Effect of Vitamin B2 and Vitamin E on Cancer-Related Sarcopenia in a Mouse Cachexia Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Sphere Assay
2.3. Animals
2.4. Diet
2.5. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.6. Protein Extraction
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Nitric Oxide (NO)
2.9. Peritoneal Macrophage
2.10. Statistical Analysis
3. Results
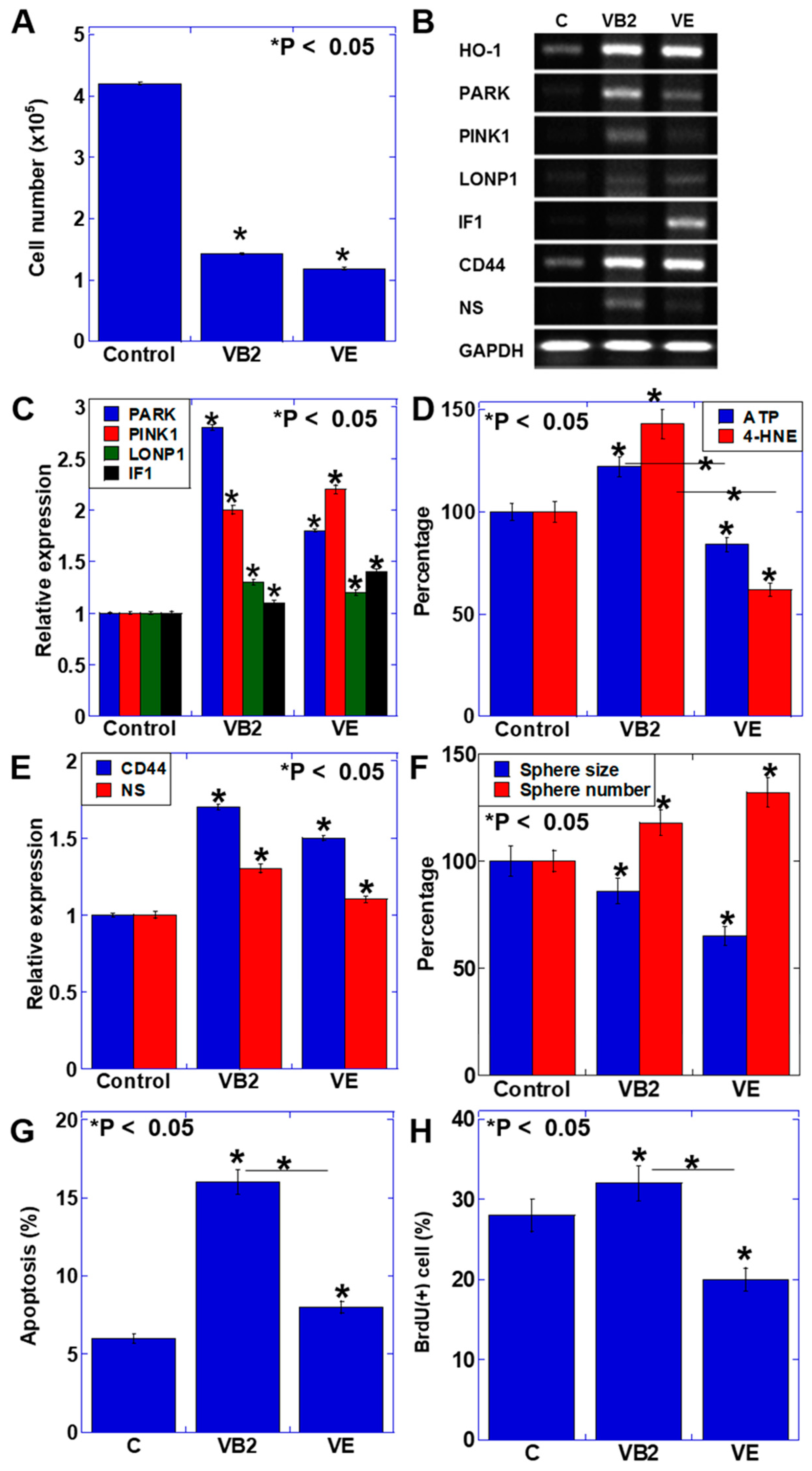
3.1. Effect of VB2 and VE on the Proliferation and Gene Expression of CT26 Cells
3.2. Effect of VB2 and VE Treatments on the Proliferation and Maturation of C2C12 Myoblasts
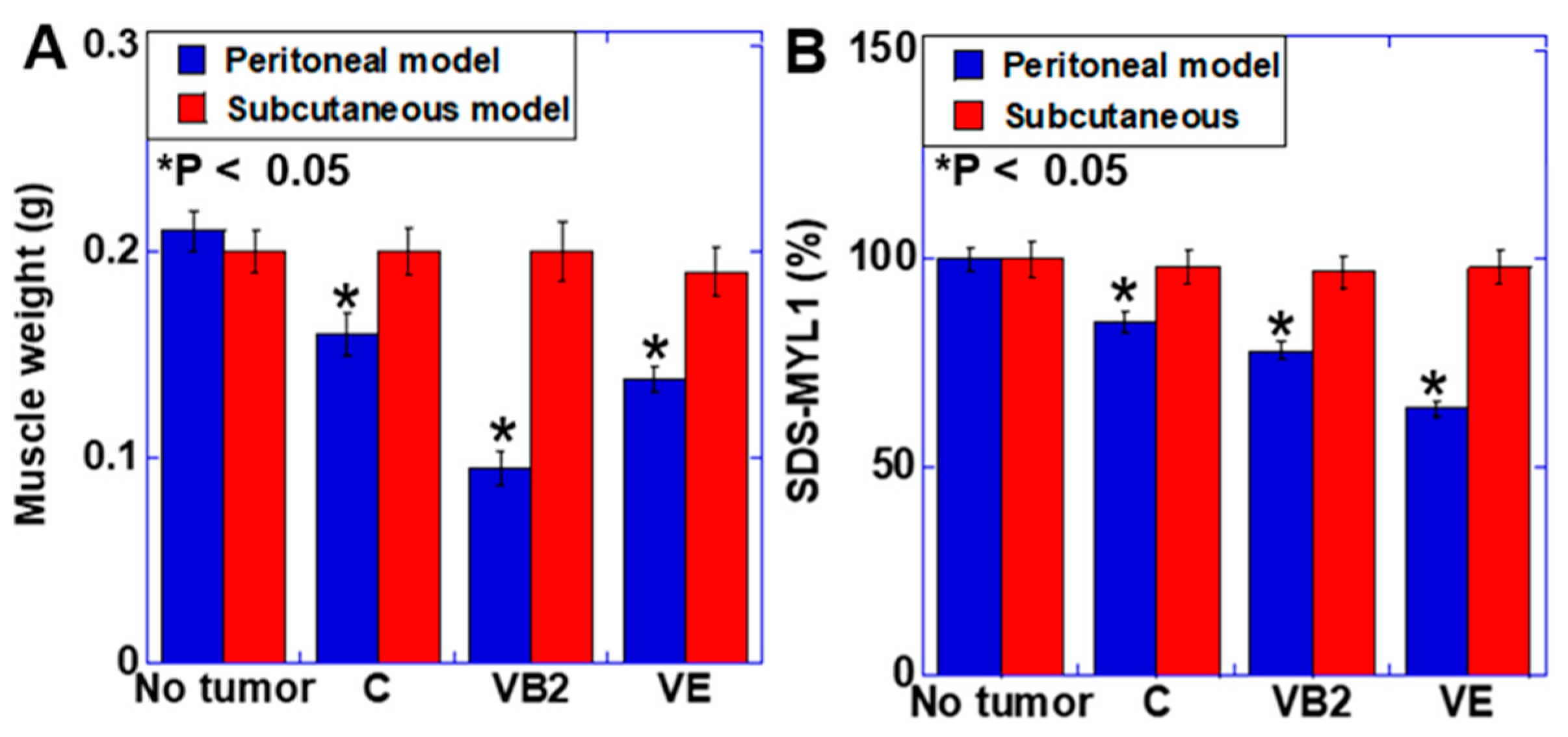
3.3. Effect of VB2 and VE Treatments on Tumors in the Peritoneal and Subcutaneous Models
3.4. Effect of VB2 and VE Treatments on Skeletal Muscle Weight and Maturation in the Peritoneal and Subcutaneous Models
3.5. Effect of VB2 and VE Treatments on Macrophages in the Peritoneal and Subcutaneous Models
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VB2 | vitamin B2 |
| VE | vitamin E |
| ATP | adenosine triphosphate |
| ROS | reactive oxygen species |
| HO | heme oxygenase |
| PARKIN | E3 ubiquitin-protein ligase parkin |
| PINK | PTEN-induced kinase |
| LONP | ON protease; IF1, inhibitor of F1; NS, nucleostemin |
| 4-HNE | 4-hydroxynonenal |
| SDS-MYL1 | sodium dodecyl sulfate-soluble myosin light chain-1 |
| MYH | myosin heavy chain |
| HMGB | high mobility group box |
| TNF | tumor necrosis factor |
| NO | nitric oxide; BrdU, 5-bromo-2’-deoxyuridine |
References
- Blum, D.; Stene, G.B.; Solheim, T.S.; Fayers, P.; Hjermstad, M.J.; Baracos, V.E.; Fearon, K.; Strasser, F.; Kaasa, S.; Koen, M.; et al. Validation of the Consensus-Definition for Cancer Cachexia and evaluation of a classification model—A study based on data from an international multicentre project (EPCRC-CSA). Ann. Oncol. 2014, 25, 1635–1642. [Google Scholar] [CrossRef]
- Fearon, K.C.; Voss, A.C.; Hustead, D.S. Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am. J. Clin. Nutr. 2006, 83, 1345–1350. [Google Scholar] [CrossRef]
- Tisdale, M.J. Cachexia in cancer patients. Nat. Rev. Cancer 2002, 2, 862–871. [Google Scholar] [CrossRef]
- Evans, W.J.; Morley, J.E.; Argiles, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Fearon, K.C. Cancer cachexia: Developing multimodal therapy for a multidimensional problem. Eur. J. Cancer 2008, 44, 1124–1132. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; López-Soriano, F.J. Cytokines as mediators and targets for cancer cachexia. Cancer Treat. Res. 2006, 130, 199–217. [Google Scholar]
- Nardone, O.M.; de Sire, R.; Petito, V.; Testa, A.; Villani, G.; Scaldaferri, F.; Castiglione, F. Inflammatory Bowel Diseases and Sarcopenia: The Role of Inflammation and Gut Microbiota in the Development of Muscle Failure. Front. Immunol. 2021, 12, 694217. [Google Scholar] [CrossRef]
- de Sire, R.; Rizzatti, G.; Ingravalle, F.; Pizzoferrato, M.; Petito, V.; Lopetuso, L.; Graziani, C.; de Sire, A.; Mentella, M.C.; Mele, M.C.; et al. Skeletal muscle-gut axis: Emerging mechanisms of sarcopenia for intestinal and extra intestinal diseases. Minerva Gastroenterol. Dietol. 2018, 64, 351–362. [Google Scholar] [CrossRef]
- Son, J.; Yu, Q.; Seo, J.S. Sarcopenic obesity can be negatively associated with active physical activity and adequate intake of some nutrients in Korean elderly: Findings from the Korea National Health and Nutrition Examination Survey (2008–2011). Nutr. Res. Pract. 2019, 13, 47–57. [Google Scholar] [CrossRef]
- Mochamat; Cuhls, H.; Marinova, M.; Kaasa, S.; Stieber, C.; Conrad, R.; Radbruch, L.; Mucke, M. A systematic review on the role of vitamins, minerals, proteins, and other supplements for the treatment of cachexia in cancer: A European Palliative Care Research Centre cachexia project. J. Cachexia Sarcopenia Muscle 2017, 8, 25–39. [Google Scholar]
- Penna, F.; Camperi, A.; Muscaritoli, M.; Filigheddu, N.; Costelli, P. The role of vitamin D in cancer cachexia. Curr. Opin. Support. Palliat Care 2017, 11, 287–292. [Google Scholar] [CrossRef]
- Mori, T.; Ohmori, H.; Luo, Y.; Mori, S.; Miyagawa, Y.; Nukaga, S.; Goto, K.; Fujiwara-Tani, R.; Kishi, S.; Sasaki, T.; et al. Giving combined medium-chain fatty acids and glucose protects against cancer-associated skeletal muscle atrophy. Cancer Sci. 2019, 110, 3391–3399. [Google Scholar] [CrossRef]
- Nukaga, S.; Mori, T.; Miyagawa, Y.; Fujiwara-Tani, R.; Sasaki, T.; Fujii, K.; Mori, S.; Goto, K.; Kishi, S.; Nakashima, C.; et al. Combined administration of lauric acid and glucose improved cancer-derived cardiac atrophy in a mouse cachexia model. Cancer Sci. 2020, 111, 4605. [Google Scholar] [CrossRef]
- Ortega, R.M.; Jiménez Ortega, A.I.; Perea Sánchez, J.M.; Cuadrado Soto, E.; Aparicio Vizuete, A.; López-Sobaler, A.M. Nutritional value of dairy products and recommended daily consumption. Nutr. Hosp. 2019, 36, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Gil, Á.; Ortega, R.M. Introduction and Executive Summary of the Supplement, Role of Milk and Dairy Products in Health and Prevention of Noncommunicable Chronic Diseases: A Series of Systematic Reviews. Adv. Nutr. 2019, 10 (Suppl. S2), S67–S73. [Google Scholar] [CrossRef]
- Saedisomeolia, A.; Ashoori, M. Riboflavin in Human Health: A Review of Current Evidences. Adv. Food Nutr. Res. 2018, 83, 57–81. [Google Scholar]
- Bémeur, C.; Butterworth, R.F. Nutrition in the management of cirrhosis and its neurological complications. J. Clin. Exp. Hepatol. 2014, 4, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishanina, T.V.; Kohen, A. Synthesis and application of isotopically labeled flavin nucleotides. J. Label. Comp. Radiopharm. 2015, 58, 370–375. [Google Scholar] [CrossRef] [Green Version]
- Molano-Arevalo, J.C.; Hernandez, D.R.; Gonzalez, W.G.; Miksovska, J.; Ridgeway, M.E.; Park, M.A.; Fernandez-Lima, F. Flavin adenine dinucleotide structural motifs: From solution to gas phase. Anal. Chem. 2014, 86, 10223–10230. [Google Scholar] [CrossRef] [Green Version]
- Giancaspero, T.A.; Busco, G.; Panebianco, C.; Carmone, C.; Miccolis, A.; Liuzzi, G.M.; Colella, M.; Barile, M. FAD synthesis and degradation in the nucleus create a local flavin cofactor pool. J. Biol. Chem. 2013, 288, 29069–29080. [Google Scholar] [CrossRef] [Green Version]
- Etchegaray, J.P.; Mostoslavsky, R. Interplay between Metabolism and Epigenetics: A Nuclear Adaptation to Environmental Changes. Mol. Cell 2016, 62, 695–711. [Google Scholar] [CrossRef] [Green Version]
- Niki, E.; Traber, M.G. A history of vitamin E. Ann. Nutr. Metab. 2012, 61, 207–212. [Google Scholar] [CrossRef]
- Jiang, Q. Natural Forms of Vitamin E as Effective Agents for Cancer Prevention and Therapy. Adv. Nutr. 2017, 8, 850–867. [Google Scholar] [CrossRef]
- Chung, E.; Mo, H.; Wang, S.; Zu, Y.; Elfakhani, M.; Rios, S.R.; Chyu, M.C.; Yang, R.S.; Shen, C.L. Potential roles of vitamin E in age-related changes in skeletal muscle health. Nutr. Res. 2018, 49, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Terawaki, K.; Sawada, Y.; Kashiwase, Y.; Hashimoto, H.; Yoshimura, M.; Suzuki, M.; Miyano, K.; Sudo, Y.; Shiraishi, S.; Higami, Y.; et al. New cancer cachexia rat model generated by implantation of a peritoneal dissemination-derived human stomach cancer cell line. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E373–E387. [Google Scholar] [CrossRef] [Green Version]
- Pin, F.; Barreto, R.; Kitase, Y.; Mitra, S.; Erne, C.E.; Novinger, L.J.; Zimmers, T.A.; Couch, M.E.; Bonewald, L.F.; Bonetto, A. Growth of ovarian cancer xenografts causes loss of muscle and bone mass: A new model for the study of cancer cachexia. J. Cachexia Sarcopenia Muscle 2018, 9, 685–700. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Yoshida, K.; Kondo, H.; Ohmori, H.; Kuniyasu, H. Heme oxygenase-1 accelerates protumoral effects of nitric oxide in cancer cells. Virchows. Arch. 2005, 446, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Kusume, A.; Sasahira, T.; Luo, Y.; Isobe, M.; Nakagawa, N.; Tatsumoto, N.; Fujii, K.; Ohmori, H.; Kuniyasu, H. Suppression of dendritic cells by HMGB1 is associated with lymph node metastasis of human colon cancer. Pathobiology 2009, 76, 155–162. [Google Scholar] [CrossRef]
- Ejma, M.; Madetko, N.; Brzecka, A.; Guranski, K.; Alster, P.; Misiuk-Hojło, M.; Somasundaram, S.G.; Kirkland, C.E.; Aliev, G. The Links between Parkinson’s Disease and Cancer. Biomedicines 2020, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, L.; Losi, L.; De Biasi, S.; Nasi, M.; Lo Tartaro, D.; Pecorini, S.; Patergnani, S.; Pinton, P.; De Gaetano, A.; Carnevale, G.; et al. LonP1 Differently Modulates Mitochondrial Function and Bioenergetics of Primary Versus Metastatic Colon Cancer Cells. Front. Oncol. 2018, 8, 254. [Google Scholar] [CrossRef]
- García-Bermúdez, J.; Cuezva, J.M. The ATPase Inhibitory Factor 1 (IF1): A master regulator of energy metabolism and of cell survival. Biochim. Biophys. Acta 2016, 1857, 1167–1182. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Prakash, M.D.; Kuol, N.; Nurgali, K.; Stojanovska, L.; Apostolopoulos, V. Anti-Tumor Effects of Vitamin B2, B6 and B9 in Promonocytic Lymphoma Cells. Int. J. Mol. Sci. 2019, 20, 3763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sylvester, P.W. Vitamin E and apoptosis. Vitam. Horm. 2007, 76, 329–356. [Google Scholar] [PubMed]
- Zingg, J.M. Vitamin E: Regulatory Role on Signal Transduction. IUBMB Life 2019, 71, 456–478. [Google Scholar] [CrossRef]
- Araki, S.; Suzuki, M.; Fujimoto, M.; Kimura, M. Enhancement of resistance to bacterial infection in mice by vitamin B2. J. Vet. Med. Sci. 1995, 57, 599–602. [Google Scholar] [CrossRef] [Green Version]
- Mazur-Bialy, A.I.; Pochec, E.; Plytycz, B. Immunomodulatory effect of riboflavin deficiency and enrichment—Reversible pathological response versus silencing of inflammatory activation. J. Physiol. Pharm. 2015, 66, 793–802. [Google Scholar]
- Ahn, H.; Lee, G.S. Riboflavin, vitamin B2, attenuates NLRP3, NLRC4, AIM2, and non-canonical inflammasomes by the inhibition of caspase-1 activity. Sci. Rep. 2020, 10, 19091. [Google Scholar] [CrossRef]
- Dey, S.; Bishayi, B. Riboflavin along with antibiotics balances reactive oxygen species and inflammatory cytokines and controls Staphylococcus aureus infection by boosting murine macrophage function and regulates inflammation. J. Inflamm. 2016, 13, 36. [Google Scholar] [CrossRef] [Green Version]
- Singh, U.; Jialal, I. Anti-inflammatory effects of alpha-tocopherol. Ann. N. Y. Acad. Sci. 2004, 1031, 195–203. [Google Scholar] [CrossRef]
- Shklar, G.; Schwartz, J.L.; Trickler, D.P.; Reid, S. Prevention of experimental cancer and immunostimulation by vitamin E (immunosurveillance). J. Oral. Pathol. Med. 1990, 19, 60–64. [Google Scholar] [CrossRef]
- Shklar, G.; Schwartz, J. Tumor necrosis factor in experimental cancer regression with alphatocopherol, beta-carotene, canthaxanthin and algae extract. Eur. J. Cancer Clin. Oncol. 1988, 24, 839–850. [Google Scholar] [CrossRef]



| Gene Name | Gene Symbol | GenBank ID | Primer Sequence | |
|---|---|---|---|---|
| Heme oxygenase-1 | HO-1 | NM_010442.2 | U | CACGCATATACCCGCTACCT |
| L | CCAGAGTGTTCATTCGAGCA | |||
| E3 ubiquitin-protein ligase | PARKIN | AB019558.1 | U | TGGAAAGCTCCGAGTTCAGT |
| parkin | L | CCTTGTCTGAGGTTGGGTGT | ||
| PTEN-induced kinase-1 | PINK1 | NM_026880.2 | U | CCCACACCCTAACATCATCC |
| L | ACTGGGAGTCTGCTCCTCAA | |||
| LON protease-1 | LONP1 | NM_028782.2 | U | GACAGAGAACCCGCTAGTGC |
| L | CTCAGTGGTTCTGGGATGGT | |||
| Inhibitor of F1 | IF1 | AF002718.1 | U | TCTGGGGTATGAAGGTCCTG |
| L | TCGAATGGTGGTCAATCTCA | |||
| CD44 | CD44 | M27130.1 | U | TGGATCCGAATTAGCTGGAC |
| L | AGCTTTTTCTTCTGCCCACA | |||
| Nucleostemin | NS | BC037996.1 | U | ATGTGGGGAAAAGCAGTGTC |
| L | TGGGGGAGTTACAAGGTGAG | |||
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | NM_001289726.1 | U | AACTTTGGCATTGTGGAAGG |
| L | ACACATTGGGGGTAGGAACA | |||
| CD68 | CD68 | BC021637.1 | U | TTCTGCTGTGGAAATGCAAG |
| L | AGAGGGGCTGGTAGGTTGAT | |||
| Basic leucine zipper transcription factor, ATF-like 2 | Batf2 | BC024521.1 | U | AGCACGAATCCTTGGAGAAA |
| L | GTTCCTGGCAGCCATTGTAT | |||
| Parasite-induced macrophage novel gene 1 protein | Fizz1 | AF316397.2 | U | CCCTTCTCATCTGCATCTCC |
| L | CAGTAGCAGTCATCCCAGCA | |||
| β-actin | BACT | NM_007393.5 | U | ACAATGAGCTGCGTGTGGCC |
| L | AGGGACAGCACAGCCTGGAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, T.; Goto, K.; Kawahara, I.; Nukaga, S.; Wakatsuki, Y.; Mori, S.; Fujiwara-Tani, R.; Kishi, S.; Sasaki, T.; Ohmori, H.; et al. Effect of Vitamin B2 and Vitamin E on Cancer-Related Sarcopenia in a Mouse Cachexia Model. BioMed 2021, 1, 50-62. https://doi.org/10.3390/biomed1010004
Mori T, Goto K, Kawahara I, Nukaga S, Wakatsuki Y, Mori S, Fujiwara-Tani R, Kishi S, Sasaki T, Ohmori H, et al. Effect of Vitamin B2 and Vitamin E on Cancer-Related Sarcopenia in a Mouse Cachexia Model. BioMed. 2021; 1(1):50-62. https://doi.org/10.3390/biomed1010004
Chicago/Turabian StyleMori, Takuya, Kei Goto, Isao Kawahara, Shota Nukaga, Yuma Wakatsuki, Shiori Mori, Rina Fujiwara-Tani, Shingo Kishi, Takamitsu Sasaki, Hitoshi Ohmori, and et al. 2021. "Effect of Vitamin B2 and Vitamin E on Cancer-Related Sarcopenia in a Mouse Cachexia Model" BioMed 1, no. 1: 50-62. https://doi.org/10.3390/biomed1010004
APA StyleMori, T., Goto, K., Kawahara, I., Nukaga, S., Wakatsuki, Y., Mori, S., Fujiwara-Tani, R., Kishi, S., Sasaki, T., Ohmori, H., Kido, A., Honoki, K., Tanaka, Y., & Kuniyasu, H. (2021). Effect of Vitamin B2 and Vitamin E on Cancer-Related Sarcopenia in a Mouse Cachexia Model. BioMed, 1(1), 50-62. https://doi.org/10.3390/biomed1010004







