Abstract
Background: Steroid-resistant nephrotic syndrome (SRNS) in adults presents a significant therapeutic challenge, often leading to end-stage kidneys. This study aims to evaluate the clinical outcomes of rituximab (RTX) administration as an alternative to traditional cytostatic therapy in adults with SRNS, focusing on its effectiveness and safety profile. Methods: This multicenter, randomized study evaluates the effects of RTX for SRNS treatment, analyzing its clinical outcomes, safety, and efficacy across 52 adults (median age 47, 52% male) over 36 months. Amyloidosis and proliferative diseases were excluded by a kidney biopsy. Results: Complete remission rates improved from 50% to 66.7% by 36 months, with variations based on the morphological types of nephrotic syndrome experienced. The number needed to treat (NNT) for complete remission decreased from indeterminate to 12 by 36 months. RTX was well tolerated, with 17.3% experiencing allergic reactions and 25% developing hypogammaglobulinemia after one year. Severe infusion reactions were managed with omalizumab. Hypogammaglobulinemia and recurrent respiratory infections (21.5%) required additional treatments. Conclusions: RTX shows promise in achieving sustained remission in SRNS, especially in MN and FSGS, with increasing effectiveness over time. While its safety profile is encouraging, extended monitoring is essential for accurate treatment assessments. Further studies are needed to refine RTX protocols and outcomes.
1. Introduction
Steroid-resistant nephrotic syndrome (SRNS) in adults presents a significant therapeutic challenge due to its failure to respond to standard corticosteroid therapy. This leads to persistent proteinuria and a high risk of progression to end-stage kidney disease [1]. SRNS can arise from a variety of underlying causes, including primary glomerular diseases such as focal segmental glomerulosclerosis (FSGS) [2] and membranous nephropathy (MN) [3]. The effective management of SRNS is critical, as the condition can severely impact patients’ quality of life and increase their long-term morbidity due to complications related to chronic kidney disease [4].
Current treatments for steroid-resistant nephrotic syndrome in adults are limited and mainly target specific conditions like FSGS. There is a critical need to develop new therapeutic approaches. Advances in our understanding of the molecular and genetic basis of SRNS may provide innovative treatments that improve outcomes for patients facing this challenging condition.
Rituximab (RTX) might be a promising treatment for refractory NS [5] as it has been used increasingly and shown efficacy in treating SRNS in pediatric [6] and adult populations [7], especially in cases where traditional immunosuppressive treatments have failed or caused significant side effects. RTX, a monoclonal antibody targeting CD20-positive B-cells, helps reduce disease activity by modulating B-cell function, which is believed to play a role in the pathogenesis of SRNS [8]. It has emerged as a promising therapeutic option for patients with SRNS who are unresponsive to traditional immunosuppressive therapies [9]. Originally developed for the treatment of B-cell lymphomas, RTX has shown efficacy in various autoimmune and inflammatory conditions by depleting B-cells, thus modulating the immune response [10,11]. Recent studies have indicated its potential to induce remission in SRNS, making it an attractive option for adults with limited treatment choices [12,13]. The exact effect of RTX as well as the new generation like Obinutuzumab on SRNS is still under investigation, but it is believed to involve both the direct and indirect modulation of immune pathways [13]. By depleting B-cells, RTX may reduce the production of pathogenic autoantibodies, inhibit T-cell activation, and alter the cytokine profiles that contribute to kidney damage [14]. However, not all patients with SRNS respond to RTX, and predictors of their response are still being explored [15]. The efficacy and safety of RTX, as well as its potential role in treatment protocols for SRNS in adults, continue to be topics of active research and debate [16].
Based on recent studies and clinical trials from 2021 to 2024 [5,9,12,16,17,18], we examine advancements in RTX therapy and its expanding role in treating SRNS, exploring its potential to redefine therapeutic strategies and improve outcomes in adult populations.
This study aims to evaluate the clinical outcomes of RTX administration as an alternative to traditional cytostatic therapy in adults with SRNS, focusing on its effectiveness, safety profile, and integration into broader therapeutic strategies.
2. Materials and Methods
2.1. Study Cohort, Definitions, and Diagnostic Criteria
This open-label, multicenter, observational study enrolled 52 adult participants (age range 18–64, median age 47 years), comprising 27 white males (52%) and 25 (48%) females, all diagnosed with steroid-resistant nephrotic syndrome (SRNS). Nephrotic syndrome (NS) was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) 2021 criteria [1] and characterized by proteinuria (>3.5 g/L), their albumin-to-creatinine ratio (ACR > 2 g/g), hypoalbuminemia (<30 g/L), hypercholesterolemia, and edema. SRNS was confirmed when no remission occurred after a 4-week standard course of glucocorticoid therapy for the first idiopathic nephrotic syndrome episode. Hypertension 1–2 grade was initially documented in 48 participants (92%).
A kidney biopsy was performed before initial treatment (38 patients, 73%) or after documenting steroid resistance (14, 27%), and glucocorticoids were tapered over one month. To confirm membranous nephropathy, in addition to a positive immunohistochemical test, the presence of a positive PLA2R or THSD7-AAK was considered mandatory. RTX treatment was initiated alongside tacrolimus for up to 6 months, due to the delayed full effect of RTX during the initial treatment phase.
2.2. Study Design
Participants were stratified into two groups based on the one-year efficacy of their RTX treatment (Figure 1). Those achieving complete or partial remission (CR/PR) continued RTX every 6–9 months for up to three years. Non-responders transitioned to alternative treatments, including mycophenolate mofetil. The study design followed the principles outlined in the RICHNESS trial, “Rituximab in Children with Nephrotic Syndromes” [19]. A kidney biopsy was performed for all subjects before treatment started, revealing various morphological subtypes (as shown in Table 1). Genetic testing was performed in 2 patients with FSGS.
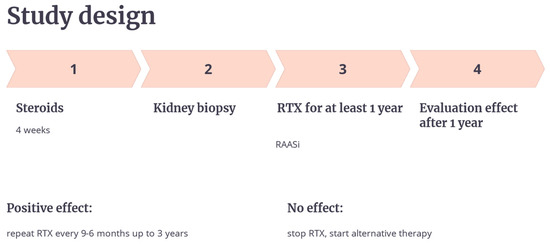
Figure 1.
Study design.

Table 1.
The morphological diagnosis of kidneys from kidney biopsies in the SRNS group.
2.3. Inclusion and Exclusion Criteria
Inclusion criteria: Adults with SRNS who received RTX following steroid resistance.
Exclusion criteria: Age < 18 years, severe allergic reactions to RTX, active infections, severe immunodeficiency, lymphoproliferative disorders, amyloidosis, uncontrolled diabetes, severe comorbidities, genetically confirmed FSGS, recent monoclonal antibody use, refusal of kidney biopsy, or the absence of PLA2R and THSD7-AAK antibodies in cases of membranous nephropathy.
These criteria ensured patient homogeneity for evaluating the efficacy of RTX.
2.4. Endpoints and Evaluation
The primary endpoint was achieving CR/PR, as per KDIGO guidelines [1]. Secondary endpoints included adverse events, assessed through eGFR (EPI formula), ACR, and CD20 levels. Adverse effects were evaluated by monitoring IgG levels and infection rates.
2.5. Treatment Protocol
RTX served as an alternative to glucocorticoid resistance and sparing agents. After their maximum doses were administered, steroids were reduced over 24 days by 2.5 mg, thus, the total duration of high doses of prednisolone exceeded 6 weeks, which is sufficient to prove steroid resistance. The primary efficacy endpoint, changes in eGFR and ACR, was evaluated six months post-RTX initiation. Secondary endpoints assessed the delayed effects at one, two, and three years. RTX re-administration, based on CD20 levels, PLA2Rab, and side effects, occurred every 6–9 months. Longitudinal follow-ups every six months over three years included eGFR-EPI and ACR assessments. Tacrolimus was co-administered for the initial 3–6 months (0.05–0.2 mg/kg/day, median 4 months), with its blood level maintained at 3.0–5.0 ng/mL. All patients received RAASi with potassium level monitoring.
2.6. RTX Protocol
The RTX infusion protocol followed standard guidelines [20]. Premedication included paracetamol, diphenhydramine, and methylprednisolone (5–6 mg/kg). RTX was infused at 375 mg/m2 (15 mg/kg, usually 1 g per infusion, twice, with a 2-week interval) in diluted saline, with infusion rates gradually increased every 30 min to 250–300 mL/h under close monitoring. Infusions were repeated twice, with a two-week interval. Subsequent RTX doses were determined by CD20 levels, IgG concentrations, and disease activity and typically administered every 6–9 months.
2.7. Criteria of Remission
According to the KDIGO 2021 guidelines [1], the criteria for the remission of glomerulonephritis are as follows:
Complete Remission (CR):
- -
- Reduction in proteinuria to less than 300 mg/day.
- -
- Stable kidney function (serum creatinine).
- -
- Normal or improved serum albumin levels.
Partial Remission (PR):
- -
- Reduction in proteinuria to less than 3.5 g/day.
- -
- At least a 50% reduction from peak values.
- -
- Stable kidney function (serum creatinine).
- -
- Normal or improved serum albumin levels.
2.8. Monitoring and Adverse Events
Adverse reactions were classified as immediate (within 24 h of infusion) or delayed (up to two weeks post-infusion). Acute side effects were managed according to predefined protocols; drug discontinuation occurred in unresolved cases [21].
2.9. Statistical Analysis
Statistical analyses were conducted using Prism 5.0 software. Data were reported as mean ± standard deviation (SD). The normality of the distribution of the data was evaluated using standard tests. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for relative and absolute risk. Statistical significance was set at p < 0.05. Absolute and relative risks and the number needed to treat (NNT) were calculated separately.
This study adheres to the Declaration of Helsinki and the Declaration of Istanbul and was approved by an ethics committee.
At the beginning of the study, 57 patients were selected, but only 52 of them (91%) completed the study, which is due to difficulty in receiving care during the period of the study due to limited opportunities within the country, which affected four people, and growing allergic reaction, which affected one. The statistical analysis was carried out on the 52 patients who completed all stages of therapy.
3. Results
In total, 52 patients finished RTX treatment. While following the study’s design we found that one year after initiating RTX therapy, four patients (7.7%) demonstrated no therapeutic response, with a persistent progression of their SRNS. Morphological findings in these cases included glomerulonephritis with crescents in the four (7.7%) patients. Among this subgroup, three patients (5.7%) succumbed to rapidly progressive glomerulonephritis. These outcomes indicate that resistance to both steroids and RTX was observed in four patients (7.7%) of the cohort, who died within the first 2–9 months. Therefore, the subsequent analysis of RTX efficacy focused on the remaining 48 (92.3%) patients, offering a broader evaluation of its clinical utility.
The primary outcome measured was complete or partial remission (CR/PR) at 12, 18, 24, and 36 months on RTX treatment. The calculated values for remission rates, the confidence interval (CI), relative risk (RR), and the number needed to treat (NNT) are presented in Table 2.

Table 2.
Treatment results with Rituximab at 12–36 months.
At 12 months, there was no difference in the remission rates between the complete and partial remission groups. From 18 to 36 months, the relative risk increased, indicating that the complete remission group had a higher likelihood of remission than the partial remission group. The Absolute Risk Reduction also increased over time, leading to a lower NNT, which signifies more effective treatment in the complete remission group. The 95% confidence intervals show that the differences in remission rates between the groups become statistically significant over time. Insights into statistical significance and Clinical Relevance, according to Fisher’s Exact Test, show that at 12 months there was no statistical significance between the groups (p = 1.000); meanwhile, at 36 months, a statistically significant difference (p = 0.037) had appeared.
The relative risk (RR) demonstrated an incremental increase from 1.00 to 2.00, indicating a progressively higher likelihood of complete remission in these patients. This evolution suggests that over time, patients in the complete remission group were increasingly more likely to achieve complete remission compared to those in the partial remission group. The ARR showed a gradual increase from 0 to 0.33, demonstrating the cumulative effectiveness of the treatment. This means that as time progressed, the treatment’s ability to reduce the risk of partial remission improved, highlighting the growing impact of the treatment regimen. The number needed to treat decreased from an indeterminate value to three, reflecting improved treatment efficiency over time. A lower NNT signifies that fewer patients need to be treated to achieve one additional complete remission, underscoring the enhanced effectiveness of the treatment as it progresses.
3.1. The RTX Efficacy Across Morphological Types
Individual analyses of the therapeutic effect of RTX revealed that complete remission was attained in cases of membranous nephropathy, whereas other morphological subtypes resulted in a delayed therapeutic response (Table 3). Table 3 summarizes the remission outcomes and their associated statistical measures, providing a comprehensive analysis of the treatment’s efficacy based on morphological subtypes.

Table 3.
The therapeutic efficacy of rituximab across various morphological subtypes.
Based on the data presented in Table 3, we can observe distinct patterns in the therapeutic efficacy of rituximab (RTX) across various morphological subtypes. In cases of membranous nephropathy (MN), the highest probability of achieving complete remission is seen, with consistently elevated rates throughout the study period.
For focal segmental glomerulosclerosis (FSGS), there is a moderate increase in complete remission rates over time, but the initial response of these patients is characterized by higher rates of partial remission. Genetic testing, which was performed due to limited logistical capabilities in only two patients out of fourteen (14%) with FSGS, revealed genetically determined type 4 nephrotic syndrome (256370; AD) in one patient, while the other patient’s FSGS was not determined by genetics.
In IgA nephropathy, complete remission rates remained stable, mirroring the number of patients achieving partial remission. MPGN demonstrated low rates of complete remission, with stable partial remission rates throughout the study. MsPGN exhibited a notable increase in complete remission rates over time, accompanied by a corresponding decrease in partial remission.
These findings highlight that while morphological characteristics contribute to predicting the efficacy of RTX therapy, they are likely not the sole determinants. In clinical practice, the primary emphasis is likely placed on the clinical characteristics of patients, which may more accurately reflect their response to treatment.
The graphical representation in Figure 2 illustrates the complete remission rate over time (at 12, 24, and 36 months) for different morphological types of kidney diseases. MN shows the highest complete remission rate, reaching 100% by 24 months and remaining stable at 36 months. FSGS exhibits a moderate response, with complete remission increasing from 38.5% at 12 months to 53.8% at 36 months. IgAN follows a similar pattern to FSGS, with remission rates fluctuating between 42.9% and 57.1% over the study period. MPGN starts with the lowest complete remission rate (28.6% at 12 months) but this improves to 57.1% at 24 and 36 months. MsPGN did not achieve complete remission at 12 months, but this slowly increased to 50% by 36 months.
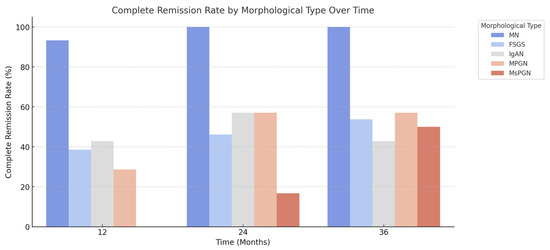
Figure 2.
Complete remission rate, by morphological type of kidney disease, over time.
The results indicate that MN has the most favorable remission trajectory, while MsPGN has the least favorable response over time. The trends in FSGS, IgAN, and MPGN suggest partial improvement but not a consistent full recovery.
3.2. The RTX Efficacy Across eGFR
This study examined the efficacy of rituximab on the dynamics of eGFR across various morphological types of kidney disease over 12, 24, and 36 months (Table 4).

Table 4.
The dynamics of eGFR (mL/min/1.73 m2) in terms of the therapeutic efficacy of rituximab towards various morphological types of kidney disease.
The data provided in this study highlight the efficacy of rituximab across various morphological types of nephropathy over 12, 24, and 36 months. The results show significant differences in the rates of complete and partial remissions among the different types of nephropathy and variations in their ARR and NNT.
MN showed a consistent and high rate of complete remission over the 36 months. With ARR values indicating a substantial reduction in the risk of non-remission, rituximab appears to be highly effective for patients with MN. The low NNT values seen (two across all periods) further support the drug’s efficacy in this subgroup. The efficacy of rituximab in FSGS was mixed. While the complete remission rates were moderate, there was a notable negative ARR and high NNT at 12 months, suggesting limited immediate efficacy. However, the ARR and NNT of this subgroup improved significantly by the 36-month mark, indicating delayed but increasing efficacy over time. Patients with IgAN showed progressive improvement in their complete remission rates over time, with particularly high ARR and low NNT values at 36 months. This suggests that rituximab provides increasing benefits with extended treatment durations in this subgroup. MPGN patients experienced moderate to high rates of complete remission across all periods. Their ARR and NNT values consistently improved over time, indicating the increasing efficacy of rituximab in these patients. MsPGN showed the least favorable outcomes, with no complete remissions at 12 months and limited partial remissions. However, there was a notable improvement by 36 months, suggesting some delayed benefits. The ARR and NNT values seen at 36 months indicate modest efficacy in this subgroup.
The analysis of eGFR dynamics across different morphological subtypes reveals varying responses to rituximab therapy. While MN shows a consistent increase in eGFR and high complete remission rates, other conditions like FSGS and IgAN demonstrate more variability. These results indicate that rituximab may be more effective in certain types of nephropathy, highlighting the need for tailored therapeutic approaches based on patients’ morphological classification.
3.3. The RTX Side Effects
Clinical status, B-cell depletion, and IgG levels were meticulously monitored before each RTX administration, and as needed if its administration was delayed. RTX led to the complete depletion of CD-20 cells in all patients, with a median value of 0.36 (95% CI 9–121).
In a cohort of 52 patients treated over 36 months, 9 patients (17.3%) experienced allergic reactions. Seven patients (13.5%) experienced mild infusion-related reactions during RTX administration, such as skin papules, decreased blood pressure, and/or tachycardia. These reactions were more frequent with increased infusion rates or temporary interruptions but were mitigated by slowing the infusion rate and steroid usage. Two additional patients had severe infusion reactions; the infusion was stopped, they received 150 mg omalizumab, and RTX was safely continued the following day. The overall rate of immediate allergic reactions was 17.3%, yielding an absolute risk of 0.173 and a number needed to treat of 6. This indicates that for every six patients treated, one is likely to experience an allergic reaction.
The most frequent serological complication was hypogammaglobulinemia, which was observed in 13 patients (25%). This condition typically appeared after 1 year of RTX treatment, with IgG levels dropping to 4 ± 1 g/L (95% CI 2.13–6.05). Adjunctive intravenous immunoglobulins were administered to 2 (15.3%) of the 13 affected patients, yielding an NNT of 17, indicating that for every 17 patients treated, 1 additional patient would require immunoglobulin therapy to manage hypogammaglobulinemia.
Recurrent respiratory infections were another complication that was documented in 11 patients (21.2%). The NNT was calculated to be 5, meaning that for every five patients treated, one patient is likely to experience recurrent respiratory infections. The risk of developing these infections was successfully reduced by prescribing dietary supplements containing Uncaria tomentosa for 2 months.
4. Discussion
Rituximab has well-established indications for treating glomerulopathies [21], with its efficacy being particularly pronounced in membranous nephropathy [22,23], where it could serve as a cornerstone of initial therapeutic regimens. In recent years, its therapeutic scope has broadened to encompass more prognostically complex glomerulonephritis variants, including steroid- and immunosuppressant-resistant nephrotic syndrome [24,25]. In this study, we specifically examined RTX’s role in SRNS management, opting to forgo traditional immunosuppressants to minimize their associated adverse effects. The inclusion criteria included biopsy-confirmed underlying kidney pathology to ensure precise characterization of the disease’s etiology. Notably, cases of amyloidosis and lymphoproliferative diseases were excluded due to the ineffectiveness of RTX in this context.
Our primary objective was to evaluate the therapeutic response to RTX in a diverse cohort of SRNS patients [26,27], irrespective of their specific biopsy-confirmed histological subtype. To better account for the potential delayed effects of RTX, therapy discontinuation decisions were only made after one year, extending beyond the customary six-month evaluation period. Additionally, recognizing the risk of relapse following treatment cessation [28], we opted for extending RTX therapy to up to three years, with rigorous monitoring for adverse effects.
The rationale for RTX use in SRNS lies in its unique immunomodulatory properties [29]. By targeting CD20-positive B-cells, RTX potentially reduces autoantibody production, modulates T-cell activity, and alters the cytokine pathways contributing to glomerular injury [30]. These mechanisms make RTX a promising agent in addressing the multifactorial pathogenesis of SRNS, particularly in cases where traditional immunosuppressive therapies have failed or are poorly tolerated.
Clinical trials and observational studies from 2021 to 2024 indicate that RTX is effective in inducing remission in many patients with SRNS, both as an initial treatment and as a maintenance therapy [9,26,30,31,32,33]. Studies have demonstrated a reduction in proteinuria and decreased dependency on steroids, which is particularly beneficial in pediatric patients as it avoids the long-term side effects of steroids [34].
4.1. Treatment Effectiveness
Our results indicate that the treatment’s effectiveness is not immediate but develops over time. Statistically significant improvements at 36 months suggest a cumulative therapeutic effect on SRNS that becomes more pronounced with prolonged treatment. The most striking improvements occur between 24 and 36 months, challenging premature evaluations of treatment success. Initially, the treatment did not significantly differ between the complete and partial remission groups. However, over time, there was a progressive improvement in complete remission rates, with statistically significant outcomes emerging in the later observation periods. This shows that the cumulative effect of treatment leads to better outcomes for patients, who achieved complete remission over time.
From a clinical perspective, these results emphasize the importance of extended treatment observations, as the benefits of the treatment may become more apparent over a longer period. This also highlights the potential for a delayed therapeutic response, suggesting that patients and healthcare providers should be aware of the long-term nature of the treatment process. Consequently, patient counseling should address long-term treatment expectations to ensure patients are prepared for their gradual progression towards complete remission.
Our results highlight the varying effectiveness of RTX across different morphological subtypes, with MN showing the highest rates of complete remission. Such robust outcomes highlight rituximab’s effectiveness in treating MN. Our data suggest that while RTX is ultimately beneficial for FSGS, the therapeutic response may be more gradual compared to MN and depends on genetic disease determination. In IgAN, the results obtained indicate a balanced and steady response to RTX, albeit without the pronounced efficacy seen in MN. This trend underscores the progressive therapeutic benefits of RTX in treating MsPGN. A limited effectiveness of RTX in inducing full remission was observed in MPGN patients. The data support the need for tailored therapeutic approaches based on the specific morphological type of glomerulonephritis a patient has. The findings underscore the critical importance of patience, persistence, and comprehensive long-term assessments in understanding complex medical interventions.
In terms of eGFR dynamics, RTX demonstrates varying degrees of efficacy across different morphological subtypes, with the most pronounced benefits observed in MN and IgAN patients. These findings support the potential of rituximab as a therapeutic option, especially for specific subgroups with membranous and IgA nephropathy. Further research is needed to explore the long-term effects and optimal treatment durations of RTX to maximize patient outcomes.
4.2. Safety Profile of RTX Therapy in SRNS
RTX has demonstrated a generally favorable safety profile, with most adverse events being mild in nature. Nevertheless, serious side effects, including an increased risk of infections due to immunosuppression, remain a notable concern. Recent studies emphasize that while RTX is not devoid of risks, its therapeutic benefits in SRNS often outweigh potential complications, particularly when the toxicity of steroids or steroid-sparing agents becomes a limiting factor and especially in children [34,35].
Our findings align with previous reports, highlighting the tolerability of RTX even during its prolonged use in SRNS populations. The incidence of adverse reactions in this study was predominantly low-to-moderate, reinforcing RTX’s safety profile. In cases of allergic reactions to RTX, desensitization protocols are essential. When conventional approaches failed, we successfully employed omalizumab as adjunctive therapy. Administering 150 mg of omalizumab significantly mitigated hypersensitivity reactions, enabling the continuation of RTX therapy across diverse clinical scenarios. This strategy has not only enhanced its tolerability but has also allowed for the nearly universal continuation of RTX treatment.
4.3. Infectious Complications and Immune Monitoring
While RTX is effective in inducing and maintaining remission, its repeated or reduced-dose administration requires caution due to the elevated risk of infectious complications. Our results suggest that RTX maintenance therapy should be withheld in patients with serum IgG levels below age-appropriate reference ranges. Low IgG levels increase patients’ vulnerability to infections, even in the absence of overt clinical symptoms. Therefore, regular monitoring of immunoglobulin levels is critical, and RTX re-administration should be avoided in patients with hypogammaglobulinemia to reduce the likelihood of immunodeficiency-related infections. This underscores the necessity of individualizing therapy to balance efficacy with safety in this vulnerable patient population [36].
4.4. Optimal Duration of Therapy and Future Directions
The optimal duration of RTX therapy remains an area of active investigation. Decisions regarding its re-administration should factor in B-cell repopulation rates and CD20 monitoring [8]. Our clinical experience suggests that extending the length of RTX therapy to three years in SRNS is both safe and effective. However, further longitudinal studies are necessary to validate these observations, refine dosing strategies, and optimize treatment intervals to minimize adverse effects while maintaining therapeutic efficacy.
4.5. Management of Allergic Reactions and Hypogammaglobulinemia
Our data emphasize the importance of carefully monitoring allergic reactions, which were observed in a minority of patients. For those experiencing hypersensitivity, interventions like omalizumab have proven invaluable [37]. Additionally, the management of hypogammaglobulinemia, a frequent complication associated with RTX, remains a critical aspect of care. Monitoring and targeted interventions, such as the use of adjunctive therapies, ensure the timely management of immunological deficiencies, safeguarding patient outcomes. We consider it extremely important that repeated administrations of rituximab be performed only in the absence of opportunistic infections and with normal levels of immunoglobulins G and CD of more than 1%.
4.6. The Role of Complementary Strategies
Emerging strategies, such as the use of Uncaria tomentosa supplements to mitigate recurrent infections, highlight the potential of adjunctive therapies. However, evidence remains insufficient to establish clear recommendations for their use. Comparative studies with well-defined control groups are necessary to better understand the risks and benefits of such interventions and to develop robust guidelines for complementary treatment modalities.
To sum up, RTX therapy in SRNS requires a carefully tailored approach, prioritizing both efficacy and safety. The continuous monitoring of allergic and immunological parameters, alongside individualized treatment regimens, is key to achieving optimal outcomes in this challenging patient population.
4.7. Limitations
In discussing the limitations of this study, it is important to acknowledge the potential for selection bias, which may have influenced the outcomes. Additionally, the exclusion of genetic FSGS cases represents a significant limitation, as this subgroup may exhibit different responses to the treatment compared to non-genetic cases. Future studies should aim to include a more diverse patient population and consider the impact of genetic variations on treatment efficacy. Recognizing these limitations will help refine future research and lead to more comprehensive and applicable findings.
4.7.1. Generalizability
The findings of this study may have limited generalizability due to the specific patient population and clinical settings involved. Therefore, caution should be exercised when applying these results to other populations or healthcare environments. Further research with diverse cohorts and multi-center trials are necessary to confirm the broader applicability of these findings.
4.7.2. Real-World Applicability of Rituximab
When considering the real-world applicability of rituximab, factors such as cost-effectiveness and accessibility play a crucial role. Rituximab, while effective, can be expensive and may not be readily available in all regions [38]. Policymakers and healthcare providers must work together to improve access to this treatment, ensuring that it is both affordable and widely available to patients who need it. Additionally, more research is needed to evaluate the long-term cost benefits of rituximab treatment in various healthcare settings.
4.7.3. Future Research Directions
Future research should focus on the potential of rituximab in SRNS, particularly with respect to nephrin antibodies [39]. Investigating the role of nephrin antibodies in the pathogenesis of SRNS could lead to the development of targeted therapies that improve patient outcomes. Additionally, exploring the molecular mechanisms and genetic factors associated with rituximab responses could provide valuable insights into optimizing treatment protocols and identifying biomarkers for better patient stratification.
By addressing these areas and pursuing further research, we can enhance our understanding and management of SRNS, ultimately leading to improved patient care and outcomes.
5. Conclusions
Overall, these observations reveal distinct therapeutic trajectories for RTX across different morphological types of kidney disease, highlighting the necessity for tailored treatment strategies to optimize patient outcomes. The data emphasize the importance of the continued evaluation and adjustment of therapeutic protocols to achieve the best possible results for each patient cohort.
While the short-term results are promising, long-term efficacy and safety data are still being collected. Ongoing research is focusing on optimizing the dosing and frequency of RTX to sustain remission and prevent relapse.
All patients with MN achieved complete remission, whereas those with SRNS related to other conditions had a lower complete remission rate. In settings where access to biomarkers and kidney biopsies are limited, rituximab therapy remains a valuable option for SRNS patients, as it can provide significant benefits despite challenges in diagnostics.
Future and ongoing research into the efficacy and safety of RTX in SRNS is expected to provide robust evidence-based practice recommendations. These studies will play a crucial role in guiding clinicians to optimize treatment strategies for this patient population, ultimately establishing definitive therapeutic outcomes.
Author Contributions
Conceptualization, D.I.; Methodology, M.I.; Formal analysis, Y.L.; Investigation, O.C., I.Z., N.B. and I.J.; Writing—original draft, M.I.; Writing—review & editing, D.I.; Supervision, D.I.; Project administration, M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the local Ethics Committee of the ”Nephrology Clinic” (Approval Code: protocol No. 1 Approval Date: 10 February 2021). Ethical review and approval were waived in part for this study, due to exemptions under 45 CFR § 46.104(d) [1], because this research involves the use of identifiable private information/biospecimens. The information used, which may include information about biospecimens, was recorded by the investigator in such a manner that the identity of the human subjects could not readily be ascertained directly or through identifiers linked to the subjects.
Informed Consent Statement
Patient consent was given orally and/or via a signed form. The information used, which may include information about biospecimens, was recorded by the investigator in such a manner that the identity of the human subjects cannot readily be ascertained directly or through identifiers linked to the subjects.
Data Availability Statement
Data supporting reported results can be found in clinic reports. Data are available on request from the authors.
Acknowledgments
The authors would like to express their gratitude for the support received from the German Society for Pediatric Nephrology (GPN), the German Society for Nephrology (DGfN), and the Board of Trustees for Dialysis and Transplantation (KfH), which generously donates medicine to some patients. It is important to note that the content of this article represents the personal experience and views of the authors and should not be construed as medical advice or a recommendation. The responsibility for the information and views expressed herein rests entirely with the authors. We kindly thank all participants, medical teams, and collaborators involved in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [CrossRef] [PubMed]
- Hansrivijit, P.; Cheungpasitporn, W.; Thongprayoon, C.; Ghahramani, N. Rituximab therapy for focal segmental glomerulosclerosis and minimal change disease in adults: A systematic review and meta-analysis. BMC Nephrol. 2020, 21, 134. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Bagga, A. Rituximab therapy in nephrotic syndrome: Implications for patients’ management. Nat. Rev. Nephrol. 2013, 9, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.P.; Wang, J.; Yuan, L.; Wang, D.G. The efficacy of rituximab in the treatment of refractory nephrotic syndrome: A meta-analysis. Int. Urol. Nephrol. 2020, 52, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Saleh, M.; Mohamed, R.; El-Kady, N. Rituximab as a treatment for steroid-resistant nephrotic syndrome: A comprehensive review. Nephrol. Dial. Transplant. 2022, 37, 230–239. [Google Scholar] [CrossRef]
- Choi, N.; Min, J.; Kim, J.H.; Kang, H.G.; Ahn, Y.H. Efficacy and safety of long-term repeated use of rituximab in pediatric patients with nephrotic syndrome. Pediatr. Nephrol. 2024, 39, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Sinha, A.; Jordan, S.C.; Hari, P.; Dinda, A.K.; Sharma, S.; Srivastava, R.N.; Moudgil, A.; Bagga, A. Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: Multicentric report. Clin. J. Am. Soc. Nephrol. 2010, 5, 2207–2212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Del Vecchio, L.; Allinovi, M.; Rocco, P.; Brando, B. Rituximab Therapy for Adults with Nephrotic Syndromes: Standard Schedules or B Cell-Targeted Therapy? J. Clin. Med. 2021, 10, 5847. [Google Scholar] [CrossRef]
- Bagga, A.; Sinha, A.; Sharma, A.; Dhingra, B. Role of rituximab in difficult-to-treat nephrotic syndrome: A review. Nephrol. Ther. 2022, 18, 15–24. [Google Scholar] [CrossRef]
- Hogan, J.; Lapeyraque, A.-L.; Howell, E. Rituximab treatment in nephrotic syndrome: Safety and efficacy updates. Am. J. Kidney Dis. 2022, 80, 952–963. [Google Scholar] [CrossRef]
- Kamei, K.; Ishikura, K.; Sako, M.; Ito, S.; Nozu, K.; Iijima, K. Rituximab therapy for refractory steroid-resistant nephrotic syndrome in children. Pediatr. Nephrol. 2020, 35, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Fang, L.; Sheng, L.; Zhou, X.; Bai, S.; Zang, X.; Wang, Y.; Li, M.; Lv, Z.; Zhong, Q.; et al. Rituximab treatment for refractory nephrotic syndrome in adults: A multicenter retrospective study. Ren. Fail. 2023, 45, 2237124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, Y.; Pan, Y.; Han, Q.; Xu, J.; Wang, J.; Lei, X.; Chen, L.; Wang, Y.; Ren, P.; Lan, L.; et al. Obinutuzumab May Be an Effective and Safe Option for Adult Minimal Change Disease and Focal Segmental Glomerulosclerosis Patients after Multitarget Therapy Including Rituximab. Am. J. Nephrol. 2025, 56, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Colucci, M.; Oniszczuk, J.; Vivarelli, M.; Audard, V. B-Cell Dysregulation in Idiopathic Nephrotic Syndrome: What We Know and What We Need to Discover. Front. Immunol. 2022, 13, 823204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahn, Y.; Yeo, S.M.; Kim, Y.H. Rituximab’s role in managing refractory nephrotic syndrome. Kidney Blood Press. Res. 2021, 46, 461–471. [Google Scholar] [CrossRef]
- Ishimoto, T.; Uesugi, T.; Takeda, S. Clinical use of rituximab in nephrology: A review of guidelines. Kidney Int. Rep. 2023, 8, 1157–1168. [Google Scholar] [CrossRef]
- Moustafa, B.; Moselhy, S.; Rabie, M.; Hammad, A.; Youssef, D.; Shouman, M.; Makar, S.; Badr, A.; Mansour, S.; Ebrahim, D.; et al. Egyptian pediatric clinical practice adapted guidelines: Evidence-based [2] steroid-resistant nephrotic syndrome (SRNS) 2022. Egypt Pediatr. Assoc. Gaz 2023, 71, 12. [Google Scholar] [CrossRef]
- Chan, E.W.; Tang, S.C.; Lai, K.N. Rituximab dosing in nephrotic syndrome treatment protocols. Nephron 2023, 147, 255–263. [Google Scholar] [CrossRef]
- Ivanov, D.; Weber, L.T.; Levtchenko, E.; Vakulenko, L.; Ivanova, M.; Zavalna, I.; Lagodych, Y.; Boiko, N. Rituximab Administration to treat Nephrotic Syndrome in Children: 2-Year Follow-Up. Biomedicines 2024, 12, 2600. [Google Scholar] [CrossRef]
- Ivanov, D. Nephrology Care in Ukraine: Almost 2 Years of Wartime Experience. Kidney 2024, 5, 266–270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, V.; Khurana, S.; Verma, M.; Mehta, S. Role of rituximab in the management of primary glomerular disease. Am. J. Nephrol. 2023, 54, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Li, H.; Zhou, T.; Zhong, Z. Clinical efficacy and safety of rituximab with membranous nephropathy: A meta-analysis. Arch. Med. Sci. 2023, 19, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Gong, S.; Li, J.; Luo, H.; Wang, Y. Efficacy and safety of rituximab in the treatment of membranous nephropathy: A systematic review and meta-analysis. Medicine 2020, 99, e19804. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watanabe, Y.; Hirai, K.; Hirata, M.; Kitano, T.; Ito, K.; Ookawara, S.; Oshiro, H.; Morishita, Y. Steroid-resistant minimal change nephrotic syndrome associated with thymoma treated effectively with rituximab following thymectomy and cyclosporine: A case report. BMC Nephrol. 2024, 25, 53. [Google Scholar] [CrossRef]
- Yokota, S.; Kamei, K.; Fujinaga, S.; Hamada, R.; Inaba, A.; Nishi, K.; Sato, M.; Ogura, M.; Sakuraya, K.; Ito, S. Efficacy of rituximab and risk factors for poor prognosis in patients with childhood-onset steroid-resistant nephrotic syndrome: A multicenter study. Pediatr. Nephrol. 2024, 39, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Lin, L.; Shen, P.; Li, X.; Xie, J.; Pan, X.; Zhang, W.; Chen, N. Rituximab treatment in adults with refractory minimal change disease or focal segmental glomerulosclerosis. Oncotarget 2017, 8, 93438–93443. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Kim, S.H.; Han, K.H.; Choi, H.J.; Cho, H.; Lee, J.W.; Shin, J.I.; Cho, M.H.; Lee, J.H.; Park, Y.S.; et al. Efficacy and safety of rituximab in childhood-onset, difficult-to-treat nephrotic syndrome: A multicenter open-label trial in Korea. Medicine 2018, 97, e13157. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Xu, Z.; Deng, H.; Yang, H.; Zhong, F. Systematic Review and Meta-Analysis of Rituximab for Steroid-Dependent or Frequently Relapsing Nephrotic Syndrome in Children. Front. Pediatr. 2021, 9, 626323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujinaga, S.; Morita, R.; Katafuchi, R. Advances in B-cell therapy in nephrotic syndrome. Clin. Kidney J. 2022, 15, 748–756. [Google Scholar] [CrossRef]
- Salehi, T.; Krishnan, A.; Al Jurdi, A.; So, P.; Lerma, E.; Wiegley, N.; GlomCon Editorial Team. Rituximab Resistance in Glomerular Diseases: A GlomCon Mini Review. Kidney Med. 2024, 6, 100791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kallash, M.; Smoyer, W.E.; Mahan, J.D. Rituximab Use in the Management of Childhood Nephrotic Syndrome. Front. Pediatr. 2019, 7, 452297. [Google Scholar] [CrossRef] [PubMed]
- Tesar, V.; Zavadil, J.; Hruskova, Z. Rituximab’s efficacy in nephrology: A systematic review. Am. J. Nephrol. 2022, 53, 245–255. [Google Scholar] [CrossRef]
- Sun, L.; Xu, H.; Shen, Q.; Cao, Q.; Rao, J.; Liu, H.M.; Fang, X.Y.; Zhou, L.J. Efficacy of rituximab therapy in children with refractory nephrotic syndrome: A prospective observational study in Shanghai. World J. Pediatr. 2014, 10, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Jellouli, M.; Charfi, R.; Maalej, B.; Mahfoud, A.; Trabelsi, S.; Gargah, T. Rituximab in The Management of Pediatric Steroid-Resistant Nephrotic Syndrome: A Systematic Review. J. Pediatr. 2018, 197, 191–197.e1. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.Y.; Sinha, A.; Yu, E.L.M.; Akhtar, N.; Angeletti, A.; Bagga, A.; Banerjee, S.; Boyer, O.; Chan, C.Y.; Francis, A.; et al. An international, multi-center study evaluated rituximab therapy in childhood steroid-resistant nephrotic syndrome. Kidney Int. 2024, 106, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.L.; Fay, M.P.; Lanning, L.L.; Hewitt, J.A. Effect of delaying treatment on efficacy of ciprofloxacin and levofloxacin in the African green monkey model of pneumonic plague. Clin. Infect. Dis. 2020, 70 (Suppl. S1), S60–S65. [Google Scholar] [CrossRef]
- Xue, C.; Yang, B.; Xu, J.; Zhou, C.; Zhang, L.; Gao, X.; Dai, B.; Yu, S.; Mao, Z.; Mei, C.; et al. Efficacy and safety of rituximab in adult frequent-relapsing or steroid-dependent minimal change disease or focal segmental glomerulosclerosis: A systematic review and meta-analysis. Clin. Kidney J. 2021, 14, 1042–1054. [Google Scholar] [CrossRef]
- Wang, X.; Cao, X.; Wu, J.; Liang, S.; Yang, J.; Wang, H. Exploration of rituximab treatment strategies for membranous nephropathy adapted to the Chinese healthcare environment. BMC Nephrol. 2025, 26, 49. [Google Scholar] [CrossRef]
- Kronbichler, A.; Barnini, C.; Matyjek, A.; Gauckler, P.; Bruchfeld, A.; Caravaca-Fontan, F.; Floege, J.; Frangou, E.; Mirioglu, S.; Moran, S.M.; et al. Antibody-mediated podocytopathies: A disease entity that implies immunotherapy. Nephrol. Dial. Transplant. 2025, 40, 218–221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).