The GooD Pregnancy Network: An Alternative Approach for Gestational Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Measurement
2.3. Intervention Strategy
- 1.
- Remodelling and implementation of a new care pathway:
- 2.
- Staff engagement and behavioural change:
- 3.
- Collaboration with patients:
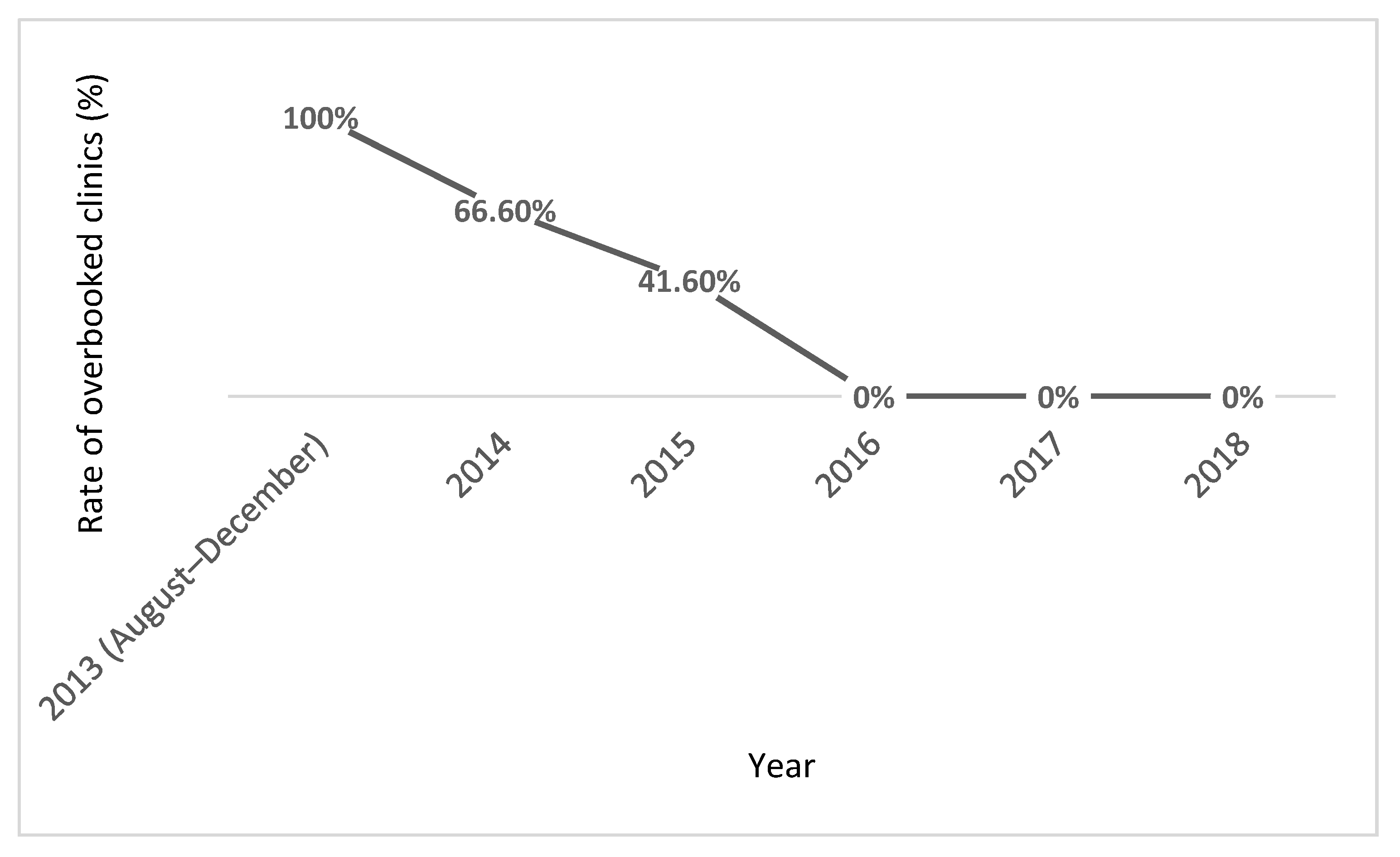
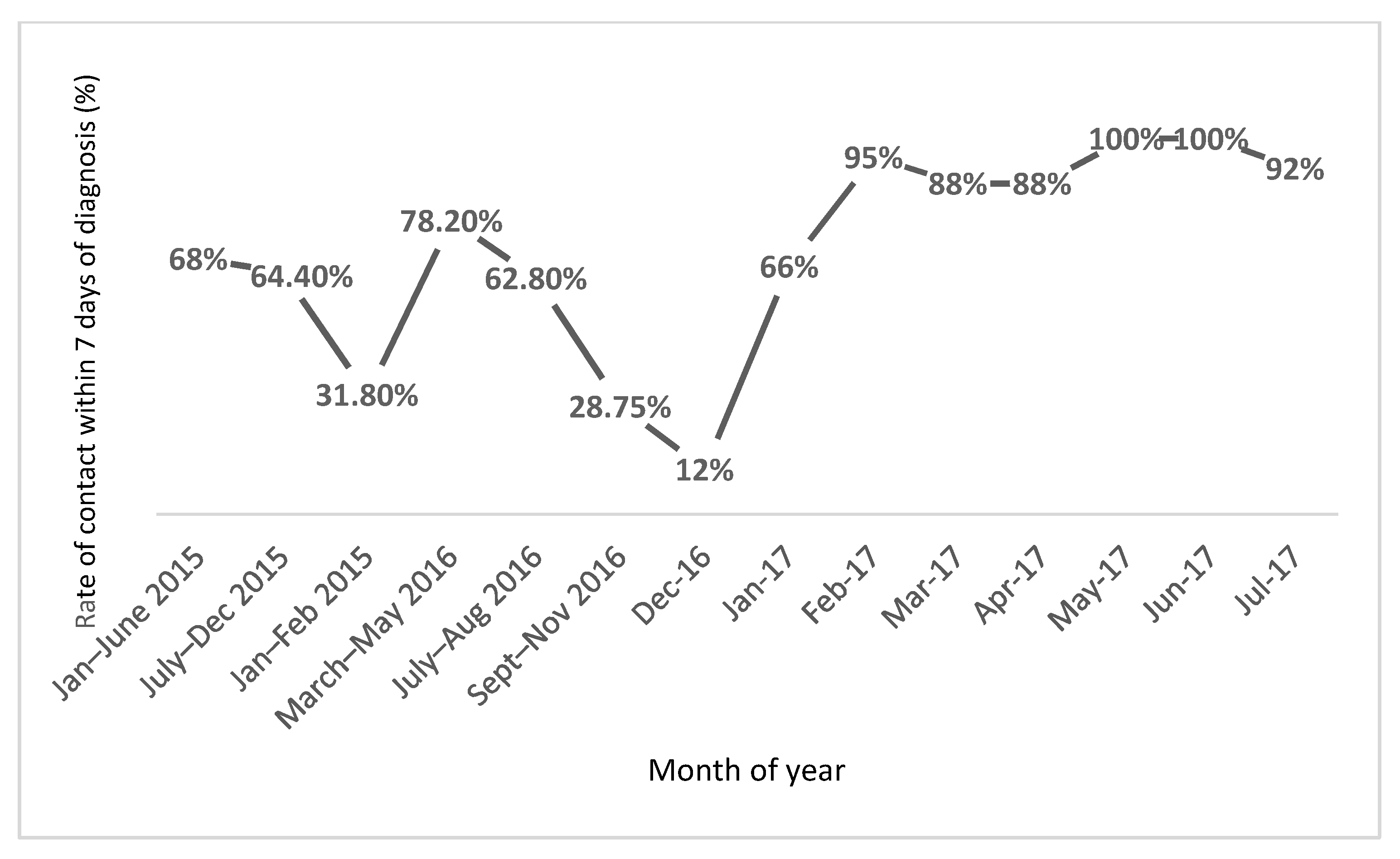
3. Results
3.1. Process Measures
3.1.1. Specialist Clinic Capacity
3.1.2. Rate of First Clinic Attendance within 7 Days of GDM Diagnosis
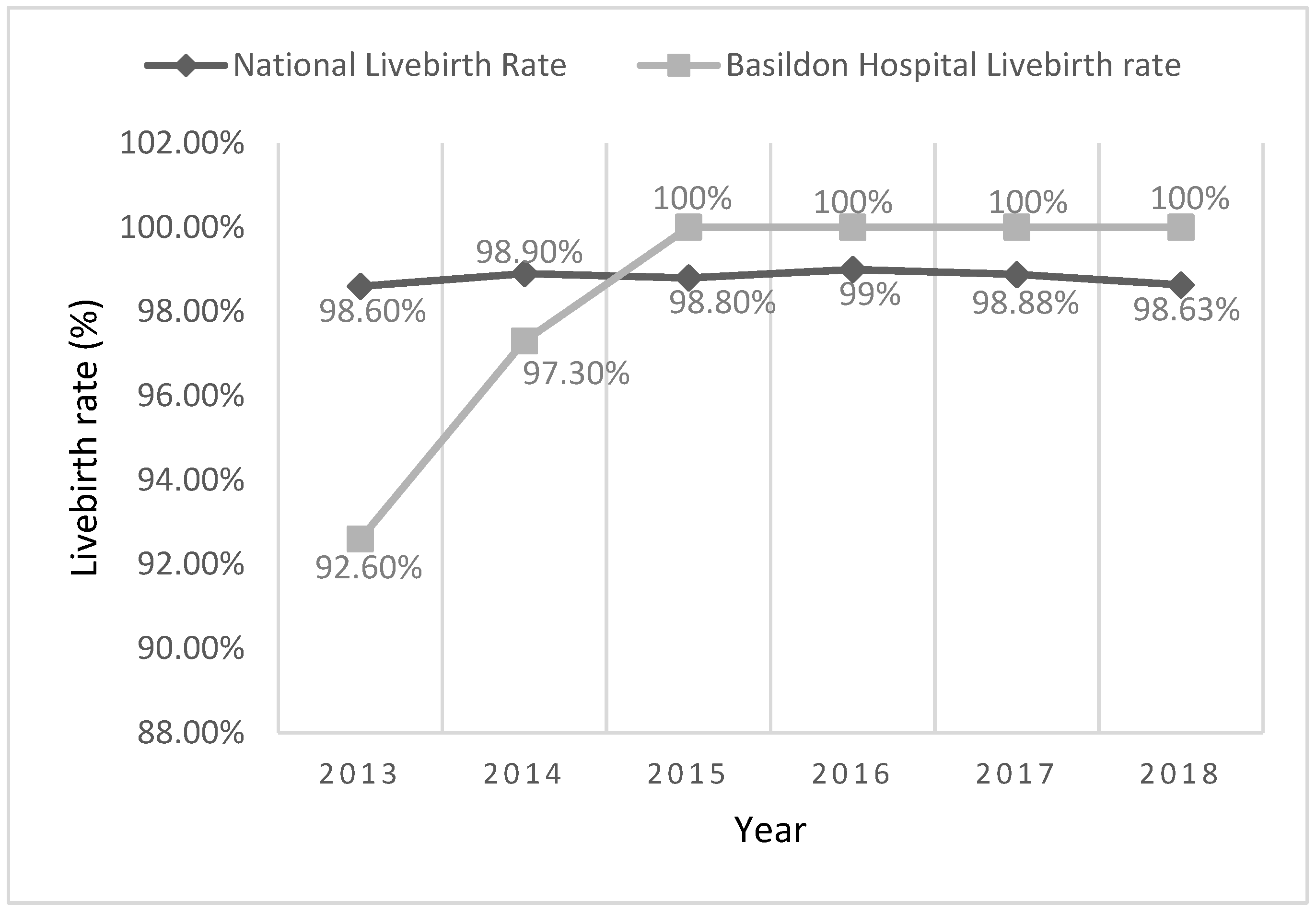
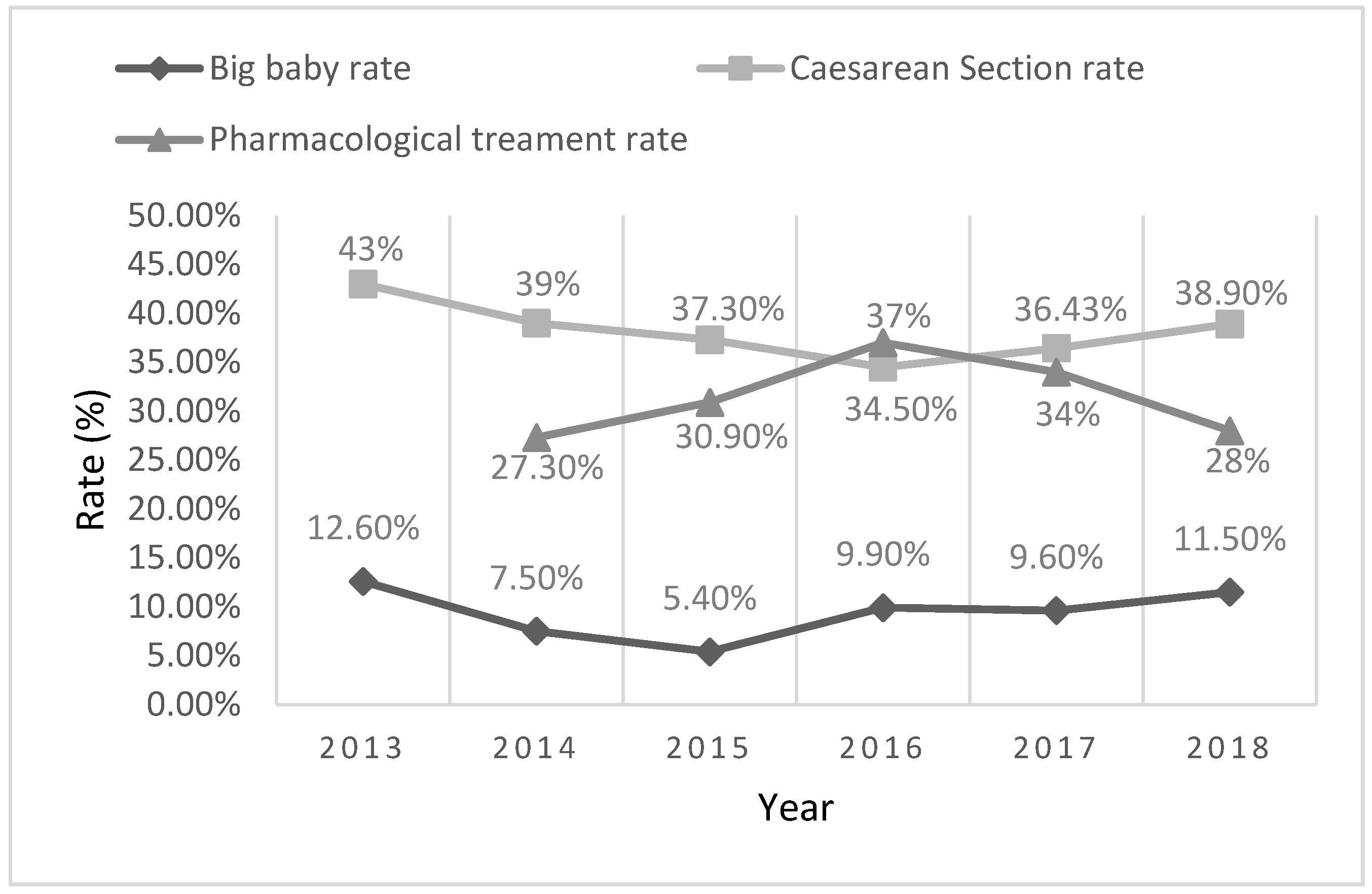
3.2. Balancing Clinical Outcome Measures
3.2.1. Live Birth Rates in Type 1 and 2 Diabetic Women
3.2.2. Caesarean Section and Big Baby Rates in GDM Women
3.2.3. Patient Satisfaction
3.2.4. Additional Observations
3.2.5. The Impact of the COVID-19 Crisis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whicher, C.; O’Neill, S.; Holt, R. Diabetes in the UK: 2019. Diabet. Med. 2020, 37, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.; Pavkov, M.; Magliano, D.; Shaw, J.; Gregg, E. Global trends in diabetes complications: A review of current evidence. Diabetologia 2018, 62, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.; Hashim, M.; King, J.; Govender, R.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2019, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alwash, S.M.; McIntyre, H.D.; Mamun, A. The association of general obesity and visceral body fat with the risk of gestational diabetes mellitus: Evidence from a systematic review and meta-analysis. Obes. Res. Clin. Pract. 2021, 15, 425–430. [Google Scholar] [CrossRef]
- Powe, C.E.; Carter, E.B. Racial and Ethnic Differences in Gestational Diabetes: Time to get serious. JAMA 2021, 326, 616–617. [Google Scholar] [CrossRef] [PubMed]
- Thurrock Joint Strategic Needs Assessment. Demograhics and Population Change, 1st ed.; Thurrock Council: Thurrock, UK, 2015; p. 15. Available online: https://www.thurrock.gov.uk/sites/default/files/assets/documents/jsna-demographics-population-v02.pdf (accessed on 29 October 2021).
- Thurrock Joint Strategic Needs Assessment. Whole Systems Obesity, 1st ed.; Thurrock Council: Thurrock, UK, 2017; p. 7. Available online: https://www.thurrock.gov.uk/sites/default/files/assets/documents/jsna-obesity-201709-v01.pdf (accessed on 29 October 2021).
- Public Health England. Thurrock Health Profile 2013, 1st ed.; Public Health England: London, UK, 2013; p. 4. Available online: https://fingertips.phe.org.uk/profile/health-profiles/data#page/13/ati/202/are/E06000034 (accessed on 29 October 2021).
- Public Health England. Basildon Health Profile 2013, 1st ed.; Public Health England: London, UK, 2013; p. 4. Available online: https://fingertips.phe.org.uk/profile/health-profiles/data#page/13/ati/201/are/E07000066 (accessed on 29 October 2021).
- Public Health England. Basildon Local Authority Health Profile 2019, 1st ed.; Public Health England: London, UK, 2019; p. 4. Available online: https://fingertips.phe.org.uk/profile/health-profiles/data#page/13/ati/201/are/E07000066 (accessed on 29 October 2021).
- Public Health England. Thurrock Local Authority Health Profile 2019, 1st ed.; Public Health England: London, UK, 2019; p. 4. Available online: https://fingertips.phe.org.uk/profile/health-profiles/data#page/13/ati/202/are/E06000034 (accessed on 29 October 2021).
- National Institute for Health and Care Excellence (NICE). Diabetes in Pregnancy: Management from Preconception to the Postnatal Period; NICE: Manchester, UK, 2015; Clinical Guideline NG3; Available online: https://www.nice.org.uk/guidance/ng3 (accessed on 29 October 2021).
- NHS England and NHS Improvement. Plan, Do, Study, Act (PDSA) Cycles and the Model for Improvement; NHS England: Leeds, UK, 2021; Available online: https://www.england.nhs.uk/wp-content/uploads/2021/03/qsir-plan-do-study-act.pdf (accessed on 29 October 2021).
- Metzger, B. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [Green Version]
- World Health Organisation (WHO). Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. Available online: https://www.who.int/publications-detail-redirect/WHO-NMH-MND-13.2 (accessed on 29 October 2021).
- Caballero, C. Shared medical appointments. Nurse Pract. 2015, 40, 1–6. [Google Scholar] [CrossRef]
- Guirguis, A.; Lugovich, J.; Jay, J.; Sanders, K.; Cioffi, S.; Jeffery, S.; Kravetz, J. Improving Diabetes Control Using Shared Medical Appointments. Am. J. Med. 2013, 126, 1043–1044. [Google Scholar] [CrossRef]
- Jhagroo, R.; Nakada, S.; Penniston, K. Shared Medical Appointments for Patients with Kidney Stones New to Medical Management Decrease Appointment Wait Time and Increase Patient Knowledge. J. Urol. 2013, 190, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Lacagnina, S.; Tips, J.; Pauly, K.; Cara, K.; Karlsen, M. Lifestyle Medicine Shared Medical Appointments. Am. J. Lifestyle Med. 2020, 15, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Hickey, G.; Chambers, M. Patient and public involvement and engagement: Mind the gap. Health Expect. 2019, 22, 607–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 7. [Google Scholar] [CrossRef]
- Noctor, E. Type 2 diabetes after gestational diabetes: The influence of changing diagnostic criteria. World J. Diabetes 2015, 6, 234. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.; Zinman, B.; Wang, X.; Hux, J. Risk of development of diabetes mellitus after diagnosis of gestational diabetes. Can. Med. Assoc. J. 2008, 179, 229–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NHS Digital. National Pregnancy in Diabetes Audit. 2013. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/national-pregnancy-in-diabetes-audit/national-pregnancy-in-diabetes-audit-2013#:~:text=Publication%2C%20Part%20of,31%20Dec%202013 (accessed on 29 October 2021).
- Diabetes Alliance for Research in England (DARE)//Diabetes Genes. Available online: https://www.diabetesgenes.org/current-research/dare/ (accessed on 29 October 2021).
- Mackillop, L.; Hirst, J.E.; Bartlett, K.J.; Birks, J.S.; Clifton, L.; Farmer, A.J.; Gibson, O.; Kenworthy, Y.; Levy, J.C.; Loerup, L.; et al. Comparing the Efficacy of a Mobile Phone-Based Blood Glucose Management System in Women with Gestational Diabetes: Randomized Controlled Trial. JMIR Mhealth Uhealth 2018, 6, e71. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Obstetricians and Gynaecologists (RCOG). Guidance for Maternal Medicine Services in the Evolving Coronavirus (COVID-19) Pandemic. Information for Healthcare Professionals Version 2.1. Published Friday 24 April 2020. Available online: https://www.rcog.org.uk/globalassets/documents/guidelines/2020-07-10-guidance-for-maternal-medicine.pdf (accessed on 29 October 2021).
- Ikomi, A.; Mannan, S.; Anthony, R.; Kiss, S. Likelihood of ‘falling through the net’ relates to contemporary prevalence of gestational diabetes. Diabetologia 2015, 58, 2671–2672. [Google Scholar] [CrossRef] [Green Version]
- Ikomi, A.; Mannan, S.; Simon, G.; Khan, R.; Smith, S.; Robbins, J.; Kavanagh, U.; Crone, D. Diagnosis of gestational diabetes during the pandemic: What is the risk of falling through the net? Diabet. Med. 2020, 37, 1782–1784. [Google Scholar] [CrossRef]
- Mannan, S.; Ikomi, A. Accuracy of Bayesian modelling in predicting COVID—19’s impact on gestational diabetes rates. In The Physician: Journal of International Health, Proceedings of BAPIO25 Silver Jubilee Conference 2021, Birmingham, UK, 22–24 October 2021; Bisht, S., Nageswaran, P., Gajanan, K., Daga, S., Chakravorty, I., Eds.; Physicianjnl.net: Bedford, UK, 2021; PP-23; p. 35. [Google Scholar]
- Lowe, W.L.; Scholtens, D.M.; Lowe, L.P.; Kuang, A.; Nodzenski, M.; Talbot, O.; Catalano, P.M.; Linder, B.; Brickman, W.J.; Clayton, P.; et al. Association of Gestational Diabetes With Maternal Disorders of Glucose Metabolism and Childhood Adiposity. J. Am. Med. Assoc. 2018, 320, 1005–1016. [Google Scholar] [CrossRef]
- Lowe, W.L.; Scholtens, D.M.; Kuang, A.; Linder, B.; Lawrence, J.M.; Lebenthal, Y.; McCance, D.; Hamilton, J.; Nodzenski, M.; Metzger, B.E.; et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Lincoln, M.; McAllister, L. Peer learning in clinical education. Med. Teach. 1993, 15, 17–26. [Google Scholar] [CrossRef]
- Nasso, J.; McCloskey, C.; Nordquist, S.; Franzese, C.; Queenan, R.A. The Gestational Diabetes Group Program. J. Perinat. Educ. 2018, 27, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Schellinger, M.; Abernathy, M.; Foxlow, L.; Carter, A.; Bastawros, D.; Hass, D. Improved outcomes for Hispanic patients with gestational diabetes using the Centering Pregnancy group prenatal care model. Am. J. Obstet. Gynaecol. 2013, 208, S128. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, S.; Huang, F.; Ma, L. Comparing the Efficacies of Telemedicine and Standard Prenatal Care on Blood Glucose Control in Women With Gestational Diabetes Mellitus: Randomized Conrolled Trial. JMIR Mhealth Uhealth 2021, 9, e22881. [Google Scholar] [CrossRef] [PubMed]
| Closed Statement | Strongly Agree or Agree | Neither Agree nor Disagree | Disagree or Strongly Disagree |
|---|---|---|---|
| My understanding of healthy eating for gestational diabetes has improved following this session. | 332/338 (98.2%) | 6/338 (1.8%) | 0/338 (0%) |
| I understand how to use a blood glucose monitor after this session. | 332/338 (98.2%) | 4/338 (1.2%) | 2/338 (0.6%) |
| The session met my expectations. | 329/338 (97.3%) | 9/338 (2.7%) | 0/338 (0%) |
| I feel confident about making positive changes for gestational diabetes. | 333/338 (98.5%) | 5/338 (1.5%) | 0/338 (0%) |
| Closed Statement | Strongly Agree or Agree | Neither Agree nor Disagree | Disagree or Strongly Disagree |
|---|---|---|---|
| My understanding of healthy eating for gestational diabetes has improved following this session. | 223/231 (96.5%) | 7/231 (3%) | 1/231 (0.5%) |
| I understand how to use a blood glucose monitor after this session. | 231/231 (100%) | 0/231 (0%) | 0/231 (0%) |
| The session met my expectations. | 226/231 (97.8%) | 5/231 (2.2%) | 0/231 (0%) |
| I feel confident about making positive changes for gestational diabetes. | 225/231 (97.4%) | 6/231 (2.6%) | 0/231 (0%) |
| Type of Appointment | Type of Antenatal Clinic | Cost Details | Cost Status |
|---|---|---|---|
| 1st contact | Group clinic | Prevented the need for 3 additional conventional 1st contact clinics a week. | Cost savings of GBP 1728/month |
| 1st follow-up | Tele-consultation clinic | Prevented the need for 8 additional multidisciplinary specialist clinics/month. | Cost savings of GBP 3804.8/month |
| 2nd follow-up | Specialist clinic | Was already running. | Cost neutral |
| Final follow-up | Standard clinic | Was already running | Cost neutral |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikomi, A.; Mannan, S. The GooD Pregnancy Network: An Alternative Approach for Gestational Diabetes. BioMed 2022, 2, 37-49. https://doi.org/10.3390/biomed2010004
Ikomi A, Mannan S. The GooD Pregnancy Network: An Alternative Approach for Gestational Diabetes. BioMed. 2022; 2(1):37-49. https://doi.org/10.3390/biomed2010004
Chicago/Turabian StyleIkomi, Amaju, and Shaheen Mannan. 2022. "The GooD Pregnancy Network: An Alternative Approach for Gestational Diabetes" BioMed 2, no. 1: 37-49. https://doi.org/10.3390/biomed2010004
APA StyleIkomi, A., & Mannan, S. (2022). The GooD Pregnancy Network: An Alternative Approach for Gestational Diabetes. BioMed, 2(1), 37-49. https://doi.org/10.3390/biomed2010004






