Definition
The stress response is a natural physiological reaction of the organism, elicited to maintain the internal environment and evoke adaptive behaviors, ultimately leading to survival. However, at the turn of the century, stress-related disorders gained increasing significance. The aim of entry is to explore the fundamental question of when the stress system changes from a beneficial to a detrimental system, contributing to a higher risk of the development of disorders and/or diseases. To develop context, here, we explore the different concepts of stress and reveal the complexities, perspectives, and multiple relationships between the neurochemistry, cerebral functional network, and associated pathologies. According to the literature, the stress response affects nearly every biological system through the close interactions between the physiological, nervous, endocrine, and immune systems when faced with a real or perceived threat. Considering today’s challenging times, where people are facing multiple unavoidable adversities in their lives and a level of uncertainty never before seen, this review emphasizes the importance of understanding the potential consequences of being unable to cope with stressful events. Susceptibility and resilience to stress have gained recognition as important areas of study. The literature presented here enhances our understanding and identifies the causes of various psychopathologies, mental health conditions, disabilities, and even mortality that are closely linked to vulnerability to stress. Experimental studies from recent decades have demonstrated the many factors affecting our ability to cope with stress, including differences between individuals due to their genetic background, epigenetic regulation, gender, and early-life experiences. Finally, there is an urgent need to change the paradigm of modern lifestyles as a potential strategy to prevent the spread of the “health epidemic of the 21st century”, which is stress. Therefore, we acknowledge different approaches to enhance resilience, focusing on perception, tolerance, and positive lifestyle behaviors.
1. Stress Overview
In today’s world, the term “stress” is widely used and often misunderstood. Stress is traditionally referred to as a state of mental or emotional strain caused by physiological arousal or negative emotions [1,2], but this has not always been the case.
The concept of stress is relatively old. It is derived from the Latin stringere, which means to squeeze, tie, or cause tension. During the 18th century, the notion of stress shifted from being linked to solely individual emotional consequences to being viewed as the cause of such reactions [3]. This shift in perspective allowed for a more thorough understanding of the concept, and at the beginning of the 20th century, the physiologist Walter Cannon introduced the term stress for the first time in medicine. Cannon made a compilation of visceral adaptive responses to different noxious stimuli. This compilation, which was compiled in the Harvard University laboratories, described the bodily changes that occurred in conjunction with painful insults, such as hunger, cold, hard exercise, or strong emotions. Cannon proposed the stress response to be the “adaptation of the organism to cope with emergencies” and characterized it as a nonspecific and generalized reaction, a state of alarm, which prepares the organism for a potential escape or attack, the “fight-or-flight” response [4]. Some years later, Cannon also introduced the concept of “homeostasis”, an inspiration based on the idea of Claude Bernard in his Introduction à la médecine experimentale (1865), which perceived that the preservation of life was critically dependent on maintaining physiological systems in equilibrium in the face of a changing environment [5]. Both concepts, “homeostasis” and “fight-or-flight response”, were fundamental to the foundation of stress research, suggesting that such a state of “alarm” was the key to explaining what happened to an organism facing an emergency and to recover the state of equilibrium.
The general characteristics of the stress response were defined some years later by Hans Selye, an endocrinologist who, as a student, observed that patients suffering from different diseases often showed similar symptoms that could constitute a single syndrome. Selye hypothesized a non-specific response of the body to stress, emphasizing that identical pathologies would result from any demand, and named it “General Adaptation Syndrome” [6]. Not all scientists agreed with Selye’s proposal from the beginning, and subsequent studies have shown the opposite, that is, the existence of different neuroendocrine responses to exposure to different stressors. While Selye spent his entire career working on physical stressors (e.g., pain, heat or cold), other academics understood that some stress factors can be quite different and induced by internal processes (e.g., anxiety). Pacak and his group presented an alternative model, a “primitive specificity” in the stress response [7], according to which each type of stress would have a neurochemical “signature” with quantitatively and perhaps qualitatively distinct central and peripheral mechanisms. These alterations would occur, not as isolated matters, but as a function of the physiological and behavioral changes and even of the experiences lived by each individual [8].
A few years later, Richard Lazarus attributed meaning to the individual’s environment, and together with Folkman, changed the concept of stress to “the relationship between the person and the environment that is appraised as personally significant and as taxing or exceeding resources for coping” [9], emphasizing, therefore, that it is neither the individual nor the environment alone that produces stress but a complex transaction between the two. Their definition formed the foundation of stress and coping theory, conceptualizing it as “the cognitive and behavioral efforts of an individual to manage the internal and external demands encountered during a specific stressful situation”. This idea was later simplified by endocrinologists Bruce McEwen and Eliot Stellar, who in 1993 proposed a term for the stress response as a process of “allostasis” [8], which literally means “to achieve stability through alteration” and for which the adaptation process was named “allostatic load” or “allostatic overload” [10,11,12]. According to these authors, this concept of allostasis could be more biologically precise than the definition of stress [12]. However, it did not facilitate its comprehension, and the scientific community kept the concepts of stress, stressor, and the stress response to be used and accepted.
It is now widely accepted that stress is a critical factor in many aspects of human life, with devastating effects on our emotional and physical health. Based on this recognition, the World Health Organization (WHO) has labeled stress the “health epidemic of the 21st century” [13]. Stress-related mental illnesses have progressively increased in recent decades, and it is estimated that over 75% of young students and adults suffer, at least at some point in their lives, from stress or anxiety disorders [14]. The Global Organization for Stress reported that stress is now the primary health concern among high school students [15] and that 80% of employers and employees feel stress at work.
In contemporary societies, there is an ever-increasing expectation placed upon individuals to attain a level of perfection and success, largely attributed to the influence of social media and work overload. This often leads to work–life imbalances, which can negatively impact both family and social relationships [16,17]. Furthermore, in the past few years, there have been several sudden, uncontrollable, and unprecedented crises that have occurred one after the other in quick succession. The negative effects of these exceptional crises, such as the COVID-19 pandemic, unexpected wars, and natural disasters, have been severe, affecting everyone at the same time [18]. These crises impacted the lives of people of all ages, and they have been a source stress, leading to forced displacement from home, isolation, uncertainty, and economic or job insecurity. Several studies demonstrated the high prevalence rates of stress-related disorders, anxiety and depression, and the increased rates of morbidity, mental illness, and suicide [18,19]. The correlation between stress and psychiatric illness is strong, stronger than with any physical or medical illness. This correlation will be the focus of our manuscript.
2. Concepts
2.1. Stress Response System and Its Modulation
Any disturbance acting on the organism is called a stressor, which could be any stimulus, external or internal (physical, chemical, acoustic, somatic, social, etc.); the result of mental anticipation of what could happen [3]; or even something that has already occurred. Subsequently, the stress response system is involved through perception (attention-dependent), processing (innate or memory-dependent responses), and transduction of that information (into specific responses that may or may not be appropriate) [20].
The effect of the stress response on the body is profound, involving a response from almost all the organ systems and tissues of higher organisms [2], resulting in myriad cardiovascular, metabolic, immune, neuroendocrine, sensory, motor, and cognitive modifications [21,22,23,24]. Such modifications allow the organism to adapt to physical, psychological, or social conditions of multiple natures. In addition, physiological processes that are not essential for short-term survival, such as digestion, reproduction, and growth, are inhibited during the stress response [10,24]. Therefore, when the stress response exceeds certain limits in terms of its intensity and/or duration, the alterations described above can, by themselves, originate functional changes and lead to pathologies [25,26,27].
2.1.1. Circuits Involved
In mammals, the brain is the organ that determines the physiological and behavior-al responses developed to face each stressor presented, with all the sensory modes being processed in the central nervous system (CNS) at multiple levels.
Once homeostasis is disrupted, the maintenance of the body’s state of equilibrium is ensured by the autonomic nervous system (ANS), which acts under the coordination of the nucleus of the solitary tract (NTS), the ventrolateral medulla (VLM) and the hypothalamus, a complex structure composed of numerous nuclei [28,29]. First, there is a rapid and direct processing that leads to reflexes and stereotyped responses generated at the spinal cord, the brainstem, or the hypothalamus. Second, sensory information is directed through multiple thalamic efferent fibers to its designated projection cortical areas and then to the associated cortical areas where connections with limbic structures (prefrontal cortex, amygdala, and hippocampus) are established to evoke a psychological response through body activation and behavioral planning (see Figure 1) [28,30].
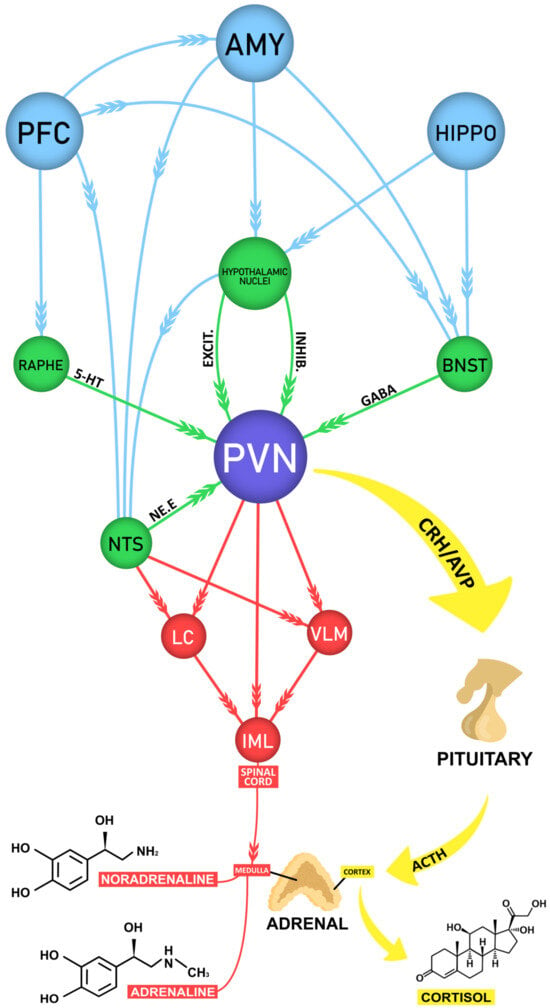
Figure 1.
Schematic diagram of the neural circuits underlying stress. Figure 1 shows in purple the paraventricular nucleus of the hypothalamus (PVN), the main output governing the activity of the sympathetic and endocrine stress response. It elaborates appropriate responses to a variety of different stressors (physical and psychological) due to its central position in a very complex neural circuit. The red arrows indicate the rapid sympathetic response to stress orchestrated by the PVN and the nucleus of the solitary tract (NTS) in the brainstem, which relays somatic and visceral sensory information. The PVN and the NTS send projections that terminate at the medulla of the adrenal gland, which secretes adrenaline and noradrenaline into the blood stream. The yellow arrows indicate the slightly slower endocrine stress response. As a result of the PVN activation, corticotrophin-releasing hormone (CRH) and vasopressin (AVP) are secreted and act at the pituitary gland, stimulating the synthesis and release of adrenocorticotrophic hormone (ACTH). Through the circulation, the ACTH reaches the cortex of the adrenal gland, where it stimulates the release of cortisol. The green arrows indicate the direct excitatory and inhibitory inputs that influence the PVN activity. This information arise from several brain regions (many not shown, nor mentioned in this review), including serotoninergic projections from the Raphe nuclei, adrenergic from the NTS, mostly GABAergic from the bed nucleus of the stria terminalis (BNST), as well as many excitatory and inhibitory inputs from hypothalamic nuclei. The blue arrows represent the influence of higher-order brain regions, including most notably the pre-frontal cortex (PFC), the amygdala (AMY) and the hippocampus (HIPPO), in a top-down fashion over the activity of the PNV through indirect pathways through the NTS, Raphe nuclei, BNST and hypothalamic nuclei. Abbreviations: LC: locus coeruleus, VLM: ventrolateral medulla, IML: intermediolateral nucleus of the spinal cord.
Despite the sympathetic response, the PVN is also responsible for eliciting the response of hypothalamic–pituitary–adrenocortical (HPA) axis [28,30]. The activity of the HPA axis is regulated by several afferents of the corticolimbic system, for example, the prefrontal cortex (PFC) in the frontal lobe, the insular and the anterior cingulate cortex (ACC), the thalamus, the hippocampus, the amygdala, the bed nucleus of the stria terminalis (BNST) and the septal nuclei [8,31,32,33,34,35,36,37,38]. These regions of the nervous system are responsible for carrying out the intermediate processes of cognitive and emotional evaluation between the stressor and the body’s response, so that all these structures receive direct or indirect sensory afferents and activate, in turn, the effector mechanisms of somatic and neuroendocrine responses [31,32,33,34,35,36], allowing, under stressful situations, the adoption of adequate behavioral responses to cope with the stressor.
The ACC plays a key role in the process of consciously selecting attentional responses and emotions. The medial PFC (mPFC) is critically involved in highly integrative functions—such as the working memory, selective attention, visceromotor control, and decision-making—mediating a cognitive top-down control of the stress response [34,39]. This area maintains connections, in addition to the limbic system, with the thalamus and other cortical areas, receiving dense inputs from the brainstem. Nevertheless, the mPFC, in addition to receiving inputs, has the unique characteristic (among cortical areas) of also sending direct projections to groups of cholinergic and monoaminergic cells in the brainstem [39,40]. As a result, the mPFC can integrate the sensory information, external or internal, and select the most appropriate behavioral strategies, leading the organism to adapt its behaviors [40,41,42].
The hippocampus is a key regulatory structure related to PFC function, acting cooperatively in the regulation of memory [37,43,44,45]. This structure also plays a key role in the sequential memory and in some aspects of learning, such as fear conditioning [46], a key feature in the development of phobias and subsequent anxiety disorders [45]. However, the amygdala, another limbic structure, is the main structure involved in the generation of emotional memories [34,38], being strongly involved in the persistence and recurrence of memories related to traumatic events [35,38,47,48]. The activation of glucocorticoid receptors (GRs) in the amygdala after a stressful event enhances the stress response, activating several other structures, such as the locus coeruleus (LC) [49] or the ACC [50], suggesting the extreme importance of these structures in relating aversive stimuli with physiological, neuroendocrine, and behavioral responses inherent to psychological stress.
Another key structure involved in mediating the effects of stress is the nucleus accumbens (NAc) [51,52,53,54,55]. The NAc is involved with the natural reward and motivation systems [52], being related to, for instance, the symptoms of anhedonia present in depressive patients, the sexual arousal, the “high” derived from certain recreational drugs, and in anxiety contexts. The stress response activates the mesolimbic dopaminergic system or the “pleasure” pathway from the ventral tegmental area (VTA) to the NAc [52], and this activation may contribute to the mediation of the homeostatic response to stress.
Extra-limbic structures are also important in modulating the stress response, such as the LC, the raphe nuclei, the periaqueductal gray matter (PGM), and the nucleus of the solitary tract (NTS), which are found in the brainstem, where the network of connections with the other brain structures are established [49,56,57]. The NTS comprises a conspicuous group of cells located in the brainstem, which relays somatic and visceral sensory information, and the PGM, a structure located around the aqueduct, which are key regions of the brain circuit involved in the coordination of the defensive and aversive response to fear, pain, and stress (see Figure 1) [56]. The importance of the PGM lies in its high density of receptors for glutamate, aspartate and serotonin, whose activity and interaction are crucial, for example, in the modulation of panic and escape behaviors, which leads to moving away from the aversive source [56]. Likewise, when faced with potentially threatening stimuli, the neurons of the LC are also activated and initiate the activation of the prosencephalic region, mainly through its vast projections in the cerebral cortex, promoting arousal, alertness, and attention [49,56]. In turn, located in the midline of the midbrain, the raphe nuclei owes its importance to the fact that it is a region of the brain that possess vast serotonergic projections to all, or almost all, the regions that mediate behavior in response to stress [27,33,42,43,57].
2.1.2. Neurochemistry
Once the brain interprets internal and/or external signals as threatening to its homeostasis, the stress response triggered is fast and acute, activating peripheral responses and allocating physiological resources to promote the most appropriate coping strategy [9]. First, activation of the sympathetic nervous system results in the release of adrenaline and noradrenaline from the adrenal medulla. The rising adrenaline allows a quick response to the stressor, in part by increasing the heart rate, blood circulation, and respiration, and itself increasing the release of noradrenaline in the brain. Slightly later, a second system is activated, the HPA axis [29]. As depicted in Figure 1, the information reaching the PVN stimulates the release of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP), stimulating the release of adrenocorticotropic hormone (ACTH) by the pituitary gland [29,58]. With this stimulation, the secretion of glucocorticoids (GCs) by the adrenal cortex (predominantly cortisol, in humans) is greatly increased. Given the catabolic properties of the GCs, there occurs a rapid mobilization of amino acids and fats, making these energy substrates available to be able to physically cope with the situation [27]. GCs also suppress the immune response, protecting the body against potential inflammation, which is of crucial importance at the time of potential danger but can have consequences in prolonged circumstances [20,33]. All these changes provide immediate beneficial responses in the organism [10,21]. However, it seems clear that once the stress situation is over, the HPA response should end.
The GCs, which control the stress response, do not act alone. They coordinate with other important neurotransmitter systems, including catecholamines (adrenaline and noradrenaline) and beta-endorphins [18,21,58,59]. The neurotransmitters that act in the modulation of behavior include cholecystokinin [60], urocortins (acting through CRH-2 receptors) [59], neurotrophic factors (BDNF) [27], enkephalins, cytokines, [61,62], neuropeptide Y, angiotensin and neurotensin (facilitators of ACTH release), endogenous opioid peptides, vasopressin (regulating the HPA axis, or together with oxytocin, enhancing the effect of CRH and regulating the stress response), and thyrotropin-releasing hormones [60,61,62]. Many other hormones critical to the effectiveness of the autonomic response (e.g., somatostatin, prolactin or glucagon) do not act at the brain level [24] and can directly control the pituitary function either as hormones or as neurotransmitters in the CNS.
Within the brain, several monoamine and amino acids systems that innervate the cortex, particularly the mPFC, are also activated in response to stress. Among the most important are the glutamate (representative of excitatory amino acids), the gamma-aminobutyric acid (GABA) (representative of inhibitory amino acids) [24,42], taurine (as inhibitor of cellular excitability) [51], and the serotonin and dopamine [52,55,63]. The serotonin and dopamine are central neurotransmitters playing a crucial role in the body’s performance in stressful situations [33,36,55,57] and stabilizing nerve activity. Both, together with their metabolites, such as melatonin (the endogenous hormone synthesized from tryptophan), influence several brain functions, such as sleep or hunger, but also most notably regulate emotional and cognitive status [56].
2.2. Determinants of the Stress Response: The Stressor
2.2.1. Origin, Repeatability, and Degree of Control over the Stressor
It is now known that depending on their origin, stressors can be classified as physiological or neurogenic, meaning a real threat or injury (e.g., heat, cold, and pain) or psychogenic (e.g., new environments and situations, excessive worrying, money problems), and being dependent on the perception and interpretation of the potential threat [64,65,66,67]. The neuronal processing of the two different subtypes of stressors is handled by the two different neurocircuits or pathways mentioned above and by different brain regions [32]. Neurogenic stressors involve a physical stimulus that constitutes a direct breakdown of homeostasis, evoking autonomic stress responses that are internally recognized. The signaling of this type of stressor occurs by direct pathways and by signals that activate the HPA axis through the brainstem directly to the hypothalamus [68]. The psychogenic stimuli, however, require cognitive processing to occur for the stimulus to gain biological meaning. Psychogenic stressors also require comparison with past experiences, which most likely implies that, together with the prosencephalic nuclei, the limbic structures are also involved [32,66]. Due to the cognitive evaluation process to which psychogenic stressful stimuli are subjected, the effects they exert depend, in addition to the stressor per se, on psychological factors that modulate the stress response, including the controllability and predictability of the stressor [35,64].
Another factor that modulates the stress response is the repeatability. Whereas repeated exposure to the same type of stressor (homotypic) can normally result in a decrease in the HPA and ANS systems’ responses to stressors—due to a process considered “habituation” [36,68,69,70], that is, the reduction of physiological responses due to “n” exposures to the same stressor and it being considered a non-associative form of learning [71]—if an individual is subjected to new or different acute stressors (heterotypic), the responses of the neuroendocrine systems could otherwise increase, inducing sensitization [36,68,71,72]. Considering the serious risk that continued exposure to high levels of corticosteroids would pose [22,33], in prolonged stress responses there is a chronic elevation of the same mediators that in the short term helped to cope with the situation but continue to be released in excess, resulting in several disorders.
The degree of control over the environment is also a determinant of psychological well-being [17,42,65]. According to several studies, the lack of control over the environment has serious detrimental effects. It contributes to the onset of various stress-related diseases, such as depression [61], post-traumatic stress disorder (PTSD) [34], and anxiety [38,73], and it increases susceptibility to brain dysfunctions, such as epilepsy [69,74], including cell necrosis and atrophy [3,36,43,75].
A series of studies in dogs conducted in the late 1960s by Seligman and Maier revealed the despair experienced when there is no control over situations. These studies focused on the phenomenon of learned helplessness [76], a feeling experienced by animals when they are unable to control the environment in which they are immersed in, to the point that they give up trying to escape from an unpleasant situation even if they can do so. It has subsequently been suggested that this phenomenon of learned helplessness has a similar mechanism to that of the development of certain types of depression in humans [36], as the characteristics of depression are shared with the learned helplessness model, such as anhedonia, passivity, loss of interest, and low motivation. In both, there is a feeling that actions are useless, with an absence of contingency between acts and consequences [36,41,43].
2.2.2. Severity and Timeframe of the Stressor
Optimal functions do not occur in a life without stimuli. A certain amount of stress is not only not aversive but can act as a stimulant [32,77]. For the proper development of the nervous system, an optimal number of stimuli is essential, as demonstrated by, e.g., the transient ability of GCs to elevate dopaminergic transmission in the mesolimbic dopaminergic system, or the “pleasure” pathway, between the VTA and the NAc [24].
The Yerkes–Dodson law, originally developed by psychologists Robert Yerkes and John Dodson in 1908 [78], states the existence of “an empirical relationship between arousal and performance”. Therefore, the fundamental question of the concepts of stress remains to be discussed. When does the stress system shift from protective to harmful, potentially leading to disorders? It was observed, according to the Yerkes–Dodson law, that performance increases with physiological or mental arousal, but only up to a point, and when the arousal levels are too high, performance decreases [78]. From this perspective, the effects of mild and transient stress exposure were considered stimulating since high stress levels were usually perceived as adverse (see Figure 2).
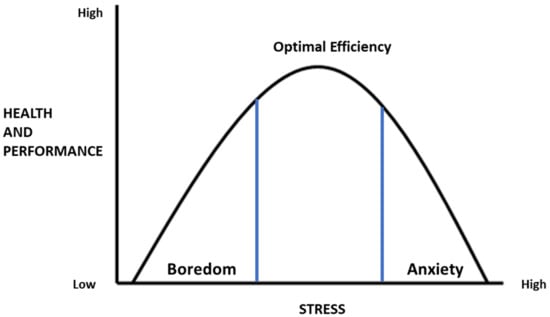
Figure 2.
The inverted-U pattern of the Yerkes–Dodson law shows that cognitive performance increases and then decreases with increasing levels of arousal.
The maintenance of heightened neural and neuroendocrine responses due to the inability to overcome stress [33], by not terminating stress responses when they are no longer needed, or because the body does not get used to the stressor [9] induce a loss of resistance and a phase of extinction of the processes aimed at maintaining homeostasis [10,60], and this has profound costs, as it is most likely compromises health.
Examples of the consequences of maladaptive stress are diverse, ranging from physical symptoms (such as coronary heart disease, hypertension, type 2 diabetes, arteriosclerosis, obesity, and pain) [21,22,23,25,26,27,28,79,80] to psychological symptoms, including psychological distress (such as insomnia, headaches, extreme fatigue, and sadness), burnout syndromes, PTSD, anxiety disorders, [51,53,81,82,83], and even an increased risk of substance abuse [30], cancer [83] and mortality [82]. All these changes are in part explained by the significant increase in proinflammatory cytokines [84,85], key mediators of inflammatory processes, and the corresponding diseases. In addition, by impairing general health, the prevalence of developing neurological or psychotic disorders, including schizophrenia [86,87], multiple sclerosis [88], Alzheimer’s disease [37] or dementia, also rises.
How stress affects health can vary depending on the different stages of life, depending on which pathways or brain regions are developing or declining [75]. Studies in animal models have demonstrated that exposure of mothers to stressful events during pregnancy can change the plasticity of the physiological systems and the functionality of their fetus. This can include the developing CNS, which is especially sensitive to external stimuli [86].
3. Impacts and Effects of Stress
3.1. Impact of Stress on the Brain
From the beginning of the stress research, it was understood that there exists a correlation between mental disorders and exposure to high levels of GCs, suggesting that these substances could reach the brain and impact behavior [89,90].
At the beginning of the 21th century, studies using animal models were conducted to explain the mechanisms underlying the pathophysiology of stress-related disorders by relating them to GC receptor occupancy levels. As an example, the Lupien group demonstrated in their laboratory that the two types of brain receptors (mineralocorticoid receptors (MRs) and GRs) differed in their affinity for GCs [65]. As the hippocampus (which is involved in memory and learning processes) contains large amounts of MRs and GRs, they found that the former were highly basally occupied and the latter (with low affinity for GCs) were barely occupied overall. With the transition from a basal to a strong stressor, as the GRs became highly occupied, they suggested that this could be the main mediator of the detrimental effects of exposure to highly stressful events, for example, on cognition [37,65,75]. Nonetheless, the effects of stress on the brain can take different forms, depending on stress intensity and duration, and on the region, timeframe, and pathways involved [91,92].
One way that stress hormones modulate brain function is by modifying the structure of neurons and reorganizing them [90,93,94,95,96,97,98], by “reprogramming” the brain, which can result in permanent changes. The hippocampus is a highly sensitive and adaptable structure in the brain that undergoes several modifications in response to chronic stress [94,98]. Previous studies with rodents have attributed the deficits in behavior to neuronal death in this cerebral structure; however, the number of hippocampal cells is preserved in animals exposed to corticosteroids for a long time [94]. However, in contrast to acute stress, chronic stress may induce changes in hippocampus neuroplasticity [21,24,26,54,94,98]. Neurochemical changes with relevance to the brain circuitry may occur in certain cases of chronic stress, increasing the hippocampus’s vulnerability to injury [98], or even suppress hippocampal neurogenesis [27] (see Table 1). Some alterations in hippocampal formation can be slowly reversed with the cessation of stress or exposure to GCs, leading to the formation of new neurons [31]. This reversal process is essential to protect the hippocampus against permanent damage and allows the recovery of fundamental competencies. Stress-induced changes in the hippocampus do not reflect the adaptive changes that occur simultaneously in other regions of the limbic system, where structural imbalances in plasticity are also observed, such as those occurring in the mPFC [41], the ACC [50], the NAc [51], the LC [49,97], and the amygdala [47].
Studies with animal models have suggested that PFC neurons are highly sensitive to stress [41]. The exposure to even brief periods of stress has been shown to be sufficient to cause significant structural remodeling in mPFC neurons [86,93], and chronic stress decreased dendritic complexity, glial and endothelial cell proliferation in rodents’ mPFC [44]. Since glia provide metabolic support to neurons, a reduction in their number may damage the morphology of mPFC cells [27], impacting their functionality.
In contrast to what happens in the above structures, in the amygdala and NAc, intense stressors can instead increase the synaptic plasticity, thus enhancing their function [28,47,59]. Prolonged stress not only increases the number and complexity of dendritic spines in these structures [48] but also their synaptic connectivity [54]. Both processes are suggested to be the result of overactivation of the neural circuits that control fear and emotion and are associated with anxiety behaviors. Such alterations do not reverse after the cessation of chronic stress and can be long-lasting [48], an effect quite distinct from the atrophy induced in the hippocampus and mPFC. Another determinant area, which is highly susceptible to damage caused by exposure to stressors, is the insular cortex, a crucial area of the brain responsible for interoception. This may shed light on the mechanisms that underlie the impact of psychological stress on brain health and, for instance, alcohol consumption [99].
3.2. Pathophysiology of Stress-Related Disorders
It has become increasingly evident that difficult experiences or a history of psychological stress, whether it was a one-time occurrence or a long-term issue, can have a significant impact on various neuroendocrine systems, such as the HPA and ANS axes [29,63,66,68,70,73,91], reward system [30,52], and immune system [85,100]. This can result in corticolimbic dysregulation, which may lead to physiological and behavioral changes in response to future challenges across different domains, including cognitive, neurological, and psychiatric domains.
Psychiatric illness correlates more strongly with a history of stress than with any physical or medical ailment [16]. Structural and functional abnormalities of the brain structures have been observed in patients with various psychiatric and neurological disorders, such as depression (e.g., with abnormalities in the hippocampus [21]), bipolar disorder (e.g., with abnormalities in the insula [31]), PTSD (e.g., with abnormalities in the ACC [50] and amygdala [53]), schizophrenia (e.g., with abnormalities in the PFC [87]), and anxiety (with abnormalities in the NAc [51]), most of which also converge with the consequences of stress. The same pathophysiological mechanisms that cause such psychiatric disorders and psychosis can be risk factors for multiple other illnesses, not directly related to brain or neuroendocrine system disruption but to the consequence of it, increasing the risk of psychiatric relapse. As depicted in the table below, several animal studies were conducted to explore the etiology of human psychopathology (Table 1). Also, non-invasive studies were conducted in humans, despite the inherent difficulties of such research, to provide meaningful brain health variations and to validate or measure neural stress processing.

Table 1.
Summary of different psychogenic stress protocols conducted in studies with animal models, key findings, and psychopathology or related condition. Abbreviations: > increased; < decreased/reduced.
Table 1.
Summary of different psychogenic stress protocols conducted in studies with animal models, key findings, and psychopathology or related condition. Abbreviations: > increased; < decreased/reduced.
| Stress Protocols | Key Findings | Brain Areas Affected | Domains | Human Condition | References |
|---|---|---|---|---|---|
| Acute stress (single stressful episode) | Long-lasting changes in synaptic transmission and neural activation | Widespread in limbic structures LC | Psychiatric | Hypervigilance, impaired cognition, PTSD mood and anxiety disorders | [86,93,97] For a review, see [75]. |
| Chronic variable stress Chronic vs. acute stress | Dysregulation HPA axis upregulation CRH mRNA expression. <cell proliferation | Hypothalamus (PVN) Hippocampus | Neurologic Psychiatric Cognitive | Increased vulnerability to later insults and neuropathology | [68,94]. |
| Chronic stress Early-life stress | Dysregulation of HPA axis <mRNA expression in GRs Seizure precipitant factor | Hippocampus widespread | Neurologic | Epilepsy | [69] For a review, see [74]. |
| Chronic stress Psychosocial stress | Dysregulation reward axis Changes in DAergic neuronal activity Plasticity changes in limbic areas | mPFC VTA NAc Insula | Neurologic | Metabolic disorder Substance abuse Alcohol use disorder | For a review, see [30,52,55]. |
| Chronic stress Uncontrollable vs. controllable stress | GABAergic disinhibition Plasticity changes in limbic areas Impaired fear extinction | Amygdala Thalamus NAc Dorsal raphe nucleus mPFC | Psychiatric | PTSD/phobias Anxiety and panic Facilitated fear Generalization of memories | [39,47,57,63] For a review, see [38]. |
| Chronic psychosocial stress (agonistic encounters) | <Cytokine mRNA levels <Cytokine Receptors <GR Receptors | Hippocampus NAc Pituitary | Psychiatric | Mood alterations | [101] |
| Chronic psychosocial stress (isolation/crowding) + acute stress | Compromised HPA axis >catecholamines plasmatic levels <GR plasmatic levels <parvalbumin expression >vulnerability to neuronal injury | Hippocampus | Psychiatric | Major depression | [98,102] |
| Early-life stress Maternal deprivation | Dysregulation HPA axis Brain development Resistance to interferon-β and neurodegeneration Neurogenesis | mPFC Hippocampus Amygdala | Cognitive | Alzheimer’s, dementia Impaired learning Impaired retrieval of memories | For a review, see [37,88]. |
| Early-life stress + adult stress Chronic unpredictable stress | Dysregulation GABAergic system Volume cell loss of limbic structures <brain and body weight gain Learned helplessness | Hippocampus | Psychiatric | Major depression Memory disorders | [44,45,78]. |
| Early-life stress + youth stress Early-life stress | <Dopamine receptor Signaling desensitization Altered DNA methylation | NAc Caudate nucleus mPFC | Psychiatric | Schizophrenia | [86,92] |
Recent research has shown that psychosocial stressors, such as early trauma and abandonment, or chronic daily stressors, like occupational stress, conflicts with in-laws, and financial problems, can trigger neurological and mental illnesses in vulnerable individuals [26]. These illnesses may include burnout, attentional or verbal memory impairments [83], schizophrenia [87], bipolar disorder [29], or alcohol craving [99].
Likewise, recent research has provided insight into the interaction between the nervous and immune systems [61,84,85,103], which must be carefully balanced to maintain overall stability. Immune alterations have been implicated in brain plasticity and mood changes, and chronic social stress has been shown to modulate the cytokines network [101]. Inflammation related to stress has been associated with various conditions, such as insomnia, late-life depression, anxiety, cognitive decline, and Alzheimer’s disease [100]. Chronic stress and heightened inflammatory biomarkers have also been linked to a reduced response to antidepressant medications [103]. Peripheral inflammation activates several brain regions, and stress can lead to immunological responses and increased brain cytokine levels [60,61]. These factors are, in turn, commonly associated with certain risk factors for impaired associative, motivational, and emotional abilities, memory impairment, and cognitive loss [38,59], including Alzheimer’s [37], insomnia, sleep disorders [73], depression [103], and schizophrenia [104]. There is growing evidence showing that even mild psychosocial stressors, such as fear of rejection, or social isolation can cause inflammatory activity and neuroinflammation [85,103]. The neural circuits that are activated in response to these interactions are the same ones that have evolved for rewarding behaviors essential to survival.
Several studies show the evident relationship between the immune system, emotional states as well as personality. GCs, GRs’ function and central/peripheral cytokines are reciprocally affected by convergent feedback mechanisms [105]. Cytokines can modulate the capacity of glucocorticoids to transmit signals to target tissues and induce GR resistance [105]. It is clear that there exists a relationship between stress and the body’s inflammatory responses and their notable overlaps at multiple levels, including the cellular and physiological pathways, by which the immune system can influence the HPA axis; nevertheless, the causal roles of these factors in the pathophysiology are not yet fully understood.
4. Vulnerability and Resilience to Stress
It is still unclear why some people are more susceptible to developing diseases in response to stress, while others seem to be more resilient and recover more quickly.
Resilience is an internal process that allows an organism to maintain its functional stability and manage threats, adversity, and trauma [106]. A big set of internal hormones, neuropeptides, and transmitters work together to promote resilience [106,107,108]. These differences between individuals define their personality traits and can make someone more susceptible to developing psychological disorders in response to stress. What is considered stressful is partly determined by one’s perception of stress [100]. The characterization of personality and stress perception, which were not included in early clinical diagnostic formulations that relied only on biomarker measurements, are now of great importance.
4.1. Genetic and Environmental Factors
The unique combinations of DNA sequences in an individual’s genes determine their susceptibility to environmental events at different stages of life. A variety of factors or conditions are suggested as “resilience factors”. Most genetic factors that play a significant role in adaptive responses to stress and resilience are involved with the central nervous system. Among these systems and genes are the neuropeptide Y, HPA axis and CRHR1 gene, the noradrenergic, dopaminergic, and serotonergic systems, and BDNF, CRH receptor 1, oxytocin receptor, and regulator of G-protein signaling 2, each with its own polymorphisms (reviewed in [106]).
However, while the initial draft of the brain and neural system’s development is specified by the genome, all the details are subtly worked out with one’s experience and environmental interaction [28]. Such genome–environment interactions are mediated by epigenetic processes, including DNA methylation or histone modifications [86]. These processes determine the body’s sensitivity and responsiveness to the environment, which can predispose individuals to various disorders [24,103]. This plasticity is highly influenced by significant early-life experiences, which shape the nervous system [22,31,107], leave their marks throughout life and can even be passed down to the next generation. As an example, any conditions upregulating the adult hippocampal neurogenesis rate have been independently described as “resilience factors” [108].
As discussed earlier, the activation of neural circuits that are genetically programmed produces adaptive responses to stress, but such circuits can be modified by experience and the environment due to their sensitivity. If adverse experiences occur early in development, they can potentially affect the maturation or organization of different components of the nervous system [26,80,86]. Their effects are likely to be lasting and profound [21,29,63,68,109,110,111,112], and they can have a great impact on the psychological well-being of individuals later in life, particularly when they encounter difficult situations in adulthood [26,87,109,110].
Studies conducted on rodents have indicated that early exposure to aversive stimuli may disrupt the proper development of newborns and their corticolimbic structures, leading to lifelong susceptibility to stress that can affect behavior, cognition, and reward feelings [26,63,111]. Similarly, research shows that children born to pregnant women who experienced stressful events, such as family conflicts, armed conflicts, or the death of their husbands, may be at a higher risk of developing behavioral abnormalities and psychiatric conditions [30,110].
Gillespie and colleagues [95] compiled data that suggested heritability would account for 30–40% of the variance, contributing to the risk of mood and anxiety disorders in humans. Research has shown that childhood exposure to abuse and other early-life adverse events, like abandonment or lack of maternal and social care, can significantly increase the risk of depression, schizophrenia, alcohol abuse, or PTSD in genetically vulnerable individuals [99,106,112].
Cumulative factors may act as genetic moderators and potentiate the effects of adversities [110]. Regarding, as an example, the early hypothesis about the etiology of schizophrenia, it was recognized that a set of adversities should occur during early development, such as malnutrition, viral infections, or genetic deficits, for the disorder to occur [87]. This hypothesis, later revised, concluded that early-life insults would not be enough for the disease to manifest, but a set of developmental insults would be required [92]. From this perspective, it is interesting to note that, in addition to having an immediate detrimental effect, early experiences seem to be critical for subsequent vulnerability to develop psychopathologies later in life [111,112]. It is now recognized that the phenotype of psychopathology in adulthood is determined by the convergence between genetics, including an individual’s sex [61,113], and all the circumstances that occurred during childhood, puberty, and youth [96,106,107,108,109,110,111,112,113,114]. Therefore, and as a conclusion, similar events can lead to different outcomes, where the environment is not necessarily the cause of the disease, but the complexity of factors (gene-mediated mechanisms and prior coping experiences) affecting how the individual responds to each experience and manage stress is what determines the outcome.
4.2. Factors Contributing to Resilience to Stress
The consideration of a possibility to “switch from vulnerability to coping with stress” was highly important in psychiatry, as it allowed clinicians to design tailored interventions to inhibit the potential consequences of the response to stressful events for their patients [107,115].
Various neurochemicals, hormones, and antidepressants can help improve resilience by acting as behavioral modulators [116,117]. They can do this by enhancing neuronal survival or neurogenesis in brain structures or by stabilizing any altered cholinergic and monoaminergic function [9,116,117,118,119]. According to a recent review by De Kloet and Joëls [107], manipulating MR-GR activation in the brain and/or peripheral tissues can help in coping with stress and improve resilience. However, such mediated actions may be supported by lifestyle factors, such as mindfulness and exercise, psychotherapy, or additional pharmacotherapy. Meaney and his colleagues [22] demonstrated 30 years ago in rodents that handling pups increases GR binding in the hippocampus and enhances negative-feedback control over HPA function.
Reflecting on McEwen’s words, “the brain is where stress begins and the one determining what is stressful” [8], suggests that any strategy aimed at increasing resilience should focus on both reducing stress and changing the way stressors are perceived. Previous investigations have shown that nonpharmacological interventions that impact lifestyle behaviors, such as exercise, self-care, dietary modification, and psychological interventions, including social support [17,120], can mitigate the adverse effects of stress [119,120] and improve well-being. Similarly, in exploring the mechanisms that link perceived stress, coping adequacy, and lifestyle behaviors, some studies suggest that perceived stress and health are connected through coping capability. When Lazarus and Folkman, in 1984, created the model of stress and coping to explain how behavioral choices are based on the appraisals (evaluations) that people make, they highlighted that events and situations are not intrinsically good or bad [9].
Resilience to a stressful event would depend on each one’s perception, tolerance, and success in coping with it. In Baratta and colleague’s work, “Understanding stress resilience”, they mentioned that it “is the presence, not the absence of control, the active ingredient” [115]. Not all individuals who experience a traumatic event develop PTSD, and the importance of perceived behavioral control in determining resilience has been often noted [41]. Coping itself would depend on each one’s capacity to find the strategies that are effective in dealing with it and to abandon those that became ineffective [119]. On the one hand, the literature shows that coping adequacy is an important factor influencing healthy lifestyle behaviors [100,120]. Some studies indicated the existence of a positive relationship between poor lifestyle behaviors and a wide spectrum of diseases or substance use [118]. On the other hand, healthy behaviors reduced the effects of stress [119], suggesting that this association between stress effects and lifestyle behaviors is bidirectional, demonstrating that the successful adoption of positive lifestyle behaviors improves resilience.
It is now clear that healthy lifestyles, positive experiences, including having a sense of purpose in life, and social support are highly effective in combating social, psychological, and general stress, especially during difficult times [17,25]. They can also help mitigate the vulnerabilities induced by stressors.
5. Conclusions and Prospects
Stress can be understood as a threat to homeostasis produced by potentially harmful stimuli that, when detected by the individual, activate specific compensatory responses in the body.
In the last 30 years, our understanding of the neurobiological alterations underlying stress-related disorders in humans has increased tremendously, thanks to the huge number of research studies conducted, including systematic molecular and cellular studies, and the relevant animal models created. It is now known that the pathophysiology of several mental and affective disorders could have its origin in the corticolimbic system dysregulation, a model that is highly sensitive to stress. However, the heterogeneous symptomatology within the wide and overlapping diagnoses of stress-related disorders—ranging from immunological, mental, or metabolic changes—makes finding a “representative sample” of features quite challenging. In humans, the genetic risk factors shared between different psychopathologies and the co-morbidity of such different diseases are evident. In contrast, less is known about the impact of perceived stress and coping adequacy. Apparently, perception of stress plays a crucial role and resilience ultimately relies on the meaning each individual assigns to it. Besides the individual’s personality, coping to stress is highly defined by a healthy lifestyle and social support. Therefore, research should ultimately shift from defining the consequences of stress on the body to finding vulnerability factors.
The possibility of the early identification of risk factors and the possibility of inhibiting the effects of stress are crucial for the design of effective psychological treatment strategies and the avoidance of long-term pathological consequences.
Author Contributions
Conceptualization, validation and visualization, I.P.-F. and E.H.L.U.; methodology, formal analysis, investigation and writing, I.P.-F. and E.H.L.U.; original draft preparation, I.P.-F.; writing, review and editing, I.P.-F. and E.H.L.U.; supervision, E.H.L.U.; project administration and funding acquisition, I.P.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created.
Acknowledgments
We are very grateful to Ilda Gomes Rosa and to Gileno Régis da Silva for their support in editing the images and reviewing the draft manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Adrenocorticotropic hormone | ACTH |
| Anterior cingulate cortex | ACC |
| Autonomic nervous system | ANS |
| Central nervous system | CNS |
| Corticotropin-releasing hormone | CRH |
| Glucocorticoids | GCs |
| Glucocorticoid receptors | GRs |
| Hypothalamic–pituitary–adrenocortical axis | HPA axis |
| Locus coeruleus | LC |
| Medial PFC | mPFC |
| Mineralocorticoid receptors | MRs |
| Nucleus accumbens | NAc |
| Paraventricular nucleus of the hypothalamus | PVN |
| Periaqueductal gray matter | PGM |
| Post-traumatic stress disorder | PTSD |
| Prefrontal cortex | PFC |
| Sympathetic nervous system | SNS |
| Ventral tegmental area | VTA |
References
- Esch, T.; Stefano, G.B. The neurobiology of stress management. Neuro Endocrinol. Lett. 2010, 31, 19–39. [Google Scholar] [PubMed]
- Karatsoreos, I.N. Stress: Common themes toward the next frontier. Front. Neuroendocr. 2018, 49, 3–7. [Google Scholar] [CrossRef] [PubMed]
- González, B.G.; Escobar, A. Neuroanatomía del estrés. Rev. Mex. Neuroci. 2002, 3, 273–282. [Google Scholar]
- Landis, C. Walter B. Cannon. Bodily Changes in Pain, Hunger, Fear and Rage. (2nd ed., revised and enlarged.) New York: Appleton, 1929. Pp. xvi+404. Pedagog. Semin. J. Genet. Psychol. 1930, 38, 527–531. [Google Scholar] [CrossRef]
- Cannon, W.B. Stresses and Strains of Homeostasis. Am. J. Med. Sci. 1935, 189, 13–14. [Google Scholar] [CrossRef]
- Selye, H. The Stress of Life; McGraw-Hill: New York, NY, USA, 1956. [Google Scholar]
- Pacak, K.; Palkovits, M.; Yadid, G.; Kvetnansky, R.; Kopin, I.J.; Goldstein, D.S. Heterogeneous neurochemical responses to different stressors: A test of Selye’s doctrine of nonspecificity. Am. J. Physiol. 1998, 275, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Stellar, E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, R.S.; Folkman, S. Stress, Appraisal and Coping; Springer: New York, NY, USA, 1984. [Google Scholar]
- McEwen, B.S. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008, 583, 174–185. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Kopin, I.J. Evolution of concepts of stress. Stress 2007, 10, 109–120. [Google Scholar] [CrossRef]
- Guidi, J.; Lucente, M.; Sonino, N.; Fava, G.A. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother. Psychosom. 2021, 90, 11–27. [Google Scholar] [CrossRef]
- World Health Organization. The World Health Report: 2001: Mental Health: New Understanding, New Hope; World Health Organization: Geneva, Switzerland, 2001; Available online: https://iris.who.int/handle/10665/42390 (accessed on 16 June 2024).
- Mustafa, M. Sources of Stress and Coping Strategies Among College Students in Ladakh. Int. J. Indian Psychol. 2024, 12, 1339–1349. [Google Scholar]
- Díez, M.; Jiménez-Iglesias, A.; Paniagua, C.; García-Moya, I. The Role of Perfectionism and Parental Expectations in the School Stress and Health Complaints of Secondary School Students. Youth Soc. 2023, 56, 885–906. [Google Scholar] [CrossRef]
- Salleh, M.R. Life event, stress and illness. Malays. J. Med. Sci. 2008, 15, 9–18. [Google Scholar] [PubMed]
- Kurtuluş, E.; Yıldırım Kurtuluş, H.; Birel, S.; Batmaz, H. The effect of social support on work-life balance: The role of psychological well-being. Int. J. Contemp. Educ. Res. 2023, 10, 239–249. [Google Scholar] [CrossRef]
- Limone, P.; Toto, G.A.; Messina, G. Impact of the COVID-19 pandemic and the Russia-Ukraine war on stress and anxiety in students: A systematic review. Front. Psychiatry 2022, 13, 1081013. [Google Scholar] [CrossRef] [PubMed]
- Pais-Ribeiro, J.; Ferreira-Valente, A.; Jarego, M.; Sánchez-Rodríguez, E.; Miró, J. COVID-19 Pandemic in Portugal: Psychosocial and Health-Related Factors Associated with Psychological Discomfort. Int. Environ. Res. Public. Health 2022, 19, 3494. [Google Scholar] [CrossRef] [PubMed]
- Vermetten, E.; Bremner, J.D. Circuits and systems in stress. I. Preclinical studies. Depress. Anxiety 2002, 15, 126–147. [Google Scholar] [CrossRef] [PubMed]
- Swaab, D.F.; Bao, A.M.; Lucassen, P.J. The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 2005, 4, 141–194. [Google Scholar] [CrossRef] [PubMed]
- Meaney, M.J.; Mitchell, J.B.; Aitken, D.H.; Bhatnagar, S.; Bodnoff, S.R.; Iny, L.J.; Sarrieau, A. The effects of neonatal handling on the development of the adrenocortical response to stress: Implications for neuropathology and cognitive deficits in later life. Psychoneuroendocrinology 1991, 16, 85–103. [Google Scholar] [CrossRef]
- Von Känel, R.; Kudielka, B.M.; Haeberli, A.; Stutz, M.; Fischer, J.E.; Patterson, S.M. Prothrombotic changes with acute psychological stress: Combined effect of hemoconcentration and genuine coagulation activation. Thromb. Res. 2009, 123, 622–630. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Davis, M.T.; Holmes, S.E.; Pietrzak, R.H.; Esterlis, I. Neurobiology of Chronic Stress-Related Psychiatric Disorders: Evidence from Molecular Imaging Studies. Chronic Stress 2017, 1, 2470547017710916. [Google Scholar] [CrossRef]
- Fumagalli, F.; Molteni, R.; Racagni, G.; Riva, M.A. Stress during development: Impact on neuroplasticity and relevance to psychopathology. Prog. Neurobiol. 2007, 81, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C. Brain-Derived Neurotrophic Factor, Depression, and Physical Activity: Making the Neuroplastic Connection. Neural Plast. 2017, 2017, 7260130. [Google Scholar] [CrossRef] [PubMed]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; Umeoka, E.H.d.L. A comprehensive overview on stress neurobiology: Basic concepts and clinical implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef]
- Umeoka, E.H.L.; van Leeuwen, J.M.C.; Vinkers, C.H.; Joëls, M. The Role of Stress in Bipolar Disorder. Curr. Top. Behav. Neurosci. 2021, 48, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Al’absi, M.; Ginty, A.T.; Lovallo, W.R. Neurobiological mechanisms of early life adversity, blunted stress reactivity and risk for addiction. Neuropharmacology 2021, 188, 108519. [Google Scholar] [CrossRef]
- Namkung, H.; Kim, S.H.; Sawa, A. The Insula: An Underestimated Brain Area in Clinical Neuroscience, Psychiatry, and Neurology. Trends Neurosci. 2017, 40, 200–207. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Stress and plasticity in the limbic system. Neurochem. Res. 2003, 28, 1735–1742. [Google Scholar] [CrossRef]
- Leonard, B.E. HPA and immune axes in stress: Involvement of the serotonergic system. Neuroimmunomodulation 2006, 13, 268–276. [Google Scholar] [CrossRef]
- Sotres-Bayon, F.; Cain, C.K.; LeDoux, J.E. Brain mechanisms of fear extinction: Historical perspectives on the contribution of prefrontal cortex. Biol. Psychiatry 2006, 60, 329–336. [Google Scholar] [CrossRef]
- Averill, L.A.; Averill, C.L.; Kelmendi, B.; Abdallah, C.G.; Southwick, S.M. Stress Response Modulation Underlying the Psychobiology of Resilience. Curr. Psychiatry Rep. 2018, 20, 27. [Google Scholar] [CrossRef]
- Jaferi, A.; Bhatnagar, S. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology 2006, 147, 4917–4930. [Google Scholar] [CrossRef]
- Caruso, A.; Nicoletti, F.; Mango, D.; Saidi, A.; Orlando, R.; Scaccianoce, S. Stress as risk factor for Alzheimer’s disease. Pharmacol. Res. 2018, 132, 130–134. [Google Scholar] [CrossRef]
- Merz, C.J.; Wolf, O.T. How stress hormones shape memories of fear and anxiety in humans. Neurosci. Biobehav. Rev. 2022, 142, 1049012022. [Google Scholar]
- Yamashita, P.S.; Spiacci , A.; Hassel, J.E., Jr.; Lowry, C.A.; Zangrossi, H., Jr. Disinhibition of the rat prelimbic cortex promotes serotonergic activation of the dorsal raphe nucleus and panicolytic-like behavioral effects. J. Psychopharmacol. 2017, 31, 704–714. [Google Scholar] [CrossRef]
- Robbins, T.W. Chemistry of the mind: Neurochemical modulation of prefrontal cortical function. J. Comp. Neurol. 2005, 493, 140–146. [Google Scholar] [CrossRef]
- Baratta, M.V.; Christianson, J.P.; Gomez, D.M.; Zarza, C.; Amat, J.; Masini, C.; Watkins, L.; Maier, S. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience 2007, 146, 1495–1503. [Google Scholar] [CrossRef]
- Maier, S.F.; Watkins, L.R. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010, 1355, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, S.M.; Wang, Y.; Msghina, M. Behavioral, cortical and autonomic effects of single-dose escitalopram on the induction and regulation of fear and disgust: Comparison with single-session psychological emotion regulation with reappraisal. Front. Psychiatry 2023, 13, 988893. [Google Scholar] [CrossRef] [PubMed]
- Czeh, B.; Simon, M.; van der Hart, M.G.; Schmelting, B.; Hesselink, M.B.; Fuchs, E. Chronic stress decreases the number of parvalbumin-immunoreactive interneurons in the hippocampus: Prevention by treatment with a substance P receptor (NK1) antagonist. Neuropsychopharmacology 2005, 30, 67–79. [Google Scholar] [CrossRef]
- Shin, H.S.; Lee, S.H.; Moon, H.J.; So, Y.H.; Jang, H.; Lee, K.-H.; Ahn, C.; Jung, E.-M. Prolonged stress response induced by chronic stress and corticosterone exposure causes adult neurogenesis inhibition and astrocyte loss in mouse hippocampus. Brain Res. Bull. 2024, 208, 110903. [Google Scholar] [CrossRef]
- Sanders, M.J.; Wiltgen, B.J.; Fanselow, M.S. The place of the hippocampus in fear conditioning. Eur. J. Pharmacol. 2003, 463, 217–223. [Google Scholar] [CrossRef]
- Vyas, A.; Jadhav, S.; Chattarji, S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience 2006, 143, 387–393. [Google Scholar] [CrossRef]
- Rosen, J.B.; Donley, M.P. Animal studies of amygdala function in fear and uncertainty: Relevance to human research. Biol. Psychol. 2006, 73, 49–60. [Google Scholar] [CrossRef]
- Ross, J.A.; Van Bockstaele, E.J. The Locus Coeruleus- Norepinephrine System in Stress and Arousal: Unraveling Historical, Current, and Future Perspectives. Front. Psychiatry 2021, 11, 601519. [Google Scholar] [CrossRef]
- Kitayama, N.; Quinn, S.; Bremner, J.D. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J. Affect. Disord. 2006, 90, 171–174. [Google Scholar] [CrossRef]
- Strasser, A.; Xin, L.; Gruetter, R.; Sandi, C. Nucleus accumbens neurochemistry in human anxiety: A 7 T1 H-MRS study. Eur. Neuropsychopharmacol. 2019, 29, 365–375. [Google Scholar] [CrossRef]
- Ironside, M.; Kumar, P.; Kang, M.S.; Pizzagalli, D.A. Brain mechanisms mediating effects of stress on reward sensitivity. Curr. Opin. Behav. Sci. 2018, 22, 106–113. [Google Scholar] [CrossRef]
- Ravindran, L.N.; Stein, M.B. Pharmacotherapy of PTSD: Premises, principles, and priorities. Brain Res. 2009, 1293, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Morales-Medina, J.C.; Sanchez, F.; Flores, G.; Dumont, Y.; Quirion, R. Morphological reorganization after repeated corticosterone administration in the hippocampus, nucleus accumbens and amygdala in the rat. J. Chem. Neuroanat. 2009, 38, 266–272. [Google Scholar] [CrossRef]
- Baik, J.H. Stress and the dopaminergic reward system. Exp. Mol. Med. 2020, 52, 1879–1890. [Google Scholar] [CrossRef]
- Graeff, F.G. Serotonin, the periaqueductal gray and panic. Neurosci. Biobehav. Rev. 2004, 28, 239–259. [Google Scholar] [CrossRef]
- Ronan, P.; Korzan, W.; Johnson, P.; Lowry, C.; Renner, K.J.; Summers, C.H. Prior stress and vasopressin promote corticotropin-releasing factor inhibition of serotonin release in the central nucleus of the amygdala. Front. Behav. Neurosci. 2023, 17, 1148292. [Google Scholar] [CrossRef]
- Goncharova, N.D.; Vaudry, H.; Carr, J.A. Stress responsiveness of the hypothalamic-pituitary-adrenal axis: Age-related features of the vasopressinergic regulation. Front. Endocrinol. 2013, 4, 37513. [Google Scholar] [CrossRef]
- Yoshii, T. The Role of the Thalamus in Post-Traumatic Stress Disorder. Int. J. Mol. Sci. 2021, 22, 1730. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef]
- Kropp, D.; Hodes, G. Sex Differences in Depression: An Immunological Perspective. Brain Res. Bull. 2023, 196, 34–45. [Google Scholar] [CrossRef]
- Bierhaus, A.; Humpert, P.M.; Nawroth, P.P. Linking stress to inflammation. Anesth. Clin. 2006, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Figueiredo, I.; Sancho, C.; Carro, J.; Castellano, O.; López, D.E. The effects of sertraline administration from adolescence to adulthood on physiological and emotional development in prenatally stressed rats of both sexes. Front. Behav. Neurosci. 2014, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Oka, T. Psychogenic fever: How psychological stress affects body temperature in the clinical population. Temp. Multidiscip. Biomed. J. 2015, 2, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; Maheu, F.; Tu, M.; Fiocco, A.; Schramek, T.E. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007, 65, 209–237. [Google Scholar] [CrossRef]
- Schneiderman, N.; Ironson, G.; Siegel, S.D. Stress and health: Psychological, behavioral, and biological determinants. Annu. Rev. Clin. Psychol. 2005, 1, 607–628. [Google Scholar] [CrossRef]
- Gorman, J.M.; Hirschfeld, R.M.; Ninan, P.T. New developments in the neurobiological basis of anxiety disorders. Psychopharmacol. Bull. 2002, 36 (Suppl. S2), 49–67. [Google Scholar]
- Ostrander, M.M.; Ulrich-Lai, Y.M.; Choi, D.C.; Richtand, N.M.; Herman, J.P. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology 2006, 147, 2008–2017. [Google Scholar] [CrossRef]
- Umeoka, E.H.L.; Robinson, E.J.; Turimella, S.L.; van Campen, J.S.; Motta-Teixeira, L.C.; Sarabdjitsingh, R.A.; Garcia-Cairasco, N.; Braun, K.; Graan, P.N.; Joëls, M. Hyperthermia-induced seizures followed by repetitive stress are associated with age-dependent changes in specific aspects of the mouse stress system. J. Neuroendocrinol. 2019, 31, 12697. [Google Scholar] [CrossRef]
- Meir Drexler, S.; Merz, C.J.; Jentsch, V.L.; Wolf, O.T. Stress modulation of fear and extinction in psychopathology and treatment. Neuroforum 2020, 26, 133–141. [Google Scholar] [CrossRef]
- Grissom, N.; Bhatnagar, S. Habituation to repeated stress: Get used to it. Neurobiol. Learn. Mem. 2009, 92, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Simpkiss, J.L.; Devine, D.P. Responses of the HPA axis after chronic variable stress: Effects of novel and familiar stressors. Neuro Endocrinol. Lett. 2003, 24, 97–103. [Google Scholar] [PubMed]
- Han, K.S.; Kim, L.; Shim, I. Stress and sleep disorder. Exp. Neurobiol. 2012, 21, 141–150. [Google Scholar] [CrossRef]
- van Campen, J.S.; Jansen, F.E.; de Graan, P.N.E.; Braun , K.P.J.; Joels, M. Early life stress in epilepsy: A seizure precipitant and risk factor for epileptogenesis. Epilepsy Behav. 2014, 38, 160–171. [Google Scholar] [CrossRef]
- Lupien, S.J.; Juster, R.-P.; Raymond, C.; Marin, M.-F. The effects of chronic stress on the human brain: From neurotoxicity, to vulnerability, to opportunity. Front. Neuroendocrin. 2018, 49, 91–105. [Google Scholar] [CrossRef]
- Alloy, L.B.; Seligman, M.E.P. On the Cognitive Component of Learned Helplessness and Depression. Psychol. Learn. Motiv. 1979, 13, 219–276. [Google Scholar] [CrossRef]
- Fowden, A.L.; Li, J.; Forhead, A.J. Glucocorticoids and the preparation for life after birth: Are there long-term consequences of the life insurance? Proc. Nutr. Soc. 1998, 57, 113–122. [Google Scholar] [CrossRef]
- Yerkes, R.M.; Dodson, J.D. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. 1908, 18, 459–482. [Google Scholar] [CrossRef]
- Stuart, M.J.; Baune, B.T. Depression and type 2 diabetes: Inflammatory mechanisms of a psychoneuroendocrine co-morbidity. Neurosci. Biobehav. Rev. 2012, 36, 658–676. [Google Scholar] [CrossRef]
- Harris, A.; Seckl, J. Glucocorticoids, prenatal stress and the programming of disease. Horm. Behav. 2011, 59, 279–289. [Google Scholar] [CrossRef]
- Folkman, S. Stress: Appraisal and Coping. In Encyclopedia of Behavioral Medicine; Gellman, M.D., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Castagné, R.; Garès, V.; Karimi, M.; ChadeauHyam, M.; Vineis, P.; Delpierre, C.; Kelly-Irving, M. Lifepath Consortium. Allostatic load and subsequent all-cause mortality: Which biological markers drive the relationship? Findings from a UK birth cohort. Eur. J. Epidemiol. 2018, 33, 441–458. [Google Scholar] [CrossRef]
- Mikołajewski, D.; Masiak, J.; Mikołajewska, E. Neurophysiological Determinants of Occupational Stress and Burnout. J. Educ. Health Sport. 2023, 21, 33–46. [Google Scholar] [CrossRef]
- Lekander, M.; Elofsson, S.; Neve, I.M.; Hansson, L.O.; Undén, A.L. Self-rated health is related to levels of circulating cytokines. Psychosom. Med. 2004, 66, 559–563. [Google Scholar] [CrossRef]
- Slavich, G.M.; Way, B.M.; Eisenberger, N.I.; Taylor, S.E. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc. Natl. Acad. Sci. USA 2010, 107, 14817–14822. [Google Scholar] [CrossRef]
- Bahari-Javan, S.; Varbanov, H.; Halder, R.; Benito, E.; Kaurani, L.; Burkhardt, S.; Anderson-Schmidt, H.; Anghelescu, I.; Budde, M.; Stilling, R.M.; et al. HDAC1 links early life stress to schizophrenia-like phenotypes. Proc. Natl. Acad. Sci. USA 2017, 114, E4686–E4694. [Google Scholar] [CrossRef]
- Popovic, D.; Schmitt, A.; Kaurani, L.; Senner, F.; Papiol, S.; Malchow, B.; Fischer, A.; Schulze, T.G.; Koutsouleris, N.; Falkai, P. Childhood Trauma in Schizophrenia: Current Findings and Research Perspectives. Front. Neurosci. 2019, 13, 274. [Google Scholar] [CrossRef]
- Schulz, M.A.; Hetzer, S.; Eitel, F.; Asseyer, S.; Meyer-Arndt, L.; Schmitz-Hübsch, T.; Bellmann-Strobl, J.; Cole, J.H.; Gold, S.M.; Paul, F.; et al. Similar neural pathways link psychological stress and brain-age in health and multiple sclerosis. iScience 2023, 26, 107679. [Google Scholar] [CrossRef]
- ter Horst, J.P.; van der Mark, M.H.; Arp, M.; Berger, S.; de Kloet, E.R.; Oitzl, M. Stress or no stress: Mineralocorticoid receptors in the forebrain regulate behavioral adaptation. Neurobiol. Learn. Mem. 2012, 98, 33–40. [Google Scholar] [CrossRef]
- Nederhof, E.; Schmidt, M.V. Mismatch or cumulative stress: Toward an integrated hypothesis of programming effects. Physiol. Behav. 2012, 106, 691–700. [Google Scholar] [CrossRef]
- Romeo, R.D.; Tang, A.C.; Sullivan, R.M. Early-Life Experiences: Enduring Behavioral, Neurological, and Endocrinological Consequences. Horm. Brain Behav. 2009, 62, 1975–2006. [Google Scholar]
- Choy, K.H.; de Visser, Y.P.; van den Buusem, M. The effect of ‘two hit’ neonatal and young-adult stress on dopaminergic modulation of prepulse inhibition and dopamine receptor density. Br. J. Pharmacol. 2009, 156, 388–396. [Google Scholar] [CrossRef]
- Sood, A.; Chaudhari, K.; Vaidya, V.A. Acute stress evokes sexually dimorphic, stressor-specific patterns of neural activation across multiple limbic brain regions in adult rats. Stress 2018, 21, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Dagyte, G.; Van der Zee, E.A.; Postema, F.; Luiten, P.G.M.; Boer, J.D.; Trentani, A.; Meerlo, P. Chronic but not acute foot-shock stress leads to temporary suppression of cell proliferation in rat hippocampus. Neuroscience 2009, 162, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, C.F.; Phifer, J.; Bradley, B.; Ressler, K.J. Risk and resilience: Genetic and environmental influences on development of the stress response. Depress. Anxiety 2009, 26, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Godoy, D.L.; Umeoka, E.H.L.; Ribeiro, E.; Santos, V.R.; Antunes-Rodrigues, J.; Joca, S.R.L.; Garcia-Cairasco, N. Multimodal early-life stress induces biological changes associated to psychopathologies. Horm. Behav. 2018, 100, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Alleva, E.; Francia, N. Psychiatric vulnerability: Suggestions from animal models and role of neurotrophins. Neurosci. Biobehav. Rev. 2009, 33, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Filipović, D.; Zlatković, J.; Gass, P.; Inta, D. The differential effects of acute vs. chronic stress and their combination on hippocampal parvalbumin and inducible heat shock protein 70 expression. Neuroscience 2013, 236, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zaiser, J.; Zimmermann, S.; Gessner, T.; Hoffmann, S.; Gerhardt, S.; Berhe, O.; Bekier, N.K.; Abel, M.; Radler, P.; Langejürgen, J.; et al. Stress-Induced Sensitization of Insula Activation Predicts Alcohol Craving and Alcohol Use in Alcohol Use Disorder. Biol. Psychiatry 2024, 95, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Lavretsky, H.; Newhouse, P.A. Stress, inflammation, and aging. Am. J. Geriatr. Psychiatry 2012, 20, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Bartolomucci, A.; Palanza, P.; Parmigiani, S.; Pederzani, T.; Merlot, E.; Neveu, P.J.; Dantzer, R. Chronic psychosocial stress down-regulates central cytokines mRNA. Brain Res. Bull. 2003, 62, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Dronjak, S.; Gavrilović, L.; Dragana, G.; Filipović, F.; Radojčicradojčić, M.B. Immobilization and cold stress affect sympatho-adrenomedullary system and pituitary-adrenocortical axis of rats exposed to long-term isolation and crowding. Physiol. Behav. 2004, 81, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Almutabagani, L.F.; Almanqour, R.A.; Alsabhan, J.F.; Alhossan, A.M.; Alamin, M.A.; Alrajeh, H.M.; Alonazi, A.S.; El-Malky, A.M.; Alrasheed, N.M. Inflammation and Treatment-Resistant Depression from Clinical to Animal Study: A Possible Link? Neurol. Int. 2023, 15, 100–120. [Google Scholar] [CrossRef]
- Altamura, A.C.; Pozzoli, S.; Fiorentini, A.; Dell’osso, B. Neurodevelopment and inflammatory patterns in schizophrenia in relation to pathophysiology. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 42, 63–70. [Google Scholar] [CrossRef]
- Pariante, C.M.; Pearce, B.D.; Pisell, T.L.; Sanchez, C.I.; Po, C.; Su, C.; Miller, A.H. The Proinflammatory Cytokine, Interleukin-1α, Reduces Glucocorticoid Receptor Translocation and Function. Endocrinology 1999, 140, 4359–4366. [Google Scholar] [CrossRef] [PubMed]
- Niitsu, K.; Rice, M.J.; Houfek, J.F.; Stoltenberg, S.F.; Kupzyk, K.A.; Barron, C.R. A Systematic Review of Genetic Influence on Psychological Resilience. Biol. Res. Nurs. 2019, 21, 61–71. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Joëls, M. The cortisol switch between vulnerability and resilience. Mol. Psychiatry 2023, 29, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, N.P.; Meijer, O.C.; Ron De Kloet, E. Mineralocorticoid receptor and glucocorticoid receptor work alone and together in cell-type-specific manner: Implications for resilience prediction and targeted therapy. Neurobiol. Stress 2022, 18, 100455. [Google Scholar] [CrossRef] [PubMed]
- Masten, A.S.; Narayan, A.J. Child development in the context of disaster, war, and terrorism: Pathways of risk and resilience. Annu. Rev. Psychol. 2012, 63, 227–257. [Google Scholar] [CrossRef] [PubMed]
- Musillo, C.; Berry, A.; Cirulli, F. Prenatal exposure to psychological or metabolic stress increases the risk for psychiatric disorders: The “funnel effect” model. Neurosci. Biobehav. Rev. 2022, 136, 104624. [Google Scholar] [CrossRef]
- Imanaka, A.; Morinobu, S.; Toki, S.; Yamawaki, S. Importance of early environment in the development of post-traumatic stress disorder-like behaviors. Behav. Brain Res. 2006, 173, 129–137. [Google Scholar] [CrossRef]
- Bale, T.; Epperson, C. Sex differences and stress across the lifespan. Nat. Neurosci. 2015, 18, 1413–1420. [Google Scholar] [CrossRef]
- Stadtler, H.; Neigh, G.N. Sex Differences in the Neurobiology of Stress. Psychiatr. Clin. N. Am. 2023, 46, 427–446. [Google Scholar] [CrossRef]
- Schneider, K.M.; Blank, N.; Alvarez, Y.; Thum, K.; Lundgren, P.; Litichevskiy, L.; Sleeman, M.; Bahnsen, K.; Kim, J.; Kardo, S.; et al. The enteric nervous system relays psychological stress to intestinal inflammation. Cell 2023, 186, 2823–2838.e20. [Google Scholar] [CrossRef]
- Baratta, M.V.; Rozeske, R.R.; Maier, S.F. Understanding stress resilience. Front. Behav. Neurosci. 2013, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Shinohara, R.; Fogaça, M.V.; Hare, B. Neurobiology of rapid-acting antidepressants: Convergent effects on GluA1-synaptic function. Mol. Psychiatry 2019, 24, 1816–1832. [Google Scholar] [CrossRef]
- Pereira-Figueiredo, I.; Castellano, O.; Riolobos, A.S.; Ferreira-Dias, G.; López, D.E.; Sancho, C. Long-term sertraline intake reverses the behavioral changes induced by prenatal stress in rats in a sex-dependent way. Front. Behav. Neurosci. 2017, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, J.L.; Castañeda, P.; Berríos, C.; Díaz-Veliz, G.; Mora, S.; Bravo, J.; Araneda, K.; Menares, C.; Morales, P.; Fiedler, J. Comparison of the antidepressant sertraline on differential depression-like behaviors elicited by restraint stress and repeated corticosterone administration. Pharmacol. Biochem. Behav. 2010, 97, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, N.; Kusano, T.; Kinoshita, S.; Nakamoto, H. Influence of Perceived Stress and Stress Coping Adequacy on Multiple Health-Related Lifestyle Behaviors. Int. J. Environ. Res. Public Health 2022, 19, 284. [Google Scholar] [CrossRef]
- Ingledew, D.K.; McDonagh, G. What Coping Functions are Served when Health Behaviours are Used as Coping Strategies? J. Health Psychol. 1998, 3, 195–213. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).