Bloodstream Infection Caused by Raoultella ornithinolytica in a Chronic Hemodialysis Patient
Abstract
1. Introduction
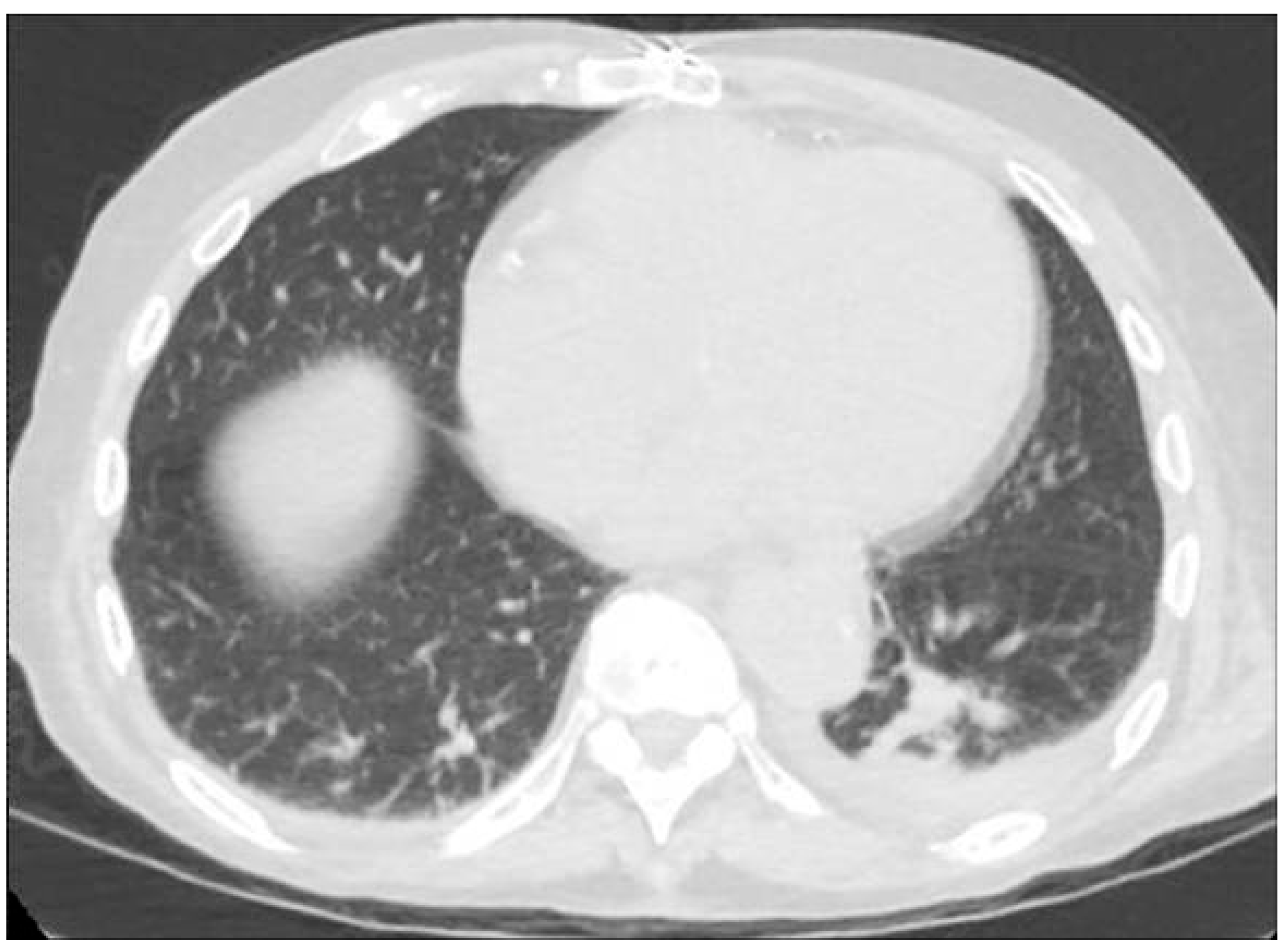
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAL | Bronchoalveolar lavage |
| blaKPC | Klebsiella pneumoniae carbapenemase resistant to broad-spectrum â-lactam antibiotics |
| BP | Blood pressure |
| BSI | Bloodstream infections |
| CoNS | Coagulase-negative staphylococci |
| CRBSI | Catheter-related bloodstream infections |
| CRP | C-reactive protein |
| VIM | Verona integron-encoded metallo-â-lactamase. |
| CT | Computer tomography |
| HD | Hemodialysis |
| HR | Heart rate |
| IV | Intravenous |
| MALDI-TOF MS | Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry |
| MDR | Multidrug-resistant |
| MIC | Minimum inhibitory concentration |
| nv | Normal values |
| OXA | Oxacillinase |
| T | Temperature |
| TMP–SMX | Trimteprim–suplhametoxazole |
| WBC | White blood cells |
| CRP | C-reactive protein |
| WBC | white blood cells |
| PCT | procalcitonin. |
References
- Pravda, M.S.; Maor, Y.; Brodsky, K.; Katkov, A.; Cernes, R.; Pravda, N.S.; Tocut, M.; Zohar, I.; Soroksky, A.; Feldman, L. Blood stream Infections in chronic hemodialysis patients—Characteristics and outcomes. BMC Nephrol. 2024, 25, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sękowska, A.; Dylewska, K.; Gospodarek, E.; Bogiel, T. Catheter-related blood stream infection caused by Raoultella ornithinolytica. Folia Microbiol. 2015, 60, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Rteil, A.; Kazma, J.M.; El Sawda, J.; Gharamti, A.; Koubar, S.H.; Kanafani, Z.A. Clinical characteristics, risk factors and microbiology of infections in patients receiving chronic hemodialysis. J. Infect. Public Health 2020, 13, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Jaber, B.L. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000, 58, 1758–1764. [Google Scholar] [CrossRef]
- Wetmore, J.B.; Li, S.; Molony, J.T.; Guo, H.; Herzog, C.A.; Gilbertson, D.T.; Peng, Y.; Collins, A.J. Insights from the 2016 peer kidney care initiative report: Still a ways to go to improve care for dialysis patients. Am. J. Kidney Dis. 2018, 71, 123–132. [Google Scholar] [CrossRef]
- Seng, P.; Boushab, B.M.; Romain, F.; Gouriet, F.; Bruder, N.; Martin, C.; Paganelli, F.; Bernit, E.; Le Treut, Y.P.; Thomas, P.; et al. Emerging role of Raoultella ornithinolytica in human infections: A series of cases and review of the literature. Int. J. Infect. Dis. 2016, 45, 65–71. [Google Scholar] [CrossRef]
- Haruki, Y.; Hagiya, H.; Sakuma, A.; Murase, T.; Sugiyama, T.; Kondo, S. Clinical characteristics of Raoultella ornithinolytica bacteremia: A case series and literature review. J. Infect. Chemother. 2014, 20, 589–591. [Google Scholar] [CrossRef]
- Chun, S.; Yun, J.W.; Huh, H.J.; Lee, N.Y. Clinical characteristics of Raoultella ornithinolytica bacteremia. Infection 2015, 43, 59–64. [Google Scholar] [CrossRef]
- Hajjar, R.; Ambaraghassi, G.; Sebajang, H.; Schwenter, F.; Su, S.-H. Raoultella ornithinolytica: Emergence and resistance. Infect. Drug Resist. 2020, 13, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Deshpande, L.M.; DiPersio, J.R.; Kang, J.; Weinstein, M.P.; Jones, R.N. First descriptions of blaKPC in Raoultella spp. (R. planticola and R. ornithinolytica): Report from the SENTRY antimicrobial surveillance program. J. Clin. Microbiol. 2009, 47, 4129–4130. [Google Scholar] [CrossRef] [PubMed]
- Tijet, N.; Sheth, P.M.; Lastovetska, O.; Chung, C.; Patel, S.N.; Melano, R.G.; Friedrich, A. Molecular characterization of Klebsiella pneumoniae Carbapenemase (KPC)-producing Enterobacteriaceae in Ontario, Canada, 2008–2011. PLoS ONE 2014, 9, 2008–2011. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.S.; Sercia, L.; Branda, J.A.; Burnham, C.-A.D.; Bythrow, M.; Ferraro, M.J.; Garner, O.B.; Ginocchio, C.C.; Jennemann, R.; Lewinski, M.A.; et al. Identification of Enterobacteriaceae by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry using the VITEK MS system. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Surani, A.; Slama, E.M.; Thomas, S.; Ross, R.W.; Cunningham, S.C. Raoultella ornithinolytica and Klebsiella oxytoca pyogenic liver abscess presenting as chronic cough. IDCases 2020, 20, e00736. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.; Kesper, M.S.; Marschner, J.A.; Konrad, L.; Ryu, M.; Kumar, V.R.S.; Kulkarni, O.P.; Mulay, S.R.; Romoli, S.; Demleitner, J.; et al. Intestinal Dysbiosis, Barrier Dysfunction, and Bacterial Translocation Account for CKD-Related Systemic Inflammation. J. Am. Soc. Nephrol. 2017, 28, 76–83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Timeline Day | Event | Laboratory Data | Vitals |
|---|---|---|---|
| Day 0 | Onset of symptoms (malaise, weakness, and dyspnea) | Unavailable | Unavailable |
| Day 2 | Admission to Emergency Department after dialysis session for worsening clinical condition with fever and chills Blood cultures taken | WBC: 4560/mmc CRP: 158.4 mg/L PCT: 4.74 µg/L | T: 38 °C BP: 70/40 mmHg |
| Day 3 | Empirical antibiotic therapy with piperacillin–tazobactam initiated (loading dose of 9 g + maintenance dose of 6.75 g IV over 24 h) | WBC: 15.750/mmc CRP: 178 mg/L PCT: 8 µg/L | T: 38.5 °C BP: 80/50 mmHg |
| Day 4 | Blood culture positive for R. ornithinolytica; treatment switched to ceftriaxone (2 g IV over 24 h) | WBC: 16.980/mmc CRP: 158.4 mg/L | T: 37.5 °C BP: 80/50 mmHg |
| Day 6–7 | Improvement in clinical condition, with resolution of fever and hypotension | WBC: 10.250/mmc CRP: 28 mg/L PCT: 4.74 µg/L | T: 36.6 °C BP: 120/60 mmHg |
| Day 10 | Normalization of infection markers Blood cultures taken | WBC: 6370/mmc CRP: 7 mg/L PCT: 1.2 µg/L | T: 36 °C BP: 120/75 mmHg |
| Day 12 | Follow-up blood cultures negative Patient discharged | WBC: 5390/mmc CRP: 3 mg/L PCT: 0.2 µg/L | T: 36.2 °C BP: 120/75 mmHg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Righini, M.; Titone, M.; Martelli, D.; Isola, E.; Tampieri, E.; Graziani, R.; Valentini, C.; De Liberali, M.; Troiano, A.; Monti, M.; et al. Bloodstream Infection Caused by Raoultella ornithinolytica in a Chronic Hemodialysis Patient. Kidney Dial. 2025, 5, 33. https://doi.org/10.3390/kidneydial5030033
Righini M, Titone M, Martelli D, Isola E, Tampieri E, Graziani R, Valentini C, De Liberali M, Troiano A, Monti M, et al. Bloodstream Infection Caused by Raoultella ornithinolytica in a Chronic Hemodialysis Patient. Kidney and Dialysis. 2025; 5(3):33. https://doi.org/10.3390/kidneydial5030033
Chicago/Turabian StyleRighini, Matteo, Martina Titone, Davide Martelli, Elisabetta Isola, Elena Tampieri, Romina Graziani, Chiara Valentini, Matteo De Liberali, Antonella Troiano, Mattia Monti, and et al. 2025. "Bloodstream Infection Caused by Raoultella ornithinolytica in a Chronic Hemodialysis Patient" Kidney and Dialysis 5, no. 3: 33. https://doi.org/10.3390/kidneydial5030033
APA StyleRighini, M., Titone, M., Martelli, D., Isola, E., Tampieri, E., Graziani, R., Valentini, C., De Liberali, M., Troiano, A., Monti, M., Minerva, V., Hu, L., Sejdiu, B., Baraldi, O., & Buscaroli, A. (2025). Bloodstream Infection Caused by Raoultella ornithinolytica in a Chronic Hemodialysis Patient. Kidney and Dialysis, 5(3), 33. https://doi.org/10.3390/kidneydial5030033






