Abstract
The incidence of kidney diseases has been increasing in the last decade due to extended lifespan, which is often related to polymorbidity. Chronic kidney disease (CKD) and acute kidney injury (AKI) are associated with high morbidity and mortality, elevated costs for renal replacement therapy, and heavy psychosomatic burden. At the same time, therapeutic options are limited to prophylactic and renoprotective medications and measurements, and they often cannot restore the impaired kidney function. With the development of cellular therapies, new perspectives arise on the horizon with promising potential, including mesenchymal stem cells (MSCs) and induced pluripotent cells (iPSCs). Here we review the current possibility of both cell types in the field of nephrology and assess their cost implication.
1. Introduction
The rising incidence of kidney diseases is an alarming trend which leads to critical health and financial consequences. It is estimated that almost one billion people in low income and low–middle income countries have kidney diseases, which are undiagnosed due to lack of secondary prevention. This is a concerning phenomenon, since the current therapy of many nephrological conditions is limited to avoiding and delaying the progression to chronic kidney disease (CKD) [1]. Many kidney diseases cause glomeruli to be replaced by fibrotic tissue and that results in impaired organ function, potentially leading to the need for renal replacement therapies (RRT) [2]. CKD, dialysis, and transplantation are related to high morbidity and mortality rates, and at the same time represent a high economic burden on the population [1]. Additionally, acute kidney injury (AKI) is another syndrome in nephrology which can be caused by different etiological factors, but most importantly it is a common complication in critically ill patients and is related to higher mortality and costs as well. In most cases blood pressure control, fluid management, exclusion of nephrotoxic agents and in severe cases RRT are among the few options that clinicians have to treat this condition [3,4]. That is to say that the two most common syndromes in the field of nephrology possess high incidence and can lead to fatal complications, while at the same time management opportunities are limited and sometimes very expensive. New etiological drugs are needed that can decrease morbidity and mortality and improve the quality of life of these patients [4,5].
The development of cellular therapies, and more precisely MSCs and iPSCs, gives us new therapeutic options. MSCs possess many beneficial effects; e.g., regenerative, anti-inflammatory, immunomodulating, antifibrotic, and others. They appear to specifically target certain common pathological processes in kidney diseases, but the main scientific data is still based on in vitro experiments, animal models, and only a few clinical trials with restricted number of patients [6].
On the other hand, iPSCs are promising models for in vitro experiments that can improve our understanding of kidney diseases. They can be used for regenerative purposes, but the main limitation remains the danger of an onset of oncological diseases [7,8].
The results of many preclinical and clinical trials indicate their effectiveness, but still there are no available cellular therapies in routine clinical practice [9]. Here, we review the applications of cellular therapies in the field of nephrology and assess their economic potential.
In the field of nephrology, there is a vast need for treatment options, since incidence rates of acute kidney injury (AKI) and chronic kidney disease (CDK) are rising and at the same time therapeutic options are limited to preventative and renoprotective measurements and renal replacement therapies (RRT) [1].
CKD is a medical condition of progressively deteriorating kidney function, which, according to KDIGO, is defined as abnormalities of kidney structure and function for more than 3 months and decline of glomerular filtration rate (GFR) under 60 mL/min/1.73 m2. Different pathological processes can induce permanent kidney impairment but the most common are diabetes mellitus, hypertension, and glomerulonephritis. CKD usually develops after loss of nephrons, which consequently leads to compensatory hyperfiltration and hypertrophy of the remaining healthy nephrons, followed by changes in glomerular structure and podocytes. Gradually, this process ends with nephrosclerosis: interstitial fibrosis, glomerulosclerosis, and decrease of renal function. Immune-mediated inflammation, epithelial-to-mesenchymal transition (EMT), and extracellular matrix (ECM) reorganization by metalloproteases are well-established pathophysiological mechanisms behind CKD development. Common management strategies are changes in diet, weight loss, avoidance of nephrotoxins, use of RAAS and SGLT inhibitors, aldosterone antagonists, and control of underlying disease [5,10]. Despite the standard care, the kidney impairment often progresses with different pace. Some CKD pathological processes can possibly be targeted by cellular therapies [2,11,12].
AKI is another nephrological syndrome that manifests with an increase in serum creatinine and decrease of urine output or both. It has a diverse etiology (e.g., ischemia and nephrotoxins) and is a usual complication affecting almost 15% of hospitalized patients and more than half of the patients in an ICU. Furthermore, it is related to higher mortality and costs. It is often classified as pre-renal (e.g., hypoperfusion), renal (e.g., glomerular diseases), or post-renal (e.g., obstruction of the voiding system of the kidney), referring to the cause. The therapeutic opportunities are limited to fluid management, blood pressure control, initiation of RRT, and prevention of complications, and thus cellular therapies can enrich the treatment modalities [3,4,13].
A key point for clinical intervention is AKI to CKD transition, when processes of cell death, inflammation, epithelial-to-mesenchymal transition (EMT), and extracellular matrix (ECM) reorganization are still ongoing and reversible. Induction of regeneration and inhibition of fibrotic processes by different cellular products may be a revolutionary innovation [14].
2. Mesenchymal Stem Cells and Kidney Diseases
A promising therapeutic perspective is mesenchymal stem cells (MSCs), which have been extensively investigated in the last two decades and clinical trials have shown multiple benefits of their usage. The International Society of Cell & Gene Therapy defines MSCs as plastic-adherent cells that express CD73, CD90, and CD105 and are lacking the expression of hematopoietic and endothelial markers CD11b, CD14, CD19, CD34, CD45, CD79a, and HLA-DR. Mesenchymal stem cells can differentiate into various tissues in vitro and in vivo and are capable of self-renewal [15,16]. They can be isolated from different tissues: adipose, bone marrow, umbilical cord, dental pulp, muscle, and many others. Although MSCs of different origins share similar fibroblast-like morphology and surface markers, they vary in their differentiation capacity and protein expression pattern and ISCT recommends annotating the tissue they are derived from. For research and clinical purposes adipose (AD), bone marrow (BM), and umbilical cord (UC) tissues are most often used since they are accessible and relatively safe [15,17,18,19].
The MSCs possess regenerative, anti-inflammatory, immunomodulating, antifibrotic, proangiogenic, and antiapoptotic effects, which make them a promising perspective for treatment of many diseases [20]. Even though in recent years MSCs have been extensively investigated, the intimate mechanism by which they affect the damaged tissues is not fully understood. Some researchers point out their homing ability to be of great importance, since it allows them to migrate to injured tissues, where they induce regeneration by cell-to-cell contact and secretion of different active molecules [21,22,23]. Others reported that most of the MSCs are trapped in lungs vessels after intravenous application and their effect is mediated by extracellular vesicles with different size and origin, including exosomes (30–150 nm in diameter), microvesicles (150–500 nm in diameter), and apoptotic bodies (800–500 nm in diameter). Thus chemokines, interleukins, and growth factors that are contained in the vesicles affect the targeted organ by paracrine mechanism [24,25].
All the characteristics mentioned above make MSCs promising therapeutic option for nephrology patients, since incidence rates of acute kidney injury (AKI) and chronic kidney disease (CDK) are rising while at the same time their management is limited to preventative and renoprotective measurements and renal replacement therapies
Figure 1 summarizes the beneficial effects that MSCs can have on damaged kidneys and their possible mechanism. It is shown in vivo and in vitro that MSC therapy reduces scarification of the kidney by suppressing a key regulator of this process—TGF-β1/TGF-βR1—and thus decreases collagen 4α1 accumulation. miR-let7c was identified as a responsible mediator, which can additionally downregulate metalloprotease 9, an enzyme known to contribute to renal ECM reorganization by fibrosis [2,26]. TGF-β is known to promote fibrosis by enhancing production of extracellular proteins and simultaneously downregulate the expression of matrix degrading plasminogen-activator inhibitor [27]. This is an important possibility for treating interstitial fibrosis and glomerulosclerosis, which are common pathological processes leading to CKD progression. Additionally, it was shown in other tissues that chemokines such as chemokine ligand 2 (CCL2) and C-X-C motif chemokine 12 (CXCL12) secreted by MSCs induce the differentiation of anti-inflammatory M2 macrophages [28]. These cells are known to contribute to tissue repair and decrease scarring by reducing inflammatory cytokines and increasing IL-10 levels in different tissues [29]. Furthermore, Moroni et al. reported that macrophages can ameliorate liver fibrosis by upregulating metalloproteases, which degrade the fibrotic ECM and decrease inflammation, thus promoting liver regeneration in patients—a finding previously observed in mouse model. This macrophage-mediated action could be another possible anti-fibrotic mechanism of MSCs in kidney diseases [30].
The immunomodulatory effect of MSCs is crucial for the management of nephrosclerosis, glomerulonephritis, and kidney transplantation, since innate and acquired immunity are a general regulator of these processes [31,32]. It was shown that injured kidneys are infiltrated by immune cells—macrophages, T and B -lymphocytes, and NK cells—and they promote interstitial fibrosis and glomerular sclerosis [11,27]. MSCs inhibit the production of proinflammatory cytokines like TNF-α, INF-γ, IL-1, and IL-6, while increasing the level of their antagonistic, IL-10 [33]. These cells can induce T regs, thus promoting kidney regeneration and simultaneously suppressing inflammation [34,35]. Additionally, data from preclinical experiments shows that MSCs can inhibit the maturation of dendritic cells and by mitochondrial transfer to macrophages can and thus promote anti-inflammatory phenotypes [36]. Swaminathan et al. reported that MSCs modulate the immune response in patients with AKI by shifting the cytokine profile from a pro-inflammatory Th1 to an anti-inflammatory Th2 cytokine profile. The group observed that in treated patients, the levels of chemo-attractants like CCL2, CCL7, and CCL8 are lower, and consequently, monocyte infiltration is significantly diminished. Furthermore, Th2 cytokines induce the infiltrating monocytes to predominantly differentiate into M2 macrophages, which promote tissue regeneration and healing. This effect is confirmed by a reduction of the kidney injury markers kidney injury molecule-1 and osteoactivin [37]. Other authors have also reported that the immunomodulatory effects of MSCs are mediated by the suppression of Th1 and Th17 cells and a shift toward Th2 and Treg cell phenotypes. MSC-generated Tregs are known to suppress the activation of almost all immune cells. However, MSCs are unable to suppress cytotoxic T cells if they are already activated [38].
Glomerulonephritis is a heterogeneous group of kidney diseases, and their etiology is mainly related to immune system dysregulation, which makes them an attractive target for MSCs therapy [39]. Rampino et al. reported that in an experimental animal model treatment with MSCs intravenously can significantly decrease proteinuria, serum levels of creatinine, IL-6 and TGF-β, while sparing glomeruli from monocyte infiltration and injury. Some of the stem cells were found in the kidney, which supports the hypothesis of their homing ability [40]. These findings were confirmed by Kunter et al. with similar demonstration, with the difference that only application in renal artery gave significant results while MSCs intravenous infusion failed to reproduce the regenerative outcomes [41]. Interestingly, another group that compared the effect of everolimus to BM-MSCs administration found that despite the reduced expression of renal T cells, IL-17, and activated macrophages, they were unable to inhibit glomerular injury and apoptosis [42]. Although these articles have controversial results at first glance, a deeper review can provide an explanation. First, Rampino et al. and Kunter et al. used higher cell number respectively 2 × 106 and 3 × 106, while Zedan et al. injected 1 × 105–1 × 106 cells. Second, the latter group did not characterize the cell suspension according to ISCT prescriptions and they probably injected a heterogeneous group of cells, not only MSCs. These differences provoke us to consider that in comparison to other chemical drugs, MSCs are a “living medicine” and the number of isolated cells, their fitness and condition, and their distinct origin, as well as their multipotent effects, make cellular therapies very challenging and well-regulated reproducible protocols are needed [25].
The immunomodulatory effects of this cellular therapy were further proved in a clinical case of a patient with vasculitis and kidney involvement, where standard care fails to induce remission, but allogenic MSCs application led to recovery with no adverse events [43].
Besides their effect on the immune system, MSCs release trophic factors like hepatocyte growth factor (HDF), insulin-like growth factor-1 (IGF-1), and microRNAs miR10a and miR486, which induce reparative processes [44]. Mitochondrial transfer towards renal cells with dysfunctional mitochondria can promote regeneration, as well [45]. In addition, by increasing level of the VEGF and decreasing HIF-1α, mesenchymal cells are reversing rarefication of the kidney vasculature, and thus encouraging declining ischemia. Upregulation of antiapoptotic genes (Bcl-2, Bcl-xL, BIRC8) with simultaneous suppression of proapoptotic genes also contribute to the protective effect of MSCs on tubular cells [33,46]. Their regenerative potential is further supported by preclinical experimental data, which shows MSCs to promote activation in tubular progenitor stem cells. By releasing miR-125b-5p they can also regulate cell cycle arrest and apoptosis of tubular cells by influencing p53 signaling pathway [47,48].
Considering the numerous benefits of this therapy, over 50 clinical trials related to various nephrological diseases are currently listed on clinicaltrials.gov. Many papers from the recent past confirm the good tolerability of MSCs but are controversial about their efficacy in CKD, AKI, immune-mediated nephritis, and kidney transplantation. These differences are related to the many variable factors like isolation from distinct tissues: BM, AD, and UC, variations between donors and protocols, and many others. An opportunity for minimization of this deviation is the use of suspension of allogenic stem cells from a few to dozen healthy donors [49]. Application methods can differ as well; e.g., intravenously [50] or in renal parenchyma [51].
In addition, there are some adverse drug reactions that need to be considered and further investigated. Ex vivo culturing of the cells is related to changes in their cytokine profile, gene expression, and even chromosomal aberrations. Longer incubation causes higher replicative stress and even spontaneous malignant transformation is reported. MSCs have a safer profile compared to iPSC and embryonic stem cells [52] regarding the risk of causing a neoplasm. However, it is reported that in study with 2372 patients treated with MSCs, 0.3% were diagnosed with cancer, but the authors disclaim relation between the two events. A further disadvantage of this type of cell therapy is the risk of contamination, mostly with viruses from the donor or by cell processing, but also with xenoantigens from culture medium. Most serious complications are related to thromboembolism, since MSCs express tissue factors that can activate coagulation by intravenous application. Especially dangerous is the infusion of higher doses. Importantly, experimental data and a single clinical case indicate that MSCs can possibly differentiate into myofibroblasts and accelerate the scarring of the kidney [53].
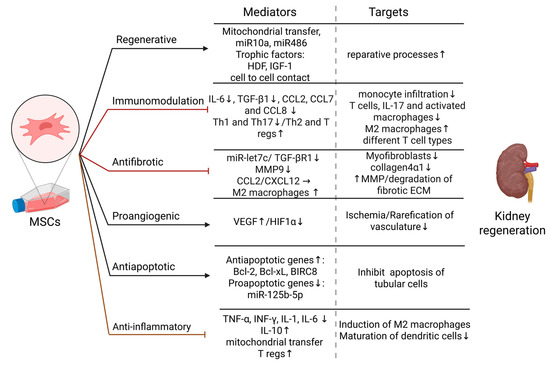
Figure 1.
Effects of MSCs on kidney regeneration—mediators and targets. Regenerative effect: Reparative processes are induced by MSCs by mitochondrial transfer, miR10a and miR486, secretion of trophic factors: HDF and IGF-1, and cell to cell interaction with kidney cells [21,44,45]; Immunomodulation: MSCs decrease the levels of IL-6 and TGF-β1, and CCL2,7,8, suppress pro-inflammatory Th-1 and Th17 cells, and induce anti-inflammatory Th2 cytokine profile and T regs. Consequently, the number of infiltrated monocytes and T cells in kidney tissue are reduced, activation of T cells is inhibited, infiltrated monocytes are differentiated into M2-like phenotype macrophages [37,38,40]; Antifibrotic effect: MSCs therapy suppresses TGF-β1 and miR-let7c and thus inhibit renal fibrosis by decreasing collagen 4α1 accumulation and MMP9 expression [26,27]. Additionally by CCL2 and CXCL12 secretion MSCs induce M2-like phenotype macrophages, which are shown to degrade fibrotic ECM by upregulation of MMPs [28,29,30]; Proangiogenic effect: By increasing the level of VEGF and decreasing HIF-1α, MSCs inhibit rarefication of the kidney vasculature and reduce ischemia [33]; Antiapoptotic effect: MSCs promote antiapoptotic gene expression while inhibiting proapoptotic genes and miRs and thus mediate tubular cells survival [33,46,47,48]; Anti-inflammatory effect: MSCs decrease proinflammatory cytokines TNF-α, INF-γ, IL-1, and IL-6, while increasing the level of their antagonistic, IL-10. Additionally, they induce T regs, inhibit the maturation of dendritic cells, and induce M2-like phenotype by mitochondrial transfer to macrophages [33,34,35,36]. Abbreviations: miR—MicroRNA, HDF—Hepatocyte growth factor, IGF-1—Insulin-like growth factor-1, IL—Interleukin, TGF-β1—Transforming growth factor beta 1, Th—T helper, T regs—T regulatory cells, CCL—Chemokine ligand, CXCL—C-X-C motif chemokine, MMP—metalloprotease, VEGF—Vascular endothelial growth factor, HIF1α—Hypoxia-inducible factor 1-alpha, TNF-α—Tumor necrosis factor-alpha, IFN-γ—Interferon-gamma.
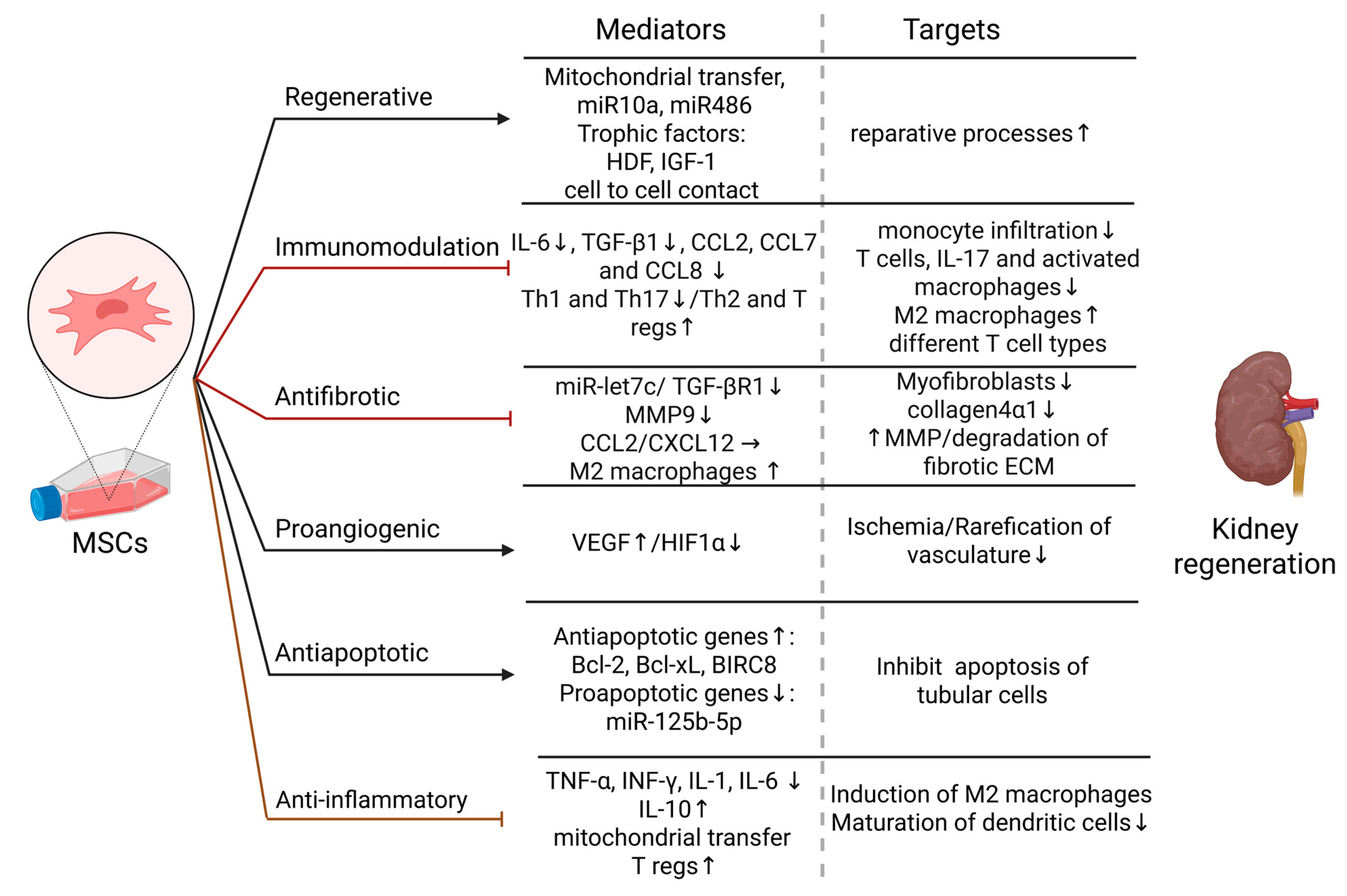
Figure 1.
Effects of MSCs on kidney regeneration—mediators and targets. Regenerative effect: Reparative processes are induced by MSCs by mitochondrial transfer, miR10a and miR486, secretion of trophic factors: HDF and IGF-1, and cell to cell interaction with kidney cells [21,44,45]; Immunomodulation: MSCs decrease the levels of IL-6 and TGF-β1, and CCL2,7,8, suppress pro-inflammatory Th-1 and Th17 cells, and induce anti-inflammatory Th2 cytokine profile and T regs. Consequently, the number of infiltrated monocytes and T cells in kidney tissue are reduced, activation of T cells is inhibited, infiltrated monocytes are differentiated into M2-like phenotype macrophages [37,38,40]; Antifibrotic effect: MSCs therapy suppresses TGF-β1 and miR-let7c and thus inhibit renal fibrosis by decreasing collagen 4α1 accumulation and MMP9 expression [26,27]. Additionally by CCL2 and CXCL12 secretion MSCs induce M2-like phenotype macrophages, which are shown to degrade fibrotic ECM by upregulation of MMPs [28,29,30]; Proangiogenic effect: By increasing the level of VEGF and decreasing HIF-1α, MSCs inhibit rarefication of the kidney vasculature and reduce ischemia [33]; Antiapoptotic effect: MSCs promote antiapoptotic gene expression while inhibiting proapoptotic genes and miRs and thus mediate tubular cells survival [33,46,47,48]; Anti-inflammatory effect: MSCs decrease proinflammatory cytokines TNF-α, INF-γ, IL-1, and IL-6, while increasing the level of their antagonistic, IL-10. Additionally, they induce T regs, inhibit the maturation of dendritic cells, and induce M2-like phenotype by mitochondrial transfer to macrophages [33,34,35,36]. Abbreviations: miR—MicroRNA, HDF—Hepatocyte growth factor, IGF-1—Insulin-like growth factor-1, IL—Interleukin, TGF-β1—Transforming growth factor beta 1, Th—T helper, T regs—T regulatory cells, CCL—Chemokine ligand, CXCL—C-X-C motif chemokine, MMP—metalloprotease, VEGF—Vascular endothelial growth factor, HIF1α—Hypoxia-inducible factor 1-alpha, TNF-α—Tumor necrosis factor-alpha, IFN-γ—Interferon-gamma.
Although MSCs may lead to some adverse events, their significant potential in treating various conditions (e.g., inducing remission in many autoimmune diseases, reversing CKD progression, improving AKI treatment, and extending the lifespan of kidney grafts) warrants further investigation [49].
3. Induced Pluripotent Stem Cells and Kidney Diseases
Of note, iPSCs offer a number of advantages over other types of stem cells, but a key one is that they can be obtained in a much less invasive and traumatic manner and in much higher numbers than, for example, bone marrow-derived MSCs, which makes them an attractive alternative and is at least one reason for the growing amount of research in the field. One therapeutic approach with iPSCs is that they can be injected directly in an attempt to slow the progression of CKD. Caldas et al. conducted an in vivo study in a rat model based on a 5/6 renal mass reduction mimicking CKD, where they aimed to compare the benefits of BM-MSCs to iPSCs. The authors showed that injection of 0.5 million stem cells in the renal parenchyma immediately after nephrectomy could effectively slow down CKD progression as measured on day 60 after the intervention by creatinine clearance, reduction of glomerulosclerosis, and lower macrophage infiltration (better score in the iPSCs group vs. the BM-MSCs one). Nevertheless, five out of eight kidneys injected with iPSCs developed Wilms’ tumors (rather than benign teratomas) originating from the injected cells (as assessed by SRY gene expression), highlighting the danger of this type of therapeutic approach. In contrast, no tumors were observed within 60 days in the BM-MSC group [54]. Shankar et al. in 2022 also showed the tumorigenic potential of both iPSCs (forming teratomas) and, more interestingly, iPSC-derived kidney organoids in a study where 5 out of 103 mice developed Wilms’ tumor upon subcutaneous injection of such pre-differentiated 3D structures [55].
Of note, in an attempt to avoid teratoma or Wilm’s tumor formation following injection of iPSCs, Ribeiro et al. validated a strategy of pretreating the cells with mitomycin C (an antimitotic drug aiming to reduce the proliferation of cells) prior implantation in nephrectomised mouse kidneys. The effect on CKD attenuation was compared to implantation of iPSC-derived renal progenitors and the authors found comparable results both in terms of efficacy and safety, re-opening the door to using iPSCs directly for cell therapy in CKD [56].
While iPSCs can be injected directly, another approach is to differentiate them into renal progenitors prior to therapy. The way to this strategy was first paved in 2014 by Taguchi et al., who demonstrated through elaborate in vitro protocols that iPSC can differentiate into intermediate mesoderm and subsequently into nephron progenitors (metanephric mesenchyme cells) [57].
It has been 10 years since the first reports of promising results with mouse models of AKI. Imberti et al. and Tayohara et al. showed, respectively, that iPSC-derived renal progenitors injected through the tail vein can engraft in cisplatin-induced AKI tubuli and reduce renal tubular damage and improve tubular function, and that implantation of in vitro generated 3D proximal renal tubule-like structures can ameliorate renal injury in mice [58,59]. Tayohara et al. also identified three renoprotective factors secreted by these differentiated iPSCs—angiopoietin, VEGF-A and hepatocyte growth factor [59]. Although MSCs may be considered as one of the first choices for cell-based therapies, there have been studies suggesting that uremia may affect MSCs’ functionality in patients with CKD (e.g., can decrease numbers and differentiation capacity of endothelia progenitor cells) [60]. Therefore, Tajiri et al. sought to investigate if induced pluripotent stem cells (iPSCs) from CKD patients would retain their potential to differentiate into nephron progenitors. Indeed, iPSCs derived from patients on hemodialysis (HD-iPSCs) had comparable differentiation abilities in vitro and could form vascularized glomeruli upon implantation in mice which demonstrated their therapeutic potential as an autologous source for CKD treatment [61]
A similar strategy using progenitors rather than iPSCs was more recently adopted by Araoka et al. as well. The research group developed and validated an expansion media for iPSC-derived nephron progenitor cells (NPCs) and used these in mouse models of aristolochic acid-induced CKD and cisplatin-induced AKI. In both conditions, iPSC-NPCs successfully improved kidney function and delayed disease progression (as judged by reduced fibrosis and senescence). Importantly, conditioned medium from these cells was also demonstrated to hold therapeutic potential and one key mediator of the improved survival in CKD and AKI was found to be VEGF-A secreted by the iPSC-NPCs [62].
Interestingly, there are more intricate co-culture protocols of separately generated nephron and ureteric duct progenitors. Bringing these two types of progenitor cells together can recapitulate kidney organoids that contain simultaneously cell differentiated into glomeruli, renal tubules, and collecting ducts. Implantation of such complex iPSCs-derived organoids under the kidney capsule of mice has been shown to result in functional vascularization of the glomerulus-like structures, which again suggests the great therapeutic potential that iPSCs have [63].
Lastly, although iPSCs’ ultimate goal would be in cell-based therapies, intermediate mesoderm to mesentheric mesenchyme or ureteric bud progenitor differentiation protocols are in fact more readily available and more widely used for in vitro disease modeling. Thus, new methods can make possible to generate different types of organoids (nephrons, collecting ducts or containing both). Such experimental systems have been available for more than 5 years and include brunching ureteric bud (Wolfian duct), collecting duct that can recapitulate aspects of multicystic dysplastic kidney (MCDK), and others [64,65] including genetic kidney diseases (when using either iPSCs derived from the actual patient or gene edited iPSCs) [7]. Models of AKI have also been established through cisplatin-mediated toxic damage of such organoids mimicking the proximal tubule [66]. Several studies have suggested that drug screening can also be carried out in such 3D systems; for example, showing that imatinib may have a renoprotective effect upon cisplatin-induced kidney injury [67,68].
In summary, even if scarce, experimental data from animal studies suggest that a clear distinction should be made between iPSCs and iPSCs that are already committed to progenitor cells, at least with regards to safety. While iPSCs have the potential to lead to malignancies in vivo, iPSC-derived progenitor cells have not been linked to adverse effects in animal models and several studies demonstrate that lab-engineered renal structures can ameliorate renal function. Many of the results of these studies should be taken with caution as the CKD animal models do not recapitulate the clinical picture in patients but are merely mimicking reduced kidney function via nephrectomy. Even though there are promising animal studies, there are no current clinical trials with iPSCs in CKD. Nevertheless, harnessing the greatness of such novel tissue engineering methods, there are several ongoing trials with iPSC-derived cardiomyocytes for the treatment of heart failure (e.g., NCT04945018, NCT04982081, NCT05647213), which shows that iPSC cell-based therapies may hold potential in CKD, AKI, and other kidney pathologies as well [8].
4. Assessment of Economic Potential of MSCs and iPSCs in Kidney Diseases
The global prevalence of CKD is increasing. This epidemiological trend leads to considerable economic burden on healthcare systems. In the United States, Medicare now spends more than USD 114 billion on CKD and end-stage renal disease (ESRD), while in England the National Health Service (NHS) devoted close to USD 1.95 billion to CKD care during the 2009–2010 fiscal year. With patient numbers continuing to climb, the challenge for healthcare systems is clear: to prioritize healthcare delivery and make more efficient use of resources to keep pace with the rising demand for CKD management [69].
The increasing incidence of AKI and CKD, coupled with their severe complications, emphasizes the necessity for more effective therapeutic strategies capable of mitigating renal damage and delaying progression to ESRD [70]. Patients with CKD are also at elevated risk of cardiovascular complications, which further amplifies the economic impact of the disease on healthcare systems [71]. Given that most acute and chronic kidney conditions are incurable and associated with significant morbidity, current therapeutic approaches are not only costly but frequently lead to adverse outcomes [72].
Evaluation of the cost-effectiveness of MSC therapy for CKD requires comparison of its potential benefits against conventional treatment modalities.
The significant financial and patient-centered burden of CKD is demonstrated by a scoping review that summarizes data from 78 studies conducted in high-income countries (Europe, North America, and Australia). According to the review, direct healthcare costs significantly increase as the disease progresses. For instance, the annual per-patient costs for early-stage CKD (stages 1–3) range from USD 1600 to USD 25,037, while those for stages 4–5 increase to USD 5367–USD 53,186. Expenses for people with ESRD also increase significantly, ranging from USD 20,110 to USD 100,593 annually. According to societal estimates, the total costs of stages 4–5 per patient in Europe range from USD 10,750 to USD 28,428 per year, with productivity losses accounting for as much as two-thirds of the total in certain contexts.
Treatment modality strongly influences expenditures. Hemodialysis is the most expensive RRT, followed by peritoneal dialysis. Home-based dialysis incurs lower costs than in-center procedures. Although kidney transplantation involves higher initial costs (USD 14,067–USD 80,876 in the first year), it is associated with reduced long-term expenditures and superior outcomes, including improved health-related quality of life (HRQoL; EQ-5D: 0.82–0.83) and increased life expectancy. In contrast, dialysis patients report lower HRQoL (EQ-5D: 0.58–0.68) and reduced survival rates [73].
The Inside CKD global burden study’s findings highlight the growing economic impact of CKD in 31 different nations and regions. As the disease progressed, average annual per-patient medical costs increased significantly, from USD 3060 at stage G3a to USD 8736 at stage G5. The initiation of RRT is associated with substantially higher expenditures, with mean annual costs of USD 57,334 for hemodialysis, USD 49,490 for peritoneal dialysis, and USD 75,326 during the first year after kidney transplantation. Even though post-transplant maintenance expenses drop to about USD 16,672 annually, they are still much higher than in earlier stages of chronic kidney disease.
With estimated costs of USD 18,294 for myocardial infarction and USD 10,168 for stroke, the management of CKD-related complications also adds to the financial burden. Significant cross-country variability and ongoing data gaps were also found in the study, especially regarding early-stage CKD and long-term transplant maintenance. These findings show the urgent need for improved health economic reporting to inform evidence-based policy-making and strategic resource allocation [71].
Although kidney transplantation represents the most cost-effective treatment option, its broader implementation is limited by significant barriers, including the increasing number of elderly or frail patients with end-stage renal disease, the persistent shortage of suitable donor organs and potential organ rejection. Given the clinical experience and evidence suggesting that dialysis may offer limited survival benefit for very elderly individuals or those with significant comorbidities and reduced physical function, the decision to opt for conservative management (active supportive care without dialysis) in such cases is understandable. This calls for a shift in the traditional treatment approach, whereby conservative care is considered a first-line option for frail, elderly patients. Such a strategy should include measures to slow the progression of kidney disease, prevent complications, and emphasize advance care planning and comprehensive supportive interventions [74,75]. Clinical trials suggest MSCs are safe, well-tolerated, and may improve renal function. Their regenerative, anti-inflammatory, and anti-fibrotic effects could delay CKD progression and onset of ESRD, making them a promising treatment strategy [49,70,72,76].
A cost-effectiveness analysis evaluated MSC therapy for diabetic kidney disease in comparison to usual care (UC) and SGLT2 inhibitors (SGLT2i) using a Markov model. In the base-case (age 71 years; eGFR 35 mL/min/1.73 m2), MSCs yielded 6.39 QALYs at USD 158,770 versus UC at 6.28 QALYs and USD 159,978, while SGLT2i provided 6.46 QALYs at USD 158,131—indicating comparable outcomes with SGLT2i slightly dominant in both effectiveness and cost. Despite this, threshold and probabilistic sensitivity analyses demonstrated that MSC therapy may become cost-effective under certain conditions—particularly among younger patients or when MSC therapy achieves a stronger effect in delaying progression to ESRD. For instance, in a scenario modelling a 40-year-old cohort (reflecting lower-bound eligibility in ongoing MSC trials), MSC therapy was found to have a 29% probability of being cost-effective compared to 0.1% for UC. The authors emphasize that MSC therapy may be valuable under specific clinical conditions—in younger patients, or when SGLT2i use is contraindicated. Additionally, SGLT2i-related adverse events like diabetic ketoacidosis or genitourinary infections may shift clinical preference toward MSC therapy, especially in high-risk patients or those with a GFR < 60 mL/min/1.73 m2. Furthermore, the pricing of SGLT2i varies by jurisdiction, indicating scenarios where MSC might be more advantageous. The study acknowledged that the model did not take into consideration all the possible advantages of MSC or SGLT2i therapy, such as the impact on body weight and cardiovascular risk. Furthermore, the estimated benefit of MSC therapy in delaying ESRD was based on surrogate data, and future evidence confirming its regenerative effects on kidney tissue may further improve its cost-effectiveness by reducing the hazard ratio of disease progression [77].
The transformative potential of both cell-based and cell-free therapeutic approaches in regenerative medicine is becoming more widely acknowledged. According to analyses, the global stem cell market, which was estimated to be worth USD 9.4 billion in 2020, is expected to increase at a projected annual growth rate of 8.8% and reach an estimated USD 16 billion by 2028. Mesenchymal stromal cells (MSCs) are becoming a significant market segment within this sector, with a projected market value of USD 6.1 billion by the same year, with a 12.6% growth rate [78]. The expanding market capitalization, combined with evidence from studies proposing cost-reduction strategies [78,79,80] suggests that MSC therapies are likely to become more economically accessible in the near future.
There are still significant financial obstacles to iPSC-based treatments. Recent analyses of pluripotent stem cell banking highlight that labor, facility maintenance, and quality control contribute significantly to manufacturing costs, with examples such as the Korea National Institute of Health reporting annual facility maintenance costs of approximately USD 2.2 million, and labor accounting for up to 50% of total expenses at other centers such as CiRA in Japan. iPSC vials are currently priced between USD 1000 and USD 1500 by professional stem cell banks [81].
Future pluripotent stem cell therapies are expected to be more economically viable and less expensive due to advances in scalable manufacturing, such as bioreactor-based production, process optimization, and automation [82].
Techno-economic analyses suggest that automated iPSC production may overcome key limitations of traditional manual methods, which are labor-intensive, expensive, and subject to operator variability. The fully automated iPSC production platform StemCellFactory (SCF) has demonstrated that automation can improve reproducibility, throughput, and standardization while achieving 42% lower total costs over an eight-year period when compared to manual production. The cost of iPSC-based regenerative treatments for chronic kidney disease may be lowered by these manufacturing advancements [83].
Important limitations should be noted even though this analysis summarizes the evidence currently available regarding the economic burden of CKD and the potential role of regenerative therapies like MSCs and iPSCs. Most data informing cost estimates for both MSC and iPSC therapies are at an early stage, reflecting projections or surrogate outcomes rather than results from large-scale, long-term clinical trials. As such, current evaluations of cost-effectiveness are preliminary and must be interpreted with caution.
In particular, the clinical application of MSCs for CKD remains experimental, with most trials focused on short-term safety and efficacy endpoints, not long-term health and economic outcomes. For iPSCs, their therapeutic use is even less mature, with no approved indications and substantial technical, regulatory, and safety barriers that must be overcome before reliable cost-effectiveness assessment is feasible.
While early findings suggest that MSCs and iPSCs hold promises as regenerative therapies for CKD, evidence supporting their comparative clinical effectiveness and economic value remains limited. Future research should address these gaps through comprehensive clinical and health-economic evaluations before definitive conclusions on their cost-effectiveness can be drawn.
5. Conclusions
Cellular therapies are promising strategies to overcome morbidity and mortality and improve quality of life of patients affected by kidney disease. Because of the safe profile, good tolerability, and their multiple beneficial effects, many clinical trials investigate the therapeutic potential of MSCs. Further research is needed to elucidate the intimate mechanisms by which these cells promote their effect. In comparison to other drugs, cellular therapy effectiveness can be influenced by many different factors; e.g., fitness of the cells, isolation and culturing methods, and route of administration.
Induced pluripotent stem cells have even greater therapeutic potential than MSCs, but their tumorigenicity remains a major limitation and more research is needed for this obstacle to be overcome.
Due to the limited application of cellular therapies in clinical practice, the assessment of cost effectiveness is based mainly on preliminary data. However, with manufacturing advancements and an expanding market projected to lower costs, this could become an economically valuable option, offering the potential to reverse tissue damage, improve quality of life, and extend life expectancy.
Author Contributions
Draft preparation—B.V., Y.S., K.Y.D. and V.Z. Writing, reviewing, editing and visualization—B.V., Y.S. and K.Y.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; et al. Chronic kidney disease and the global public health agenda: An international consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef]
- Hewitson, T.D. Renal tubulointerstitial fibrosis: Common but never simple. Am. J. Physiol. Physiol. 2009, 296, F1239–F1244. [Google Scholar] [CrossRef]
- Tamargo, C.; Hanouneh, M.; Cervantes, C.E. Treatment of Acute Kidney Injury: A Review of Current Approaches and Emerging Innovations. J. Clin. Med. 2024, 13, 2455. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Traitteur, T.; Zhang, C.; Morizane, R. The application of iPSC-derived kidney organoids and genome editing in kidney disease modeling. iPSCs State Sci. 2022, 111–136. [Google Scholar]
- Osafune, K. iPSC technology-based regenerative medicine for kidney diseases. Clin. Exp. Nephrol. 2021, 25, 574–584. [Google Scholar] [CrossRef]
- Salybekov, A.A.; Kinzhebay, A.; Kobayashi, S. Cell therapy in kidney diseases: Advancing treatments for renal regeneration. Front. Cell Dev. Biol. 2024, 12, 1505601. [Google Scholar] [CrossRef]
- Charles, C.; Ferris, A.H. Chronic Kidney Disease. Prim. Care Clin. Off. Pr. 2020, 47, 585–595. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Mechanisms of Tubulointerstitial Fibrosis. J. Am. Soc. Nephrol. 2010, 21, 1819–1834. [Google Scholar] [CrossRef]
- Boor, P.; Ostendorf, T.; Floege, J. Renal fibrosis: Novel insights into mechanisms and therapeutic targets. Nat. Rev. Nephrol. 2010, 6, 643–656. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pr. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Kurzhagen, J.T.; Dellepiane, S.; Cantaluppi, V.; Rabb, H. AKI: An increasingly recognized risk factor for CKD development and progression. J. Nephrol. 2020, 33, 1171–1187. [Google Scholar] [CrossRef]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A.; International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Jeon, Y.; Kim, J.; Cho, J.H.; Chung, H.; Chae, J. Comparative Analysis of Human Mesenchymal Stem Cells Derived From Bone Marrow, Placenta, and Adipose Tissue as Sources of Cell Therapy. J. Cell. Biochem. 2015, 117, 1112–1125. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Nakajima, K.; Awada, T.; Tsuka, Y.; Abe, T.; Ando, K.; Hiraki, T.; Kimura, A.; Tanimoto, K. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow–derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 501, 193–198. [Google Scholar] [CrossRef]
- Li, C.Y.; Wu, X.-Y.; Tong, J.-B.; Yang, X.-X.; Zhao, J.-L.; Zheng, Q.-F.; Zhao, G.-B.; Ma, Z.-J. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 1–22. [Google Scholar] [CrossRef]
- Popielarczyk, T.L.; Huckle, W.R.; Barrett, J.G. Human Bone Marrow-Derived Mesenchymal Stem Cells Home via the PI3K-Akt, MAPK, and Jak/Stat Signaling Pathways in Response to Platelet-Derived Growth Factor. Stem Cells Dev. 2019, 28, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Shahror, R.A.; Ali, A.A.A.; Wu, C.-C.; Chiang, Y.-H.; Chen, K.-Y. Enhanced Homing of Mesenchymal Stem Cells Overexpressing Fibroblast Growth Factor 21 to Injury Site in a Mouse Model of Traumatic Brain Injury. Int. J. Mol. Sci. 2019, 20, 2624. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-S.; Lin, C.-Y.; Chiu, Y.-H.; Chen, C.-P.; Tsai, P.-J.; Wang, H.-S. IL-1β-Induced Matrix Metalloprotease-1 Promotes Mesenchymal Stem Cell Migration via PAR1 and G-Protein-Coupled Signaling Pathway. Stem Cells Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 1–18. [Google Scholar] [CrossRef]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef]
- Wang, B.; Yao, K.; Huuskes, B.M.; Shen, H.-H.; Zhuang, J.; Godson, C.; Brennan, E.P.; Wilkinson-Berka, J.L.; Wise, A.F.; Ricardo, S.D. Mesenchymal Stem Cells Deliver Exogenous MicroRNA-let7c via Exosomes to Attenuate Renal Fibrosis. Mol. Ther. 2016, 24, 1290–1301. [Google Scholar] [CrossRef]
- Eardley, K.S.; Cockwell, P. Macrophages and progressive tubulointerstitial disease. Kidney Int. 2005, 68, 437–455. [Google Scholar] [CrossRef]
- Giri, J.; Das, R.; Nylen, E.; Chinnadurai, R.; Galipeau, J. CCL2 and CXCL12 Derived from Mesenchymal Stromal Cells Cooperatively Polarize IL-10+ Tissue Macrophages to Mitigate Gut Injury. Cell Rep. 2020, 30, 1923–1934.e4. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 1–19. [Google Scholar] [CrossRef]
- Moroni, F.; Dwyer, B.J.; Graham, C.; Pass, C.; Bailey, L.; Ritchie, L.; Mitchell, D.; Glover, A.; Laurie, A.; Doig, S.; et al. Safety profile of autologous macrophage therapy for liver cirrhosis. Nat. Med. 2019, 25, 1560–1565. [Google Scholar] [CrossRef]
- Li, N.; Hua, J. Interactions between mesenchymal stem cells and the immune system. Cell. Mol. Life Sci. 2017, 74, 2345–2360. [Google Scholar] [CrossRef]
- Mudrabettu, C.; Kumar, V.; Rakha, A.; Yadav, A.K.; Ramachandran, R.; Kanwar, D.B.; Nada, R.; Minz, M.; Sakhuja, V.; Marwaha, N.; et al. Safety and efficacy of autologous mesenchymal stromal cells transplantation in patients undergoing living donor kidney transplantation: A pilot study. Nephrology 2014, 20, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Birtwistle, L.; Chen, X.-M.; Pollock, C. Mesenchymal Stem Cell-Derived Extracellular Vesicles to the Rescue of Renal Injury. Int. J. Mol. Sci. 2021, 22, 6596. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E2 and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25Highforkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Gandolfo, M.T.; Jang, H.R.; Bagnasco, S.M.; Ko, G.-J.; Agreda, P.; Satpute, S.R.; Crow, M.T.; King, L.S.; Rabb, H. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009, 76, 717–729. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, P.; Wuren, T. Narrative Review of Mesenchymal Stem Cell Therapy in Renal Diseases: Mechanisms, Clinical Applications, and Future Directions. Stem Cells Int. 2024, 2024. [Google Scholar] [CrossRef]
- Swaminathan, M.; Kopyt, N.; Atta, M.G.; Radhakrishnan, J.; Umanath, K.; Nguyen, S.; O’ROurke, B.; Allen, A.; Vaninov, N.; Tilles, A.; et al. Pharmacological Effects of Ex Vivo Mesenchymal Stem Cell Immunotherapy in Patients with Acute Kidney Injury and Underlying Systemic Inflammation. Stem Cells Transl. Med. 2021, 10, 1588–1601. [Google Scholar] [CrossRef]
- Duffy, M.M.; Ritter, T.; Ceredig, R.; Griffin, M.D. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res. Ther. 2011, 2, 1–9. [Google Scholar] [CrossRef]
- Jin, M.; Xie, Y.; Li, Q.; Chen, X. Stem Cell-Based Cell Therapy for Glomerulonephritis. BioMed Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Rampino, T.; Gregorini, M.; Bedino, G.; Piotti, G.; Gabanti, E.; Ibatici, A.; Sessarego, N.; Piacenza, C.; Balenzano, C.T.; Esposito, P.; et al. Mesenchymal stromal cells improve renal injury in anti-Thy 1 nephritis by modulating inflammatory cytokines and scatter factors. Clin. Sci. 2010, 120, 25–36. [Google Scholar] [CrossRef]
- Kunter, U.; Rong, S.; Djuric, Z.; Boor, P.; Müller-Newen, G.; Yu, D.; Floege, J. Transplanted Mesenchymal Stem Cells Accelerate Glomerular Healing in Experimental Glomerulonephritis. J. Am. Soc. Nephrol. 2006, 17, 2202–2212. [Google Scholar] [CrossRef]
- Zedan, M.M.; Mansour, A.K.; Bakr, A.A.; Sobh, M.A.; Khodadadi, H.; Salles, E.L.; Alhashim, A.; Baban, B.; Golubnitschaja, O.; Elmarakby, A.A. Effect of Everolimus versus Bone Marrow-Derived Stem Cells on Glomerular Injury in a Rat Model of Glomerulonephritis: A Preventive, Predictive and Personalized Implication. Int. J. Mol. Sci. 2021, 23, 344. [Google Scholar] [CrossRef] [PubMed]
- Gregorini, M.; Maccario, R.; Avanzini, M.A.; Corradetti, V.; Moretta, A.; Libetta, C.; Esposito, P.; Bosio, F.; Canton, A.D.; Rampino, T. Antineutrophil Cytoplasmic Antibody-Associated Renal Vasculitis Treated With Autologous Mesenchymal Stromal Cells: Evaluation of the Contribution of Immune-Mediated Mechanisms. Mayo Clin. Proc. 2013, 88, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Tapparo, M.; Bruno, S.; Collino, F.; Togliatto, G.; Deregibus, M.C.; Provero, P.; Wen, S.; Quesenberry, P.J.; Camussi, G. Renal Regenerative Potential of Extracellular Vesicles Derived from miRNA-Engineered Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2019, 20, 2381. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Khryapenkova, T.G.; Galkina, S.I.; Sukhikh, G.T.; Zorov, D.B. Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Exp. Cell Res. 2010, 316, 2447–2455. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, H.; Xu, W.; Wang, B.; Wu, H.; Tao, Y.; Zhang, B.; Wang, M.; Mao, F.; Yan, Y.; et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res. Ther. 2013, 4, 34. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, M.; Ren, X.; Song, Y.; Xu, Y.; Zhuang, K.; Xiao, T.; Guo, X.; Wang, S.; Hong, Q.; et al. Single-cell dissection of cellular and molecular features underlying mesenchymal stem cell therapy in ischemic acute kidney injury. Mol. Ther. 2023, 31, 3067–3083. [Google Scholar] [CrossRef]
- Cao, J.-Y.; Wang, B.; Tang, T.-T.; Wen, Y.; Li, Z.-L.; Feng, S.-T.; Wu, M.; Liu, D.; Yin, D.; Ma, K.-L.; et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics 2021, 11, 5248–5266. [Google Scholar] [CrossRef]
- Perico, N.; Casiraghi, F.; Remuzzi, G. Clinical Translation of Mesenchymal Stromal Cell Therapies in Nephrology. J. Am. Soc. Nephrol. 2017, 29, 362–375. [Google Scholar] [CrossRef]
- Perico, N.; Remuzzi, G.; Griffin, M.D.; Cockwell, P.; Maxwell, A.P.; Casiraghi, F.; Rubis, N.; Peracchi, T.; Villa, A.; Todeschini, M.; et al. Safety and Preliminary Efficacy of Mesenchymal Stromal Cell (ORBCEL-M) Therapy in Diabetic Kidney Disease: A Randomized Clinical Trial (NEPHSTROM). J. Am. Soc. Nephrol. 2023, 34, 1733–1751. [Google Scholar] [CrossRef]
- Stavas, J.; Silva, A.L.; Wooldridge, T.D.; Aqeel, A.; Saad, T.; Prakash, R.; Bakris, G. Rilparencel (Renal Autologous Cell Therapy-REACT®) for Chronic Kidney Disease and Type 1 and Type 2 Diabetes: Phase 2 Trial Design Evaluating Bilateral Kidney Dosing and Redosing Triggers. Am. J. Nephrol. 2024, 55, 389–398. [Google Scholar] [CrossRef]
- Reinders, M.E.; Rabelink, T.J. Adipose tissue-derived stem cells: Can impure cell preparations give pure results? Nephrol. Dial. Transplant. 2010, 25, 3805–3807. [Google Scholar] [CrossRef] [PubMed]
- Baranovskii, D.S.; Klabukov, I.D.; Arguchinskaya, N.V.; Yakimova, A.O.; Kisel, A.A.; Yatsenko, E.M.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Adverse events, side effects and complications in mesenchymal stromal cell-based therapies. Stem Cell Investig. 2022, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Caldas, H.C.; Lojudice, F.H.; Dias, C.; Fernandes-Charpiot, I.M.M.; Baptista, M.A.S.F.; Kawasaki-Oyama, R.S.; Sogayar, M.C.; Takiya, C.M.; Abbud-Filho, M. Induced Pluripotent Stem Cells Reduce Progression of Experimental Chronic Kidney Disease but Develop Wilms’ Tumors. Stem Cells Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.S.; Du, Z.; Mora, H.T.; Boers, R.; Cao, W.; Bosch, T.P.P.v.D.; Korevaar, S.S.; Boers, J.; van Ijcken, W.F.J.; Bindels, E.M.J.; et al. Kidney Organoids Are Capable of Forming Tumors, but Not Teratomas. Stem Cells 2022, 40, 577–591. [Google Scholar] [CrossRef]
- Ribeiro, P.d.C.; Lojudice, F.H.; Fernandes-Charpiot, I.M.M.; Baptista, M.A.S.F.; Araújo, S.d.A.; Mendes, G.E.F.; Sogayar, M.C.; Abbud-Filho, M.; Caldas, H.C. Therapeutic potential of human induced pluripotent stem cells and renal progenitor cells in experimental chronic kidney disease. Stem Cell Res. Ther. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Taguchi, A.; Kaku, Y.; Ohmori, T.; Sharmin, S.; Ogawa, M.; Sasaki, H.; Nishinakamura, R. Redefining the In Vivo Origin of Metanephric Nephron Progenitors Enables Generation of Complex Kidney Structures from Pluripotent Stem Cells. Cell Stem Cell 2014, 14, 53–67. [Google Scholar] [CrossRef]
- Imberti, B.; Tomasoni, S.; Ciampi, O.; Pezzotta, A.; Derosas, M.; Xinaris, C.; Rizzo, P.; Papadimou, E.; Novelli, R.; Benigni, A.; et al. Renal progenitors derived from human iPSCs engraft and restore function in a mouse model of acute kidney injury. Sci. Rep. 2015, 5, srep08826. [Google Scholar] [CrossRef]
- Toyohara, T.; Mae, S.-I.; Sueta, S.-I.; Inoue, T.; Yamagishi, Y.; Kawamoto, T.; Kasahara, T.; Hoshina, A.; Toyoda, T.; Tanaka, H.; et al. Cell Therapy Using Human Induced Pluripotent Stem Cell-Derived Renal Progenitors Ameliorates Acute Kidney Injury in Mice. Stem Cells Transl. Med. 2015, 4, 980–992. [Google Scholar] [CrossRef]
- De Groot, K.; Bahlmann, F.H.; Sowa, J.; Koenig, J.; Menne, J.; Haller, H.; Fliser, D. Uremia causes endothelial progenitor cell deficiency. Kidney Int. 2004, 66, 641–646. [Google Scholar] [CrossRef]
- Tajiri, S.; Yamanaka, S.; Fujimoto, T.; Matsumoto, K.; Taguchi, A.; Nishinakamura, R.; Okano, H.J.; Yokoo, T. Regenerative potential of induced pluripotent stem cells derived from patients undergoing haemodialysis in kidney regeneration. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Araoka, T.; Toyohara, K.; Ryosaka, M.; Inui, C.; Matsuura, M.; Ma, C.; Watahiki, J.; Li, Z.; Iwasaki, M.; Watanabe, A.; et al. Human iPSC–derived nephron progenitor cells treat acute kidney injury and chronic kidney disease in mouse models. Sci. Transl. Med. 2025, 17, eadt5553. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, H.; Kasahara, T.; Sueta, S.-I.; Araoka, T.; Sakamoto, S.; Okada, C.; Mae, S.-I.; Nakajima, T.; Okamoto, N.; Taura, D.; et al. A Modular Differentiation System Maps Multiple Human Kidney Lineages from Pluripotent Stem Cells. Cell Rep. 2020, 31, 107476. [Google Scholar] [CrossRef] [PubMed]
- Mae, S.-I.; Ryosaka, M.; Sakamoto, S.; Matsuse, K.; Nozaki, A.; Igami, M.; Kabai, R.; Watanabe, A.; Osafune, K. Expansion of Human iPSC-Derived Ureteric Bud Organoids with Repeated Branching Potential. Cell Rep. 2020, 32, 107963. [Google Scholar] [CrossRef] [PubMed]
- Mae, S.-I.; Ryosaka, M.; Toyoda, T.; Matsuse, K.; Oshima, Y.; Tsujimoto, H.; Okumura, S.; Shibasaki, A.; Osafune, K. Generation of branching ureteric bud tissues from human pluripotent stem cells. Biochem. Biophys. Res. Commun. 2018, 495, 954–961. [Google Scholar] [CrossRef]
- Kishi, S.; Brooks, C.R.; Taguchi, K.; Ichimura, T.; Mori, Y.; Akinfolarin, A.; Gupta, N.; Galichon, P.; Elias, B.C.; Suzuki, T.; et al. Proximal tubule ATR regulates DNA repair to prevent maladaptive renal injury responses. J. Clin. Investig. 2019, 129, 4797–4816. [Google Scholar] [CrossRef]
- Ning, N.; Liu, Z.; Li, X.; Liu, Y.; Song, W. Progress of Induced Pluripotent Stem Cell-Derived Renal Organoids in Clinical Application. Kidney Dis. 2024, 11, 1–10. [Google Scholar] [CrossRef]
- Oishi, H.; Tabibzadeh, N.; Morizane, R. Advancing preclinical drug evaluation through automated 3D imaging for high-throughput screening with kidney organoids. Biofabrication 2024, 16, 035003. [Google Scholar] [CrossRef]
- Darlington, O.; Dickerson, C.; Evans, M.; McEwan, P.; Sörstadius, E.; Sugrue, D.; van Haalen, H.; Sanchez, J.J.G. Costs and Healthcare Resource Use Associated with Risk of Cardiovascular Morbidity in Patients with Chronic Kidney Disease: Evidence from a Systematic Literature Review. Adv. Ther. 2021, 38, 994–1010. [Google Scholar] [CrossRef]
- Nargesi, A.A.; Lerman, L.O.; Eirin, A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: Current status and looming challenges. Stem Cell Res. Ther. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Jha, V.; Al-Ghamdi, S.M.G.; Li, G.; Wu, M.-S.; Stafylas, P.; Retat, L.; Card-Gowers, J.; Barone, S.; Cabrera, C.; Sanchez, J.J.G. Global Economic Burden Associated with Chronic Kidney Disease: A Pragmatic Review of Medical Costs for the Inside CKD Research Programme. Adv. Ther. 2023, 40, 4405–4420. [Google Scholar] [CrossRef]
- Hickson, L.J.; Herrmann, S.M.; McNicholas, B.A.; Griffin, M.D. Progress toward the Clinical Application of Mesenchymal Stromal Cells and Other Disease-Modulating Regenerative Therapies: Examples from the Field of Nephrology. Kidney360 2021, 2, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Elshahat, S.; Cockwell, P.; Maxwell, A.P.; Griffin, M.; O’Brien, T.; O’Neill, C. The impact of chronic kidney disease on developed countries from a health economics perspective: A systematic scoping review. PLoS ONE 2020, 15, e0230512. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cheng, F.; Pan, S.; Liu, Z. Stem cells: A potential treatment option for kidney diseases. Stem Cell Res. Ther. 2020, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- on behalf of the European Kidney Health Alliance; Vanholder, R.; Annemans, L.; Brown, E.; Gansevoort, R.; Gout-Zwart, J.J.; Lameire, N.; Morton, R.L.; Oberbauer, R.; Postma, M.J.; et al. Reducing the costs of chronic kidney disease while delivering quality health care: A call to action. Nat. Rev. Nephrol. 2017, 13, 393–409. [Google Scholar] [CrossRef]
- Chen, F.; Chen, N.; Xia, C.; Wang, H.; Shao, L.; Zhou, C.; Wang, J. Mesenchymal Stem Cell Therapy in Kidney Diseases: Potential and Challenges. Cell Transplant. 2023, 32. [Google Scholar] [CrossRef]
- Barry, L.E.; Crealey, G.E.; Cockwell, P.; Elliman, S.J.; Griffin, M.D.; Maxwell, A.P.; O’bRien, T.; Perico, N.; O’nEill, C. Mesenchymal stromal cell therapy compared to SGLT2-inhibitors and usual care in treating diabetic kidney disease: A cost-effectiveness analysis. PLoS ONE 2022, 17, e0274136. [Google Scholar] [CrossRef]
- Silva, R.M.; Rosa, S.S.; Santos, J.A.L.; Azevedo, A.M.; Fernandes-Platzgummer, A. Enabling Mesenchymal Stromal Cells and Their Extracellular Vesicles Clinical Availability—A Technological and Economical Evaluation. J. Extracell. Biol. 2025, 4, e70037. [Google Scholar] [CrossRef]
- Childs, P.G.; Reid, S.; Salmeron-Sanchez, M.; Dalby, M.J. Hurdles to uptake of mesenchymal stem cells and their progenitors in therapeutic products. Biochem. J. 2020, 477, 3349–3366. [Google Scholar] [CrossRef]
- Russell, A.L.; Lefavor, R.C.; Zubair, A.C. Characterization and cost–benefit analysis of automated bioreactor-expanded mesenchymal stem cells for clinical applications. Transfusion 2018, 58, 2374–2382. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kawase, E.; Bharti, K.; Karnieli, O.; Arakawa, Y.; Stacey, G. Perspectives on the cost of goods for hPSC banks for manufacture of cell therapies. npj Regen. Med. 2022, 7, 1–8. [Google Scholar] [CrossRef]
- Mills, J.; Donnelly, T.; Brennan, M.; Marques, B. Transitioning to Scalable Bioreactors for Allogeneic iPSC-Derived Cell Therapies: Cost Savings and Global Patient Access. Cytotherapy 2025, 27, S148. [Google Scholar] [CrossRef]
- Nießing, B.; Kiesel, R.; Herbst, L.; Schmitt, R.H. Techno-Economic Analysis of Automated iPSC Production. Processes 2021, 9, 240. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
