to patients with chronic back pain [
59].
These treatments and their varying effectiveness led to different guideline recommendations for managing acute [
50] and chronic low back pain [
60].
6. Conclusions
We found that LBP is defined as pain in a specified anatomical area and lasting a certain period. Therefore, can “pain” be defined as a “disease”? Of course, it is “just” a symptom affecting an individual’s functional health status for a certain time. Additionally, not every pain results in disability or affects an individual’s quality of life.
It is doubtful whether primary prevention can be successful since one of the well-known major risk factors is previous periods of LBP; this health condition has already been found in younger populations [
61]. Other risk factors (modifiable and nonmodifiable) were not identified for a true first episode of LBP [
22] except for exercise programs [
62].
Individual management programs of LBP must be based on a “BIO-PSYCHO-SOCIOECONOMIC-CULTURAL MODEL” [
10] that can be classified with the terminology of ICF and an assessment by adequate instruments (tests, scores, and questionnaires).
A recent literature review concerning the causality of LBP did not differentiate between the LBP “disease” and its recurring episodes mainly due to an unclear definition of LBP absence at baseline. Therefore, the authors could not provide a valid answer on whether LBP can be defined as a disease or episode [
62].
LBP being seen as a condition rather than a disease supports individual comprehensive care where interventions are applied for any kind of health component. This approach does not imply that disease does not have high priority in the management of health problems, but rather, in absence of disease priority, should be defined based on the risk of developing restrictions to activity and/or participation. Reducing disability by targeting the structural/functional conditions defining a disease cannot be achieved since the relationship between pathological changes and restriction to activity/participation is indirect in most cases.