Modular Strategies for Nephron Replacement and Clinical Translation
Abstract
1. Introduction
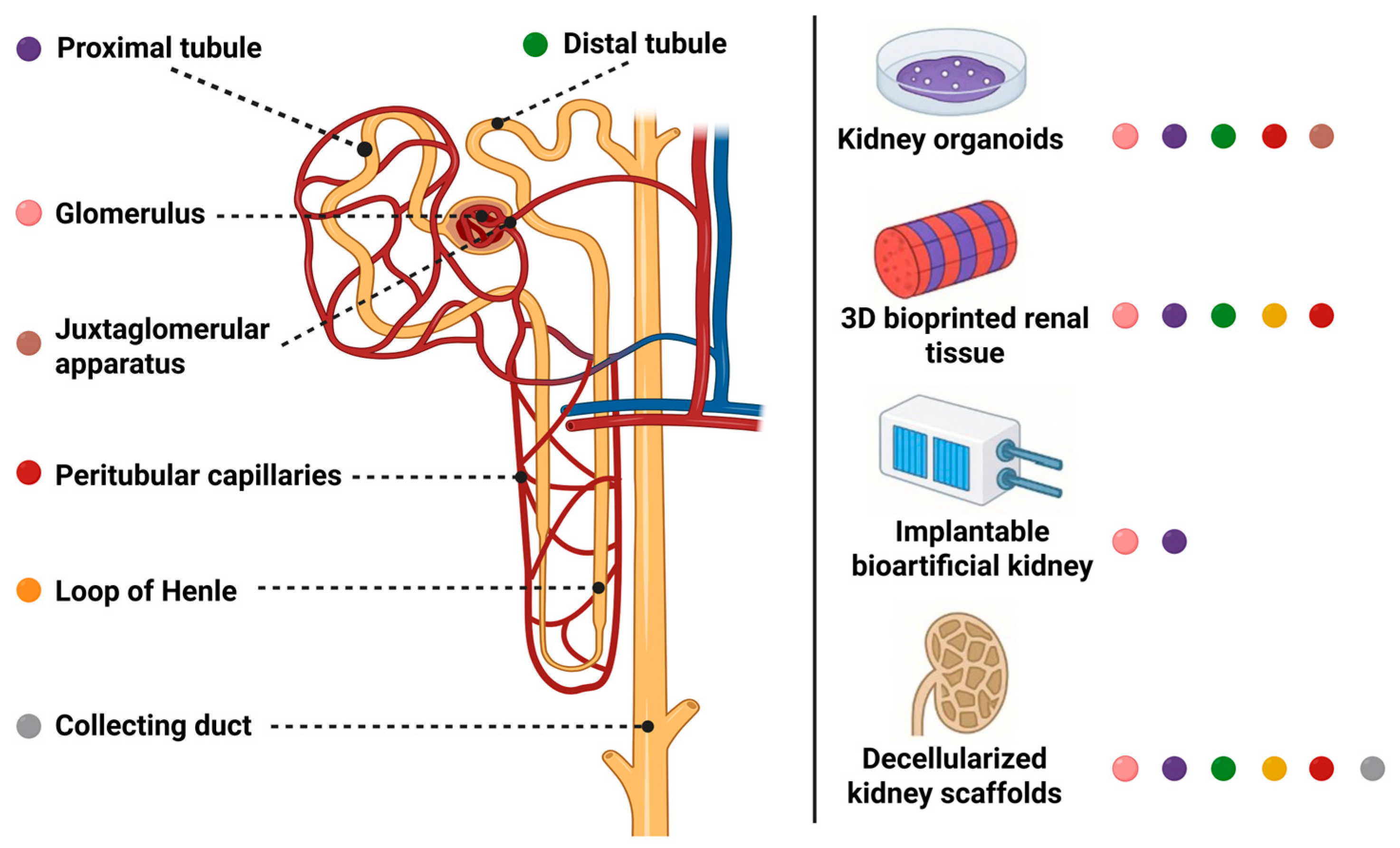
2. Modular Building Blocks for Nephron Replacement
2.1. Kidney Organoids
- Absence of a vascular network, which restricts nutrient delivery and size;
- Lack of a urine drainage system, precluding physiological excretion;
2.2. iBK Devices
- Sustaining epithelial cell viability and function under physiological shear stress;
- Preventing fibrosis and immune rejection in the absence of systemic immunosuppression;
- Ensuring long-term durability of membrane materials and preventing device fouling or occlusion;
2.3. 3D Bioprinted Renal Tissues
- Achieving hierarchical vascularization;
- Maintaining long-term cell viability under physiological pressure;
- Scaling constructs to clinically relevant sizes without compromising function;
2.4. Decellularized Kidney Scaffolds
- Efficient and selective recellularization of glomerular, tubular, and vascular compartments;
- Sourcing sufficient quantities of autologous or immunocompatible cells;
- Achieving functional integration and perfusion after implantation;
3. The Nephron as a Blueprint for Modular Kidney Replacement
3.1. Glomerulus (Filtration)
3.2. Proximal Tubule (Reabsorption and Secretion)
3.3. Loop of Henle (Countercurrent Concentration)
3.4. Distal Tubule (Electrolyte Regulation)
3.5. Collecting Duct (Water and Acid–Base Regulation)
3.6. Juxtaglomerular Apparatus (Endocrine and Autoregulatory Function)
3.7. Peritubular Capillaries (Microvascular Support)
4. From Platform to Patient: Strategic Pathways for Modular Nephron Replacement
5. Outlook: Clinical Integration, Equity, and Ethical Imperatives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.-J.; Liu, H.-Y.; Zhang, Y.-Q.; Li, S.-R.; Zhang, J.-H.; Li, R. Global Burden of Chronic Kidney Disease and Its Attributable Risk Factors (1990–2021): An Analysis Based on the Global Burden of Disease Study. Front. Endocrinol. 2025, 16, 1563246. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; et al. Chronic Kidney Disease and the Global Public Health Agenda: An International Consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Nkunu, V.; Tungsanga, S.; Diongole, H.M.; Sarki, A.; Arruebo, S.; Caskey, F.J.; Damster, S.; Donner, J.A.; Jha, V.; Levin, A.; et al. Landscape of Kidney Replacement Therapy Provision in Low- and Lower-Middle Income Countries: A Multinational Study from the ISN-GKHA. PLoS Glob. Public Health 2024, 4, e0003979. [Google Scholar] [CrossRef]
- Bello, A.K.; Okpechi, I.G.; Osman, M.A.; Cho, Y.; Htay, H.; Jha, V.; Wainstein, M.; Johnson, D.W. Epidemiology of Haemodialysis Outcomes. Nat. Rev. Nephrol. 2022, 18, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Lanini, I.; Samoni, S.; Husain-Syed, F.; Fabbri, S.; Canzani, F.; Messeri, A.; Mediati, R.D.; Ricci, Z.; Romagnoli, S.; Villa, G. Palliative Care for Patients with Kidney Disease. J. Clin. Med. 2022, 11, 3923. [Google Scholar] [CrossRef]
- Kupiec-Weglinski, J.W. Grand Challenges in Organ Transplantation. Front. Transplant. 2022, 1, 897679. [Google Scholar] [CrossRef]
- Kolesnyk, M.; Korol, L.; Shifris, I.; Stepanova, N.; Voroniak, O.; Shuba, I. Immunological determinants of long-term kidney graft survival as therapeutic targets. Ukr. J. Nephrol. Dialys. 2025, 2, 98–111. [Google Scholar] [CrossRef]
- Salani, M.; Roy, S.; Fissell, W.H. Innovations in Wearable and Implantable Artificial Kidneys. Am. J. Kidney Dis. 2018, 72, 745–751. [Google Scholar] [CrossRef]
- Copur, S.; Tanriover, C.; Yavuz, F.; Soler, M.J.; Ortiz, A.; Covic, A.; Kanbay, M. Novel Strategies in Nephrology: What to Expect from the Future? Clin. Kidney J. 2023, 16, 230–244. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Hurst, F.P.; West, M.; Wu, I.; Roy-Chaudhury, P.; Sheldon, M. A Technology Roadmap for Innovative Approaches to Kidney Replacement Therapies: A Catalyst for Change. Clin. J. Am. Soc. Nephrol. 2019, 14, 1539–1547. [Google Scholar] [CrossRef]
- Chambers, B.E.; Weaver, N.E.; Wingert, R.A. The “3Ds” of Growing Kidney Organoids: Advances in Nephron Development, Disease Modeling, and Drug Screening. Cells 2023, 12, 549. [Google Scholar] [CrossRef]
- Tekguc, M.; Gaal, R.C.V.; Uzel, S.G.M.; Gupta, N.; Riella, L.V.; Lewis, J.A.; Morizane, R. Kidney Organoids: A Pioneering Model for Kidney Diseases. Transl. Res. 2022, 250, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tabibzadeh, N.; Morizane, R. Advancements in Therapeutic Development: Kidney Organoids and Organs-on-a-Chip. Kidney Int. 2024, 105, 702708. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yue, L.; Deng, J.; Tan, Y.; Wu, C. Progress and Breakthroughs in Human Kidney Organoid Research. Biochem. Biophys. Rep. 2024, 39, 101736. [Google Scholar] [CrossRef]
- Wilson, S.B.; Vanslambrouck, J.M.; Murphy, A.; Neavin, D.R.; Powell, J.E.; Howden, S.E.; Little, M.H. Classification of Indeterminate and Off-Target Cell Types within Human Kidney Organoid Differentiation. bioRxiv 2025. [Google Scholar] [CrossRef]
- Goux Corredera, I.; Amato, G.; Moya-Rull, D.; Garreta, E.; Montserrat, N. Unlocking the Full Potential of Human Pluripotent Stem Cell–Derived Kidney Organoids through Bioengineering. Kidney Int. 2025, 108, 38–47. [Google Scholar] [CrossRef]
- Papamichail, L.; Koch, L.S.; Veerman, D.; Broersen, K.; van der Meer, A.D. Organoids-on-a-Chip: Microfluidic Technology Enables Culture of Organoids with Enhanced Tissue Function and Potential for Disease Modeling. Front. Bioeng. Biotechnol. 2025, 13, 1515340. [Google Scholar] [CrossRef]
- Menéndez, A.B.C.; Du, Z.; van den Bosch, T.P.P.; Othman, A.; Gaio, N.; Silvestri, C.; Quirós, W.; Lin, H.; Korevaar, S.; Merino, A.; et al. Creating a Kidney Organoid-Vasculature Interaction Model Using a Novel Organ-on-Chip System. Sci. Rep. 2022, 12, 20699. [Google Scholar] [CrossRef]
- Maggiore, J.C.; LeGraw, R.; Przepiorski, A.; Velazquez, J.; Chaney, C.; Vanichapol, T.; Streeter, E.; Almuallim, Z.; Oda, A.; Chiba, T.; et al. A Genetically Inducible Endothelial Niche Enables Vascularization of Human Kidney Organoids with Multilineage Maturation and Emergence of Renin Expressing Cells. Kidney Int. 2024, 106, 1086–1100. [Google Scholar] [CrossRef]
- Du, Z.; Bas-Cristóbal Menéndez, A.; Urban, M.; Hartley, A.; Ratsma, D.; Koedam, M.; van den Bosch, T.P.P.; Clahsen-van Groningen, M.; Gribnau, J.; Mulder, J.; et al. Erythropoietin delivery through kidney organoids engineered with an episomal DNA vector. Stem Cell Res. Ther. 2025, 16, 174. [Google Scholar] [CrossRef]
- Raykhel, I.; Nishikawa, M.; Sakai, Y.; Vainio, S.J.; Skovorodkin, I. Vascularization of Kidney Organoids: Different Strategies and Perspectives. Front. Urol. 2024, 4, 1355042. [Google Scholar] [CrossRef]
- Wang, R.; Sui, Y.; Liu, Q.; Xiong, Y.; Li, S.; Guo, W.; Xu, Y.; Zhang, S. Recent Advances in Extracellular Matrix Manipulation for Kidney Organoid Research. Front. Pharmacol. 2024, 15, 1472361. [Google Scholar] [CrossRef]
- Nalesso, F.; Garzotto, F.; Cattarin, L.; Bettin, E.; Cacciapuoti, M.; Silvestre, C.; Stefanelli, L.F.; Furian, L.; Calò, L.A. The Future for End-Stage Kidney Disease Treatment: Implantable Bioartificial Kidney Challenge. Appl. Sci. 2024, 14, 491. [Google Scholar] [CrossRef]
- Karageorgos, F.F.; Neiros, S.; Karakasi, K.-E.; Vasileiadou, S.; Katsanos, G.; Antoniadis, N.; Tsoulfas, G. Artificial Kidney: Challenges and Opportunities. World J. Transplant. 2024, 14, 89025. [Google Scholar] [CrossRef]
- Corridon, P.R.; Ko, I.K.; Yoo, J.J.; Atala, A. Bioartificial Kidneys. Curr. Stem Cell Rep. 2017, 3, 68–76. [Google Scholar] [CrossRef]
- Van Gelder, M.K.; Mihaila, S.M.; Jansen, J.; Wester, M.; Verhaar, M.C.; Joles, J.A.; Stamatialis, D.; Masereeuw, R.; Gerritsen, K.G.F. From Portable Dialysis to a Bioengineered Kidney. Expert. Rev. Med. Devices 2018, 15, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Chen, C.; Gologorsky, R.; Santandreu, A.; Torres, A.; Wright, N.; Goodin, M.S.; Moyer, J.; Chui, B.W.; Blaha, C.; et al. Feasibility of an Implantable Bioreactor for Renal Cell Therapy Using Silicon Nanopore Membranes. Nat. Commun. 2023, 14, 4890. [Google Scholar] [CrossRef] [PubMed]
- Groth, T.; Stegmayr, B.G.; Ash, S.R.; Kuchinka, J.; Wieringa, F.P.; Fissell, W.H.; Roy, S. Wearable and implantable artificial kidney devices for end-stage kidney disease treatment: Current status and review. Artif. Organs. 2023, 47, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Jena, R.; Aggarwal, A.; Choudhary, G.R.; Bajpai, N.K.; Jena, R.; Aggarwal, A.; Choudhary, G.R.; Bajpai, N.K. Current Status and Future of Artificial Kidney in Humans. Indian. J. Nephrol. 2022, 32, 531–538. [Google Scholar] [CrossRef]
- Rayat Pisheh, H.; Haghdel, M.; Jahangir, M.; Hoseinian, M.S.; Rostami Yasuj, S.; Sarhadi Roodbari, A. Effective and New Technologies in Kidney Tissue Engineering. Front. Bioeng. Biotechnol. 2024, 12, 1476510. [Google Scholar] [CrossRef]
- Fransen, M.F.J.; Addario, G.; Bouten, C.V.C.; Halary, F.; Moroni, L.; Mota, C. Bioprinting of Kidney in Vitro Models: Cells, Biomaterials, and Manufacturing Techniques. Essays Biochem. 2021, 65, 587–602. [Google Scholar] [CrossRef]
- Mirshafiei, M.; Rashedi, H.; Yazdian, F.; Rahdar, A.; Baino, F. Advancements in Tissue and Organ 3D Bioprinting: Current Techniques, Applications, and Future Perspectives. Mater. Des. 2024, 240, 112853. [Google Scholar] [CrossRef]
- Yuan, Z.; Bai, X.; Li, S.; Fu, Y.; Wan, Z.; Guo, X.; Zhai, M.; Yi, J.; Liu, Y.; Zhou, Y.; et al. Multimaterial and Multidimensional Bioprinting in Regenerative Medicine: Advances, Limitations, and Future Directions. Adv. Healthc. Mater. 2025, 14, 2500475. [Google Scholar] [CrossRef]
- Mou, X.; Shah, J.; Roye, Y.; Du, C.; Musah, S. An Ultrathin Membrane Mediates Tissue-Specific Morphogenesis and Barrier Function in a Human Kidney Chip. Sci. Adv. 2024, 10, eadn2689. [Google Scholar] [CrossRef]
- Carreno-Caleano, G.; Ali, M.; Yoo, J.J.; Lee, S.J.; Atala, A. 3D Bioprinted Renal Constructs Using Kidney-Specific ECM Bioink System on Kidney Regeneration. Adv. Healthc. Mater. 2025, e2502576. [Google Scholar] [CrossRef]
- Eghosasere, E.; Osasumwen, E.; Emmanuella, O. 3D Bioprinting in Tissue Engineering: Advancements, Challenges, and Pathways to Clinical Translation. JSM Regen. Med. Bio Eng. 2025, 7, 1023. [Google Scholar] [CrossRef]
- de Haan, M.J.A.; Witjas, F.M.R.; Engelse, M.A.; Rabelink, T.J. Have We Hit a Wall with Whole Kidney Decellularization and Recellularization: A Review. Curr. Opin. Biomed. Eng. 2021, 20, 100335. [Google Scholar] [CrossRef]
- Destefani, A.C.; Sirtoli, G.M.; Nogueira, B.V. Advances in the Knowledge about Kidney Decellularization and Repopulation. Front. Bioeng. Biotechnol. 2017, 5, 255314. [Google Scholar] [CrossRef] [PubMed]
- Khosropanah, M.H.; Torabinavid, P.; Azimzadeh, A.; Tanourlouee, S.B.; Kajbafzadeh, A.M. Efficient Decellularization of Human Fetal Kidneys through Optimized SDS Exposure. Sci. Rep. 2024, 14, 21545. [Google Scholar] [CrossRef] [PubMed]
- Torabinavid, P.; Khosropanah, M.H.; Azimzadeh, A.; Kajbafzadeh, A.M. Current Strategies on Kidney Regeneration Using Tissue Engineering Approaches: A Systematic Review. BMC Nephrol. 2025, 26, 66. [Google Scholar] [CrossRef]
- Luque-Badillo, A.C.; Monjaras-Avila, C.U.; Adomat, H.; So, A.; Chavez-Muñoz, C. Evaluating Different Methods for Kidney Recellularization. Sci. Rep. 2024, 14, 23520. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Al Fahad, M.A.; Shanto, P.C.; Park, S.-s.; Lee, B.T. Surface Modification of Decellularized Kidney Scaffold with Chemokine and AKI-CKD Cytokine Juice to Increase the Recellularization Efficiency of Bio-Engineered Kidney. Biomaterials 2025, 316, 123007. [Google Scholar] [CrossRef] [PubMed]
- Abolbashari, M.; Agcaoili, S.M.; Lee, M.K.; Ko, I.K.; Aboushwareb, T.; Jackson, J.D.; Yoo, J.J.; Atala, A. Repopulation of porcine kidney scaffold using porcine primary renal cells. Acta Biomater. 2016, 29, 52–61. [Google Scholar] [CrossRef]
- Theodorou, C.; Leatherby, R.; Dhanda, R. Function of the Nephron and the Formation of Urine. Anaesth. Intensive Care Med. 2021, 22, 434–438. [Google Scholar] [CrossRef]
- Koulouridis, E.; Koulouridis, I. The loop of Henle as the milestone of mammalian kindey concentrating ability: A historical review. Acta Med. Hist. Adriat. 2014, 12, 413–428. [Google Scholar] [PubMed]
- Olde Hanhof, C.J.A.; Yousef Yengej, F.A.; Rookmaaker, M.B.; Verhaar, M.C.; Van Der Wijst, J.; Hoenderop, J.G. Modeling Distal Convoluted Tubule (Patho)Physiology: An Overview of Past Developments and an Outlook Toward the Future. Tissue Eng. Part. C Methods 2021, 27, 200–212. [Google Scholar] [CrossRef]
- Lam, A.Q.; Bonventre, J.V. Regenerating the Nephron with Human Pluripotent Stem Cells. Curr. Opin. Organ. Transplant. 2015, 20, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Haraldsson, B.; Nyström, J.; Deen, W.M. Properties of the Glomerular Barrier and Mechanisms of Proteinuria. Physiol. Rev. 2008, 88, 451–487. [Google Scholar] [CrossRef]
- van den Berg, C.W.; Dumas, S.J.; Little, M.H.; Rabelink, T.J. Challenges in Maturation and Integration of Kidney Organoids for Stem Cell–Based Renal Replacement Therapy. Kidney Int. 2025, 107, 262–270. [Google Scholar] [CrossRef]
- Mazloomnejad, R.; Babajani, A.; Kasravi, M.; Ahmadi, A.; Shariatzadeh, S.; Bahrami, S.; Niknejad, H. Angiogenesis and Re-Endothelialization in Decellularized Scaffolds: Recent Advances and Current Challenges in Tissue Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1103727. [Google Scholar] [CrossRef]
- Wang, K.; Kestenbaum, B. Proximal Tubular Secretory Clearance: A Neglected Partner of Kidney Function. Clin. J. Am. Soc. Nephrol. 2018, 13, 1291. [Google Scholar] [CrossRef]
- Wagner, C.A. Beyond SGLT2: Proximal Tubule Transporters as Potential Drug Targets for Chronic Kidney Disease. Nephrol. Dial. Transplant. 2025, 40, i18–i28. [Google Scholar] [CrossRef]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci. Rep. 2016, 6, 34845. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, Y.Y.; He, F.F.; Zhang, C. Revolutionizing Nephrology Research: Expanding Horizons with Kidney-on-a-Chip and Beyond. Front. Bioeng. Biotechnol. 2024, 12, 1373386. [Google Scholar] [CrossRef]
- Ross, E.J.; Gordon, E.R.; Sothers, H.; Darji, R.; Baron, O.; Haithcock, D.; Prabhakarpandian, B.; Pant, K.; Myers, R.M.; Cooper, S.J.; et al. Three Dimensional Modeling of Biologically Relevant Fluid Shear Stress in Human Renal Tubule Cells Mimics in Vivo Transcriptional Profiles. Sci. Rep. 2021, 11, 14053. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.B.; Kim, K.; Sung, G.Y. Effect of Shear Stress on the Proximal Tubule-on-a-Chip for Multi-Organ Microphysiological System. J. Ind. Eng. Chem. 2022, 115, 279–286. [Google Scholar] [CrossRef]
- Karp, S.; Pollak, M.R.; Subramanian, B. Disease Modeling with Kidney Organoids. Micromachines 2022, 13, 1384. [Google Scholar] [CrossRef]
- Li, S.R.; Gulieva, R.E.; Helms, L.; Cruz, N.M.; Vincent, T.; Fu, H.; Himmelfarb, J.; Freedman, B.S. Glucose Absorption Drives Cystogenesis in a Human Organoid-on-Chip Model of Polycystic Kidney Disease. Nat. Commun. 2022, 13, 7918. [Google Scholar] [CrossRef]
- Yu, Y.; Alkhawaji, A.; Ding, Y.; Mei, J. Decellularized Scaffolds in Regenerative Medicine. Oncotarget 2016, 7, 58671. [Google Scholar] [CrossRef] [PubMed]
- Marulli, M.; Edwards, A.; Milišić, V.; Vauchelet, N. On the Role of the Epithelium in a Model of Sodium Exchange in Renal Tubules. Math. Biosci. 2020, 321, 108308. [Google Scholar] [CrossRef] [PubMed]
- Pahuja, A.; Goux Corredera, I.; Moya-Rull, D.; Garreta, E.; Montserrat, N. Engineering Physiological Environments to Advance Kidney Organoid Models from Human Pluripotent Stem Cells. Curr. Opin. Cell Biol. 2024, 86, 102306. [Google Scholar] [CrossRef]
- Liu, M.; Cardilla, A.; Ngeow, J.; Gong, X.; Xia, Y. Studying Kidney Diseases Using Organoid Models. Front. Cell Dev. Biol. 2022, 10, 845401. [Google Scholar] [CrossRef]
- McCormick, J.A.; Ellison, D.H. Distal Convoluted Tubule. Compr. Physiol. 2015, 5, 2147–2163. [Google Scholar] [CrossRef]
- Dilmen, E.; Olde Hanhof, C.J.A.; Yousef Yengej, F.A.; Ammerlaan, C.M.E.; Rookmaaker, M.B.; Orhon, I.; Jansen, J.; Verhaar, M.C.; Hoenderop, J.G. A Semi-Permeable Insert Culture Model for the Distal Part of the Nephron with Human and Mouse Tubuloid Epithelial Cells. Exp. Cell Res. 2025, 444, 114342. [Google Scholar] [CrossRef]
- Yousef Yengej, F.A.; Pou Casellas, C.; Ammerlaan, C.M.E.; Olde Hanhof, C.J.A.; Dilmen, E.; Beumer, J.; Begthel, H.; Meeder, E.M.G.; Hoenderop, J.G.; Rookmaaker, M.B.; et al. Tubuloid Differentiation to Model the Human Distal Nephron and Collecting Duct in Health and Disease. Cell Rep. 2024, 43, 113614. [Google Scholar] [CrossRef]
- Zeng, Z.; Huang, B.; Parvez, R.K.; Li, Y.; Chen, J.; Vonk, A.C.; Thornton, M.E.; Patel, T.; Rutledge, E.A.; Kim, A.D.; et al. Generation of Patterned Kidney Organoids That Recapitulate the Adult Kidney Collecting Duct System from Expandable Ureteric Bud Progenitors. Nat. Commun. 2021, 12, 3641. [Google Scholar] [CrossRef] [PubMed]
- Howden, S.E.; Wilson, S.B.; Groenewegen, E.; Starks, L.; Forbes, T.A.; Tan, K.S.; Vanslambrouck, J.M.; Holloway, E.M.; Chen, Y.H.; Jain, S.; et al. Plasticity of Distal Nephron Epithelia from Human Kidney Organoids Enables the Induction of Ureteric Tip and Stalk. Cell Stem Cell 2021, 28, 671.e6–684.e6. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, G.; Li, G.; Li, P.-L.; Li, N. Collecting Duct-Specific Deletion of Acid Ceramidase Promoted Sodium Retention and Salt-Sensitive Hypertension. Physiology 2025, 40, 0632. [Google Scholar] [CrossRef]
- Higashihara, E.; Harada, T.; Fukuhara, H. Juxtaglomerular Apparatus-Mediated Homeostatic Mechanisms: Therapeutic Implication for Chronic Kidney Disease. Expert. Opin. Pharmacother. 2024, 25, 819–832. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Gomez, R.A.; Sequeira-Lopez, M.L.S. Renin Cells, From Vascular Development to Blood Pressure Sensing. Hypertension 2023, 80, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.S.; Du, Z.; Mora, H.T.; van den Bosch, T.P.P.; Korevaar, S.S.; Van den Berg-Garrelds, I.M.; Bindels, E.; Lopez-Iglesias, C.; Clahsen-van Groningen, M.C.; Gribnau, J.; et al. Human Kidney Organoids Produce Functional Renin. Kidney Int. 2021, 99, 134–147. [Google Scholar] [CrossRef]
- Freedman, B.S. Physiology Assays in Human Kidney Organoids. Am. J. Physiol. Renal Physiol. 2022, 322, F625–F638. [Google Scholar] [CrossRef]
- Yanofsky, S.M.; Dugas, C.M.; Katsurada, A.; Liu, J.; Saifudeen, Z.; El-Dahr, S.S.; Satou, R. Angiotensin II Biphasically Regulates Cell Differentiation in Human IPSC-Derived Kidney Organoids. Am. J. Physiol. Renal Physiol. 2021, 321, F559–F571. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y. Peritubular Capillary Rarefaction: An Underappreciated Regulator of CKD Progression. Int. J. Mol. Sci. 2020, 21, 8255. [Google Scholar] [CrossRef]
- Choi, Y.J.; Chakraborty, S.; Nguyen, V.; Nguyen, C.; Kim, B.K.; Shim, S.I.; Suki, W.N.; Truong, L.D. Peritubular Capillary Loss Is Associated with Chronic Tubulointerstitial Injury in Human Kidney: Altered Expression of Vascular Endothelial Growth Factor. Hum. Pathol. 2000, 31, 1491–1497. [Google Scholar] [CrossRef]
- Meng, X.; Xing, Y.; Li, J.; Deng, C.; Li, Y.; Ren, X.; Zhang, D. Rebuilding the Vascular Network: In Vivo and in Vitro Approaches. Front. Cell Dev. Biol. 2021, 9, 639299. [Google Scholar] [CrossRef]
- Shin, Y.J.; Safina, D.; Zheng, Y.; Levenberg, S. Microvascularization in 3D Human Engineered Tissue and Organoids. Annu. Rev. Biomed. Eng. 2025, 27, 473–498. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ying, G.; Hu, C.; Du, L.; Zhang, H.; Wang, Z.; Yue, H.; Yetisen, A.K.; Wang, G.; Shen, Y.; et al. Engineering in Vitro Vascular Microsystems. Microsyst. Nanoeng. 2025, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Lebedenko, C.G.; Banerjee, I.A. Enhancing Kidney Vasculature in Tissue Engineering—Current Trends and Approaches: A Review. Biomimetics 2021, 6, 40. [Google Scholar] [CrossRef]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.J.; Ferguson, C.; Parton, R.G.; Wolvetang, E.J.; Roost, M.S.; De Sousa Lopes, S.M.C.; et al. Kidney Organoids from Human IPS Cells Contain Multiple Lineages and Model Human Nephrogenesis. Nature 2015, 526, 564–568. [Google Scholar] [CrossRef]
- Wieringa, F.P.; Suran, S.; Søndergaard, H.; Ash, S.; Cummins, C.; Chaudhuri, A.R.; Irmak, T.; Gerritsen, K.; Vollenbroek, J. The Future of Technology-Based Kidney Replacement Therapies: An Update on Portable, Wearable, and Implantable Artificial Kidneys. Am. J. Kidney Dis. 2025, 85, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Araoka, T.; Toyohara, K.; Ryosaka, M.; Inui, C.; Matsuura, M.; Ma, C.; Watahiki, J.; Li, Z.; Iwasaki, M.; Watanabe, A.; et al. Human IPSC–Derived Nephron Progenitor Cells Treat Acute Kidney Injury and Chronic Kidney Disease in Mouse Models. Sci. Transl. Med. 2025, 17, eadt5553. [Google Scholar] [CrossRef]
- Tsokas, K.; McFarland, R.; Burke, C.; Lynch, J.L.; Bollenbach, T.; Callaway II, D.A.; Siegel, J. Reducing Risks and Delays in the Translation of Cell and Gene Therapy Innovations into Regulated Products. NAM Perspect. 2019, 2019, 10-31478. [Google Scholar] [CrossRef]
- Lowdell, M.W. Considerations for Manufacturing of Cell and Gene Medicines for Clinical Development. Cytotherapy 2025, 27, 874–883. [Google Scholar] [CrossRef]
- Iglesias-Lopez, C.; Agustí, A.; Vallano, A.; Obach, M. Current Landscape of Clinical Development and Approval of Advanced Therapies. Mol. Ther. Methods Clin. Dev. 2021, 23, 606. [Google Scholar] [CrossRef]
- Sekar, M.P.; Budharaju, H.; Zennifer, A.; Sethuraman, S.; Vermeulen, N.; Sundaramurthi, D.; Kalaskar, D.M. Current standards and ethical landscape of engineered tissues-3D bioprinting perspective. J. Tissue Eng. 2021, 12, 20417314211027677. [Google Scholar] [CrossRef]
- Zheng, Y.L. Some Ethical Concerns About Human Induced Pluripotent Stem Cells. Sci. Eng. Ethics 2016, 22, 1277–1284. [Google Scholar] [CrossRef]
- Arifa, N. Induced Pluripotent Stem Cells (IPSCs): The Ethical Alternative? Stem Cell Res. Reg. Med. 2024, 7, 251–252. [Google Scholar] [CrossRef]
- Cintron Pregosin, N.; Bronstein, R.; Mallipattu, S.K. Recent Advances in Kidney Bioengineering. Front. Pediatr. 2021, 9, 743301. [Google Scholar] [CrossRef] [PubMed]

| Platform | Key Advances | Major Barriers |
|---|---|---|
| Kidney Organoids | Multi-segment architecture; organ-on-chip vascular cues; in vivo maturation | No vasculature; no urine outflow; immaturity; heterogeneity |
| iBK Devices | Functional hemofilter with tubule bioreactor; stable in large animals; no external power | Cell viability under shear; fibrosis/immune risk; membrane durability |
| 3D Bioprinting | Custom geometry; epithelial–endothelial constructs; ECM-based bioinks | Poor vascularization; limited scaling; host integration issues |
| Decellularized Scaffolds | Native ECM and vasculature preserved; recellularization with renal cells; perfusion bioreactors | Incomplete reseeding; cell sourcing; immune rejection/thrombosis |
| Nephron Function | Physiological Role | Cellular/Molecular Requirements | Replacement Technologies | Current Status |
|---|---|---|---|---|
| Glomerulus (filtration) | Initiates urine formation via selective, high-pressure filtration of blood; retains proteins and cells while allowing passage of water and small solutes | Podocytes, glomerular endothelial cells, specialized GBM (collagen IV, laminin), slit diaphragm proteins (nephrin, podocin) | Decellularized glomerular scaffolds; bioprinted glomerular units; PSC-derived organoid glomeruli | Proof-of-concept filtration achieved in vitro and in small animal models; physiological selectivity and sustained filtration rates remain suboptimal. Integration with vascular networks is a key challenge. |
| Proximal tubule (reabsorption/secretion) | Reabsorbs ~65% of filtered water, glucose, amino acids, bicarbonate, and ions; secretes organic solutes and drugs | Proximal tubular epithelial cells with brush border, SGLT2, NHE3, rich mitochondrial content, tight junctions | 3D bioprinted tubules; organoid-derived proximal segments; recellularized scaffolds; microfluidic kidney-on-chip platforms | Functional reabsorption and secretion are demonstrated in vitro; models support drug screening and nephrotoxicity studies. Long-term maturation, polarity, and integration with downstream segments are active areas of research. |
| Loop of Henle (countercurrent concentration) | Establishes medullary osmotic gradient via countercurrent multiplication, enabling urine concentration | Thin and thick limb epithelial cells, aquaporins (AQP1, AQP2), Na-K-2Cl cotransporter (NKCC2), medullary interstitium | Microengineered loop modules; segment-specific differentiation in organoids | Early stage prototypes: partial recapitulation of countercurrent function. Full osmotic gradient generation and integration with adjacent segments remain to be achieved. |
| Distal tubule (electrolyte fine-tuning) | Regulates sodium, potassium, calcium, and acid–base balance under hormonal control (aldosterone and PTH) | Distal tubular epithelial cells, ENaC, NCC, calcium channels, hormone receptors | Segment-specific cell sheets; responsive bioartificial modules; engineered distal tubule constructs | Segment identity and hormonal responsiveness were demonstrated in vitro. Integration with upstream and downstream modules and dynamic regulation is under development. |
| Collecting duct (water reabsorption/excretion) | Final site for water reabsorption (ADH-regulated), acid–base homeostasis, and urine excretion | Principal and intercalated cells, aquaporins (AQP2), ADH and aldosterone receptors, tight junctions | Engineered collecting duct arrays; organoid-derived collecting duct segments; responsive bioartificial modules | Functional water reabsorption and hormone response are shown in vitro. Full integration with nephron modules and urine drainage systems remains a challenge. |
| Juxtaglomerular apparatus (endocrine/autoregulation) | Senses tubular flow and sodium; regulates renin secretion and blood pressure (RAAS system); autoregulates GFR | Juxtaglomerular cells (renin), macula densa, afferent arteriole, paracrine signaling molecules | Organoid-based models; microfluidic feedback systems | Experimental models recapitulate some aspects of renin secretion and feedback. Full endocrine and autoregulatory function has not yet been achieved. |
| Peritubular capillaries (microvascular support) | Supplies oxygen/nutrients, removes reabsorbed solutes, supports tubule metabolism and function; mediates oxygen sensing and EPO production | Endothelial cells, pericytes, angiogenic factors, basement membrane; peritubular interstitial fibroblast-like cells (EPO), HIF pathway components | Vascularized scaffolds; endothelialized microfluidic chips; co-culture systems; microfluidic models; scaffold-derived interstitial cell populations | Microvascular networks are established in vitro; perfusion and stability over time are improving. Full integration with nephron modules and host vasculature remains a barrier. EPO expression is reported in organoid and scaffold studies under hypoxia, but physiologic oxygen sensing and regulated EPO secretion have not yet been achieved. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, N.; Tamazenko, Y. Modular Strategies for Nephron Replacement and Clinical Translation. Kidney Dial. 2025, 5, 41. https://doi.org/10.3390/kidneydial5030041
Stepanova N, Tamazenko Y. Modular Strategies for Nephron Replacement and Clinical Translation. Kidney and Dialysis. 2025; 5(3):41. https://doi.org/10.3390/kidneydial5030041
Chicago/Turabian StyleStepanova, Natalia, and Yevheniia Tamazenko. 2025. "Modular Strategies for Nephron Replacement and Clinical Translation" Kidney and Dialysis 5, no. 3: 41. https://doi.org/10.3390/kidneydial5030041
APA StyleStepanova, N., & Tamazenko, Y. (2025). Modular Strategies for Nephron Replacement and Clinical Translation. Kidney and Dialysis, 5(3), 41. https://doi.org/10.3390/kidneydial5030041







