Physical Frailty/Sarcopenia as a Key Predisposing Factor to Coronavirus Disease 2019 (COVID-19) and Its Complications in Older Adults
Abstract
:1. Introduction
2. Overview of Age-Related Effects on Skeletal Muscle
3. Definitions of Sarcopenia
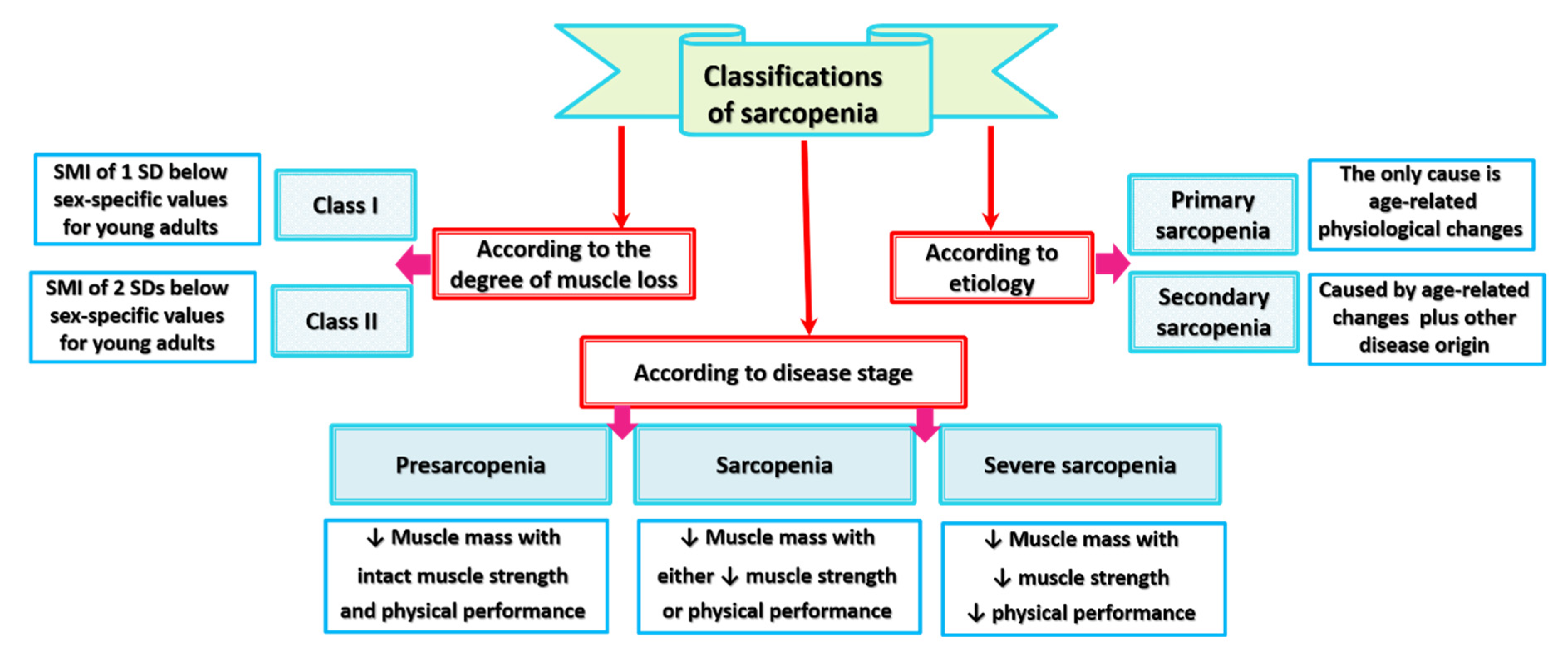
4. Classifications of Sarcopenia
5. Sarcopenia and Related Syndromes
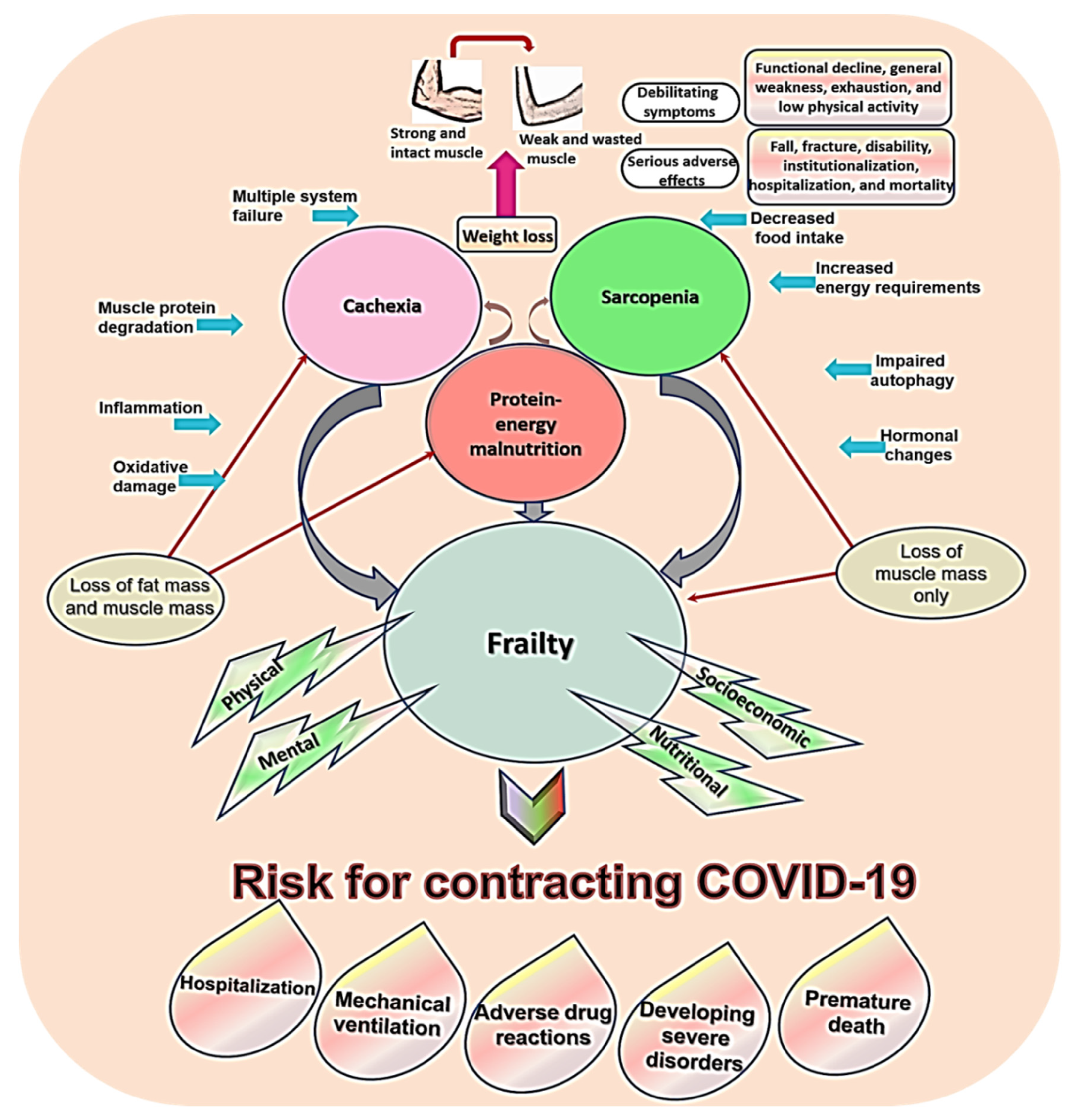
5.1. Sarcopenia, Frailty, Cachexia, and Malnutrition
5.2. Sarcopenic Obesity
5.3. Risk for COVID-19 in Sarcopenia and Related Conditions
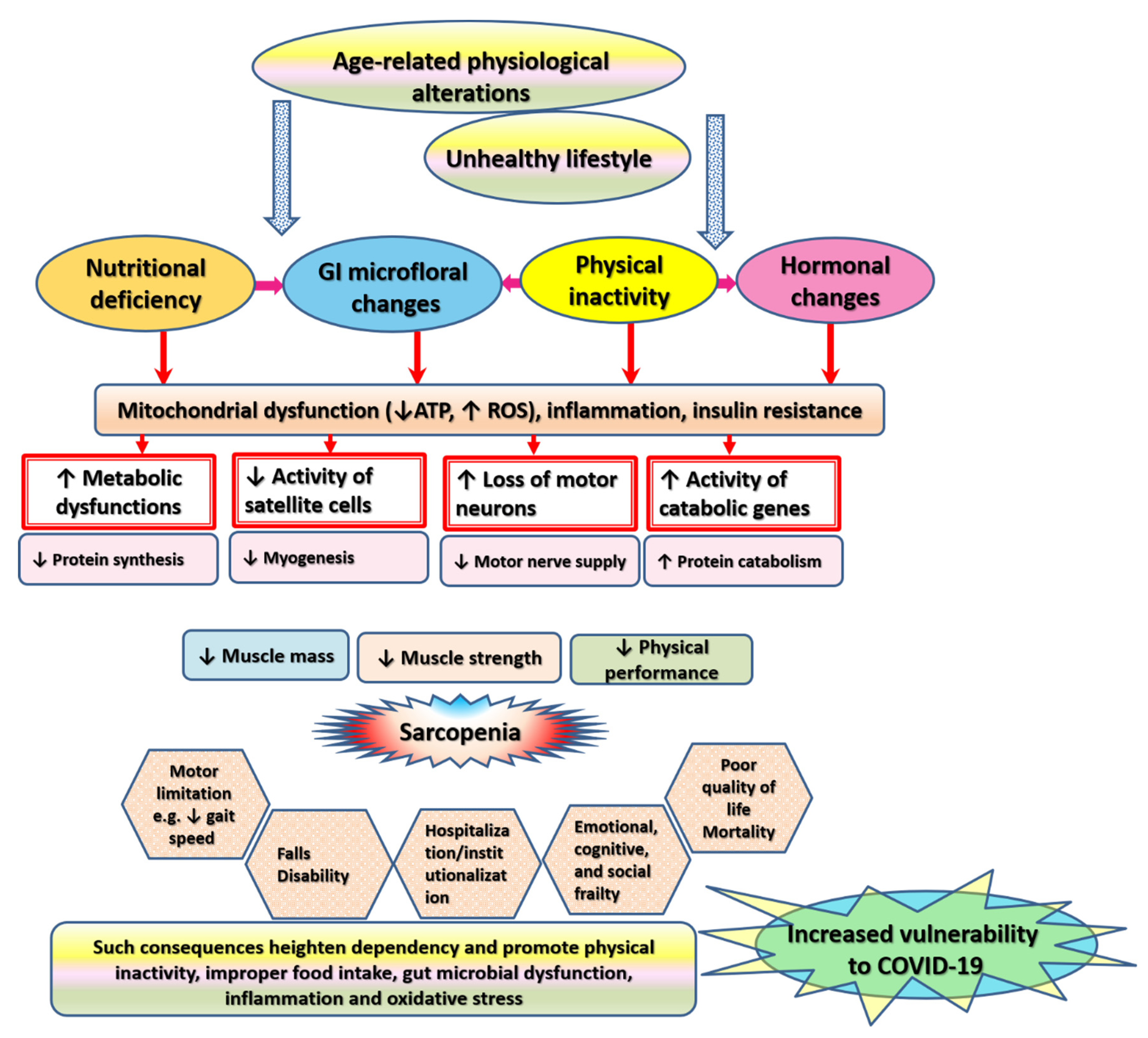
6. Pathophysiological Alterations Underlying Sarcopenia
6.1. Inflammation
6.2. Oxidative Stress
6.3. Metabolic Alterations
6.4. Glycation Stress
6.5. Physical Inactivity
6.6. Neuromuscular Failure
6.7. Hormonal Dysregulations
6.8. Nutritional Deficiency
6.9. Satellite Cells Senescence
6.10. Microbiome Alterations
7. Health-Related Consequences of Sarcopenia
8. Current Pharmacological Management of Sarcopenia
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1,25(OH)D | 1,25-dihydroxyvitamin D |
| AChRs | Acetylcholine receptors |
| ActR2B | Activin type IIB receptor |
| AGEs | Advanced glycation end products |
| AKT | Protein kinase B—A serine/threonine nutrient sensing protein kinase |
| AMPK | Adenosine monophosphate activated protein kinase |
| AOR | Adjusted odds ratio |
| ASM | Appendicular skeletal muscle mass |
| ARE | Antioxidant response element |
| ARDS | Severe acute pneumonia-associated respiratory syndrome |
| ATP | Adenosine tri-phosphate |
| AWGS | Asian Working Group for Sarcopenia |
| BMI | Body mass index |
| CPT-1 | Carnitine:palmitoyl transferase-1 |
| CK | Creatine kinase |
| CNS | Central nervous system |
| COVID-19 | Coronavirus disease 2019 |
| COX7A1 | Cytochrome C Oxidase Subunit 7A1 |
| CRP | C-reactive protein |
| CREB | cAMP response element binding protein |
| DAPK3 | Death-associated protein kinase 3 |
| ERK | Extracellular signal-regulated kinase |
| ER | Endoplasmic reticulum |
| EUGMS | European Geriatric Medical Society |
| EWGSOP | European Working Group on Sarcopenia in Older People |
| FOXO | Forkhead box O |
| GI | Gastrointestinal |
| GLUT4 | Glucose transporter 4 |
| GDF-8 | Growth differentiation factor-8 |
| GPRs | G-protein-coupled receptors |
| HLH | Helix-loop-helix |
| HR | Hazard ratio |
| IGFs | Insulin-like growth factors |
| ICFSR | International Conference on Frailty and Sarcopenia Research |
| IL | Interleukin |
| IWGS | International Working Group on Sarcopenia |
| LBM | Lean body mass |
| MAPK | Mitogen-activated protein kinase |
| MIP-1α | Macrophage inflammatory protein 1-α |
| mTOR | Mammalian target of rapamycin |
| MCP-1 | Monocyte chemotactic protein 1 |
| MRFs | Myogenic regulatory transcription factors |
| MPO | Myeloperoxidase |
| MRF4 | Myogenic regulatory factor 4 |
| Myf5 | Myogenic factor 5 |
| MyoD | Myogenic differentiation 1 |
| NAD+ | Pyridine nucleotide nicotinamide adenine dinucleotide |
| NADPH | Nicotinamide adenine dinucleotide phosphate hydrogen |
| NF-kB | Nuclear factor kappa-B |
| NRF2 | Nuclear factor erythroid 2/Nuclear respiratory factor 2 |
| Pax3 | Paired domain homeobox 3 |
| PDGF | Platelet-derived growth factor |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1 alpha |
| QoL | Quality of life |
| RAGE | Receptor for Advanced Glycation End products |
| ROS | Reactive oxygen species |
| SARS-CoV-2 | Severe acute respiratory syndrome-coronavirus-2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| SDs | Standard deviations |
| SMI | Skeletal muscle mass index |
| TGF-β | Transforming growth factor-β |
| TNF | Tumor necrosis factor |
| NDUFB6 | Ubiquinone Oxidoreductase Subunit B6 |
| UPS | Ubiquitin-proteasome system |
References
- Vandewoude, M.F.; Alish, C.J.; Sauer, A.C.; Hegazi, R.A. Malnutrition-sarcopenia syndrome: Is this the future of nutrition screening and assessment for older adults? J. Aging Res. 2012, 2012, 651570. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Gao, M.; Bai, X.; Chen, Z. Neuroprotective Effect of Several Phytochemicals and Its Potential Application in the Prevention of Neurodegenerative Diseases. Geriatrics 2016, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, N.; Lim, J.Y.; Miljkovic, I.; Frontera, W.R. Aging of skeletal muscle fibers. Ann. Rehabil. Med. 2015, 39, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, M.; Reggiani, C.; Richardson, R.S.; Schena, F. Skeletal Muscle Function in the Oldest-Old: The Role of Intrinsic and Extrinsic Factors. Exerc. Sport Sci. Rev. 2018, 46, 188–194. [Google Scholar] [CrossRef]
- Okumura, N.; Toda, T.; Ozawa, Y.; Watanabe, K.; Ikuta, T.; Tatefuji, T.; Hashimoto, K.; Shimizu, T. Royal Jelly Delays Motor Functional Impairment During Aging in Genetically Heterogeneous Male Mice. Nutrients 2018, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Aran, L.; Bulli, G.; Curcio, F.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Sarcopenia: Assessment of disease burden and strategies to improve outcomes. Clin. Interv. Aging 2018, 13, 913–927. [Google Scholar] [CrossRef] [Green Version]
- Perkisas, S.; Vandewoude, M. Where frailty meets diabetes. Diabetes Metab. Res. 2016, 32, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Drey, M.; Hasmann, S.E.; Krenovsky, J.P.; Hobert, M.A.; Straub, S.; Elshehabi, M.; von Thaler, A.K.; Fallgatter, A.J.; Eschweiler, G.W.; Suenkel, U.; et al. Associations between Early Markers of Parkinson’s Disease and Sarcopenia. Front. Aging Neurosci. 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Niu, K.; Guo, H.; Guo, Y.; Ebihara, S.; Asada, M.; Ohrui, T.; Furukawa, K.; Ichinose, M.; Yanai, K.; Kudo, Y.; et al. Royal jelly prevents the progression of sarcopenia in aged mice in vivo and in vitro. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 1482–1492. [Google Scholar] [CrossRef] [Green Version]
- Pinedo-Villanueva, R.; Westbury, L.D.; Syddall, H.E.; Sanchez-Santos, M.T.; Dennison, E.M.; Robinson, S.M.; Cooper, C. Health Care Costs Associated With Muscle Weakness: A UK Population-Based Estimate. Calcif. Tissue Int. 2019, 104, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Rong, S.; Wang, L.; Peng, Z.; Liao, Y.; Li, D.; Yang, X.; Nuessler, A.K.; Liu, L.; Bao, W.; Yang, W. The mechanisms and treatments for sarcopenia: Could exosomes be a perspective research strategy in the future? J. Cachexia Sarcopenia Muscle 2020, 11, 348–365. [Google Scholar] [CrossRef] [Green Version]
- Hardee, J.P.; Lynch, G.S. Current pharmacotherapies for sarcopenia. Expert Opin. Pharmacother. 2019, 20, 1645–1657. [Google Scholar] [CrossRef]
- Mori, H.; Kuroda, A.; Ishizu, M.; Ohishi, M.; Takashi, Y.; Otsuka, Y.; Taniguchi, S.; Tamaki, M.; Kurahashi, K.; Yoshida, S.; et al. Association of accumulated advanced glycation end-products with a high prevalence of sarcopenia and dynapenia in patients with type 2 diabetes. J. Diabetes Investig. 2019, 10, 1332–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakuma, K.; Aoi, W.; Yamaguchi, A. Molecular mechanism of sarcopenia and cachexia: Recent research advances. Pflug. Arch. Eur. J. Phys. 2017, 469, 573–591. [Google Scholar] [CrossRef]
- Keller, K. Sarcopenia. Wien. Med. Wochenschr. 2019, 169, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Volpato, S.; Bianchi, L.; Cherubini, A.; Landi, F.; Maggio, M.; Savino, E.; Bandinelli, S.; Ceda, G.P.; Guralnik, J.M.; Zuliani, G.; et al. Prevalence and clinical correlates of sarcopenia in community-dwelling older people: Application of the EWGSOP definition and diagnostic algorithm. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 438–446. [Google Scholar] [CrossRef]
- Vatic, M.; von Haehling, S.; Ebner, N. Inflammatory biomarkers of frailty. Exp. Gerontol. 2020, 133, 110858. [Google Scholar] [CrossRef]
- Gensous, N.; Bacalini, M.G.; Franceschi, C.; Meskers, C.G.M.; Maier, A.B.; Garagnani, P. Age-Related DNA Methylation Changes: Potential Impact on Skeletal Muscle Aging in Humans. Front. Physiol. 2019, 10, 996. [Google Scholar] [CrossRef]
- Goyal, B.; Goyal, D. Targeting the Dimerization of the Main Protease of Coronaviruses: A Potential Broad-Spectrum Therapeutic Strategy. ACS Comb. Sci. 2020, 22, 297–305. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Propolis, bee honey, and their components protect against coronavirus disease 2019 (Covid-19): A review of in silico, in vitro, and clinical studies. Molecular 2021, 26, 1232. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Baylink, D.J.; Chen, C.S.; Reeves, M.E.; Xiao, J.; Lacy, C.; Lau, E.; Cao, H. The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J. Transl. Med. 2020, 18, 322. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Kunugi, H. Approaches to nutritional screening in patients with Coronavirus Disease 2019 (COVID-19). Int. J. Environ. Res. Public Health 2021, 18, 2772. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Hypoproteinemia predicts disease severity and mortality in COVID-19: A call for action. Diagn. Pathol. 2021, 16, s13000–s13021. [Google Scholar] [CrossRef]
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients 2020, 12, 1466. [Google Scholar] [CrossRef]
- Carter, S.J.; Baranauskas, M.N.; Fly, A.D. Considerations for Obesity, Vitamin D, and Physical Activity Amid the COVID-19 Pandemic. Obesity 2020, 28, 1176–1177. [Google Scholar] [CrossRef] [PubMed]
- Laviano, A.; Koverech, A.; Zanetti, M. Nutrition support in the time of SARS-CoV-2 (COVID-19). Nutrition 2020, 74, 110834. [Google Scholar] [CrossRef] [PubMed]
- Labenz, C.; Kremer, W.M.; Schattenberg, J.M.; Wörns, M.A.; Toenges, G.; Weinmann, A.; Galle, P.R.; Sprinzl, M.F. Clinical Frailty Scale for risk stratification in patients with SARS-CoV-2 infection. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2020, 68, 1199–1202. [Google Scholar] [CrossRef]
- Al Rihani, S.B.; Smith, M.K.; Bikmetov, R.; Deodhar, M.; Dow, P.; Turgeon, J.; Michaud, V. Risk of Adverse Drug Events Following the Virtual Addition of COVID-19 Repurposed Drugs to Drug Regimens of Frail Older Adults with Polypharmacy. J. Clin. Med. 2020, 9, 2591. [Google Scholar] [CrossRef] [PubMed]
- De Smet, R.; Mellaerts, B.; Vandewinckele, H.; Lybeert, P.; Frans, E.; Ombelet, S.; Lemahieu, W.; Symons, R.; Ho, E.; Frans, J.; et al. Frailty and Mortality in Hospitalized Older Adults With COVID-19: Retrospective Observational Study. J. Am. Med. Dir. Assoc. 2020, 21, 928–932.e1. [Google Scholar] [CrossRef]
- Bellelli, G.; Rebora, P.; Valsecchi, M.G.; Bonfanti, P.; Citerio, G. Frailty index predicts poor outcome in COVID-19 patients. Intensive Care Med. 2020, 46, 1634–1636. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, J.; Carter, B.; Vilches-Moraga, A.; Quinn, T.J.; Braude, P.; Verduri, A.; Pearce, L.; Stechman, M.; Short, R.; Price, A.; et al. The effect of frailty on survival in patients with COVID-19 (COPE): A multicentre, European, observational cohort study. Lancet Public Health 2020, 5, e444–e451. [Google Scholar] [CrossRef]
- Riuzzi, F.; Sorci, G.; Sagheddu, R.; Chiappalupi, S.; Salvadori, L.; Donato, R. RAGE in the pathophysiology of skeletal muscle. J. Cachexia Sarcopenia Muscle 2018, 9, 1213–1234. [Google Scholar] [CrossRef] [Green Version]
- Egawa, T.; Ohno, Y.; Yokoyama, S.; Yokokawa, T.; Tsuda, S.; Goto, K.; Hayashi, T. The Protective Effect of Brazilian Propolis against Glycation Stress in Mouse Skeletal Muscle. Foods 2019, 8, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suetta, C.; Maier, A.B. Is muscle failure a better term than sarcopenia? J. Cachexia Sarcopenia Muscle 2019, 10, 1146–1147. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Kunugi, H. Screening for sarcopenia (physical frailty) in the COVID-19 era. Int. J. Endocrinol. 2021, 2021, 5563960. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.A.; Hayhoe, R.P.G.; Cameron, D. The relationships between sarcopenic skeletal muscle loss during ageing and macronutrient metabolism, obesity and onset of diabetes. Proc. Nutr. Soc. 2020, 79, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Argiles, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Manas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990s–991s. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Phung, L.A.; Karvinen, S.M.; Colson, B.A.; Thomas, D.D.; Lowe, D.A. Age affects myosin relaxation states in skeletal muscle fibers of female but not male mice. PLoS ONE 2018, 13, e0199062. [Google Scholar] [CrossRef] [Green Version]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut-Muscle Axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favaro-Moreira, N.C.; Krausch-Hofmann, S.; Matthys, C.; Vereecken, C.; Vanhauwaert, E.; Declercq, A.; Bekkering, G.E.; Duyck, J. Risk Factors for Malnutrition in Older Adults: A Systematic Review of the Literature Based on Longitudinal Data. Adv. Nutr. 2016, 7, 507–522. [Google Scholar] [CrossRef]
- Jeejeebhoy, K.N. Malnutrition, fatigue, frailty, vulnerability, sarcopenia and cachexia: Overlap of clinical features. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 213–219. [Google Scholar] [CrossRef]
- Gingrich, A.; Volkert, D.; Kiesswetter, E.; Thomanek, M.; Bach, S.; Sieber, C.C.; Zopf, Y. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. 2019, 19, 120. [Google Scholar] [CrossRef] [Green Version]
- Zopf, Y.; Schink, K.; Reljic, D.; Herrmann, H.J.; Dieterich, W.; Kiesswetter, E.; Sieber, C.C.; Neurath, M.F.; Volkert, D. Assessing cachexia in older patients: Different definitions—But which one is the most practical for clinical routine? Arch. Gerontol. Geriatr. 2020, 86, 103943. [Google Scholar] [CrossRef]
- Ali, A.M.; Ali, E.M.; Ahmed, M.S.; Hendawy, A.O. Targeting gut microbiome and the recovery of muscle loss associated with cancer (cachexia): An overview of the possible effect of bee products. Med. Leg. Update 2021, 21, 163–171. [Google Scholar] [CrossRef]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Pedone, C.; Costanzo, L.; Cesari, M.; Bandinelli, S.; Ferrucci, L.; Antonelli Incalzi, R. Are Performance Measures Necessary to Predict Loss of Independence in Elderly People? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, K.; Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Intermittent fasting, dietary modifications, and exercise for the control of gestational diabetes and maternal mood dysregulation: A review and a case report. Int. J. Environ. Res. Public Health 2020, 17, 9379. [Google Scholar] [CrossRef]
- Ali, A.M.; Ahmed, A.H.; Smail, L. Psychological Climacteric Symptoms and Attitudes toward Menopause among Emirati Women. Int. J. Environ. Res. Public Health 2020, 17, 5028. [Google Scholar] [CrossRef]
- Breucker, S.D.; Luce, S.; Njemini, R.; Bautmans, I.; Decoster, L.; Mets, T.; Pepersack, T. Analysis of inflammatory markers and hormones in old cancer patients: A descriptive study. Exp. Gerontol. 2020, 130, 110787. [Google Scholar] [CrossRef]
- Consitt, L.A.; Clark, B.C. The Vicious Cycle of Myostatin Signaling in Sarcopenic Obesity: Myostatin Role in Skeletal Muscle Growth, Insulin Signaling and Implications for Clinical Trials. J. Frailty Aging 2018, 7, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Ponziani, F.R.; Calvani, R.; Marini, F.; Biancolillo, A.; Coelho-Junior, H.J.; Gervasoni, J.; Primiano, A.; Putignani, L.; Del Chierico, F.; et al. Gut Microbial, Inflammatory and Metabolic Signatures in Older People with Physical Frailty and Sarcopenia: Results from the BIOSPHERE Study. Nutrients 2019, 12, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut Microbiota Contribute to Age-Related Changes in Skeletal Muscle Size, Composition, and Function: Biological Basis for a Gut-Muscle Axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleau, C.; Karelis, A.D.; St-Pierre, D.H.; Lamontagne, L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes Metab. Res. Rev. 2015, 31, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Park, S.W.; Jun, J.E.; Jin, S.-M.; Hur, K.Y.; Lee, M.-K.; Kang, M.; Kim, G.; Kim, J.H. Relationship between low skeletal muscle mass, sarcopenic obesity and left ventricular diastolic dysfunction in Korean adults. Diabetes/Metab. Res. Rev. 2021, 37, e3363. [Google Scholar] [CrossRef]
- Cipolloni, L.; Sessa, F.; Bertozzi, G.; Baldari, B.; Cantatore, S.; Testi, R.; D’Errico, S.; Di Mizio, G.; Asmundo, A.; Castorina, S.; et al. Preliminary Post-Mortem COVID-19 Evidence of Endothelial Injury and Factor VIII Hyperexpression. Diagnostics 2020, 10, 575. [Google Scholar] [CrossRef]
- Sawaya, Y.; Ishizaka, M.; Kubo, A.; Shiba, T.; Hirose, T.; Onoda, K.; Maruyama, H.; Urano, T. Association between skeletal muscle mass index and lung function/respiratory muscle strength in older adults requiring long-term care or support. J. Phys. Ther. Sci. 2020, 32, 754–759. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Skeletal muscle damage in COVID-19: A call for action. Medicina 2021, 57, 372. [Google Scholar] [CrossRef]
- Yoo, J.H.; Kim, G.; Park, S.W.; Choi, M.S.; Ahn, J.; Jin, S.M.; Hur, K.Y.; Lee, M.K.; Kang, M.; Kim, J.H. Effects of low skeletal muscle mass and sarcopenic obesity on albuminuria: A 7-year longitudinal study. Sci. Rep. 2020, 10, 5774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann-Podczaska, A.; Al-Saad, S.R.; Karbowski, L.M.; Chojnicki, M.; Tobis, S.; Wieczorowska-Tobis, K. COVID 19—Clinical Picture in the Elderly Population: A Qualitative Systematic Review. Aging Dis. 2020, 11, 988–1008. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S.; Killander, A.; Åstrand, P.; Jakobsson, J.; Gille-Johnson, P. Risk factors for death in adult COVID-19 patients: Frailty predicts fatal outcome in older patients. Int. J. Infect. Dis. 2021, 102, 415–421. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Royal jelly as an intelligent anti-aging—a focus on cognitive aging and Alzheimer’s disease: A review. Antioxidants 2020, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Corona Virus Disease 2019 (COVID-19): A pandemic that threatens physical and mental health by promoting physical inactivity. Sports Med. Health Sci. 2020, 2, 221–223. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Ge, Y.; Shi, Y.; Lv, P.; Zhang, J.; Fu, G.; Zhou, Y.; Jiang, K.; Lin, N.; et al. Evaluation of Nutrition Risk and Its Association With Mortality Risk in Severely and Critically Ill COVID-19 Patients. J. Parenter. Enter. Nutr. 2020, 45, 32–42. [Google Scholar] [CrossRef]
- Jiménez-Pavón, D.; Carbonell-Baeza, A.; Lavie, C.J. Physical exercise as therapy to fight against the mental and physical consequences of COVID-19 quarantine: Special focus in older people. Prog. Cardiovasc. Dis. 2020, 63, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Paoli, A. When COVID-19 affects muscle: Effects of quarantine in older adults. Eur. J. Transl. Myol. 2020, 30, 9069. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Apitherapy for age-related skeletal muscle dysfunction (sarcopenia): A review on the effects of royal jelly, propolis, and bee pollen. Foods 2020, 9, 1362. [Google Scholar] [CrossRef]
- Bhasker, A.G.; Greve, J.W. Are Patients Suffering from Severe Obesity Getting a Raw Deal During COVID-19 Pandemic? Obes. Surg. 2020, 30, 4107–4108. [Google Scholar] [CrossRef]
- Azzolino, D.; Saporiti, E.; Proietti, M.; Cesari, M. Nutritional Considerations in Frail Older Patients with COVID-19. J. Nutr. Health Aging 2020, 24, 696–698. [Google Scholar] [CrossRef]
- Ma, Y.; Hou, L.; Yang, X.; Huang, Z.; Yang, X.; Zhao, N.; He, M.; Shi, Y.; Kang, Y.; Yue, J.; et al. The association between frailty and severe disease among COVID-19 patients aged over 60 years in China: A prospective cohort study. BMC Med. 2020, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Aw, D.; Woodrow, L.; Ogliari, G.; Harwood, R. Association of Frailty with Mortality in Older Inpatients with Covid-19: A Cohort Study. Age Ageing 2020, 49, 915–922. [Google Scholar] [CrossRef]
- Sriram, S.; Subramanian, S.; Sathiakumar, D.; Venkatesh, R.; Salerno, M.S.; McFarlane, C.D.; Kambadur, R.; Sharma, M. Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-kappaB. Aging Cell 2011, 10, 931–948. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Kunugi, H. The effects of royal jelly acid, 10-hydroxy-trans-2-decenoic acid, on neuroinflammation and oxidative stress in astrocytes stimulated with lipopolysaccharide and hydrogen peroxide. Immuno 2021, 1, 212–222. [Google Scholar] [CrossRef]
- Miko, A.; Poto, L.; Matrai, P.; Hegyi, P.; Furedi, N.; Garami, A.; Illes, A.; Solymar, M.; Vincze, A.; Balasko, M.; et al. Gender difference in the effects of interleukin-6 on grip strength; a systematic review and meta-analysis. BMC Geriatr. 2018, 18, 107. [Google Scholar] [CrossRef]
- Marzetti, E.; Picca, A.; Marini, F.; Biancolillo, A.; Coelho-Junior, H.J.; Gervasoni, J.; Bossola, M.; Cesari, M.; Onder, G.; Landi, F.; et al. Inflammatory signatures in older persons with physical frailty and sarcopenia: The frailty “cytokinome” at its core. Exp. Gerontol. 2019, 122, 129–138. [Google Scholar] [CrossRef]
- Miyatake, S.; Shimizu-Motohashi, Y.; Takeda, S.; Aoki, Y. Anti-inflammatory drugs for Duchenne muscular dystrophy: Focus on skeletal muscle-releasing factors. Drug Des. Dev. Ther. 2016, 10, 2745–2758. [Google Scholar] [CrossRef] [Green Version]
- Saleh, J.; Peyssonnaux, C.; Singh, K.K.; Edeas, M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion 2020, 54, 1–7. [Google Scholar] [CrossRef]
- Anagnostou, M.E.; Hepple, R.T. Mitochondrial Mechanisms of Neuromuscular Junction Degeneration with Aging. Cells 2020, 9, 197. [Google Scholar] [CrossRef] [Green Version]
- Genin, E.C.; Madji Hounoum, B.; Bannwarth, S.; Fragaki, K.; Lacas-Gervais, S.; Mauri-Crouzet, A.; Lespinasse, F.; Neveu, J.; Ropert, B.; Auge, G.; et al. Mitochondrial defect in muscle precedes neuromuscular junction degeneration and motor neuron death in CHCHD10(S59L/+) mouse. Acta Neuropathol. 2019, 138, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Menzies, K.; Zaldivar-Jolissaint, J.F.; Auwerx, J. Sirtuins as Metabolic Modulators of Muscle Plasticity. In Sirtuins, Proteins and Cell Regulation; Houtkooper, R., Ed.; Springer: Dordrecht, The Netherlands, 2016; Volume 10. [Google Scholar]
- Becker, C.; Lord, S.R.; Studenski, S.A.; Warden, S.J.; Fielding, R.A.; Recknor, C.P.; Hochberg, M.C.; Ferrari, S.L.; Blain, H.; Binder, E.F.; et al. Myostatin antibody (LY2495655) in older weak fallers: A proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015, 3, 948–957. [Google Scholar] [CrossRef]
- Camporez, J.P.; Petersen, M.C.; Abudukadier, A.; Moreira, G.V.; Jurczak, M.J.; Friedman, G.; Haqq, C.M.; Petersen, K.F.; Shulman, G.I. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc. Natl. Acad. Sci. USA 2016, 113, 2212–2217. [Google Scholar] [CrossRef] [Green Version]
- Zarbafian, M.; Dayan, S.; Fabi, S.G. Teachings from COVID-19 and aging—An oxidative process. J. Cosmet. Dermatol. 2020, 19, 3171–3176. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, T.W.; Clark, J.P.; Gustafson, G.E.; Miller, K.N.; Conklin, M.W.; DeMuth, T.M.; Berres, M.E.; Eliceiri, K.W.; Vaughan, L.K.; Lary, C.W.; et al. Molecular and Functional Networks Linked to Sarcopenia Prevention by Caloric Restriction in Rhesus Monkeys. Cell Syst. 2020, 10, 156–168. [Google Scholar] [CrossRef] [Green Version]
- Bindels, L.B.; Delzenne, N.M. Muscle wasting: The gut microbiota as a new therapeutic target? Int. J. Biochem. Cell Biol. 2013, 45, 2186–2190. [Google Scholar] [CrossRef]
- Kim, Y.; Triolo, M.; Hood, D.A. Impact of Aging and Exercise on Mitochondrial Quality Control in Skeletal Muscle. Oxid. Med. Cell. Longev. 2017, 2017, 3165396. [Google Scholar] [CrossRef]
- Mudry, J.M.; Lassiter, D.G.; Nylen, C.; Garcia-Calzon, S.; Naslund, E.; Krook, A.; Zierath, J.R. Insulin and Glucose Alter Death-Associated Protein Kinase 3 (DAPK3) DNA Methylation in Human Skeletal Muscle. Diabetes 2017, 66, 651–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laker, R.C.; Ryall, J.G. DNA Methylation in Skeletal Muscle Stem Cell Specification, Proliferation, and Differentiation. Stem Cells Int. 2016, 2016, 5725927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giresi, P.G.; Stevenson, E.J.; Theilhaber, J.; Koncarevic, A.; Parkington, J.; Fielding, R.A.; Kandarian, S.C. Identification of a molecular signature of sarcopenia. Physiol. Genom. 2005, 21, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Begue, G.; Raue, U.; Jemiolo, B.; Trappe, S. DNA methylation assessment from human slow- and fast-twitch skeletal muscle fibers. J. Appl. Phys. 2017, 122, 952–967. [Google Scholar] [CrossRef] [Green Version]
- Davegardh, C.; Hall Wedin, E.; Broholm, C.; Henriksen, T.I.; Pedersen, M.; Pedersen, B.K.; Scheele, C.; Ling, C. Sex influences DNA methylation and gene expression in human skeletal muscle myoblasts and myotubes. Stem Cell Res. Ther. 2019, 10, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, D.L.; Vlietstra, L.; Qualls, C.; Morley, J.E.; Vellas, B. Sex-specific muscle and metabolic biomarkers associated with gait speed and cognitive transitions in older adults: A 9-year follow-up. GeroScience 2020, 42, 585–593. [Google Scholar] [CrossRef]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959–9981. [Google Scholar] [CrossRef]
- Boisard, S.; Shahali, Y.; Aumond, M.-C.; Derbré, S.; Blanchard, P.; Dadar, M.; Le Ray, A.-M.; Richomme, P. Anti-AGE activity of poplar-type propolis: Mechanism of action of main phenolic compounds. Int. J. Food Sci. 2020, 55, 453–460. [Google Scholar] [CrossRef]
- Egawa, T.; Ohno, Y.; Yokoyama, S.; Goto, A.; Ito, R.; Hayashi, T.; Goto, K. The effect of advanced glycation end products on cellular signaling molecules in skeletal muscle. J. Phys. Fit. Sports Med. 2018, 7, 229–238. [Google Scholar] [CrossRef]
- He, T.; Qu, R.; Qin, C.; Wang, Z.; Zhang, Y.; Shao, X.; Lu, T. Potential mechanisms of Chinese Herbal Medicine that implicated in the treatment of COVID-19 related renal injury. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2020, 28, 1138–1148. [Google Scholar] [CrossRef]
- Pinto-Junior, D.C.; Silva, K.S.; Michalani, M.L.; Yonamine, C.Y.; Esteves, J.V.; Fabre, N.T.; Thieme, K.; Catanozi, S.; Okamoto, M.M.; Seraphim, P.M.; et al. Advanced glycation end products-induced insulin resistance involves repression of skeletal muscle GLUT4 expression. Sci. Rep. 2018, 8, 8109. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Broccatelli, M.; Savera, G.; D’Elia, M.; Pahor, M.; et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Szentesi, P.; Csernoch, L.; Dux, L.; Keller-Pinter, A. Changes in Redox Signaling in the Skeletal Muscle with Aging. Oxidative Med. Cell. Longev. 2019, 2019, 4617801. [Google Scholar] [CrossRef]
- Bressa, C.; Bailen-Andrino, M.; Perez-Santiago, J.; Gonzalez-Soltero, R.; Perez, M.; Montalvo-Lominchar, M.G.; Mate-Munoz, J.L.; Dominguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmassani, Z.S.; Reidy, P.T.; McKenzie, A.I.; Stubben, C.; Howard, M.T.; Drummond, M.J. Disuse-induced insulin resistance susceptibility coincides with a dysregulated skeletal muscle metabolic transcriptome. J. Appl. Phys. 2019, 126, 1419–1429. [Google Scholar] [CrossRef]
- Latham, C.M.; Wagner, A.L.; Urschel, K.L. Effects of dietary amino acid supplementation on measures of whole-body and muscle protein metabolism in aged horses. J. Anim. Physiol. Anim. Nutr. 2019, 103, 283–294. [Google Scholar] [CrossRef]
- Bocco, B.M.; Louzada, R.A.; Silvestre, D.H.; Santos, M.C.; Anne-Palmer, E.; Rangel, I.F.; Abdalla, S.; Ferreira, A.C.; Ribeiro, M.O.; Gereben, B.; et al. Thyroid hormone activation by type 2 deiodinase mediates exercise-induced peroxisome proliferator-activated receptor-gamma coactivator-1alpha expression in skeletal muscle. J. Physiol. 2016, 594, 5255–5269. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Sanchez, J.L.; Manas, A.; Garcia-Garcia, F.J.; Ara, I.; Carnicero, J.A.; Walter, S.; Rodriguez-Manas, L. Sedentary behaviour, physical activity, and sarcopenia among older adults in the TSHA: Isotemporal substitution model. J. Cachexia Sarcopenia Muscle 2019, 10, 188–198. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Shiels, P.G. The role of the microbiota in sedentary lifestyle disorders and ageing: Lessons from the animal kingdom. J. Intern. Med. 2020, 287, 271–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [Green Version]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Apitherapy for Parkinson’s disease: A focus on the effects of propolis and royal jelly. Oxid. Med. Cell. Longev. 2020, 2020, 1727142. [Google Scholar] [CrossRef]
- Yazar, T.; Yazar, H.O.; Zayimoglu, E.; Cankaya, S. Incidence of sarcopenia and dynapenia according to stage in patients with idiopathic Parkinson’s disease. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2018, 39, 1415–1421. [Google Scholar] [CrossRef]
- Peball, M.; Mahlknecht, P.; Werkmann, M.; Marini, K.; Murr, F.; Herzmann, H.; Stockner, H.; de Marzi, R.; Heim, B.; Djamshidian, A.; et al. Prevalence and Associated Factors of Sarcopenia and Frailty in Parkinson’s Disease: A Cross-Sectional Study. Gerontology 2018, 65, 216–228. [Google Scholar] [CrossRef]
- Rempe, R.G.; Hartz, A.M.S.; Bauer, B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metab. 2016, 36, 1481–1507. [Google Scholar] [CrossRef] [Green Version]
- Inoue, Y.; Hara, H.; Mitsugi, Y.; Yamaguchi, E.; Kamiya, T.; Itoh, A.; Adachi, T. 4-Hydroperoxy-2-decenoic acid ethyl ester protects against 6-hydroxydopamine-induced cell death via activation of Nrf2-ARE and eIF2α-ATF4 pathways. Neurochem. Int. 2018, 112, 288–296. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; Shu, X.; Bai, L.; Xu, W.; Wang, A.; Chen, A.; Tu, W.Y.; Wang, J.; Zhang, K.; et al. Loss of mitochondrial protein CHCHD10 in skeletal muscle causes neuromuscular junction impairment. Hum. Mol. Genet. 2019, 29, 1784–1796. [Google Scholar] [CrossRef]
- Romero-Suarez, S.; Shen, J.; Brotto, L.; Hall, T.; Mo, C.; Valdivia, H.H.; Andresen, J.; Wacker, M.; Nosek, T.M.; Qu, C.K.; et al. Muscle-specific inositide phosphatase (MIP/MTMR14) is reduced with age and its loss accelerates skeletal muscle aging process by altering calcium homeostasis. Aging 2010, 2, 504–513. [Google Scholar] [CrossRef] [Green Version]
- Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 2018, 70, 87–94. [Google Scholar] [CrossRef]
- Kunugi, H.; Ali, A.M. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pronsato, L.; Milanesi, L.; Vasconsuelo, A. Testosterone induces up-regulation of mitochondrial gene expression in murine C2C12 skeletal muscle cells accompanied by an increase of nuclear respiratory factor-1 and its downstream effectors. Mol. Cell. Endocrinol. 2020, 500, 110631. [Google Scholar] [CrossRef]
- Church, D.D.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Acute testosterone administration does not affect muscle anabolism. Nutr. Metab. 2019, 16, 56. [Google Scholar] [CrossRef] [Green Version]
- Landi, F.; Camprubi-Robles, M.; Bear, D.E.; Cederholm, T.; Malafarina, V.; Welch, A.A.; Cruz-Jentoft, A.J. Muscle loss: The new malnutrition challenge in clinical practice. Clin. Nutr. 2019, 38, 2113–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walrand, S.; Gryson, C.; Salles, J.; Giraudet, C.; Migne, C.; Bonhomme, C.; Le Ruyet, P.; Boirie, Y. Fast-digestive protein supplement for ten days overcomes muscle anabolic resistance in healthy elderly men. Clin. Nutr. 2016, 35, 660–668. [Google Scholar] [CrossRef]
- Boirie, Y.; Guillet, C. Fast digestive proteins and sarcopenia of aging. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Tsintzas, K.; Jones, R.; Pabla, P.; Mallinson, J.; Barrett, D.A.; Kim, D.H.; Cooper, S.; Davies, A.; Taylor, T.; Chee, C.; et al. Effect of acute and short-term dietary fat ingestion on postprandial skeletal muscle protein synthesis rates in middle-aged, overweight, and obese men. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E417–E429. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; D’Angelo, E.; Sisto, A.; Marzetti, E. Protein Intake and Muscle Health in Old Age: From Biological Plausibility to Clinical Evidence. Nutrients 2016, 8, 295. [Google Scholar] [CrossRef]
- Salles, J.; Cardinault, N.; Patrac, V.; Berry, A.; Giraudet, C.; Collin, M.L.; Chanet, A.; Tagliaferri, C.; Denis, P.; Pouyet, C.; et al. Bee pollen improves muscle protein and energy metabolism in malnourished old rats through interfering with the Mtor signaling pathway and mitochondrial activity. Nutrients 2014, 6, 5500. [Google Scholar] [CrossRef] [Green Version]
- Belančić, A. Gut microbiome dysbiosis and endotoxemia—Additional pathophysiological explanation for increased COVID-19 severity in obesity. Obes. Med. 2020, 20, 100302. [Google Scholar] [CrossRef] [PubMed]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef] [Green Version]
- Inostroza Flores, G.; Francino Barrera, G.; Jiménez Torres, S. How does vitamin D influence body composition, sarcopenia and lifespan in older persons? A retrospective study of nine years. Nutr. Hosp. 2019, 36, 1067–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, S.V.; Fischer, K.; Dawson-Hughes, B.; Freystaetter, G.; Beuschlein, F.; Schietzel, S.; Egli, A.; Bischoff-Ferrari, H.A. Association between 25-Hydroxyvitamin D Status and Components of Body Composition and Glucose Metabolism in Older Men and Women. Nutrients 2018, 10, 1826. [Google Scholar] [CrossRef] [Green Version]
- Perna, S.; Alalwan, T.A.; Al-Thawadi, S.; Negro, M.; Parimbelli, M.; Cerullo, G.; Gasparri, C.; Guerriero, F.; Infantino, V.; Diana, M.; et al. Evidence-Based Role of Nutrients and Antioxidants for Chronic Pain Management in Musculoskeletal Frailty and Sarcopenia in Aging. Geriatric 2020, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Kunugi, H.; Abdelmageed, H.A.; Mandour, A.S.; Ahmed, M.E.; Ahmad, S.; Hendawy, A.O. Vitamin K in COVID-19—Potential Anti-COVID-19 Effects of Vitamin K Antagonists (VKA) and Fermented Milk Fortified with Bee Honey as a Natural Source of Vitamin K and Probiotics. Fermentation 2021, submitted. [Google Scholar]
- Mahajan, A.; Donovan, L.E.; Vallee, R.; Yamamoto, J.M. Evidenced-Based Nutrition for Gestational Diabetes Mellitus. Curr. Diabetes Rep. 2019, 19, 94. [Google Scholar] [CrossRef]
- Konishi, K.; Wada, K.; Yamakawa, M.; Goto, Y.; Mizuta, F.; Koda, S.; Uji, T.; Tsuji, M.; Nagata, C. Dietary Soy Intake Is Inversely Associated with Risk of Type 2 Diabetes in Japanese Women but Not in Men. J. Nutr. 2019, 149, 1208–1214. [Google Scholar] [CrossRef]
- Konya, J.; Sathyapalan, T.; Kilpatrick, E.S.; Atkin, S.L. The Effects of Soy Protein and Cocoa With or Without Isoflavones on Glycemic Control in Type 2 Diabetes. A Double-Blind, Randomized, Placebo-Controlled Study. Front. Endocrinol. 2019, 10, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.E. The Effects of Dietary Supplements That Overactivate the Nrf2/ARE System. Curr. Med. Chem. 2019, 27, 2077–2094. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, C.; Fares, S.; Chardigny, J.M.; Boirie, Y.; Walrand, S. Vitamin D supplementation and muscle strength in pre-sarcopenic elderly Lebanese people: A randomized controlled trial. Arch. Osteoporos. 2018, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Schnell, D.M.; Redzic, M.; Zhao, M.; Abraha, H.; Jones, D.; Brim, H.; Yu, G. Local In Vivo Measures of Muscle Lipid and Oxygen Consumption Change in Response to Combined Vitamin D Repletion and Aerobic Training in Older Adults. Nutrients 2019, 11, 930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, L.E.; McClester Brown, M.; Hawes, E.M. Vitamin D Supplementation for Extraskeletal Indications in Older Persons. J. Am. Med. Dir. Assoc. 2020, 21, 164–171. [Google Scholar] [CrossRef]
- Chiche, A.; Le Roux, I.; von Joest, M.; Sakai, H.; Aguin, S.B.; Cazin, C.; Salam, R.; Fiette, L.; Alegria, O.; Flamant, P.; et al. Injury-Induced Senescence Enables In Vivo Reprogramming in Skeletal Muscle. Cell Stem Cell 2017, 20, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Hernandez, J.M.; Garcia-Gonzalez, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Wang, H.; Pei, Y.; Li, Y.; Wu, H.; Song, Y.; Guo, Q.; Guo, H.; Fukushima, S.; Tatefuji, T.; et al. Effects of protease-treated royal jelly on muscle strength in elderly nursing home residents: A randomized, double-blind, placebo-controlled, dose-response study. Sci. Rep. 2017, 7, 11416. [Google Scholar] [CrossRef] [Green Version]
- Madaro, L.; Torcinaro, A.; De Bardi, M.; Contino, F.F.; Pelizzola, M.; Diaferia, G.R.; Imeneo, G.; Bouche, M.; Puri, P.L.; De Santa, F. Macrophages fine tune satellite cell fate in dystrophic skeletal muscle of mdx mice. PLoS Genet. 2019, 15, e1008408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jaber, V.; Lukiw, W.J. Secretory Products of the Human GI Tract Microbiome and Their Potential Impact on Alzheimer’s Disease (AD): Detection of Lipopolysaccharide (LPS) in AD Hippocampus. Front. Cell. Infect. Microbiol. 2017, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.B.; Lynch, D.B.; O’Toole, P.W. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016, 10, 170–182. [Google Scholar] [CrossRef] [Green Version]
- Siddharth, J.; Chakrabarti, A.; Pannerec, A.; Karaz, S.; Morin-Rivron, D.; Masoodi, M.; Feige, J.N.; Parkinson, S.J. Aging and sarcopenia associate with specific interactions between gut microbes, serum biomarkers and host physiology in rats. Aging 2017, 9, 1698–1720. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.E.; Lim, S.M.; Jeong, J.J.; Jang, H.M.; Lee, H.J.; Han, M.J.; Kim, D.H. Gastrointestinal inflammation by gut microbiota disturbance induces memory impairment in mice. Mucosal Immunol. 2018, 11, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Pretorius, L.; Kell, D.B.; Pretorius, E. Iron Dysregulation and Dormant Microbes as Causative Agents for Impaired Blood Rheology and Pathological Clotting in Alzheimer’s Type Dementia. Front. Neurosci. 2018, 12, 851. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Yan, H.; Diao, H.; Xiao, Y.; Li, W.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Mao, X.; Luo, Y.; et al. Gut microbiota can transfer fiber characteristics and lipid metabolic profiles of skeletal muscle from pigs to germ-free mice. Sci. Rep. 2016, 6, 31786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ticinesi, A.; Lauretani, F.; Tana, C.; Nouvenne, A.; Ridolo, E.; Meschi, T. Exercise and immune system as modulators of intestinal microbiome: Implications for the gut-muscle axis hypothesis. Exerc. Immunol. Rev. 2019, 25, 84–95. [Google Scholar] [PubMed]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef]
- Bindels, L.B.; Beck, R.; Schakman, O.; Martin, J.C.; De Backer, F.; Sohet, F.M.; Dewulf, E.M.; Pachikian, B.D.; Neyrinck, A.M.; Thissen, J.P.; et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS ONE 2012, 7, e37971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, X.; Liu, S.; Liao, J.F.; Wen, W.; Long, Z.Q.; Lu, Z.J.; Guo, L.; Lin, H.X. When the Loss Costs Too Much: A Systematic Review and Meta-Analysis of Sarcopenia in Head and Neck Cancer. Front. Oncol. 2019, 9, 1561. [Google Scholar] [CrossRef]
- Bruyere, O.; Beaudart, C.; Ethgen, O.; Reginster, J.Y.; Locquet, M. The health economics burden of sarcopenia: A systematic review. Maturitas 2019, 119, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, J.E. Treatment of sarcopenia: The road to the future. J. Cachexia Sarcopenia Muscle 2018, 9, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Rooks, D.; Roubenoff, R. Development of Pharmacotherapies for the Treatment of Sarcopenia. J. Frailty Aging 2019, 8, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Hendawy, A.O. Royal Jelly Acid, 10-Hydroxy-Trans-2-Decenoic Acid, for Psychiatric and Neurological Disorders: How helpful could it be?! Edelweiss J. Food Sci. Technol. 2019, 1, 1–4. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Bee honey protects astrocytes against oxidative stress: A preliminary in vitro investigation. Neuropsychopharmacol. Rep. 2019, 39, 312–314. [Google Scholar] [CrossRef] [Green Version]
- Mossalayi, M.D.; Rambert, J.; Renouf, E.; Micouleau, M.; Mérillon, J.M. Grape polyphenols and propolis mixture inhibits inflammatory mediator release from human leukocytes and reduces clinical scores in experimental arthritis. Phytomedicine 2014, 21, 290–297. [Google Scholar] [CrossRef]
- Tomasin, R.; de Andrade, R.S.; Gomes-Marcondes, M.C. Oral Administration of Aloe vera (L.) Burm. f. (Xanthorrhoeaceae) and Honey Improves the Host Body Composition and Modulates Proteolysis Through Reduction of Tumor Progression and Oxidative Stress in Rats. J. Med. Food 2015, 18, 1128–1135. [Google Scholar] [CrossRef]
- Santos, N.W.; Yoshimura, E.H.; Mareze-Costa, C.E.; Machado, E.; Agustinho, B.C.; Pereira, L.M.; Brito, M.N.; Brito, N.A.; Zeoula, L.M. Supplementation of cow milk naturally enriched in polyunsaturated fatty acids and polyphenols to growing rats. PLoS ONE 2017, 12, e0172909. [Google Scholar] [CrossRef]
- Maaroufi, H. The Spike Protein S1 Subunit of SARS-CoV-2 Contains an LxxIxE-like Motif that is Known to Recruit the Host PP2A-B56 Phosphatase. bioRxiv 2020. [Google Scholar] [CrossRef]
- Jahrami, H.A.; Alsibai, J.; Clark, C.C.T.; Faris, M.e.A.-I.E. A systematic review, meta-analysis, and meta-regression of the impact of diurnal intermittent fasting during Ramadan on body weight in healthy subjects aged 16 years and above. Eur. J. Nutr. 2020, 59, 2291–2316. [Google Scholar] [CrossRef] [PubMed]
- Domaszewski, P.; Konieczny, M.; Pakosz, P.; Bączkowicz, D.; Sadowska-Krępa, E. Effect of a Six-Week Intermittent Fasting Intervention Program on the Composition of the Human Body in Women over 60 Years of Age. Int. J. Environ. Res. Public Health 2020, 17, 4138. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, P.C.; Seeds, W.A.; Miller, A.C.; Mahajan, V.R.; Curtis, W.M. COVID-19: Proposing a Ketone-Based Metabolic Therapy as a Treatment to Blunt the Cytokine Storm. Oxid. Med. Cell. Longev. 2020, 2020, 6401341. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.M.; Kunugi, H. Physical Frailty/Sarcopenia as a Key Predisposing Factor to Coronavirus Disease 2019 (COVID-19) and Its Complications in Older Adults. BioMed 2021, 1, 11-40. https://doi.org/10.3390/biomed1010002
Ali AM, Kunugi H. Physical Frailty/Sarcopenia as a Key Predisposing Factor to Coronavirus Disease 2019 (COVID-19) and Its Complications in Older Adults. BioMed. 2021; 1(1):11-40. https://doi.org/10.3390/biomed1010002
Chicago/Turabian StyleAli, Amira Mohammed, and Hiroshi Kunugi. 2021. "Physical Frailty/Sarcopenia as a Key Predisposing Factor to Coronavirus Disease 2019 (COVID-19) and Its Complications in Older Adults" BioMed 1, no. 1: 11-40. https://doi.org/10.3390/biomed1010002
APA StyleAli, A. M., & Kunugi, H. (2021). Physical Frailty/Sarcopenia as a Key Predisposing Factor to Coronavirus Disease 2019 (COVID-19) and Its Complications in Older Adults. BioMed, 1(1), 11-40. https://doi.org/10.3390/biomed1010002






