Abstract
Due to the increasing world population and environmental considerations, there has been a tremendous interest in alternative energy sources. Hydrogen plays a major role as an energy carrier due to its environmentally benign nature. The combustion of hydrogen releases water vapor while it also has a vast industrial application in aerospace, pharmaceutical, and metallurgical industries. Although promising, hydrogen faces storage challenges. Underground hydrogen storage (UHS) presents a promising method of safely storing hydrogen. The selection of the appropriate cushion gas for UHS is a critical aspect of ensuring the safety, efficiency, and reliability of the storage system. Cushion gas plays a pivotal role in maintaining the necessary pressure within the storage reservoir, thereby enabling consistent injection and withdrawal rates of hydrogen. One of the key functions of the cushion gas is to act as a buffer, ensuring that the storage pressure remains within the desired range despite fluctuations in hydrogen demand or supply. This is achieved by alternately expanding and compressing the cushion gas during the injection and withdrawal cycles, thereby effectively regulating the overall pressure dynamics within the storage facility. Furthermore, the choice of cushion gas can have significant implications on the performance and long-term stability of the UHS system. Factors such as compatibility with hydrogen, cost-effectiveness, availability, and environmental impact must be carefully considered when selecting the most suitable cushion gas. The present study provides a comprehensive review of different types of cushion gases commonly used in UHS, including nitrogen, methane, and carbon dioxide. By examining the advantages, limitations, and practical considerations associated with each option, the study aims to offer valuable insights into optimizing the performance and reliability of UHS systems. Ultimately, the successful implementation of UHS hinges not only on technological innovation but also on strategic decisions regarding cushion gas selection and management. By addressing these challenges proactively, stakeholders can unlock the full potential of hydrogen as a clean and sustainable energy carrier, thereby contributing to the global transition towards a low-carbon future.
1. Introduction
Fossil fuels have come under increased scrutiny in recent years due to various factors such as high greenhouse gas emissions, fluctuating prices, and a rise in energy needs [1]. As the world’s population continues to rise, these issues are expected to become even more pressing. Considering this, numerous initiatives have been taken to develop alternative sources of energy that are self-sustaining and renewable [1]. Although wind and solar are among the most known renewable energy resources, they have notable limitations. While these options can produce clean usable energy, they are not reliable. Wind and sunlight are not consistently available each day [2]. This creates gaps in energy production, which can strain the ever-increasing demand for energy. On the other hand, Hydrogen is a reliable and clean energy carrier [3]. Hydrogen can be used in industries known to be high GHG emitters such as aviation, chemical manufacturing, and iron and steel production [4]. The combustion of hydrogen mostly releases water vapor as a byproduct, making it a clean energy source with no carbon emissions.
The majority of hydrogen generated today is from the well-established method of steam-methane reforming (SMR), which uses high-temperature steam (700–1000 °C) to produce hydrogen from a methane (CH4) source, such as natural gas [5]. Although promising, SMR releases lots of GHG. Therefore, there have been several research interests in alternative hydrogen production methods from waste materials [6] and electrolysis technology [7,8].
Another issue with hydrogen technology is its storage. The challenges of hydrogen storage are primarily due to its low ambient temperature density, resulting in a low energy-per-unit volume [9]. This necessitates the development of advanced storage methods to achieve higher energy density. Hydrogen can be stored physically as either a gas or a liquid. However, each method has its drawbacks [10]. It should be mentioned that hydrogen could be stored aboveground and underground. Currently, there are several aboveground options for storing hydrogen such as in cryogenic tanks, using a metal–organic framework (MOF), and direct conversion to energy carriers or liquefied hydrogen, all depicted below in Figure 1. However, there are fewer options for storing hydrogen in underground deposits. Regarding aboveground cryogenic storage, it is worth noting that while these tanks can hold both liquid and compressed gaseous hydrogen, there are many requirements to keep the hydrogen viable.
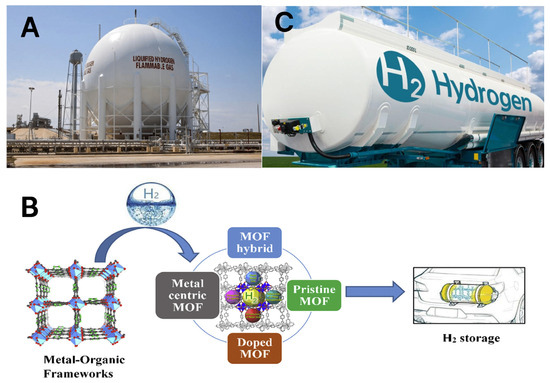
Figure 1.
A schematic representation of the above-ground hydrogen storage options: (A) depicts a cryogenic tank, (B) depicts a metal–organic framework (MOF), and (C) depicts direct conversion to energy carriers or liquefied hydrogen.
Ahluwalia and Peng [11] address a crucial aspect of hydrogen storage. Recent strides have been achieved in producing robust composite fiber tanks capable of containing compressed hydrogen (H2) at pressures ranging from 350 to 700 bar. Nevertheless, the aspiration of achieving a system volumetric capacity of 45–60 g/L encounters limitations because, at room temperature, the density of hydrogen is merely 23.5 g/L at 350 bars and 39.5 g/L at 700 bars [11]. An additional requirement of cryogenic tanks is they must be fitted with an in-tank heater as hydrogen has a low critical point of 13.15 bar and −239.96 °C. With these strict requirements in mind, this makes cryogenic tanks challenging both economically and in terms of accessibility.
Metal–organic frameworks (MOFs) are also promising hydrogen storage mediums; however, they are characterized by low volumetric density, slow hydrogen uptake and release, and high cost and scalability issues [1]. In contrast, the direct conversion of hydrogen to its liquid carriers such as ammonia is restricted by cost and environmental impacts. Alternatively, hydrogen could be stored safely and cheaply in various underground deposits.
The top three contenders for underground hydrogen storage (UHS) are salt caverns, aquifers, and depleted hydrocarbon reservoirs [12]. It is worth noting that salt caverns must be excavated and prepared for hydrogen storage, unlike the other storage options, which creates an economic and time disadvantage. On the other hand, aquifers are naturally occurring geological formations that are made up of porous media such as sandstone, limestone, shale, and conglomerates [13]. Surrounding this porous media is nonporous media, also referred to as cap rocks. A drawback of aquifers is the possible reactions of hydrogen with mineral constituents and microbes [13]. Lastly, depleted hydrocarbon reservoirs are described as oil and gas wells in petroleum fields. This option is feasible due to sufficient depth and bottom-hole temperatures that are suitable for thermal energy extraction [13]. However, due to these reservoirs being drilled into various geological formations and media, there is no constant measurement of permeability. This means that different reservoirs will exhibit differing values of gas leakage and loss. The fluctuating value per reservoir creates an economic drawback as the potential for hydrogen gas loss each day could be high [14]. Optimizing and reducing the cost of UHS requires the selection of an effective cushion gas. It should be mentioned that cushion gas in UHS refers to a permanent gas layer used to maintain pressure and ensure the efficient extraction of hydrogen when needed [12,13].
There have been various studies and simulations performed to determine the best cushion gas for UHS such as Iloejesi and Beckinghams [15], who reported the reservoir modeling and performance of carbon dioxide, CO2, as a cushion gas for UHS. Along with CO2, other cushion gases that are under consideration by several researchers include nitrogen (N2), CH4, and residual native gases. Depending on which gas is chosen for an underground storage cavity, possible interactions must be evaluated. When discussing CO2 as a cushion gas, Iloejesi and Beckinghams [15] explain that once injected, CO2 creates conditions often suitable for the dissolution of carbonate and aluminosilicate minerals [15]. However, the possible interactions change with differing storage methods. Porous saline aquifers, for example, involve additional hazards and complications compared to storage in a medium such as caverns including multiphase flow and geochemical reactions. These complexities are not well understood and could potentially impact system operation or efficiency. The interaction between CO2 and different UHS media is not yet fully understood. Further research is needed to determine how CO2 may impact the performance and safety of UHS options such as salt caverns, aquifers, and depleted hydrocarbon reservoirs. Furthermore, the economic and environmental impacts of using different cushion gases in various hydrogen storage media are not yet fully understood. To the authors’ knowledge, there are relatively few studies related to cushion gas considerations for UHS. Table 1 outlines a few reviews in this area, especially those related to UHS. To address the knowledge gaps, the present study provides a comprehensive review of different cushion gases used for hydrogen storage. The study also delves into various mediums for UHS, as well as their advantages and limitations.

Table 1.
Summary of previous studies related to underground hydrogen storage.
2. Overview of Underground Hydrogen Storage
As mentioned earlier, hydrogen can be stored underground in various mediums illustrated in Figure 2. The different media are discussed in this section.
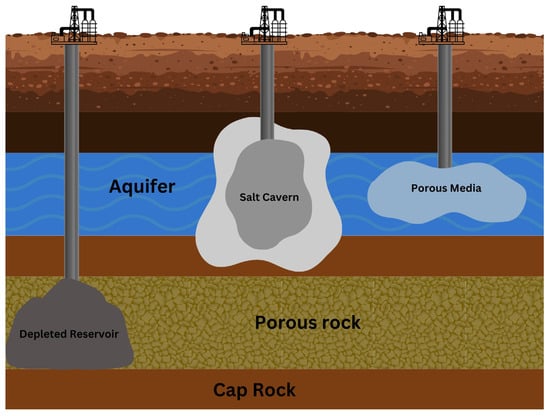
Figure 2.
A diagram showing various UHS mediums.
2.1. Porous Media
Porous media for UHS refer to geological formations with permeable characteristics, allowing for the storage of hydrogen gas [23]. These formations include depleted oil and gas reservoirs, saline aquifers, and engineered salt caverns, each offering unique properties and capacities for storing hydrogen. The porosity of these media is essential for enabling the adsorption and containment of hydrogen, providing a buffer for energy storage in renewable energy systems. The suitability and efficiency of a particular porous medium for hydrogen storage depends on factors like porosity, permeability, and geological stability, essential for ensuring safe and effective long-term storage.
Other high-permeable media include sandstone formations, limestone, shale, and conglomerates [23]. These geological formations hold onto the hydrogen in the porous and permeable media. The hydrogen will then diffuse and move throughout the storage site until it reaches a confining bed or “cap rock”. The cap rock caps off the possible exit points from the media allowing the hydrogen to be held in place with minimal seepage and loss.
An issue that porous media storage faces is the growth of unwanted bacteria and microbes. These microbes may come from a reaction of hydrogen with preexisting minerals in structural storage. They could also come from a contaminated aquifer. Furthermore, a chemical reaction of hydrogen can interfere with the pressure inside the cavity. This could cause seepage of hydrogen and potentially toxic gases. The change in pressure could also put the structural storage at risk of collapsing or even explosion.
2.2. Salt Caverns
Salt caverns are widely regarded as ideal locations for UHS, primarily because of their mechanical properties and the low permeability of salt rock [24]. These caverns are artificial underground cavities found within salt domes or salt layers. They are characterized by their exceptional gas tightness and inertness, attributes that are critical for the secure storage of hydrogen. The creation of these caverns involves the controlled injection of fresh water from the surface into the salt deposits, a process that dissolves the salt and forms spacious cavities. This method not only ensures the structural integrity of the storage site but also minimizes the risk of gas leakage, making salt caverns a preferred choice for hydrogen storage projects. The primary issue with favorable salt formations is that they are not found everywhere. Current operational salt caverns storing hydrogen are located in Teesside, UK, Epe, Germany, and Texas, USA [4].
Salt caverns are known to contain natural impurities that can be both soluble and insoluble. Laban’s research on cavern S43, located in Epe, Germany, details the types of impurities found within this particular cavern [4]. The most common insoluble impurities in the Z1 layer include anhydrite (CaSO4), gypsum (CaSO4·2H2O), dolomite (CaMg(CO3)2), calcite (CaCO3), pyrite (FeS2), and quartz (SiO2); additionally, the presence of clay deposits is also possible. Regarding soluble impurities, the most frequently encountered ions are calcium (Ca2+), iron in both its divalent (Fe2+) and trivalent (Fe3+) forms, magnesium (Mg2+), potassium (K+), chloride (Cl−), carbonate (CO32−), and sulfate (SO42−). Furthermore, barium (Ba2+), strontium (Sr2+), boron (B3+), and bromide (Br−) may also be present, albeit in minor quantities. This comprehensive identification of impurities is crucial for understanding the chemical environment within salt caverns and for assessing their suitability and safety for hydrogen storage or any other proposed uses [4,25].
The presence of these impurities or isotopes of metal within salt caverns can interact with hydrogen to form hydrides or gases, potentially creating an imbalance in the metal structure. Such interactions are significant because they can lead to embrittlement—a condition where the metal becomes brittle and more likely to fracture [26,27]. This phenomenon is a critical concern when considering the structural integrity and safety of engineered cavities used for hydrogen storage or other purposes. The issue of embrittlement and its implications for the stability and longevity of engineered cavities is discussed in greater detail in the section titled “Engineered Cavities”. This discussion is vital for understanding how impurities within salt caverns can affect the materials used in the construction and maintenance of these storage sites, thereby influencing their overall efficacy and safety [4,28,29].
3. Cushion Gas in UHS
Cushion gas is the volume of gas that remains in a storage reservoir to maintain adequate pressure and ensure the continuous extraction of the working gas [19,30,31]. The primary goal is to keep the storage pressure high enough to enable constant, appropriate injection and withdrawal rates. To maintain the necessary pressure and deliverability rate, it alternately expands and compresses during the injection and withdrawal cycles [32].
Depending on the method of storage, different amounts of cushion gas are needed. For instance, cushion gas makes up around 50% of the total volume of depleted gas reserves. Comparatively, salt caverns usually need approximately 25% of the overall volume. More is needed for aquifer reservoirs—up to 80% of the total capacity. To put it briefly, the precise amount needed relies on the precise storage requirements as well as the necessary withdrawal rates [33].
The role of cushion gas for UHS is not only necessary for the injection and extraction of the hydrogen but is also used to preserve the reservoir at an adequate pressure that is high enough as the hydrogen is being retrieved [12]. Hence, if the pressure in the reservoir is not maintained at proper conditions, the efficiency of the withdrawal cycle will be jeopardized. A general depiction of where cushion gas is in relation to the hydrogen being stored in an underground reservoir is shown below in Figure 3.
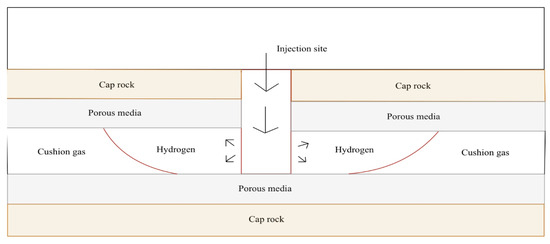
Figure 3.
An idealized single-well natural gas storage schematic that depicts working gas (hydrogen) and cushion gas.
During the injection and extraction cycles, the cushion gas can readily expand and be compressed to maintain the desired hydrogen pressure and flow rates without being consumed [13]. This has been tested and found to be successful for natural gas storage (NGS) in both porous rocks and salt caverns [16,17]. The flow rate of hydrogen in the extraction process is arguably one of the most important factors in UHS. Because the flow rate is a crucial step in UHS, the cushion gas that is chosen for a reservoir must have certain properties. Due to the possible variations of gas used for cushion gas, the differences in the gas properties such as density, viscosity, and solubility influence the flow behavior, thus influencing the hydrogen recovery performance [34].
UHS will involve losses that must be measured and carefully monitored. Storage facilities such as salt caverns and deep aquifers must pass leakage tests before they are allowed to operate [16]. These tests are put into place due to faults and fractures and underground chemical and biological reactions that cause leaks, which in turn, lead to significant losses that are related to cushion gas [16]. Depending on the type of cushion gas used in the storage reservoir, the gas may exhibit large changes in pressure due to changing temperatures and possible interactions. The large change in pressure can cause faults and fractures in the underground reservoirs, allowing gas seepage, both cushion and hydrogen. The possible interactions of cushion gas, as stated previously, are caused by preexisting bacteria, oil, water, and natural gases, which can lead to the production of large amounts of CH4 [16,35]. The environmental impact of UHS is low to none. However, potential CH4 production puts GHG into the atmosphere and poses a safety threat, as CH4 is highly flammable.
Preexisting oil, water, and natural gas, shown below in Figure 4, are a common occurrence in porous media reservoirs. Formations such as shale and carbonate rocks show amounts of organic matter disseminated in three forms: soluble hydrocarbons, soluble asphalt, and insoluble organic matter (kerogen) [18]. These components are integral constituents of petroleum (oil) and natural gas [36]. The existence of these preexisting constituents renders porous media reservoirs potentially hazardous for use in UHS. When cushion gas is injected, it can significantly affect the oil in several ways that must be considered: (1) gas components may condense into the oil, leading to an increase in oil volume; (2) oil viscosity may decrease due to the addition of lighter gas components; (3) oil volatility may increase under high temperatures and pressures; and (4) the oil may be physically displaced by the gas [37].
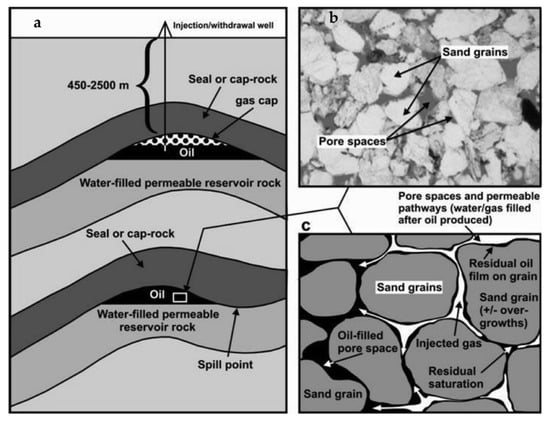
Figure 4.
(a) An overview of a depleted reservoir condition, (b) a microscopic image of reservoir pores filled with oil, and (c) graphic showing how injected H2 occupies the pores while oil evacuates the pores. Adapted from [38] with permission from Elsevier.
The potential for hazardous interactions necessitates a thorough understanding of the physical properties of the gases chosen for cushioning, as these properties can vary significantly with temperature changes [16]. The temperatures within storage reservoirs will also vary depending on the underground depth and geographic location. Considering these factors, three gases—CO2, N2, and natural gas (CH4)—will be discussed. These gases were selected based on their availability, ease of procurement, physical properties, and environmental impact. Heinemann et al. [28] conducted simulations to study how the hydrodynamic behavior of cushion gas and hydrogen during UHS in saline aquifers. Their goal was to optimize hydrogen recovery during the process of back production. They noted that cushion gas plays a crucial role in managing both the injectivity and productivity of hydrogen, directly influencing the efficiency of utilizing storage capacity. While cushion gas does not increase the maximum storage capacity of a site, it is essential for maximizing the effective use of the existing capacity.
Table 2 presents a comparative analysis of the advantages and limitations of these different cushion gases for UHS, providing essential insights into their suitability for various storage conditions.

Table 2.
Advantages and limitations of different cushion gases for underground hydrogen storage.
When discussing the advantages of each potential cushion gas, there are key factors to consider such as cost, availability, chemical composition and interactions, and engineering. CO2 provides an economic advantage due to the potential use of carbon that has been captured via carbon sequestration. Currently, tax credits are being offered to industries using carbon capture technology as well as geological storage of the carbon being captured [11]. In addition to a potential economic advantage, climate change mitigation is inherent when using CO2 produced via carbon capture. N2 shows significant advantages when considering chemical interactions. N2 shows high gas wettability, meaning that it is easier to separate N2 from H2 during production [12]. Easier separation leads to a higher H2 recovery rate, increasing profit and efficiency. When compared to CO2, N2 has fewer corrosive tendencies [13]. N2 exhibits a reduced risk of chemical reactions. CH4 also shows high hydrogen recovery like N2, but, in addition, it is compatible with most current infrastructures. This poses an economic advantage as there does not need to be much alteration in preexisting equipment. Lastly, CH4 and N2 are both widely and readily available.
As noted in Table 2, the limitations of each cushion gas are also comprehensively discussed. As previously mentioned, CO2 produced from carbon capture offers a tax credit; however, it can be costly to transport and inject. Due to the potential costs, it may outweigh the advantages of the tax credit. Another risk involved is the possibility of gas leakage, leading to CO2 emissions from UHS. N2 has a high economic risk as it is costly to separate from air and cannot be used as a cushion gas until completed. Like leakage of CO2, N2 poses environmental risks as excessive release can disrupt local N2 cycles as well as ecosystems [14]. Similarly, the leakage of CH4 can contribute to climate change as CH4 is a major GHG.
4. Different Types of Cushion Gas for UHS
Numerous cushion gases, including CH4, CO2, and N2, have been studied for subterranean hydrogen storage [29,39,40,41,42]. The selection of an appropriate cushion gas depends on its inherent physical characteristics, specifically density, as well as its availability and related costs [15]. The cushion gas’ chemical stability is a crucial prerequisite since any reactivity with the working gas or the reservoir matrix under the current circumstances could result in unwanted byproducts or jeopardize the integrity of the rock formation [5,15]. This section describes studies on various cushion gases used for UHS, specifically focusing on CO2, N2, and CH4.
4.1. Carbon Dioxide (CO2)
CO2 has been used as a reliable cushion gas for subsurface gas storage. However, its main use/actual deployment has been associated with NGS [17]. While there are experimental limited data on the use of CO2 as a cushion gas for UHS, there are considerable research data on its use for NGS. Natural gas is held underground in three types of facilities: (1) depleted reservoirs in oil and/or gas fields, (2) salt cavern formations, and (3) aquifers [39]. Since natural gas and hydrogen are typically stored in the same type of underground reservoirs, this provides some insight into the potential behavior of CO2 as a cushion gas for UHS applications [39].
CO2 shows benefits such as lower cost as well as lower gas mixing with H2 [17]. The mixing of H2 with cushion gas is one of the top issues of concern to UHS. Mixing is known to reduce the quality of recovered working gas from the reservoir, in turn leading to a reduction in the usability of the working gas [17]. The limited mixing of CO2 can be attributed to its molecular properties such as high density and viscosity [40]. These properties are extremely beneficial in reservoirs that can hold CO2 at a pressure range of 60 to 100 bar, as well as a temperature of 40 °C and below [40]. Recent numerical simulation backs up these expected behavioral properties. Adsorption results show that CO2 will accumulate in nanopores, aiding in improving the purity of extracted H2 [41]. However, in the same study, when nanopores of 2 nm are present, the storage capacity is reduced. This is due to the dilution of H2 while in the bulk phase, as well as competitive adsorption leading to surface depletion [41].
CO2 can be obtained via carbon sequestration, which is described as a transfer of atmospheric CO2 into other long-lived global pools including oceanic, pedologic, and geological strata to reduce the net rate of the increase in atmospheric CO2 [42]. These environmental benefits align with the overall goal of UHS and hold an economic benefit shown by carbon tax credits [15].
CO2 shows downsides as well, one of which is its high solubility in water. If the subsurface reservoir being used has a high initial water saturation, the injected CO2 has the potential to interact with it. This can lead to a decrease in reservoir pressure, which can cause H2 loss or leakage as well as challenges with H2 retrieval. CO2 can show poor recovery rates when compared to N2 and CH4. Recent research that used the black oil simulator Eclipse 100 shows that this poor recovery rate can be attributed to the methanation reaction that takes place [43]. A methanation reaction can occur under certain reservoir conditions, commonly occurring in aquifers, causing H2 and CO2 to convert to CH4 [43]. This ultimately leads to a reduction in recoverable H2 gas.
Studies using CMG-GEMTM have been recently used to investigate the behaviors of CO2 when used as a cushion gas for UHS. The numerical study found results that back up previous findings such as CO2’s higher solubility causes a higher volume of H2 stored. This is further explained as high solubility shows a higher occupiable volume in an aquifer, allowing for a higher volume of H2 to be stored [43]. Furthermore, the cumulative H2 injected showed a direct correlation to the cushion gas density. CO2 has a higher density (1.98 g/L) compared to N2 (1.25 g/L) and CH4 (0.657 g/L), allowing a higher H2 volume injected [43]. However, the density difference between CO2 and H2 produced a fingering phenomenon (i.e., an increase in mixing between the gases), showing that the higher mobility of H2 could cause it to penetrate the CO2 gas phase [43]. This can consequently cause a lower H2 recovery efficiency [43].
Abdellatif et al. [29] compared the performance of CO2 as a cushion gas with other gases such as N2, CH4, and He in a real Viking oil field. The total gas production profile and the hydrogen production rate are shown in Figure 5. Their findings suggest that when CO2 is used as a cushion gas, approximately 40% of the injected hydrogen remains and cannot be produced back. Compared to other cushion gases, CH4 has the optimal performance with 30% remaining hydrogen [29].
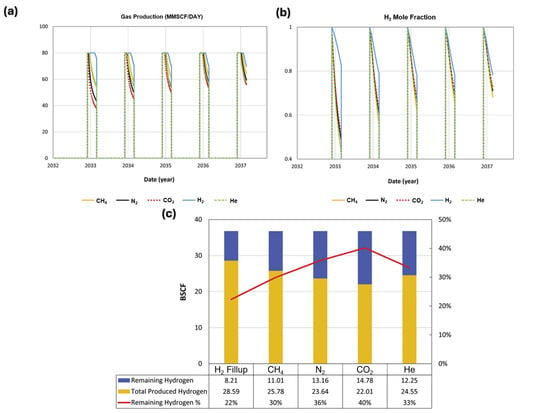
Figure 5.
(a) Total gas production profile; (b) hydrogen mole fraction; (c) total produced and remaining hydrogen after five cycles of injection. All for different cushion gases. Adapted from Ref. [29].
4.2. Nitrogen (N2)
While there are few experimental studies detailing the performance of N2 as a cushion gas for UHS (a similar challenge as CO2), many researchers have included N2 as a potential cushion gas for NGS [17]. Similarly, with CO2, we can closely compare these findings to those of UHS [17].
N2 as a cushion gas has a high potential for success as it shows the highest amount of H2 recovery as well as purity when compared to CH4 and CO2 [44]. When observing the behaviors of N2, we see that N2 increases the reservoir pressure more than CH4 and CO2. The higher pressure causes the H2 to show behaviors like those of the cushion gas. Due to these factors, when injecting hydrogen as the cushion, we can produce a higher hydrogen recovery factor [44]. In addition, N2 holds characteristics that are preferred for UHS such as fewer corrosive behaviors compared to those of CO2. Corrosion can be dangerous as well as costly due to causing an increase in leakage or explosion risk [43]. Factors like these must be kept in mind as they can become a safety concern. Corrosion can be dangerous as well as costly due to causing an increase in leakage or explosion risk [43]. Factors like these must be kept in mind as they can become a safety concern.
Another important factor when considering the chosen cushion gas is the gas wettability. Gas wettability is similar to rock wettability in that it defines the displacement ability of the gas phase in a gas–liquid–solid system [45]. N2 displays a higher gas wettability, which makes the gas easier to separate from H2 during production cycles [46]. This simultaneously reduces the overall cost of production and increases the profits from producing a cleaner H2 [46].
However, N2 may not be a sustainable cushion gas in some engineered cavities and porous media due to the potential of preexisting oil within them. In petroleum-contaminated environments, N2 can cause degradation and bio-stimulation, causing a potential production of unwanted byproducts and processes such as aggregation [47]. Aggregation of oil from N2 could potentially produce a plugging phenomenon in the porous media as well as production facilities [48]. Using N2 as a cushion gas in porous media poses additional issues such as when an increase in the reservoir’s permeability happens, it can lead to the concentration of N2 in the produced well stream to increase. This can be attributed to the increase in the fingering phenomenon. However, by increasing the porosity, the concentration of N2 in the produced well stream decreases. This decrease in the mixing phenomenon may be caused by the increased volume of the injection gas arising from the increased porosity [49].
Recent studies have been performed using molecular dynamic simulations to predict interfacial tension to bridge gaps in knowledge [50]. Studies like these are important for learning how N2 may behave in differing underground reservoirs. Another proposed UHS cushion gas scenario suggested an initial N2 injection, keeping in mind the density difference between H2 and N2 [44]. When simulated, the density difference between H2 and N2 produced a H2 accumulation at the top of the reservoir, while N2 stayed towards the bottom [44]. This shows promising data toward higher H2 recovery as well as storage efficiency. This same simulation produced data that back up previous findings that N2 increases reservoir pressure causing an increase in H2 recovery when compared to CO2 and CH4 [44]. Huang et al. [30] noted that N2 showed a superior performance as a cushion gas compared to CO2. Their observations account for both the hydrogen recovery factor as well as the purity of the produced gas. Carbon dioxide is denser than nitrogen when both gases are under the same thermo-physical conditions. As a result, the same amount of injected nitrogen will occupy a larger volume compared to carbon dioxide. This leads to a significant expansion of the gas phase with nitrogen, whereas the change in the gas phase distribution with carbon dioxide is minimal. Additionally, the high solubility of carbon dioxide in fluids reduces the amount of carbon dioxide available in its gaseous form.
Injecting cushion gas can enhance the recovery factor of hydrogen, but it also unavoidably raises the level of impurities in the produced gas, particularly when maintaining constant bottom-hole pressure during production. Huang et al. [30] also presented results from the produced gas stream with the use of different cushion gases. Initially, most of the gas produced is hydrogen, which gathers near the production well. As production continues, other gases begin to mix with the hydrogen. In the base scenario without any cushion gas, the gas is mostly hydrogen and CH4, with hydrogen making up approximately half of the total at the end. When N2 is used as cushion gas, hydrogen accounts for only approximately 20% of the gas, with N2 making up 75%. CH4 remains a minor component in both cases. However, when CO2 is used as cushion gas, both CH4 and CO2 percentages are higher at around 30% and 45%, respectively, because they mix well during injection. This scenario indicates a need for several purification steps to extract hydrogen, increasing the overall cost of the process.
4.3. Methane (CH4)
CH4 has a shorter atmospheric lifespan than CO2 (around 12 years compared to centuries for CO2), but it is a much more potent GHG as it absorbs higher amounts of energy while in the atmosphere [51]. Currently, a portion of CH4 emissions is captured to be sold in the natural gas market or to be converted to other chemicals [52]. Since these emissions are already being captured, this could help to reduce the overall cost of UHS operations while using CH4 as a cushion gas.
When using CH4 as the injected cushion gas, maximum hydrogen recovery (89.7%) was anticipated [12]. This is backed up by additional research performed with the use of dual-porosity and dual-permeability models, showing that CH4 provided the maximum productivity index [17]. This can be attributed to the CH4 wettability of storage rock being higher than hydrogen wettability, suggesting that hydrogen penetration into the rock pore space will be reduced in the presence of CH4. Hence, it will be easier to separate and retrieve the injected hydrogen during the withdrawal cycles [47]. These properties suggest that CH4 is a good option for cushion gas in depleted oil and gas reservoirs [53]. However, there has been very little research performed on the interactions of CH4, CO2, and N2. This can become an issue in real-world implementation as these gases may be present in substantial or trace amounts within the reservoir.
More recent studies have used a fully coupled multi-physic framework of Delft Advanced Reservoir Simulation to produce the results of behaviors when using CH4 as a cushion gas. The results were consistent with others, showing that CH4 produces a higher H2 production rate as well as mobility [31]. Furthermore, the GERG-2008 equation of state has been used due to its ability to predict the thermodynamic properties of H2 when combined with other gases [31]. Even with the equation’s ability of high accuracy, it does not account for a water-rich aqueous phase [31].
A numerical study was performed using core samples that were prepared with an initial vacuum and then flooded with a brine solution. The study produced results showing that CH4, when injected as a cushion gas, was able to displace the brine within the pore spaces, creating an additional storage capacity for H2 [54]. Additionally, the study shows that CH4 accelerates core pressurization at significant rates, and this results in a reduction of 30.3% of gas volume that is required to achieve 1000 psi pressurization [54]. This efficient pressurization of the reservoir can produce a sufficiently higher-pressure formation during H2 retrieval, producing a more controlled extraction from the reservoir [54].
5. Challenges and Prospects
The challenges and prospects of using cushion gas for underground hydrogen storage are significant and multifaceted. A few key points when discussing the successful implementation of UHS are social issues, geological, financial, and engineering design issues. Each of these key points must be addressed before full-scale UHS can be launched internationally. As such, the responsibility falls on the government and non-governmental entities, research institutions, and universities [35]. While each has different roles, the end goal is shared, that is, to safely and effectively introduce UHS. Currently, there is extensive research including reservoir simulations [43] and economic assessments [55] being published, however, there is an obvious knowledge gap when addressing the previously mentioned social issues. A clear next step towards the successful implementation of UHS is to take note of public concern as well as provide educational materials to improve public awareness of the technology.
In terms of the economic viability of UHS, Zivar et al. [56] provided a comprehensive review of the economic viability of UHS. The authors noted that the cost of UHS is dependent on several factors including the capture cost, transportation cost, storage cost, withdrawal cost, and monitoring cost. Additionally, pressure change, maintenance, cyclic operations costs, and annual throughput are significant components and considerations while evaluating storage costs. Tarkowski et al. [35] noted that storing hydrogen in aquifers incurs higher construction and operational expenses compared to caverns and depleted hydrocarbon reservoirs. Specifically, depleted oil reservoirs have higher storage costs than depleted natural gas reservoirs. Meanwhile, the cost of using a cavern system is lower than that of aquifers but higher than that of depleted reservoirs. Among various underground storage options, abandoned natural gas reservoirs are the least expensive, followed by solution salt caverns and hard rock caverns [57]. Lord et al. [58,59] studied the capital costs for different UHS methods and found that depleted reservoirs are the most cost-effective at 1.23 USD/kg of stored hydrogen, with aquifers close behind at 1.29 USD/kg. The costs for caverns are higher, at 1.61 USD/kg for salt caverns and 2.77 USD/kg for hard rock caverns [58,59]. However, comprehensive lifecycle costs for each storage mode, including both pure hydrogen and gas mixtures, have not yet been fully reported and remain uncertain. More importantly, the economic and environmental impact of various cushion gases for UHS is scarcely reported and should be the focus of future studies.
Sambo et al. [60] presented a comprehensive review of UHS potential fields and operating facilities worldwide. The authors noted that a key difference between porous media including aquifers and depleted hydrocarbon reservoirs is the percentage of cushion gas required. A range of 50–60% is reported for depleted hydrocarbon compared to the 80% requirement for the aquifer [60]. Therefore, future studies should also focus on evaluating the relationship between different porous media and their cushion gas percentage requirements. This would aid the optimization and screening of various cushion gases for UHS.
The injection and withdrawal cycles could affect the storage capacity and integrity over time. It should be mentioned that advances in materials science may lead to better sealing and barrier technologies that minimize gas migration and enhance the economic viability of UHS. Innovations in recycling cushion gas or employing synthetic or alternative gases could also mitigate environmental and cost concerns, thereby making underground hydrogen storage more practical and sustainable in the long term.
It should be mentioned that the effect of the physical properties of gases including CH4, CO2, and H2 on the underground storage tightness and H2 leakage is different [61]. Table 3 compares the physical properties of various cushion gases with H2. As seen in the table, the different cushion gases exhibit vast disparities in their physical properties compared to H2. These differences could influence the storage capacity and tightness of UHS. Therefore, future studies should evaluate the interconnection between the physical properties of cushion gases, hydrogen leakage, tightness, and storage capacity during UHS.

Table 3.
Comparison between the physical properties of different cushion gases and hydrogen at ambient temperature and 0.1 MPa. Data extracted from Refs. [61,62,63,64].
Some researchers recently studied the impact of cushion gas injection on hydrogen loss in depleted oil reservoirs [12]. This was motivated by the fact that at the end of each injection and withdrawal cycle for a prolonged production period, some hydrogen tends to remain in the reservoir gas phase or is trapped from within the oil or water phase. Compared to N2 and CO2, the residual hydrogen was lower with CH4 as a cushion gas. The performance of CH4 was linked to its lower molecular weights compared to other gases (Table 3). If the molecular weight of the cushion gas is significantly different from that of hydrogen (which is very light), it can lead to inefficient mixing and an uneven distribution of gases within the storage site. For instance, a heavier cushion gas will tend to settle at the bottom of the storage formation, potentially leading to less effective displacement of hydrogen during extraction. This uneven distribution can affect the overall performance and efficiency of the storage system, impacting the deliverability rate, the rate at which hydrogen can be withdrawn, and the integrity of the hydrogen purity upon retrieval.
Safety and efficiency are also critical issues during UHS [65,66]. There are several factors that directly or indirectly contribute to the safety and efficiency of hydrogen storage in underground deposits, and these include the geochemical and microbiological interactions [67], geomechanical properties [68], and the petrophysical properties of the porous media [61]. Future studies should explore the influence of cushion gas on the geochemical and geomechanical properties of rocks as well as the well and reservoir integrity.
6. Conclusions
Underground hydrogen storage (UHS) is an encouraging technology for enabling the transition to a low-carbon energy system. However, UHS faces several technical, economic, and environmental challenges, such as the choice of cushion gas, the risk of co-production of CH4, and the need for the separation of hydrogen and CH4. There are also environmental concerns as CH4 is a potent greenhouse gas and contributes significantly to global warming. Assessing and mitigating the potential environmental impacts associated with CH4 production and subsequent separation is crucial to minimizing the overall carbon footprint of the hydrogen storage system. Furthermore, while utilizing CH4 as cushion gas may initially seem cost-effective due to its apparent viability, the potential co-production of large amounts of CH4 raises concerns. For instance, in examining the risk of co-producing CH4 cushion gas, it is important to consider the safety aspects. Thorough risk assessments should be conducted to evaluate the potential hazards associated with the storage, handling, and separation of CH4. The economic impact of separating CH4 from hydrogen after storage should be thoroughly analyzed to ensure the viability of this strategy. Various methods of separation exist, such as membranes, adsorption, and cryogenic distillation. However, these methods have their limitations and trade-offs, such as energy consumption, selectivity, and scalability. Therefore, the feasibility of developing efficient and cost-effective separation technologies should be explored to ensure the practicality of this approach. Further research is needed to optimize the design and operation of UHS systems, taking into account the interactions and trade-offs between cushion gas, co-production, and separation. The environmental and economic impacts of UHS should be evaluated holistically and comprehensively, considering the entire life cycle and value chain of hydrogen production, storage, and utilization.
Author Contributions
Conceptualization, S.P. and J.A.O.; methodology, S.P., E.E. and J.A.O.; software, J.A.O.; validation, E.E., S.P. and C.C.O.; formal analysis, J.A.O., E.E. and P.U.O.; investigation, S.P., J.A.O., E.E. and P.U.O.; resources, J.A.O.; data curation, S.P. and I.P.E.; writing—original draft preparation, S.P., O.A.O.-A., I.P.E., C.C.O., P.U.O. and J.A.O.; writing—review and editing, S.P., C.C.O., E.E. and J.A.O.; visualization, S.P., E.E. and J.A.O.; supervision, J.A.O. and E.E.; project administration, J.A.O.; funding acquisition, J.A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. However, this project was supported by the Provost’s Summer Undergraduate Research and Creative Activities (UReCA) Fellowship and the Gallogly College of Engineering Summer Research Fellowship at the University of Oklahoma. Its contents, including findings, conclusions, opinions, and recommendations, are solely attributed to the author(s) and do not necessarily represent the views of the Provost’s Office.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This is a review article so there are no data available in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Okolie, J.A.; Patra, B.R.; Mukherjee, A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Futuristic applications of hydrogen in energy, biorefining, aerospace, pharmaceuticals and metallurgy. Int. J. Hydrogen Energy 2021, 46, 8885–8905. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew. Sustain. Energy Rev. 2020, 119, 109546. [Google Scholar] [CrossRef]
- Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Laban, M.P. Hydrogen Storage in Salt Caverns: Chemical Modelling and Analysis of Large-Scale Hydrogen Storage in Underground Salt Caverns. 2020. Available online: https://repository.tudelft.nl/islandora/object/uuid%3Ad647e9a5-cb5c-47a4-b02f-10bc48398af4 (accessed on 1 April 2023).
- Wittich, K.; Kraemer, M.; Bottke, N.; Schunk, S.A. Catalytic Dry Reforming of Methane: Insights from Model Systems. ChemCatChem 2020, 12, 2130–2147. [Google Scholar] [CrossRef]
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-hydrogen: A review of main routes production, processes evaluation and techno-economical assessment. Biomass Bioenergy 2021, 144, 105920. [Google Scholar] [CrossRef]
- Yates, J.; Daiyan, R.; Patterson, R.; Egan, R.; Amal, R.; Ho-Baille, A.; Chang, N.L. Techno-economic Analysis of Hydrogen Electrolysis from Off-Grid Stand-Alone Photovoltaics Incorporating Uncertainty Analysis. Cell Rep. Phys. Sci. 2020, 1, 100209. [Google Scholar] [CrossRef]
- Michalski, J.; Bünger, U.; Crotogino, F.; Donadei, S.; Schneider, G.-S.; Pregger, T.; Cao, K.-K.; Heide, D. Hydrogen generation by electrolysis and storage in salt caverns: Potentials, economics and systems aspects with regard to the German energy transition. Int. J. Hydrogen Energy 2017, 42, 13427–13443. [Google Scholar] [CrossRef]
- Møller, K.T.; Sheppard, D.; Ravnsbæk, D.B.; Buckley, C.E.; Akiba, E.; Li, H.-W.; Jensen, T.R. Complex Metal Hydrides for Hydrogen, Thermal and Electrochemical Energy Storage. Energies 2017, 10, 1645. [Google Scholar] [CrossRef]
- Ennis-King, J.; Michael, K.; Strand, J.; Sander, R.; Green, C. Underground Storage of Hydrogen: Mapping Out the Options for Australia; Future Fuel CRC: Wollongong, Australia, 2021. [Google Scholar]
- Ahluwalia, R.; Peng, J. Dynamics of cryogenic hydrogen storage in insulated pressure vessels for automotive applications. Int. J. Hydrogen Energy 2008, 33, 4622–4633. [Google Scholar] [CrossRef]
- Kanaani, M.; Sedaee, B.; Asadian-Pakfar, M. Role of Cushion Gas on Underground Hydrogen Storage in Depleted Oil Reservoirs. J. Energy Storage 2021, 45, 103783. [Google Scholar] [CrossRef]
- Epelle, E.I.; Obande, W.; Udourioh, G.A.; Afolabi, I.C.; Desongu, K.S.; Orivri, U.; Gunes, B.; Okolie, J.A. Perspectives and prospects of underground hydrogen storage and natural hydrogen. Sustain. Energy Fuels 2022, 6, 3324–3343. [Google Scholar] [CrossRef]
- Duggal, R.; Rayudu, R.; Hinkley, J.; Burnell, J.; Wieland, C.; Keim, M. A comprehensive review of energy extraction from low-temperature geothermal resources in hydrocarbon fields. Renew. Sustain. Energy Rev. 2021, 154, 111865. [Google Scholar] [CrossRef]
- Iloejesi, C.O.; Beckingham, L.E. Assessment of Geochemical Limitations to Utilizing CO2 as a Cushion Gas in Compressed Energy Storage Systems. Environ. Eng. Sci. 2021, 38, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, R.; Uliasz-Misiak, B. Towards underground hydrogen storage: A review of barriers. Renew. Sustain. Energy Rev. 2022, 162, 112451. [Google Scholar] [CrossRef]
- Sadeghi, S.; Sedaee, B. Mechanistic simulation of cushion gas and working gas mixing during underground natural gas storage. J. Energy Storage 2021, 46, 103885. [Google Scholar] [CrossRef]
- Hunt, J.M.; Jamieson, G.W. Oil and Organic Matter in Source Rocks of Petroleum. AAPG Bull. 1956, 40, 477–488. [Google Scholar] [CrossRef]
- WG, P.K.; Ranjith, P.G. An overview of underground hydrogen storage with prospects and challenges for the Australian context. Geoenergy Sci. Eng. 2023, 231, 212354. [Google Scholar] [CrossRef]
- Miocic, J.; Heinemann, N.; Edlmann, K.; Scafidi, J.; Molaei, F.; Alcalde, J. Underground hydrogen storage: A review. Geol. Soc. Lond. Spéc. Publ. 2022, 528, 73–86. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Muhammed, N.S.; Patil, S.; Al Shehri, D.; Haq, B.; Epelle, E.I.; Mahmoud, M.; Kamal, M.S. Underground hydrogen storage: A critical assessment of fluid-fluid and fluid-rock interactions. J. Energy Storage 2023, 72, 108473. [Google Scholar] [CrossRef]
- Bin Navaid, H.; Emadi, H.; Watson, M. A comprehensive literature review on the challenges associated with underground hydrogen storage. Int. J. Hydrogen Energy 2023, 48, 10603–10635. [Google Scholar] [CrossRef]
- Bünger, U.; Michalski, J.; Crotogino, F.; Kruck, O. Large-scale underground storage of hydrogen for the grid integration of renewable energy and other applications. Compend. Hydrog. Energy 2016, 4, 133–163. [Google Scholar] [CrossRef]
- Małachowska, A.; Łukasik, N.; Mioduska, J.; Gębicki, J. Hydrogen Storage in Geological Formations—The Potential of Salt Caverns. Energies 2022, 15, 5038. [Google Scholar] [CrossRef]
- Liu, W.; Li, Q.; Yang, C.; Shi, X.; Wan, J.; Jurado, M.J.; Li, Y.; Jiang, D.; Chen, J.; Qiao, W.; et al. The role of underground salt caverns for large-scale energy storage: A review and prospects. Energy Storage Mater. 2023, 63, 103045. [Google Scholar] [CrossRef]
- Minougou, J.D.; Gholami, R.; Andersen, P. Underground hydrogen storage in caverns: Challenges of impure salt structures. Earth-Sci. Rev. 2023, 247, 104599. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen embrittlement in different materials: A review. Int. J. Hydrogen Energy 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Heinemann, N.; Scafidi, J.; Pickup, G.; Thaysen, E.M.; Hassanpouryouzband, A.; Wilkinson, M.; Satterley, A.K.; Booth, M.G.; Edlmann, K.; Haszeldine, R.S. Hydrogen storage in saline aquifers: The role of cushion gas for injection and production. Int. J. Hydrogen Energy 2021, 46, 39284–39296. [Google Scholar] [CrossRef]
- Abdellatif, M.; Hashemi, M.; Azizmohammadi, S. Large-scale underground hydrogen storage: Integrated modeling of res-ervoir-wellbore system. Int. J. Hydrogen Energy 2023, 48, 19160–19171. [Google Scholar] [CrossRef]
- Huang, T.; Moridis, G.J.; Blasingame, T.A.; Afifi, A.M.; Yan, B. Feasibility Analysis of Hydrogen Storage in Depleted Natural Reservoirs Through a Multi-Phase Reservoir Simulator. In Proceedings of the SPE Reservoir Characterisation and Simulation Conference and Exhibition, Abu Dhabi, United Arab Emirates, 24–26 January 2023; p. D031S017R001. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Chen, C. Numerical simulation of the impact of different cushion gases on underground hydrogen storage in aquifers based on an experimentally-benchmarked equation-of-state. Int. J. Hydrogen Energy 2024, 50, 495–511. [Google Scholar] [CrossRef]
- Zamehrian, M.; Sedaee, B. A comparative analysis of gas mixing during the underground hydrogen storage in a conventional and fractured reservoir. Gas Sci. Eng. 2024, 122, 205217. [Google Scholar] [CrossRef]
- Jahanbakhsh, A.; Potapov-Crighton, A.L.; Mosallanezhad, A.; Kaloorazi, N.T.; Maroto-Valer, M.M. Underground hydrogen storage: A UK perspective. Renew. Sustain. Energy Rev. 2024, 189, 114001. [Google Scholar] [CrossRef]
- Wang, G.; Pickup, G.E.; Sorbie, K.S.; Mackay, E.J. Driving Factors for Purity of Withdrawn Hydrogen: A Numerical Study of Underground Hydrogen Storage with Various Cushion Gases. In Proceedings of the Society of Petroleum Engineers—SPE EuropEC—Europe Energy Conference featured at the 83rd EAGE Annual Conference and Exhibition, EURO 2022, Madrid, Spain, 6–9 June 2022. [Google Scholar] [CrossRef]
- Tarkowski, R. Underground hydrogen storage: Characteristics and prospects. Renew. Sustain. Energy Rev. 2019, 105, 86–94. [Google Scholar] [CrossRef]
- Hydrocarbon|Definition, Types, & Facts|Britannica. Available online: https://www.britannica.com/science/hydrocarbon (accessed on 1 April 2023).
- Welge, H.J.; Johnson, E.F.; Ewing, S.P., Jr.; Brinkman, F.H. The Linear Displacement of Oil from Porous Media by Enriched Gas. onepetro.org. 1961. Available online: https://onepetro.org/JPT/article-abstract/13/08/787/162253 (accessed on 1 April 2023).
- Muhammed, N.S.; Haq, B.; Al Shehri, D.; Al-Ahmed, A.; Rahman, M.M.; Zaman, E. A review on underground hydrogen storage: Insight into geological sites, influencing factors and future outlook. Energy Rep. 2021, 8, 461–499. [Google Scholar] [CrossRef]
- Van Der Meer, L.G.H.; Assignor, A.O. Is Carbon Dioxide in Case of Natural Gas Storage a Feasible Cushion Gas? Classification Report Title Abstract Report Text Appendices Number of Pages 17 (incl. appendices) Number of Appendices. 2008. Available online: www.tno.nl (accessed on 27 April 2024).
- Oldenburg, C.M. Carbon Dioxide as Cushion Gas for Natural Gas Storage. Energy Fuels 2002, 17, 240–246. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Pan, B.; Liu, Z.; Jin, Z.; Iglauer, S. Molecular simulation on H2 adsorption in nanopores and effects of cushion gas: Implications for underground hydrogen storage in shale reservoirs. Fuel 2024, 361, 130621. [Google Scholar] [CrossRef]
- Lal, R. Carbon sequestration. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Jadhawar, P. Optimizing underground hydrogen storage in aquifers: The impact of cushion gas type. Int. J. Hydrogen Energy 2024, 52, 1537–1549. [Google Scholar] [CrossRef]
- Zamehrian, M.; Sedaee, B. Underground hydrogen storage in a partially depleted gas condensate reservoir: Influence of cushion gas. J. Pet. Sci. Eng. 2022, 212, 110304. [Google Scholar] [CrossRef]
- Jiang, G.; Li, Y.; Zhang, M. Evaluation of gas wettability and its effects on fluid distribution and fluid flow in porous media. Pet. Sci. 2013, 10, 515–527. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Haq, B.; Al Shehri, D.A. Hydrogen storage in depleted gas reservoirs using nitrogen cushion gas: A contact angle and surface tension study. Int. J. Hydrogen Energy 2023, 48, 38782–38807. [Google Scholar] [CrossRef]
- Khalaf, M.H.; Mansoori, G. Asphaltenes aggregation during petroleum reservoir air and nitrogen flooding. J. Pet. Sci. Eng. 2019, 173, 1121–1129. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Chen, X.; Sun, Y.; Zhao, L.; Han, T.; Li, T.; Weng, L.; Li, Y. A nitrogen supplement to regulate the degradation of petroleum hydrocarbons in soil microbial electrochemical remediation. Chem. Eng. J. 2021, 426, 131202. [Google Scholar] [CrossRef]
- Namdar, H.; Khodapanah, E.; Tabatabaei-Nejad, S.A. Comparison of base gas replacement using nitrogen, flue gas and air during underground natural gas storage in a depleted gas reservoir. Energy Sources Part. A Recover. Util. Environ. Eff. 2019, 42, 2778–2793. [Google Scholar] [CrossRef]
- Doan, Q.T.; Keshavarz, A.; Miranda, C.R.; Behrenbruch, P.; Iglauer, S. A prediction of interfacial tension by using molecular dynamics simulation: A study on effects of cushion gas (CO2, N2 and CH4) for Underground Hydrogen Storage. Int. J. Hydrogen Energy 2024, 50, 1607–1615. [Google Scholar] [CrossRef]
- Methane and Climate Change—Methane Tracker 2021—Analysis—IEA. Available online: https://www.iea.org/reports/methane-tracker-2021/methane-and-climate-change (accessed on 19 April 2023).
- Kim, J.; Maiti, A.; Lin, L.-C.; Stolaroff, J.K.; Smit, B.; Aines, R.D. New materials for methane capture from dilute and medium-concentration sources. Nat. Commun. 2013, 4, 1694. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Haq, B.; Al Shehri, D. Role of methane as a cushion gas for hydrogen storage in depleted gas reservoirs. Int. J. Hydrogen Energy 2023, 48, 29663–29681. [Google Scholar] [CrossRef]
- Mirchi, V.; Dejam, M.; Alvarado, V.; Akbarabadi, M. Effect of Cushion Gas on Hydrogen/Brine Flow Behavior in Oil-Wet Rocks with Application to Hydrogen Storage in Depleted Oil and Gas Reservoirs. Energy Fuels 2023, 37, 15231–15243. [Google Scholar] [CrossRef]
- Zeng, L.; Sander, R.; Chen, Y.; Xie, Q. Hydrogen Storage Performance During Underground Hydrogen Storage in Depleted Gas Reservoirs: A Review. Engineering 2024, in press. [Google Scholar] [CrossRef]
- Zivar, D.; Kumar, S.; Foroozesh, J. Underground hydrogen storage: A comprehensive review. Int. J. Hydrogen Energy 2021, 46, 23436–23462. [Google Scholar] [CrossRef]
- Amo, W.A. Costs of Storing and Transporting Hydrogen; National Renewable Energy Laboratory: Golden, CO, USA, 1999.
- Kobos, P.H.; Lord, A.S.; Borns, D.J.; Klise, G.T. A Life Cycle Cost Analysis Framework for Geologic Storage of Hydrogen: A User’s Tool (No. SAND2011-6221); Sandia National Laboratories (SNL): Albuquerque, NM, USA; Livermore, CA, USA, 2011.
- Lord, A.S.; Kobos, P.H.; Borns, D.J. Geologic storage of hydrogen: Scaling up to meet city transportation demands. Int. J. Hydrogen Energy 2014, 39, 15570–15582. [Google Scholar] [CrossRef]
- Sambo, C.; Dudun, A.; Samuel, S.A.; Esenenjor, P.; Muhammed, N.S.; Haq, B. A review on worldwide underground hydrogen storage operating and potential fields. Int. J. Hydrogen Energy 2022, 47, 22840–22880. [Google Scholar] [CrossRef]
- Song, Y.; Song, R.; Liu, J. Hydrogen tightness evaluation in bedded salt rock cavern: A case study of Jintan, China. Int. J. Hydrogen Energy 2023, 48, 30489–30506. [Google Scholar] [CrossRef]
- Kentish, S.E.; Scholes, C.A.; Stevens, G.W. Carbon dioxide separation through polymeric membrane systems for flue gas applications. Recent Pat. Chem. Eng. 2008, 1, 52–66. [Google Scholar] [CrossRef]
- Available online: https://www.engineeringtoolbox.com/ (accessed on 27 April 2024).
- Available online: http://www.peacesoftware.de/einigewerte/stickstoff_e.html (accessed on 27 April 2024).
- Smith, E.K.; Barakat, S.M.; Akande, O.; Ogbaga, C.C.; Okoye, P.U.; Okolie, J.A. Subsurface combustion and gasification for hydrogen production: Reaction mechanism, techno-economic and lifecycle assessment. Chem. Eng. J. 2024, 480, 148095. [Google Scholar] [CrossRef]
- Amiri, I.I.; Zivar, D.; Ayatollahi, S.; Mahani, H. The effect of gas solubility on the selection of cushion gas for underground hydrogen storage in aquifers. J. Energy Storage 2024, 80, 110264. [Google Scholar] [CrossRef]
- Aslannezhad, M.; Ali, M.; Kalantariasl, A.; Sayyafzadeh, M.; You, Z.; Iglauer, S.; Keshavarz, A. A review of hydrogen/rock/brine interaction: Implications for Hydrogen Geo-storage. Prog. Energy Combust. Sci. 2023, 95, 101066. [Google Scholar] [CrossRef]
- Thiyagarajan, S.R.; Emadi, H.; Hussain, A.; Patange, P.; Watson, M. A comprehensive review of the mechanisms and efficiency of underground hydrogen storage. J. Energy Storage 2022, 51, 104490. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).