Abstract
We report the case of a 44-year-old man with end-stage kidney disease (ESKD) who has been on chronic hemodialysis via a radio-cephalic arteriovenous fistula (RC-AVF) for one year. The patient arrived at the emergency department due to an inability to continue dialysis through the AVF. Clinical and ultrasound exams reveal an aneurysm in the cephalic vein, measuring 2.3 cm (cm) in diameter and 5 cm long, located in the middle third of the forearm, with intraluminal thrombosis. A surgical procedure is planned to exclude the aneurysmal segment and reconnect the vein, using a graft made from a bovine pericardium patch. Immediately after surgery, a thrill is detectable, and ultrasound shows a flow rate of 651 mL/min. On the second day, dialysis is performed through the distal cephalic vein segment under ultrasound guidance, avoiding the median forearm zone. At one month, the fistula remains functional, with no signs of thrombosis or stenosis. The bovine pericardium tubular graft has shown complete integration. This case supports the feasibility, safety, and potential advantages of using a tubularized bovine pericardial graft as an alternative conduit for RC-AVF reconstruction in select patients. However, further studies on larger cohorts and with extended follow-up are necessary to validate its reproducibility and long-term patency.
1. Introduction
According to the European Society of Vascular Surgery guidelines, the preferred initial vascular access (VA) for dialysis in patients with end-stage kidney disease (ESKD) is the radio-cephalic arteriovenous fistula (RC-AVF) [1]. Unfortunately, in the long term, the performance of RC-AVF is suboptimal, with a higher incidence of early failure reaching up to 40%, and a one-year secondary patency rate ranging from 42% to 71% [1]. The primary causes include intimal hyperplasia (IH), in situ thrombosis, and aneurysmal degeneration [2,3,4,5]. For treating these complications, both open surgical revision and endovascular procedures have shown effective outcomes [4,6,7]. However, aneurysmal AVF necessitates open revision surgery involving remodeling and reshaping of the aneurysmal vein [8,9,10,11]. Various techniques have been proposed and employed for the salvage of aneurysmal AVF, including aneurysmorrhaphy [8,9], aneurysm resection with end-to-end anastomosis [12], and partial aneurysm resection with patch angioplasty [13,14,15]. Additionally, in cases of severe stenosis, the use of a synthetic patch or bovine pericardium is often necessary to enlarge the local venous diameter and preserve the AVF [16,17,18].
Recently, considerable attention has been directed toward biological grafts specifically engineered for vascular access. The bovine carotid artery graft (BCAG) [19,20,21] remains the most extensively utilized option, demonstrating satisfactory long-term performance. Additionally, numerous studies have compared the performance of BCAG with expanded polytetrafluoroethylene (ePTFE) hemodialysis access grafts [22,23,24,25], indicating that BCAG exhibits superior primary and primary-assisted patency, as well as a lower incidence of infections. Furthermore, Abdoli et al. [26] and GnanaDev et al. [27] have shown that BCAG can be effectively employed in the revision of aneurysmal AVFs and can be cannulated early without an increased rate of complications or adverse effects on patency. Nonetheless, the primary limitation of BCAG is its high cost and limited availability across healthcare institutions.
The main scope of this study is to present the surgical management of reconstructing a thrombosed aneurysmal RC-AVF using a custom-made conduit, including a detailed step-by-step description of the process used to create a tubular bovine pericardial conduit.
2. Case Report
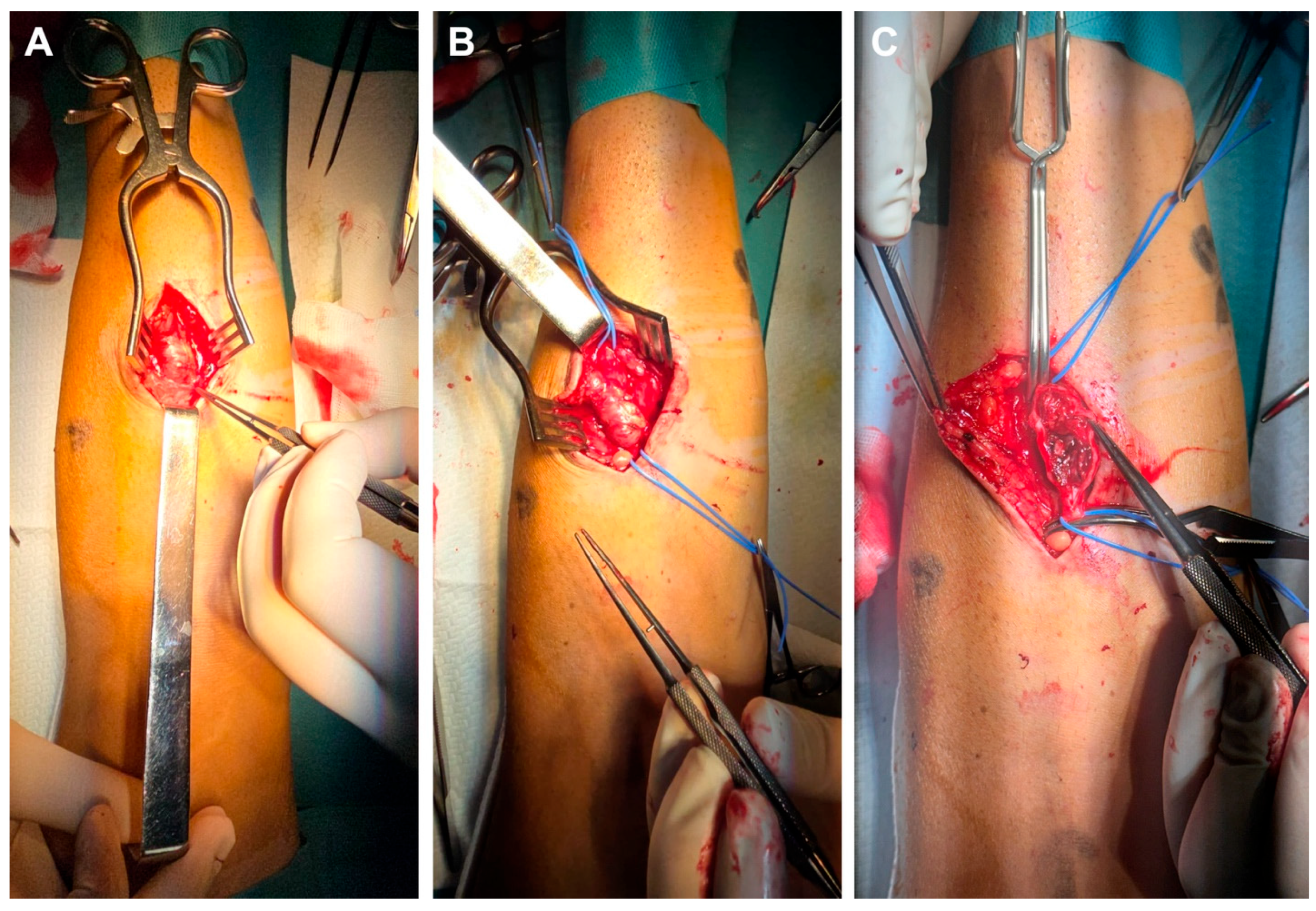
We present a 44-year-old male patient diagnosed with chronic kidney disease (CKD) in 2013, with progressive deterioration of renal function for 10 years, who was referred to the vascular surgery service for surgical creation of an AVF. In September 2023, the patient was admitted to the Vascular Surgery Clinic, where an RC-AVF was created on the left upper limb. Immediately post-operatively, a thrill was detected, and the vein diameter increased from 2.3 mm to 3.5 mm within the first 12 h after surgery. In evolution, the RC-AVF matures around 6 weeks after surgery, at which point dialysis is initiated. During follow-up, a slight dilation at the puncture site is noted, along with the appearance of an IH that progresses. By October 2024, the patient presents in the emergency department without a palpable thrill at the level of the AVF. During clinical examinations, a thrill was detected at the juxta-anastomotic region of the RC-AVF, which then diminished along the entire forearm. Ultrasound reveals blood flow at the RC-AVF anastomosis and extends over the first 2 cm of the vein, beyond which a thrombosed aneurysmal segment with a maximal diameter of 2.3 cm and length of 5 cm is identified. Distally, the cephalic vein is free of thrombus and compressible. In regional anesthesia, the aneurysmal segment is carefully dissected (Figure 1A) with the proximal and distal segments of the cephalic vein sufficiently exposed and isolated on a vessel loop (Figure 1B). Following the placement of the proximal and distal clamps, the aneurysm was opened lengthwise, and the lumen was irrigated with heparinized saline. The aneurysmal wall, along with attached thrombi, was partially removed (Figure 1C). Due to the poor quality of the remaining wall and the presence of IH, a direct aneurysmorrhaphy or patch angioplasty was deemed inappropriate. Therefore, the decision was made to remove the aneurysmal section and reconstruct the cephalic vein.
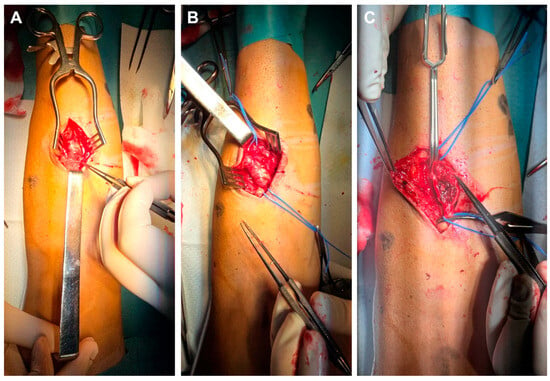
Figure 1.
Intraoperative view showing careful dissection of the aneurysmal segment of the RC-AVF (A), then vessel loop isolation of the proximal and distal segments of the non-aneurysmal cephalic vein (B), followed by clamping and opening the aneurysmal sac (C).
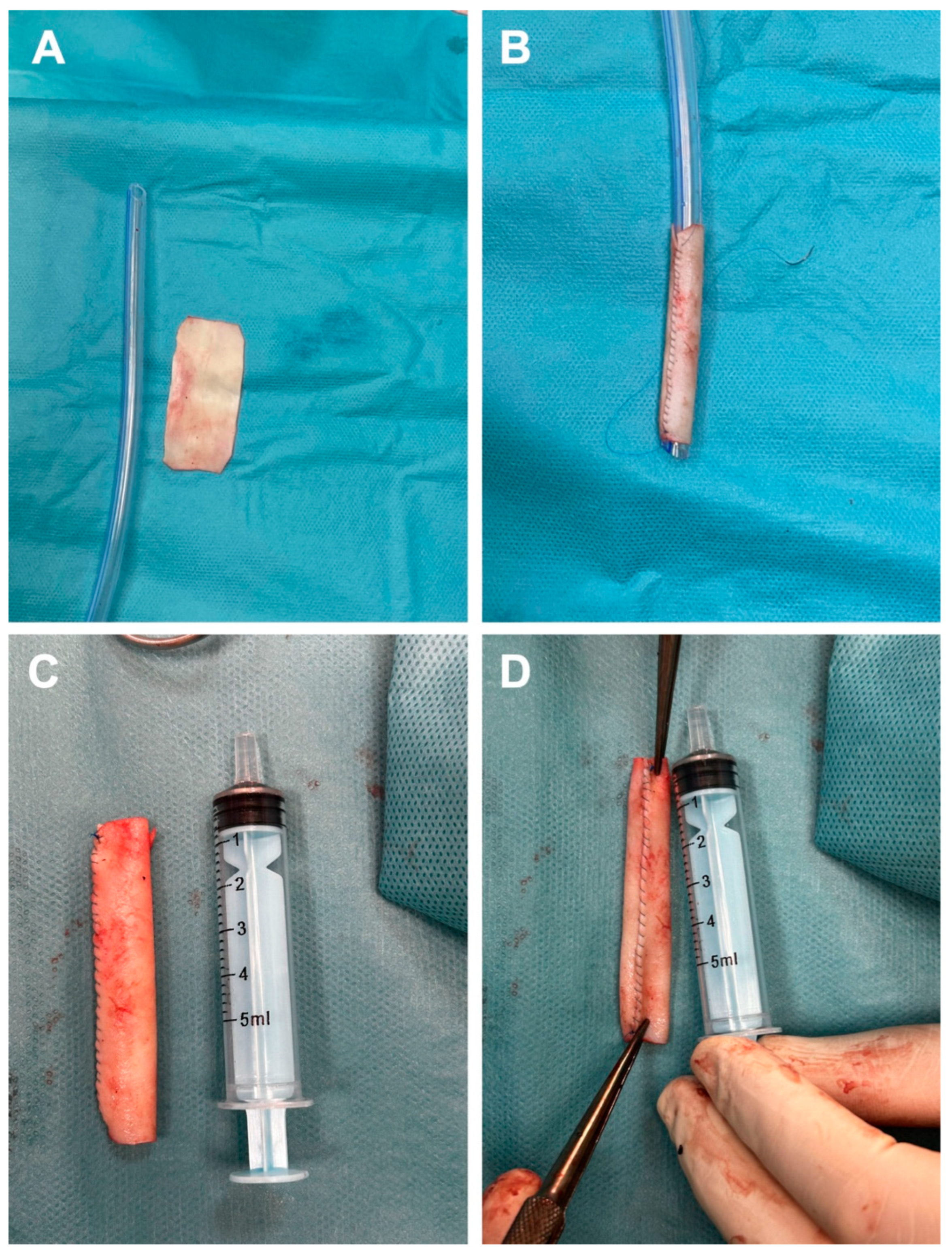
To avoid harvesting a venous graft that would leave a new scar and to minimize the risk of infection from a synthetic graft, we decided to create a tubular graft using bovine pericardium. Consequently, a rectangular patch measuring 5 cm in length and 2.5 cm in width (Figure 2A) was prepared and subsequently tabularized over a 24 Fr chest tube employing a continuous running suture with 5-0 Prolene (Figure 2B). Additionally, to facilitate more effective end-to-end anastomosis, the diameter of the newly fashioned graft was reduced at the ends to correspond with the size of the cephalic vein stumps (Figure 2C). For enhanced safety following the creation of the graft, the suture line was meticulously examined (Figure 2D).
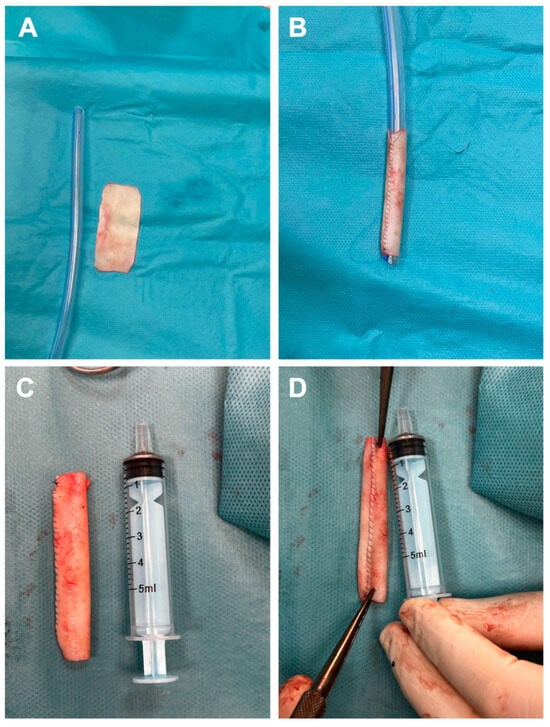
Figure 2.
Steps for the fabrication of bovine tubular pericardial duct include preparation of the rectangular specimen (A), subsequent tubularization of the specimen onto a 24 Fr thoracic tube (B), the final appearance of the neograft (C), and the appearance of the new conduit suture line (D).
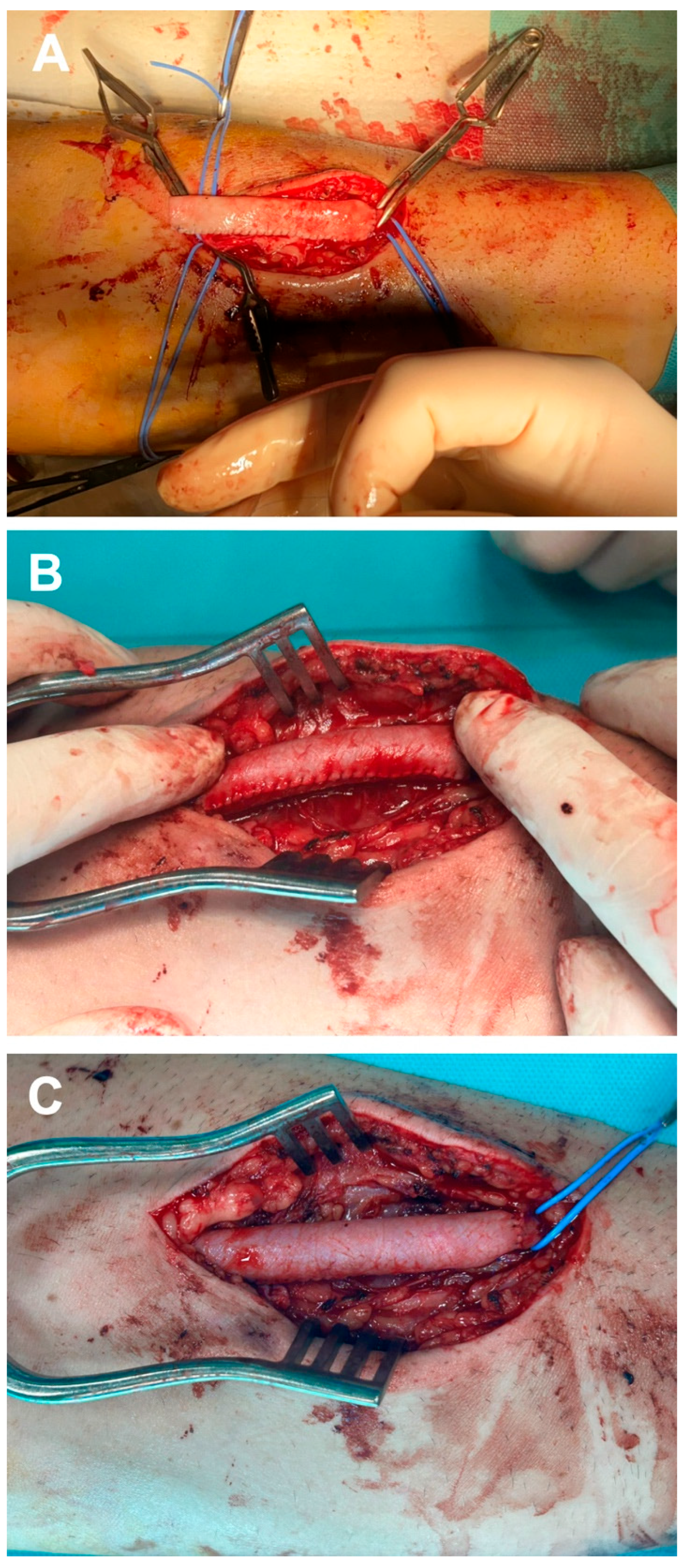
To prevent cannulation of the bovine pericardium suture line during dialysis, the newly created graft was positioned with the suture line laterally relative to the principal surgeon. Additionally, a continuous running suture was employed to perform an end-to-end anastomosis at the proximal stump of the cephalic vein using 5-0 Prolene sutures, followed by a distal anastomosis in an identical manner (Figure 3A). After removing the clamps, the thrill distal to the graft is confirmed, and the anastomoses and sutures of the tubular bovine pericardium graft are inspected for potential bleeding (Figure 3B). Once hemostasis is achieved (Figure 3C), the skin flap is closed using a simple interrupted 4-0 nylon suture. Anticoagulant therapy was not administered during the surgical procedure or in the postoperative period. Moreover, given that the procedure was conducted under regional anesthesia, the patient was mobilized eight hours after surgery, and consequently, prophylactic anticoagulant treatment was deemed unnecessary. Nevertheless, patients received prophylactic antibiotics, specifically cefuroxime 1.5 g administered intravenously intraoperatively and in three subsequent doses postoperatively.
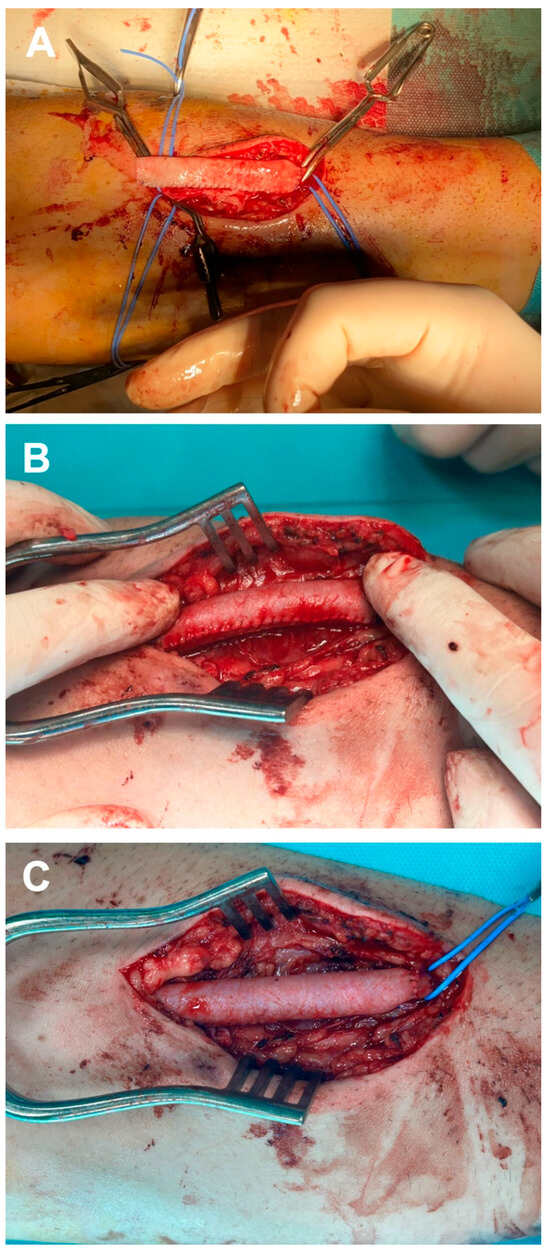
Figure 3.
Intraoperative aspect of the stages regarding the implantation of tubular bovine pericardial conduit: proximal anastomosis (A), assessment for potential bleeding at the suture line (B), and the final appearance of the neo-graft (C).
Immediately following the operation, a brachial artery flow of 651 mL/min was observed, with a diameter of at least 6 mm along the entire length of the cephalic vein. The subsequent day, the patient underwent dialysis via the RC-AVF, utilizing cannulation of the distal segment of the cephalic vein under ultrasound guidance, specifically avoiding the median forearm zone and the site where the bovine pericardium tubular graft had been implanted. The dialysis session was completed without complications, and the patient was discharged on the same day, continuing dialysis at the chronic center where he is registered.
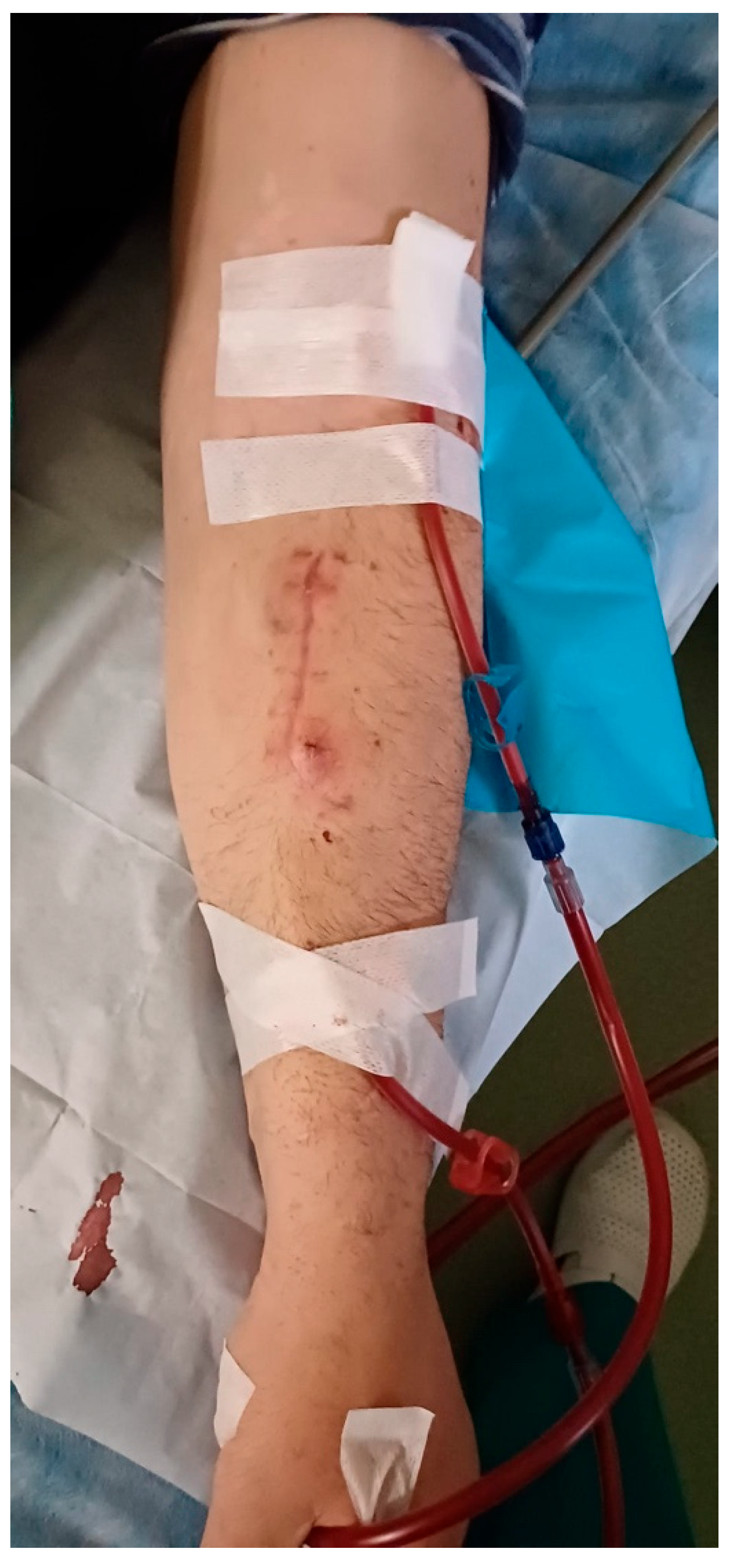
One month post-operatively, the patient is undergoing dialysis via the RC-AVF, with no issues encountered during the dialysis sessions (Figure 4). Furthermore, the surgical wound has healed without any complications, and there are no signs of inflammation or infection. The bovine pericardium tubular graft has shown complete integration.
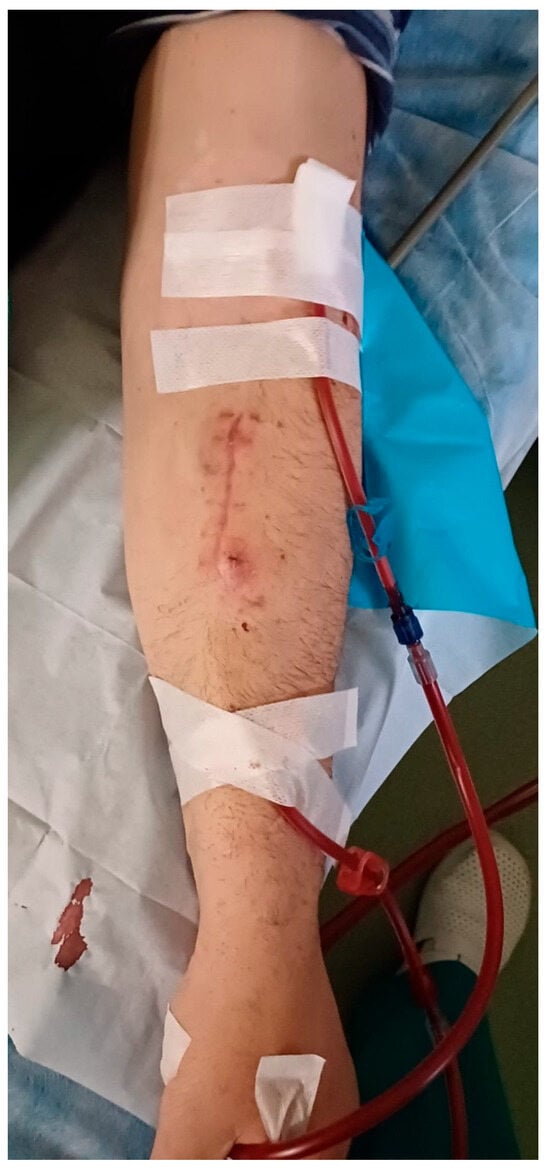
Figure 4.
Performing dialysis on the RC-AVF one month after surgery without any issues.
3. Discussion
The revision of aneurysmal AVFs continues to pose significant challenges for specialists in the field, as multiple surgical techniques are currently employed without a well-established protocol. Initially, Peter Balaz and Martin Björck [28] proposed that an aneurysmal AVF should possess a diameter exceeding 1.8 cm or be three times the diameter of the aneurysmal segment relative to the non-aneurysmal venous segment. Additionally, when describing AVF aneurysms, it is essential to consider the type of AVF, the number of aneurysms, and the specific classification of the aneurysm. Furthermore, Valenti et al. [29] proposed a novel classification system predicated on aneurysm morphology, categorizing them into four types, with the initial two types subdivided into two classes each. Thus, type 1a denotes a uniform aneurysmal dilation along the entire length of the venous segment, whereas type 1b indicates a dilation in the juxta-anastomotic area. For type 2a, the presence of at least one isolated segment, most often two portions, dilated aneurysmally, is observed; accordingly, type 2b is characterized as a combination of types 1b and 2a. In cases of heterogeneous and complex aneurysms, the AVF is classified as type 3. Lastly, the presence of false aneurysms is categorized as type 4 [29]. Several studies have investigated the use of acellular extracellular matrix (ECM) as a biological scaffold for AVF reconstruction [30,31,32]. Two studies by DuBose et al. [30,31] and Leskovar et al. [32], each enrolling six and fifteen patients, respectively, demonstrated that ECM can serve as an alternative conduit to salvage an AVF failure.
Several studies have validated the safety of bovine pericardium patches in dialysis AVF procedures, demonstrating their effectiveness in salvage, maturation, and reconstruction of AVFs. Fisher et al. [17] reported that patch angioplasty for recurrent stenosis in mature AVFs resulted in excellent patency rates (91% primary patency at 6 months), with no cases of infections or aneurysm development. In a recent study, Parker et al. [16] employed long-segment bovine pericardial patches to salvage non-maturing fistulas, achieving a maturation success rate of 92% and a 1-year patency of approximately 87%. These results are comparable to those of native fistulas. Importantly, no major complications related to the patches occurred, and more than half of the patients did not require any additional interventions [16]. In the study by Meecham et al. [33], central venous occlusive disease was managed by patch dilating the iliac vein, resulting in a patent dialysis graft after 3 years. Likewise, Bae and Kim [34] utilized a “tongue”-shaped bovine patch to dilate a stenotic segment near the anastomosis, thereby preserving the native vein for cannulation and achieving long-term success over a two-year period. Although one comparative study found no difference in patency rates between patch angioplasty and endovascular stenting in thrombosed AV access, it noted that surgical patching is more invasive [35].
Overall, the literature suggests that bovine pericardial patch angioplasty is a reliable and effective technique for AVF surgery, utilizing a biological scaffold that resists infection and facilitates early needle cannulation. It serves as a useful addition for stenosis repair, aneurysm reinforcement, and maturation in complex dialysis access procedures.
In our case, we chose to preserve the RC-AVF to maximize the possibility of performing a more proximal AVF later, especially since our patient is young. The major limitation of the presented case is the limited duration of follow-up. Therefore, prospective studies involving large cohorts are necessary to validate these findings and to assess the risk of restenosis and the long-term performance of the tubular conduit made from bovine pericardium. Each patient with an aneurysmal AVF should be evaluated individually and monitored by a multidisciplinary team, including a vascular surgeon and a nephrologist, to determine the best management approach. If there is no clear cause for the aneurysmal AVF, such as subclavian vein or cephalic arch stenosis that can be addressed endovascularly, surgical intervention remains a viable option with good outcomes. This case may represent an ideal candidate due to the patient’s anatomy, the morphological characteristics of the aneurysmal AVF, and limited comorbidities; considerations that may not apply to all patients.
4. Conclusions
This case demonstrates the successful reconstruction of a thrombosed aneurysmal RC-AVF using a tubular bovine pericardial conduit. The use of bovine pericardium enabled effective vascular reconstruction while minimizing the morbidity associated with autologous vein harvesting or the use of synthetic grafts. The graft provided immediate post-operative functionality, enabling early resumption of dialysis without complications. At one month, postoperatively, the graft remains patent and fully integrated, with no signs of infection, inflammation, or access dysfunction. This case supports the feasibility, safety, and potential advantages of using a tubularized bovine pericardial graft as an alternative conduit for RC-AVF reconstruction in select patients. However, further studies on larger cohorts and with extended follow-up are necessary to validate its reproducibility and long-term patency.
Author Contributions
Conceptualization, methodology, writing—original draft preparation, A.-A.U.-N., L.-A.S. and E.-M.A. (Emil-Marian Arbănași); software, formal analysis, investigation, resources, data curation, I.H., M.M.H. and C.C.C.; validation, A.-A.U.-N., L.-A.S., E.F., N.-A.L., S.-V.Ș., A.V.M., E.R., R.B., E.-M.A. (Eliza-Mihaela Arbănași), I.H., M.M.H., C.C.C. and E.-M.A. (Emil-Marian Arbănași); writing—review and editing, R.B. and E.-M.A. (Eliza-Mihaela Arbănași); visualization, supervision, project administration, E.F., N.-A.L., S.-V.Ș.; funding acquisition, A.V.M., E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the George Emil Palade University of Medicine, Pharmacy, Science and Technology of Târgu Mureș, Romania, research grant number 170/3/09.01.2024.
Institutional Review Board Statement
The present study was approved by the Medical Ethics Committee for the Clinical Study of the Drug within the framework of the Târgu Mureș County Emergency Clinical Hospital, Romania, decision no. 28622/18.11.2024; approval date: 18 November 2024.
Informed Consent Statement
Informed consent was obtained from the patient involved in the study.
Data Availability Statement
The original contributions of this study are included in the article. For further inquiries, please contact the corresponding author.
Acknowledgments
This work was supported by the George Emil Palade University of Medicine, Pharmacy, Science and Technology of Târgu Mureș, Romania, with research grant number 170/3/09.01.2024.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| VA | vascular access |
| ESKD | end-stage kidney disease |
| RC-AVF | radio-cephalic arteriovenous fistula |
| IH | intimal hyperplasia |
| CKD | chronic kidney disease |
| ECM | extracellular matrix |
References
- Schmidli, J.; Widmer, M.K.; Basile, C.; de Donato, G.; Gallieni, M.; Gibbons, C.P.; Haage, P.; Hamilton, G.; Hedin, U.; Kamper, L.; et al. Editor’s Choice—Vascular Access: 2018 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 757–818. [Google Scholar] [CrossRef]
- Inston, N.; Mistry, H.; Gilbert, J.; Kingsmore, D.; Raza, Z.; Tozzi, M.; Azizzadeh, A.; Jones, R.; Deane, C.; Wilkins, J.; et al. Aneurysms in Vascular Access: State of the Art and Future Developments. J. Vasc. Access 2017, 18, 464–472. [Google Scholar] [CrossRef]
- Heindel, P.; Yu, P.; Feliz, J.D.; Hentschel, D.M.; Burke, S.K.; Al-Omran, M.; Bhatt, D.L.; Belkin, M.; Ozaki, C.K.; Hussain, M.A. Radiocephalic Arteriovenous Fistula Patency and Use: A Post Hoc Analysis of Multicenter Randomized Clinical Trials. Ann. Surg. Open 2022, 3, e199. [Google Scholar] [CrossRef]
- Kim, H.K.; Kwon, T.-W.; Cho, Y.-P.; Moon, K.-M. Outcomes of Salvage Procedures for Occluded Autogenous Radiocephalic Arteriovenous Fistula. Ther. Apher. Dial. 2011, 15, 448–453. [Google Scholar] [CrossRef]
- Kordzadeh, A.; ISRAF Collaborators; Mohaghegh, V.; Inston, N. International Survey of Radiocephalic Arteriovenous Fistula: ISRAF Survey. J. Vasc. Access 2025, 26, 477–486. [Google Scholar] [CrossRef]
- Chan, P.G.; Goh, G.S. Safety and Efficacy of the AngioJet Device in the Treatment of Thrombosed Arteriovenous Fistula and Grafts: A Systematic Review. J. Vasc. Access 2018, 19, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.; Freedman, J.; Jaffe, S.; Wilmink, T. Comparison of Surgical and Radiological Interventions for Thrombosed Arteriovenous Access. J. Vasc. Access 2018, 19, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Pourriyahi, H.; Pourriahi, H.; Najd Sepas, H. Salvage Aneurysmorrhaphy as an Adaptable and Still Pertinent Technique in the Management of Challenging True Aneurysms of Arteriovenous Fistulas: A Case Series of Different Variations, With Illustrative Surgical Pictures. EJVES Vasc. Forum 2024, 61, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Płoński, A.; Płoński, A.F.; Głowiński, J. Surgical Management, Prevention and Outcomes for Aneurysms of Arteriovenous Dialysis Fistulas: A Case Series Study and Review. Int. J. Environ. Res. Public Health 2023, 20, 6256. [Google Scholar] [CrossRef] [PubMed]
- Arbănaşi, E.-M.; Russu, E.; Mureşan, A.V.; Arbănaşi, E.-M.; Kaller, R. Ulnar-Basilic Arteriovenous Fistula with Multilocular Gigantic Aneurysmal Dilatation: A Case Report. Acta Marisiensis-Ser. Medica 2021, 67, 244–246. [Google Scholar] [CrossRef]
- Kaller, R.; Mureșan, A.V.; Arbănași, E.M.; Arbănași, E.M.; Kovács, I.; Horváth, E.; Suciu, B.A.; Hosu, I.; Russu, E. Uncommon Surgical Management by AVF between the Great Saphenous Vein and Anterior Tibial Artery for Old Radiocephalic AVF Failure. Life 2022, 12, 529. [Google Scholar] [CrossRef]
- Hejna, E.E.; Chang, J.; Terranella, S.L.; Trawczynski, M.H.; Hollinger, E.F.; Jensik, S.C.; Olaitan, O.; Hertl, M.; Chan, E.Y. End-to-End Anastomosis as a Superior Repair Type to Prevent Recurrence of Arteriovenous Fistula Aneurysms and Improve Patency Outcomes. J. Vasc. Access 2024, 25, 531–537. [Google Scholar] [CrossRef]
- Piccolo, C.; Madden, N.; Famularo, M.; Domer, G.; Mannella, W. Partial Aneurysmectomy of Venous Aneurysms in Arteriovenous Dialysis Fistulas. Vasc. Endovasc. Surg. 2015, 49, 124–128. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.S. Successful Use of Partial Aneurysmectomy and Repair Approach for Managing Complications of Arteriovenous Fistulas and Grafts. J. Vasc. Surg. 2017, 66, 545–553. [Google Scholar] [CrossRef]
- Wan, Z.; Lai, Q.; Zhou, Y.; Chen, L.; Tu, B. Partial Aneurysmectomy for Treatment of Autologous Hemodialysis Fistula Aneurysm Is Safe and Effective. J. Vasc. Surg. 2019, 70, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.H.; Hashemi, H.; Grant, C.; Colpitts, D.K.; Kfoury, E.; Mukherjee, D.; Neville, R. A Novel Technique Using Long Segment Patch Angioplasty Maturation to Increase the Maturation Rate of Arteriovenous Fistulas. J. Vasc. Surg. 2021, 74, 230–236. [Google Scholar] [CrossRef]
- Fisher, O.; Meecham, L.; Buxton, P.; Legge, J.; Fairhead, J.; Rajagopalan, S.; Asquith, J.; Pherwani, A. Long-Term Outcomes of Bovine Pericardial Patch Angioplasty for Recurrent Stenosis in Vascular Access: A UK Single-Centre Experience. J. Vasc. Access 2018, 19, 658–662. [Google Scholar] [CrossRef]
- Sowa, P.; Halandras, P.; Blecha, M. The Safety of Bovine Tissue Arterial Repair in Removal of Infected Prosthetic Hemodialysis Grafts. J. Vasc. Access 2021, 22, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Katzman, H.E.; Schild, A.F.; Vanderwerf, B.A. Bovine artegraft arteriovenous fistulas for hemodialysis in one-hundred patients after “conventional” arteriovenous fistulas failed. Vasc. Surg. 1976, 10, 169–175. [Google Scholar] [CrossRef]
- Harlander-Locke, M.; Jimenez, J.C.; Lawrence, P.F.; Gelabert, H.A.; Derubertis, B.G.; Rigberg, D.A.; Farley, S.M. Bovine carotid artery (Artegraft) as a hemodialysis access conduit in patients who are poor candidates for native arteriovenous fistulae. Vasc. Endovasc. Surg. 2014, 48, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Pineda, D.M.; Dougherty, M.J.; Wismer, M.C.; Carroll, C.; Tyagi, S.; Troutman, D.A.; Calligaro, K.D. Bovine carotid artery xenografts for hemodialysis access. J. Vasc. Surg. 2017, 65, 1729–1734. [Google Scholar] [CrossRef]
- Kostakis, I.D.; Loukopoulos, I. Comparison between bovine carotid artery graft and polytetrafluoroethylene graft for haemodialysis vascular access: A systematic review and meta-analysis. J. Vasc. Access 2021, 22, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Marcus, P.; Echeverria, A.; Cheung, M.; Kfoury, E.; Shim, K.; Lin, P.H. Early Cannulation of Bovine Carotid Artery Graft Reduces Tunneled Dialysis Catheter-Related Complications: A Comparison of Bovine Carotid Artery Graft Versus Expanded Polytetrafluoroethylene Grafts in Hemodialysis Access. Vasc. Endovasc. Surg. 2019, 53, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Kraai, T.W.; Looman, R.S.; Drouven, J.W.; Gareb, B.; Saleem, B.R.; Zeebregts, C.J. Bovine Carotid Artery Grafts versus PTFE grafts for hemodialysis access: A systematic review and meta-analysis. Ann. Vasc. Surg. 2025, 122, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Albert, P.; Connelly, Z.M.; Lairmore, T.C.; Virk, C. A Retrospective Review of Bovine Artery Graft Patency: A Single-Site Study. Ann. Vasc. Surg. 2023, 94, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Abdoli, S.; Mahajan, A.; Han, S.M.; Rowe, V.L.; Katz, S.G.; Ochoa, C.J. Early cannulation of bovine carotid artery grafts (Artegraft) after primary vascular access and fistula revision procedures. J. Vasc. Surg. 2018, 68, 1865–1871. [Google Scholar] [CrossRef]
- GnanaDev, R.; Malkoc, A.; Jeney, A.B.; Mikael, A.; Andacheh, I. A Multicenter Analysis of Revision of Aneurysmal Dialysis Access Using Bovine Carotid Artery Conduit. Ann. Vasc. Surg. 2023, 97, 399–404. [Google Scholar] [CrossRef]
- Balaz, P.; Björck, M. True Aneurysm in Autologous Hemodialysis Fistulae: Definitions, Classification and Indications for Treatment. J. Vasc. Access 2015, 16, 446–453. [Google Scholar] [CrossRef]
- Valenti, D.; Mistry, H.; Stephenson, M. A Novel Classification System for Autogenous Arteriovenous Fistula Aneurysms in Renal Access Patients. Vasc. Endovasc. Surg. 2014, 48, 491–496. [Google Scholar] [CrossRef]
- DuBose, J.J.; Azizzadeh, A. Utilization of a Tubularized Cormatrix Extracellular Matrix for Repair of an Arteriovenous Fistula Aneurysm. Ann. Vasc. Surg. 2015, 29, 366.e1–366.e4. [Google Scholar] [CrossRef]
- DuBose, J.J.; Fortuna, G.R.; Charlton-Ouw, K.M.; Saqib, N.; Miller, C.C.; Estrera, A.L.; Safi, H.J.; Azizzadeh, A. Utility of a Tubularized Extracellular Matrix as an Alternative Conduit for Arteriovenous Fistula Aneurysm Repair. J. Vasc. Surg. 2016, 63, 446–452. [Google Scholar] [CrossRef][Green Version]
- Leskovar, B.; Furlan, T.; Poznic, S.; Hrastelj, M.; Adamlje, A. Using CorMatrix for Partial and Complete (Re)Construction of Arteriovenous Fistulas in Haemodialysis Patients: (Re)Construction of Arteriovenous Fistulas with CorMatrix. J. Vasc. Access 2019, 20, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Meecham, L.; Fisher, O.; Kirby, G.; Evans, R.; Buxton, P.; Legge, J.; Rajagopalan, S.; Asquith, J.; Pherwani, A. Simultaneous Iliac Vein Bovine Pericardial Patch Venoplasty and Creation of PTFE Lower Limb Arteriovenous Fistula Graft for Rescue Vascular Access. Ann. Vasc. Surg. 2016, 36, 292.e9–292.e11. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, J. Tongue patch angioplasty for small-caliber vein segments directly connected to juxta-anastomotic stenosis. J. Vasc. Access 2025, 26, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Trinh, K.N.; Wilson, S.E.; Gordon, I.L.; Williams, R.A. Postintervention Patency: A Comparison of Stenting versus Patch Angioplasty for Dysfunctional Hemodialysis Access Sites. Ann. Vasc. Surg. 2016, 33, 120–125. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).