The Role of ADMA as an Indicator of Progression in Early Stage of CKD
Abstract
1. Introduction
2. Material and Methods
2.1. Design
2.2. Inclusion and Exclusion
2.3. Sample Collection and Assay
2.4. Sample Size
2.5. Statistical Analysis
2.6. Ethical Clearance
3. Results
3.1. Patients Characteristics
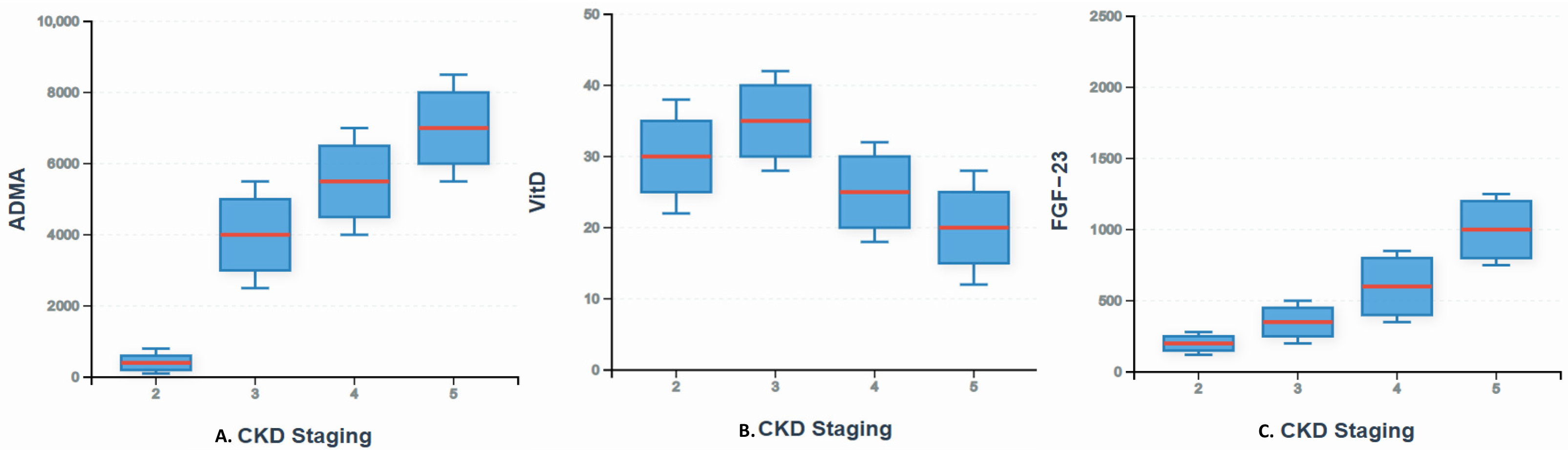
3.2. Correlation Between FGF-23, 25(OH)D, and ADMA Levels with the Degree of Albuminuria
3.3. Multivariate Analysis of Albuminuria with FGF-23, 25(OH)D, ADMA Levels Along with Concomitant Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic Kidney Disease |
| RAAS | Renin–Angiotensin–Aldosterone System |
| FGF-23 | Fibroblast Growth Factor-23 |
| 1,25(OH)2D3 | 1,25-dihydroxy vitamin D |
| FGFRs | Fibroblast Growth Factor Receptor Complexes |
| eGFR | Estimated Glomerular Filtration Rate |
| 25(OH)D | 25-hydroxyvitamin D |
| SDMA | Symmetric Dimethylarginine |
| ADMA | Asymmetric Dimethylarginine |
| ESRD | End-Stage Renal Disease |
| Rac1 | Ras-related C3 botulinum substrate 1 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PAK1 | p21 protein-activated kinase |
| SOCE | Store-Operated Ca2+ Entry |
| DDAH | Dimethylarginine–Dimethylamino–Hydrolase |
References
- Van der Velde, M.; Matsushita, K.; Coresh, J.; Astor, B.C.; Woodward, M.; Levey, A.; De Jong, P.; Gansevoort, R.T.; El-Nahas, M.; Eckardt, K.U.; et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011, 79, 1341–1352. [Google Scholar] [CrossRef]
- Gorriz, J.L.; Martinez-Castelao, A. Proteinuria: Detection and role in native renal disease progression. Transplant. Rev. 2012, 26, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Gansevoort, R.T.; Matsushita, K.; van der Velde, M.; Woodward, M.; Levey, A.; De Jong, P.; Coresh, J. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011, 80, 93–104. [Google Scholar] [CrossRef] [PubMed]
- de Borst, M.H. Interaction between inflammation, mineral metabolism and the renin–angiotensin system: Implications for cardiorenal outcomes in chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 547–551. [Google Scholar] [CrossRef]
- Vogt, I.; Haffner, D.; Leifheit-Nestler, M. FGF 23 and Phosphate-Cardiovascular Toxins in CKD. Toxins 2019, 11, 647. [Google Scholar] [CrossRef]
- De Jong, M.A.; Eisenga, M.F.; van Ballegooijen, A.J.; Beulens, J.W.J.; Vervloet, M.G.; Navis, G.; Gansevoort, R.T.; Bakker, S.J.L.; De Borst, M.H. Fibroblast growth factor 23 and new-onset chronic kidney disease in the general population: The Prevention of Renal and Vascular Endstage Disease (PREVEND) study. Nephrol. Dial. Transplant. 2021, 36, 121–128. [Google Scholar] [CrossRef]
- Liang, Q.; Hu, H.; Wu, H.; Chen, X.; Wang, W.; Le, Y.; Yang, S.; Jia, L. A Nonlinear Relationship Between Serum 25-Hydroxyvitamin D and Urine Albumin to Creatinine Ratio in Type 2 Diabetes: A Cross-Sectional Study in China. Diabetes Metab. Syndr. Obes. 2021, 14, 2581–2593. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; Gutiérrez, O.M.; Patel, N.M.; Andress, D.L.; Wolf, M.; Levin, A. Vitamin D deficiency, inflammation, and albuminuria in chronic kidney disease: Complex interactions. J. Ren. Nutr. 2011, 21, 295–302. [Google Scholar] [CrossRef]
- Oliva-Damaso, E.; Oliva-Damaso, N.; Rodriguez-Esparragon, F.; Payan, J.; Baamonde-Laborda, E.; Gonzales-Cabrera, F.; Santana Estupinan, R.; Rodrigues-Perez, J.C. Asymmetric (ADMA) and Symmetric (SDMA) Dimethylarginines in Chronic Kidney Disease: A Clinical Approach. Int. J. Mol. Sci. 2019, 20, 3668. [Google Scholar] [CrossRef]
- Zoccali, C.; Bode-Böger, S.; Mallamaci, F.; Benedetto, F.; Tripepi, G.; Malatino, L.; Cataliotti, A.; Bellanuova, I.; Fermo, I.; Frölich, J.; et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: A prospective study. Lancet 2001, 358, 2113–2117. [Google Scholar] [CrossRef]
- Leone, A.; Moncada, S.; Vallance, P.; Calver, A.; Collier, J. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar] [CrossRef]
- Bergmann, I.P.; Böger, R.H.; Marti, E.; Frey, F.J.; Schwedhelm, E.; Eisenberger, U. Renal Resistance Index in renal allograft Recipients: A role for ADMA. Am. J. Kidney Dis. 2009, 54, 327–333. [Google Scholar] [CrossRef]
- Eiselt, J.; Rajdl, D.; Racek, J.; Vostry, M.; Rulcová, K.; Wirth, J. Asymmetric dimethylarginine and progression of chronic kidney disease: A one-year follow-up study. Kidney Blood Press. Res. 2014, 39, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Tripepi, G.; Kollerits, B.; Leonardis, D.; Yilmaz, M.I.; Postorino, M.; Filser, D.; Mallamaci, F.; Kronenberg, F.; Zoccali, C. Competitive interaction between fibroblast growth factor 23 and asymmetric dimethylarginine in patients with CKD. J. Am. Soc. Nephrol. 2015, 26, 935–944. [Google Scholar] [CrossRef][Green Version]
- Humalda, J.K.; Lambers Heerspink, H.J.; Kwakernaak, A.J.; Slagman, M.C.J.; Waanders, F.; Vervlooet, M.G.; Ter Wee, P.M.; Navis, G.; De Borst, M.H.; NIGRAM Consortium. Fibroblast growth factor 23 and the antiproteinuric response to dietary sodium restriction during renin-angiotensin-aldosterone system blockade. Am. J. Kidney Dis. 2015, 65, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Kono, K.; Nakai Goto, S.; Kitazawa, R.; Fukugawa, M.; Nishi, S. Renin-Angiotensin System Inhibitors Reduce Serum Asymmetric Dimethylarginine Levels and Oxidative Stress in Normotensive Patients with Chronic Kidney Disease. Nephron Extra 2014, 4, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Uwaezuoke, S.N. Vitamin D Analogs Can Retard the Onset or Progression of Diabetic Kidney Disease: A Systematic review. Front. Clin. Diabetes Healthc. 2021, 2, 763844. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.; Nam, K.H.; Jhee, J.H.; Yun, H.R.; Park, J.T.; Han, S.H.; Chung, W.; Oh, K.H.; Park, S.K.; et al. The effect of interactions between proteinuria, activity of fibroblast growth factor 23 and serum phosphate on renal progression in patients with chronic kidney disease: A result from the KoreaN cohort study for Outcome in patients With Chronic Kidney Disease study. Nephrol. Dial. Transplant. 2020, 35, 438–446. [Google Scholar]
- Isakova, T.; Wahl, P.; Vargas, G.S.; Gutiérrez, O.M.; Scialla, J.; Xie, H.; Appleby, D.; Nessel, L.; Bellovich, K.; Chen, J.; et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011, 79, 1370–1378. [Google Scholar] [CrossRef]
- Haghikia, A.; Podewski, E.; Libhaber, E.; Labidi, S.; Fischer, D.; Roentgen, P.; Tsikas, D.; Jordan, J.; Lichtinghagen, R.; Von Kaisenberg, C.S.; et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic. Res. Cardiol. 2013, 108, 366. [Google Scholar] [CrossRef]
- Hulley, S.B.; Cummings, S.R.; Browner, W.S.; Grady, D.G.; Newman, T.B. Designing Clinical Research, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Bargman, J.M.; Skorecki, K.L. Chronic Kidney Disease. In Harrison’s Principles of Internal Medicine, 20th ed.; Jameson, J.L., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Prasad, R.M.; Bali, A.; Tikaria, R. Microalbuminuria. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Zhuo, M.; Jiang, M.Y.; Song, R.; Mothi, S.S.; Bellou, S.; Polding, L.C.; Li, J.; Cho, A.; Hsiao, L.L. High Prevalence and Low Awareness of Albuminuria in the Community Setting in the KDSAP. Kidney Int. Rep. 2020, 5, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.H.; Oh, I.H.; Park, J.S.; Lee, C.H.; Kang, C.M.; Kom, G.H. Evaluation of Urinary Indices for Albuminuria and Proteinuria in Patients with Chronic Kidney Disease. Kidney Blood Press. Res. 2016, 41, 258–266. [Google Scholar] [CrossRef]
- Erben, R.G. Physiological Actions of Fibroblast Growth Factor-23. Front. Endocrinol. 2018, 9, 267. [Google Scholar] [CrossRef]
- Lang, F.; Leibrock, C.; Pandyra, A.A.; Stournaras, C.; Wagner, C.A.; Föller, M. Phosphate Homeostasis, Inflammation and the Regulation of FGF-23. Kidney Blood Press. Res. 2018, 43, 1742–1748. [Google Scholar] [CrossRef]
- Secondo, A.; Bagetta, G.; Amantea, D. On the Role of Store-Operated Calcium Entry in Acute and Chronic Neurodegenerative Diseases. Front. Mol. Neurosci. 2018, 11, 87. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Juárez, G.; Luño, J.; Barrio, V.; De Vinuesa, S.G.; Praga, M.; Goicoechea, M.; Lahera, V.; Casas, L.; Oliva, J. 25 (OH) Vitamin D Levels and Renal Disease Progression in Patients with Type 2 Diabetic Nephropathy and Blockade of the Renin-Angiotensin System. Clin. J. Am. Soc. Nephrol. 2013, 8, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Toyoda, M.; Miyatake, H.; Tanaka, E.; Koizumi, M.; Komaba, H.; Kimura, M.; Umezono, T.; Fukugawa, M. The Prevalence of 25-hydroxyvitamin D Deficiency in Japanese Patients with Diabetic Nephropathy. Intern. Med. 2016, 55, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Echida, Y.; Mochizuki, T.; Uchida, K.; Tsuchiya, K.; Nitta, K. Risk factors for vitamin D deficiency in patients with chronic kidney disease. Intern. Med. 2012, 51, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Shroff, A.; Kupfer, J.; Gilchrist, I.C.; Caputo, R.; Speiser, B.; Bertrand, O.F.; Pancholy, S.B.; Rao, S.V. Same-Day Discharge After Percutaneous Coronary Intervention: Current Perspectives and Strategies for Implementation. JAMA Cardiol. 2016, 1, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Larson, B.; Ashrafzadeh-Kian, S.; Ito, N.; Kato, H.; Bornhorst, J.A.; Algeciras-Schimnich, A. Intact Fibroblast Growth Factor 23 Concentrations in Hypophosphatemic Disorders. Endocr. Pract. 2023, 29, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Melamed, M.L.; Thadhani, R.I. Vitamin D therapy in chronic kidney disease and end stage renal disease. Clin. J. Am. Soc. Nephrol. 2012, 7, 358–365. [Google Scholar] [CrossRef]
- Thaha, M.; Mochamad, Y.; Maulana, E.; Kadariswantiningsih, I. SUN-154 Oxidative Stress Associates with Elevated Asymetric and Symmetric Dimethylarginine in CKD Patients receiving Hemodyalisis. Kidney Int. Rep. 2020, 5 (Suppl. 3), S263–S264. [Google Scholar] [CrossRef]
- Sakin, A. The Evaluation of the Relationship Between Albuminuria and Serum Asymmetric Dimethyl Arginine Level in Type-2 Diabetes Mellitus. Eurasian J. Med. Investig. 2021, 5, 16–20. [Google Scholar] [CrossRef]
- Raptis, V.; Kapoulas, S.; Grekas, D. Role of asymmetrical dimethylarginine in the progression of renal disease. Nephrology 2013, 18, 11–21. [Google Scholar] [CrossRef]
- Thaha, M.; Yusuf, M.; Empitu, M.A.; Bakarman, A.; Tomino, Y. Distribution of dimethylarginine-dimethylaminohydrolase-II (DDAH2) gene polymorphism in hemodialysis patients. Acta Med. Indones. 2013, 45, 83–88. [Google Scholar]
- Lu, T.M.; Chung, M.Y.; Lin, C.C.; Hsu, C.P.; Lin, S.J. Asymmetric dimethylarginine and clinical outcomes in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 1566. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Results | |
|---|---|---|
| Age (years) | Median (min–max) | 59 (32–70) |
| Gender | ||
| Male | % (n) | 54.2% (n = 58) |
| Female | % (n) | 45.8% (n = 49) |
| Blood pressure (mmHg) | ||
| Systole | Mean ± SD | 145.59 ± 23.31 |
| Diastole | Median (min–max) | 81 (51−121) |
| Risk factor | ||
| Diabetes mellitus type 2 | % (n) | 72% (n = 77) |
| Hypertension | % (n) | 86.9% (n = 93) |
| Smoking | % (n) | 27.1% (n = 29) |
| Obesity | % (n) | 16.8% (n = 18) |
| Renal function | ||
| Creatinine (mg/dL) | Median (min–max) | 2.12 (1.15–11.82) |
| eGFR (mL/min/1.73 m2) | Median (min–max) | 31 (4–68) |
| Albuminuria (mg/g) | Median (min–max) | 289.7 (2.29–5849.50) |
| FGF-23 (RU/mL) | Median (min–max) | 136 (21.59–1054.70) |
| Vitamin D (ng/mL) | Mean ± SD | 23.44 ± 11.95 |
| ADMA (µmol/L) | Median (min–max) | 0.626 (0.040–4.345) |
| CKD stage | ||
| Stage 2 | % (n) | 4.7% (n = 5) |
| Stage 3 | % (n) | 47.7% (n = 51) |
| Stage 4 | % (n) | 27.1% (n = 29) |
| Stage 5 | % (n) | 20.6% (n = 22) |
| Variable 1 | Variable 2 | r Spearman | p-Value |
|---|---|---|---|
| FGF-23 | Albuminuria | 0.252 | 0.009 |
| 25(OH)D | Albuminuria | −0.375 | 0.000 |
| ADMA | Albuminuria | 0.687 | 0.000 |
| Variable | Albuminuria | p-Value Bivariate | OR (95% CI) | p-Value Multivariate | Adjusted OR (95% CI) | |
|---|---|---|---|---|---|---|
| ≥300 mg/g | <300 mg/g | |||||
| FGF-23 (RU/mL) | ||||||
| ≥100 | 40 | 39 | 0.702 | 0.92 (0.58–1.44) | ||
| <100 | 13 | 15 | ||||
| 25(OH)D (ng/mL) | ||||||
| ≥30 | 14 | 28 | 0.007 * | 1.67 (1.16–2.41) | ||
| <30 | 39 | 26 | ||||
| ADMA (µmol/L) | ||||||
| ≥0.69 | 44 | 19 | 0.00 * | 2.64 (1.76–3.95) | 0.000 ** | 9.49 (3.18–28.25) |
| <0.69 | 9 | 35 | ||||
| Age (years) | ||||||
| ≥60 | 15 | 31 | 0.002 * | 2.03 (1.25–3.29) | ||
| <60 | 38 | 23 | ||||
| Gender | ||||||
| Male | 24 | 34 | 0.066 * | 1.39 (0.97–1.99) | 0.041 ** | 3.13 (1.05–9.32) |
| Female | 29 | 20 | ||||
| Diabetes mellitus | ||||||
| Yes | 44 | 33 | 0.012 * | 2.29 (1.16–4.53) | 0.031 ** | 3.73 (1.12–12.38) |
| No | 9 | 21 | ||||
| Hypertension | ||||||
| Yes | 40 | 24 | 0.001 * | 2.27 (1.33–3.84) | 0.003 ** | 5.29 (1.75–15.99) |
| No | 13 | 30 | ||||
| Obesity | ||||||
| Yes | 10 | 8 | 0.56 | 1.05 (0.89–1.25) | ||
| No | 43 | 46 | ||||
| Smoking | ||||||
| Yes | 14 | 15 | 0.87 | 0.98 (0.78–1.24) | ||
| No | 39 | 39 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suryantoro, S.D.; Thaha, M.; Mahdi, B.A.; Haryati, M.R.; A’yunin, U.Q. The Role of ADMA as an Indicator of Progression in Early Stage of CKD. Kidney Dial. 2025, 5, 42. https://doi.org/10.3390/kidneydial5030042
Suryantoro SD, Thaha M, Mahdi BA, Haryati MR, A’yunin UQ. The Role of ADMA as an Indicator of Progression in Early Stage of CKD. Kidney and Dialysis. 2025; 5(3):42. https://doi.org/10.3390/kidneydial5030042
Chicago/Turabian StyleSuryantoro, Satriyo Dwi, Mochamad Thaha, Bagus Aulia Mahdi, Mutiara Rizky Haryati, and Ulinnuha Qurrota A’yunin. 2025. "The Role of ADMA as an Indicator of Progression in Early Stage of CKD" Kidney and Dialysis 5, no. 3: 42. https://doi.org/10.3390/kidneydial5030042
APA StyleSuryantoro, S. D., Thaha, M., Mahdi, B. A., Haryati, M. R., & A’yunin, U. Q. (2025). The Role of ADMA as an Indicator of Progression in Early Stage of CKD. Kidney and Dialysis, 5(3), 42. https://doi.org/10.3390/kidneydial5030042






