Disseminated Cryptococcosis Complicating Severe SARS-CoV-2 Infection
Abstract
:1. Introduction
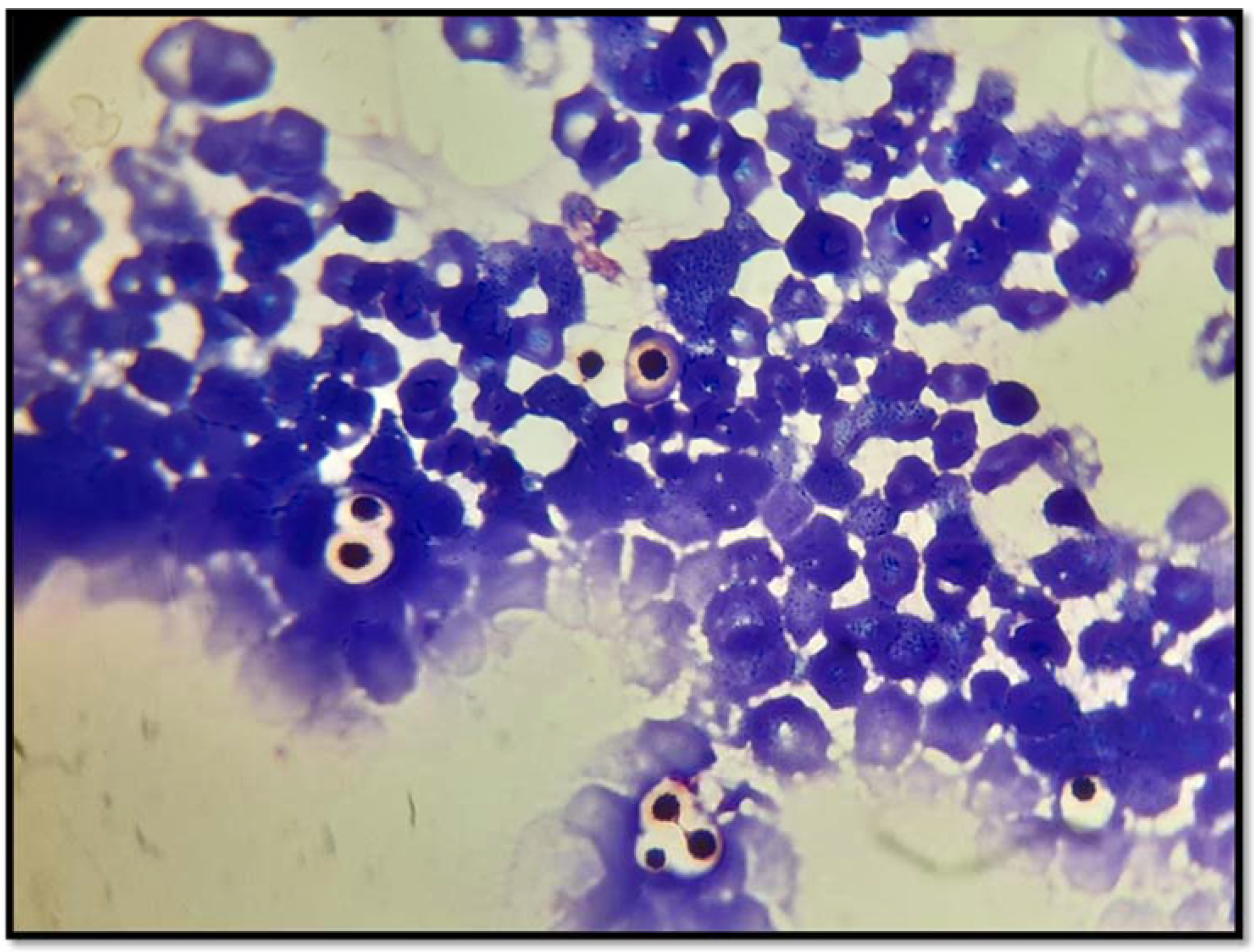
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amin, A.; Vartanian, A.; Poladian, N.; Voloshko, A.; Yegiazaryan, A.; Al-Kassir, A.L.; Venketaraman, V. Root Causes of Fungal Coinfections in COVID-19 Infected Patients. Infect. Dis. Rep. 2021, 13, 1018–1035. [Google Scholar] [CrossRef] [PubMed]
- Henao-Martínez, A.F.; Chastain, D.B.; Franco-Paredes, C. Treatment of cryptococcosis in non-HIV immunocompromised patients. Curr. Opin. Infect. Dis. 2018, 31, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Brizendine, K.D.; Baddley, J.W.; Pappas, P.G. Predictors of Mortality and Differences in Clinical Features among Patients with Cryptococcosis According to Immune Status. PLoS ONE 2013, 8, e60431. [Google Scholar] [CrossRef] [PubMed]
- Baddley, J.W.; Perfect, J.R.; Oster, R.A.; Larsen, R.A.; Pankey, G.A.; Henderson, H.; Haas, D.W.; Kauffman, C.A.; Patel, R.; Zaas, A.K.; et al. Pulmonary cryptococcosis in patients without HIV infection: Factors associated with disseminated disease. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 937–943. [Google Scholar] [CrossRef]
- Bhatt, K.; Agolli, A.; Patel, M.H.; Garimella, R.; Devi, M.; Garcia, E.; Amin, H.; Domingue, C.; Del Castillo, R.G.; Sanchez-Gonzalez, M. High mortality co-infections of COVID-19 patients: Mucormycosis and other fungal infections. Discoveries 2021, 9, e126. [Google Scholar] [CrossRef]
- Peng, X.; Ouyang, J.; Isnard, S.; Lin, J.; Fombuena, B.; Zhu, B.; Routy, J.-P. Sharing CD4+ T Cell Loss: When COVID-19 and HIV Collide on Immune System. Front. Immunol. 2020, 11, 596631. [Google Scholar] [CrossRef]
- Pericolini, E.; Cenci, E.; Monari, C.; De Jesus, M.; Bistoni, F.; Casadevall, A.; Vecchiarelli, A. Cryptococcus neoformans capsular polysaccharide component galactoxylomannan induces apoptosis of human T-cells through activation of caspase-8. Cell Microbiol. 2006, 8, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Kozel, T.R.; Gotschlich, E.C. The capsule of cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J. Immunol. 1982, 129, 1675–1680. [Google Scholar]
- Feldmesser, M.; Tucker, S.; Casadevall, A. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 2001, 9, 273–278. [Google Scholar] [CrossRef]
- Delfino, D.; Cianci, L.; Lupis, E.; Celeste, A.; Petrelli, M.L.; Curró, F.; Cusumano, V.; Teti, G. Interleukin-6 production by human monocytes stimulated with Cryptococcus neoformans components. Infect. Immun. 1997, 65, 2454–2456. [Google Scholar] [CrossRef] [Green Version]
- Retini, C.; Vecchiarelli, A.; Monari, C.; Tascini, C.; Bistoni, F.; Kozel, T.R. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infect. Immun. 1996, 64, 2897–2903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chastain, D.B.; Henao-Martínez, A.F.; Dykes, A.C.; Steele, G.M.; Stoudenmire, L.L.; Thomas, G.M.; Kung, V.; Franco-Paredes, C. Missed opportunities to identify cryptococcosis in COVID-19 patients: A case report and literature review. Ther. Adv. Infect. Dis. 2022, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Traver, E.C.; Sánchez, M.M. Pulmonary aspergillosis and cryptococcosis as a complication of COVID-19. Med. Mycol. Case Rep. 2022, 35, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Gil, Y.; Gil, Y.D.; Markou, T. The Emergence of Cryptococcemia in COVID-19 Infection: A Case Report. Cureus 2021, 13, e19761. [Google Scholar] [CrossRef]
- Alegre-González, D.; Herrera, S.; Bernal, J.; Soriano, A.; Bodro, M. Disseminated Cryptococcus neoformans infection associated to COVID-19. Med. Mycol. Case Rep. 2021, 34, 35–37. [Google Scholar] [CrossRef]
- Passerini, M.; Terzi, R.; Piscaglia, M.; Passerini, S.; Piconi, S. Disseminated Cryptococcosis in a Patient with Metastatic Prostate Cancer Who Died in the Coronavirus Disease 2019 (COVID-19) Outbreak. Cureus 2020, 12, e8254. [Google Scholar] [CrossRef]
- Thota, D.R.; Ray, B.; Hasan, M.; Sharma, K. Cryptococcal Meningoencephalitis During Convalescence from Severe COVID-19 Pneumonia. Neurohospitalist 2021, 12, 96–99. [Google Scholar] [CrossRef]
- Woldie, I.L.; Brown, I.G.; Nwadiaro, N.F.; Patel, A.; Jarrar, M.; Quint, E.; Khokhotva, V.; Hugel, N.; Winger, M.; Briskin, A. Autoimmune Hemolytic Anemia in a 24-Year-Old Patient With COVID-19 Complicated by Secondary Cryptococcemia and Acute Necrotizing Encephalitis: A Case Report and Review of Literature. J. Med. Cases 2020, 11, 362–365. [Google Scholar] [CrossRef]
- Thyagarajan, R.V.; Mondy, K.E.; Rose, D.T. Cryptococcus neoformans blood stream infection in severe COVID-19 pneumonia. IDCases 2021, 26, e01274. [Google Scholar] [CrossRef]
- Passarelli, V.C.; Perosa, A.H.; de Souza Luna, L.K.; Conte, D.D.; Nascimento, O.A.; Ota-Arakaki, J.; Bellei, N. Detected SARS-CoV-2 in ascitic fluid followed by cryptococcemia: A case report. SN Compr. Clin. Med. 2020, 2, 2414–2418. [Google Scholar] [CrossRef]
- Khatib, M.Y.; Ahmed, A.A.; Shaat, S.B.; Mohamed, A.S.; Nashwan, A.J. Cryptococcemia in a patient with COVID-19: A case report. Clin. Case Rep. 2020, 9, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Heller, H.M.; Gonzalez, R.G.; Edlow, B.L.; Ard, K.L.; Gogakos, T. Case 40-2020: A 24-Year-Old Man with Headache and COVID-19. N. Engl. J. Med. 2020, 383, 2572–2580. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, H.; Sivasubramanian, G. Cryptococcus neoformans meningoencephalitis in an immunocompetent patient after COVID-19 infection. Case Rep. Infect. Dis. 2021, 2021, 5597473. [Google Scholar] [CrossRef] [PubMed]
- Cafardi, J.; Haas, D.; Lamarre, T.; Feinberg, J. Opportunistic Fungal Infection Associated With COVID-19. Open Forum Infect. Dis. 2021, 8, ofab016. [Google Scholar] [CrossRef]
- Messina, F.; Marìn, E.; Valerga, M.; Depardo, R. Infecciones fùngicas en pacientes con COVID-19 Actualizaciones en sida e infectivologìa. Buenos Aires 2021, 29, 6–16. [Google Scholar] [CrossRef]
- Kronstad, J.W.; Attarian, R.; Cadieux, B.; Choi, J.; D’Souza, C.A.; Griffiths, E.J.; Geddes, J.M.H.; Hu, G.; Jung, W.H.; Kretschmer, M.; et al. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat. Rev. Genet. 2011, 9, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef] [Green Version]
- Ruan, Q.; Zhu, Y.; Chen, S.; Zhu, L.; Zhang, S.; Zhang, W. Disseminated cryptococcosis with recurrent multiple abscesses in an immunocompetent patient: A case report and literature review. BMC Infect. Dis. 2017, 17, 369. [Google Scholar] [CrossRef]
- Yuanjie, Z.; Jianghan, C.; Nan, X.; Xiaojun, W.; Hai, W.; Wanqing, L.; Julin, G. Cryptococcal meningitis in immunocompetent children. Mycoses 2012, 55, 168–171. [Google Scholar] [CrossRef]
- Correa, K.; Craver, S.; Sandhu, A. An Uncommon Presentation of Cryptococcal Meningitis in an Immunocompetent Patient: A Case Report. Clin. Pr. Cases Emerg. Med. 2021, 5, 450–454. [Google Scholar] [CrossRef]
- Choe, Y.H.; Moon, H.; Park, S.J.; Kim, S.R.; Han, H.J.; Lee, K.S.; Lee, Y.C. Pulmonary cryptococcosis in asymptomatic immunocompetent hosts. Scandinavian. J. Infect. Dis. 2009, 41, 602–607. [Google Scholar]
- Hou, X.; Kou, L.; Han, X.; Zhu, R.; Song, L.; Liu, T. Pulmonary cryptococcosis characteristics in immunocompetent patients-A 20-year clinical retrospective analysis in China. Mycoses 2019, 62, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, Y.; Gu, J.; Chen, J.; Liao, W.; Zhu, Y. Systemic Review of Published Reports on Primary Cutaneous Cryptococcosis in Immunocompetent Patients. Mycopathologia 2015, 180, 19–25. [Google Scholar] [CrossRef] [PubMed]
| Day 0 | Day 3 | Day 7 | Day 14 | Day 21 | Day 28 | Day 33 | |
|---|---|---|---|---|---|---|---|
| Total WBC count (×109/L) | 7.6 | 10.8 | 16.8 | 9.4 | 16.8 | 13.1 | 9.0 |
| Lymphocytes (absolute count) | 510 | 580 | 360 | 420 | 280 | 220 | 130 |
| Monocytes (absolute count) | 440 | 740 | 780 | 300 | 330 | 320 | 80 |
| Neutrophils (absolute count) | 6670 | 9480 | 15, 690 | 15, 430 | 16, 210 | 12, 540 | 8570 |
| Platelets (×109/L) | 218 | 218 | 146 | 76 | 47 | 47 | 49 |
| D-dimer (mcg/mL) | 2.99 | 1.29 | 1.24 | 1.13 | 1.22 | 1.43 | 2.91 |
| LDH (U/L) | 806 | 736 | 802 | 816 | 966 | 823 | 599 |
| GPT/GOT (U/L) | 121/131 | 76/144 | 70/129 | 83/111 | 85/111 | 93/110 | 113/101 |
| Serum creatinine (mg/dL) | 1.96 | 1.63 | 1.96 | 2.47 | 2.99 | 3.33 | 1.21 |
| BUN(mg/dL) | 89 | NA | NA | 207 | NA | 304 | 82 |
| CRP (mg/L) | 132 | 28 | 16 | 25 | 97 | 123 | 116 |
| PCT (ng/mL) | 0.63 | 0.95 | 0.82 | 1.27 | 1.86 | 1.67 | 1.95 |
| IL-6 (pg/mL) | 6.1 | 8.5 | 6.5 | NA | NA | 166 | 174 |
| Ferritin (ng/mL) | 401 | 311 | NA | 232 | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupia, T.; Gaviraghi, A.; Concialdi, E.; Penna, M.; Corcione, S.; De Rosa, F.G. Disseminated Cryptococcosis Complicating Severe SARS-CoV-2 Infection. BioMed 2022, 2, 127-132. https://doi.org/10.3390/biomed2010014
Lupia T, Gaviraghi A, Concialdi E, Penna M, Corcione S, De Rosa FG. Disseminated Cryptococcosis Complicating Severe SARS-CoV-2 Infection. BioMed. 2022; 2(1):127-132. https://doi.org/10.3390/biomed2010014
Chicago/Turabian StyleLupia, Tommaso, Alberto Gaviraghi, Erika Concialdi, Maurizio Penna, Silvia Corcione, and Francesco Giuseppe De Rosa. 2022. "Disseminated Cryptococcosis Complicating Severe SARS-CoV-2 Infection" BioMed 2, no. 1: 127-132. https://doi.org/10.3390/biomed2010014
APA StyleLupia, T., Gaviraghi, A., Concialdi, E., Penna, M., Corcione, S., & De Rosa, F. G. (2022). Disseminated Cryptococcosis Complicating Severe SARS-CoV-2 Infection. BioMed, 2(1), 127-132. https://doi.org/10.3390/biomed2010014







