Abstract
Small interfering RNAs (siRNAs) are short, double-stranded RNA molecules that play a crucial role in the regulation of gene expression, particularly through a natural process called RNA interference (RNAi). Their discovery, about 25 years ago, paved the way for a whole series of research leading to synthetic molecules. The gene silencing potential of these siRNAs was initially oriented towards diseases resulting from genetic dysfunctions. This led to the development of the first synthetic siRNAs approved for human use in hereditary transthyretin amyloidosis. Subsequently, the field of application expanded beyond the confines of genetic diseases. The refinement of pharmacological techniques has led to the synthesis of a variety of siRNAs capable of blocking the production of individual proteins responsible for various disease conditions, thus expanding their field of therapeutic application. The kidney has also been affected by this new therapeutic tool, largely indirectly but also, with some difficulty, directly. The structural complexity of the kidney has made the search for siRNAs targeting its individual components very challenging. Nevertheless, the first results of the application of this new therapeutic technology to the kidney are beginning to be seen in experimental animals and in humans. siRNAs have been approved for the treatment of amyloidosis with patisiran and oxalosis with lumasiran and nedosiran. Studies are ongoing for the use of siRNAs as anti-complement drugs in IgA nephropathy, as angiotensinogen inhibitors in hypertension, or against some mediators of acute kidney injury. In this review, the biological mechanisms underlying the use of siRNAs are briefly exposed. The results of the therapeutic application of RNA interference to the kidney and its diseases are also analyzed and discussed.
1. Introduction
In recent years, the emergence and availability of a new type of therapeutic tools, potentially affecting many fields of internal medicine, has given us a glimpse of great treatment possibilities that were previously unimaginable. We refer to small interfering RNA (siRNA). In the present review, we focus on the advances that have allowed the clinical use of siRNA. In fact, thanks to the creation of synthetic structures of short double-stranded RNA, discovered a few years ago, we have witnessed the expansion of the spectrum of indications for their use. Some of these applications are, directly or indirectly, addressed to the kidney. In order to carry out an in-depth examination of the current uses, prospects and limitations of this new class of agents in a nephrological setting, a brief excursus on their nature and their mode of action is essential.
2. RNA Interference (RNAi)
RNAi is a natural defense mechanism of eukaryotic cells responsible for control gene activity []. It works through post-transcriptional silencing of the expression of specific genes. The first report of this naturally occurring phenomenon came from a botanic study []. This was immediately followed by studies carried out in the animal world. A decisive step forward, that was also worth the Nobel Prize, was made by Fire and Mello when they demonstrated in a nematode that the most effective mechanism of post-transcribing gene silencing is operated by double-stranded RNA, which is 100 times more effective than the single-stranded molecule []. On that occasion, the mechanism was defined for the first time as “RNA interference”. Subsequent studies have demonstrated the presence of RNAi in mammalian cells []. Based on these findings, it is therefore established that RNAi plays an important dual role in the life of mammals. It represents a natural biological response to foreign or native double-stranded RNA. This mechanism provides a defense toward both endogenous parasitic and exogenous pathogenic nucleic acids. It also can regulate the expression of protein-coding genes acting as post-transcriptional gene silencing.
The explanation of how natural siRNA operates in mammalian cells can be summarized in an extreme simplification with the following steps []. The presence in the cell of foreign DNA, double-stranded RNA of viral origin, abnormal segments of DNA or micro-RNA triggers the RNAi pathway. Firstly, the double-stranded molecules are processed into a short siRNA by RNase enzymes called Dicer and Drosha. Enzymatic cleavage of dsRNA within the cell produces the short siRNA fragments typically around 20–25 base pairs (bp), of 21–23 nucleotides in length. This shorter double-stranded molecule becomes part of a multi-protein complex, the RNA-induced silencing complex (RISC). This complex is made of several proteins, not yet fully identified []. These dsRNA fragments, loaded into the RISC, are separated in two strands with different fates. One strand takes the role of a guide. The other strand, known as the passenger strand, is degraded by the RISC.
In the next step, the activated RISC complex locates the mRNA target in the cell. The RISC uses the guide strand of siRNA to target complementary mRNA transcripts. In the final step, the complex bound to mRNA operates the cleavage of its molecule. The degradation of target mRNA reduces the levels of transcript available to be translated by ribosomes, thus blocking the synthesis of the target protein. Therefore, by degrading the mRNA, siRNA effectively “silences” the gene by inhibiting protein synthesis. This process is sequence-specific, ensuring minimal off-target effects if the siRNA is well-designed.
The discovery of this natural mechanism was immediately followed by attempts to reproduce it synthetically. In fact, the therapeutic potential of these agents to reversibly block or stimulate the synthesis of any protein with direct or indirect effects on organs or biological systems had emerged. The demonstration that synthetic siRNAs could be introduced into mammalian cells to silence gene expression without producing local and systemic innate immune responses to double-stranded RNA led the way []. It was shown that synthetic siRNAs acted by the same mechanism as natural ones (Figure 1).
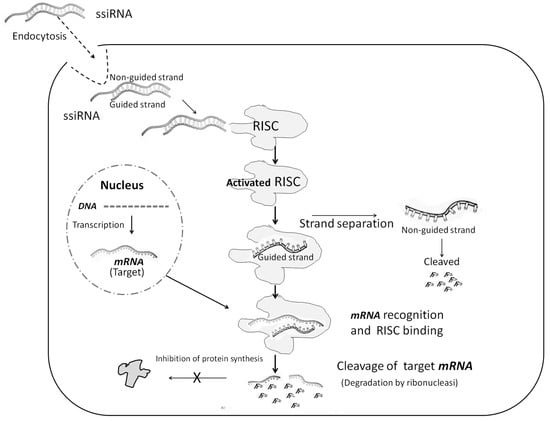
Figure 1.
Molecular mechanism of siRNA. See text for explanation.
The progressive refinement of the technique allowed the rapid transition to human experimentation []. In 2010, the first clinical trial of local delivery of a synthetic siRNA in age-related macular degeneration was published []. Systemic delivery was a bigger challenge, though, because of the presence of physiological barriers and potential toxicities. Furthermore, some of the first synthetic molecules suffered from stability problems. The use of some techniques such as chemical modification of the siRNA, direct conjugation to bioactive moieties, and nanoparticle formulations has allowed the majority of the obstacles to be overcome, such as the delivery to specific cells or tissues, the off-target effects and the stability of siRNAs in the bloodstream.
The siRNA delivery systems are categorized as non-viral and viral delivery systems. The non-viral delivery systems include polymers, lipids and peptides, while N-acetylgalacto-samine (GalNAc) conjugates are among the most widely studied delivery systems for siRNAs. Small interfering siRNA molecules that are administered without any protective support or delivery system are called naked siRNAs. While they can be effective for localized applications, they are highly susceptible to degradation and have poor cellular uptake, limiting their broader use in therapeutic settings. The breakthrough came in 2014 when the first synthetic targeted siRNA to be systemically administered to humans was reported [,]. The formal conclusion of this 20-year race was the approval in 2018 by the United States Food and Drug Administration (FDA) and the European Commission (EC) of patisiran as the first commercial RNAi-based therapeutic for the treatment of hereditary transthyretin (hATTR) amyloidosis with polyneuropathy in adults [,]. With this agent, the suffix siran was also introduced, which characterizes all synthetic siRNAs. To date, the FDA has approved six siRNA agents: patisiran, vutrisiran, givosiran, lumasiran, nedosiran and inclisiran. The six approved siRNA drugs are mainly in the therapeutic areas of neurology, cardiovascular system and metabolism, such as for amyloidosis, familial amyloid polyneuropathies [FAPs], hepatic porphyria, primary hyperoxaluria and hyperlipidemia (Table 1). Many more molecules are in the pipeline, with phase 1 or phase 3 studies already published.

Table 1.
List of siRNAs with therapeutic potential for the kidney.
3. Synthetic siRNA and the Kidney
Unlike other organs, such as the liver, the kidney presents a challenge for the use of siRNAs due to its structural complexity and the variety of cells it contains []. These diverse cell types of the kidneys make it difficult to optimize and deliver drugs to specific cell types []. Moreover, the glomerular filtration barrier hinders the delivery of larger siRNA agents. Conversely, since siRNAs with large support molecules tend to be trapped by the mesangium, this is considered a major potential target for these agents []. Naked siRNAs, smaller than 6 nm, can pass the filtration barrier; therefore, they could act directly on the structures downstream of the glomerulus. Despite the above-described intrinsic complexity of delivering siRNAs to individual kidney components, recently many advances have been made in the chemical synthesis of siRNAs targeting the kidney [,]. These advances have demonstrated the numerous renal targets for this new class of drugs in kidney disease []. In fact, the kidney was seen as an organ that offered considerable potential for the use of siRNAs []. Moreover, since shortly after their discovery, siRNAs have demonstrated their role in some experimental kidney diseases []. Consequently, their possible therapeutic use was studied first in experimental animals and then in humans [,]. In theory, siRNAs can be employed to target specific genes or pathways implicated in the development of kidney conditions, such as inflammation, fibrosis and oxidative stress, and their mediators. Meanwhile, agents that act indirectly on the kidney are already in use with interesting results. In fact, the first RNA authorized for therapeutic use acts only indirectly on the kidney. It is patisiran.
4. Patisiran in Kidney Amyloidosis
This siRNA employs a delivery carrier. Specifically, it uses lipid nanoparticles as a delivery system. These nanoparticles improve the stability of siRNA in the bloodstream and facilitate its delivery into target cells.
Patisiran works by using RNA interference to silence the gene responsible for producing the transthyretin (TTR) protein in the liver, which, when mutated, misfolds and aggregates, leading to hereditary transthyretin-mediated amyloidosis. The accumulation of these misfolded proteins damages various organs and tissues, including heart, thyroid, kidney, liver and nerves. Various studies have demonstrated improvements across a wide range of cardiac biomarkers following treatment with patisiran and have changed the perception of ATTR-CM from being thought of as a terminal disease process to now being regarded as a treatable condition [].
Initially, kidney involvement in ATTR was underappreciated as proteinuria usually lags behind peripheral neuropathy by about 10 years []. Later studies, more focused on the kidney side of the disease, have shown a significant organ involvement []. One survey on 402 ATTR patients found that one-third of patients eventually developed proteinuria, and 10% end-stage renal disease (ESRD) []. It has been shown that CKD affects almost one-third of patients with symptomatic ATTR [,]. In this setting, the availability and the use of patisiran in ATTR amyloidosis, approved in 2019, has allowed the assessment of its effects on the kidney.
Its administration, via iv infusion once every 3 weeks, reduces up to 80% of the circulating transthyretin and its deposition in parenchymal organs, kidney included []. With the diffuse use of this new agent, some of its potential beneficial renal effects soon became apparent. In sporadic cases, a remission of nephrotic syndrome was observed []. More interestingly, in a small group of patients followed for up to 30 months, the introduction of patisiran therapy improved the slope of decline of estimated glomerular filtration rate (eGFR) []. Overall, the results of patisiran therapy obtained to date lead to optimism regarding its beneficial renal effects in ATTR amyloidosis [].
5. Lumasiran and Nedosiran in Primary Hyperoxaluria
Lumasiran was the second synthetic siRNA approved for human use, as the first treatment for primary hyperoxaluria type 1 (PH1). Primary hyperoxaluria (PH) is a rare autosomal recessive genetic condition, characterized by increased production and renal excretion of oxalate. Based on the responsible genetic alteration, it is differentiated into three types. PH1 is caused by mutations of the AGXT gene causing dysfunction of the liver-specific peroxisomal enzyme alanine glyoxylate aminotransferase (AGT), which catalyzes the transamination of glyoxylate to glycine. Patients with a defect in AGXT fail to properly detoxify glyoxylate through its conversion to glycine. This results in a build-up of glyoxylate, which is then oxidized to oxalate by lactate. It is therefore a hepatic metabolic defect that determines the increase in blood levels and urinary excretion of oxalate. In PH type 2 (PH2), the mutation of the GHRPR gene causes dysfunction of the cytosolic enzyme glyoxylate/hydroxypyruvate reductase []. In PH type 3 (PH3), the alteration affects the HOGA1 gene which controls the expression of the mitochondrial enzyme 4-hydroxy-2- oxoglutarate aldolase.
PH1 accounts for approximately 80% of disease burden and has a population prevalence between 1–3 per million population. Kidney failure occurs in 19% and 57% of patients by the ages of 10 and 40 years, respectively []. More than one-third of patients with PH2 progress to kidney failure by the age of 40 years. In PH3, 14% to 29% of affected individuals develop chronic renal disease, and 3% to 4% develop kidney failure by the age of 40 years. The causal element of organ damage in PH is the increased concentration of oxalates in the urine. The consequent urolithiasis, recurrent and bilateral, causes the progressive reduction of renal filtration and a progressive accumulation of oxalate in the blood. When the concentration of oxalic acid reaches supersaturation, precipitation of insoluble crystals of calcium oxalate occurs in the kidney (nephrocalcinosis) and in other organs (systemic oxalosis). Up to now, the only therapeutic approach to this condition was supportive care, which could not stop the evolution towards ESRD. The recent availability of two new siRNAs, lumasiran and nedosiran, has completely changed the perspective [].
Lumasiran is a synthetic small interfering RNA that targets and silences the HAO1 gene. The HAO1 gene encodes the enzyme hydroxyacid oxidase 1, also known as glycolate oxidase, an enzyme upstream of peroxisomal alanine-glyoxylate aminotransferase (AGT) in the liver. By blocking the production of glycolate oxidase, lumasiran reduces levels of glyoxylate, the substrate for oxalate synthesis, and consequently decreases hepatic oxalate production (Figure 2) [].
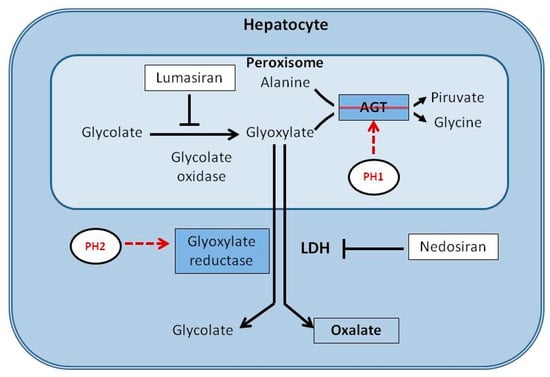
Figure 2.
Schematic representation of the metabolic pathway of glyoxylate in the liver and the mechanism of action of lumasiran and nedosiran.
Primary hyperoxaluria type 1 is a genetic alteration that leads to a deficiency of the peroxisomal alanine-glyoxylate aminotransferase (AGT). Type 2 results from a genetic deficiency of glyoxylate reductase. These defects result in accumulation of glyoxylate, which is converted to oxalate by liver-specific lactate dehydrogenase A (LDHA). Lumasiran silences the hydroxyacid oxidase 1 gene, reducing the production of glycolate oxidase and the levels of glyoxylate available for conversion into oxalate. This leads to a significant reduction in the production of oxalate.
Nedosiran, by silencing the gene that encodes LDH-A, prevents the conversion of glyoxylate to oxalate.
The approval of lumasiran was based on studies that definitively showed its efficacy. In the first, a double-blind, phase 3 trial (lLLUMINATE-A trial), patients with PH1 who were 6 years of age or older were randomly assigned in a 2:1 ratio to receive subcutaneous lumasiran or placebo for 6 months. Inclusion criteria were an estimated glomerular filtration rate (eGFR) of at least 30 mL per minute per 1.73 m2 of body-surface area, 24 h urinary oxalate excretion of at least 0.70 mmol per 24 h per 1.73 m2, and no clinical evidence of extrarenal systemic oxalosis. Lumasiran was administered once monthly for three doses, followed by maintenance doses given once every 3 months. Treated patients had a rapid and sustained decrease in 24 h urinary oxalate excretion, reaching −65.4% at 6 months. Plasma oxalate levels decreased by 39.8% []. In the ILLUMINATE-B trial, the study was extended to children aged <6 years and it lasted 12 months. At the end, all the measured parameters were improved. Additional improvements were also seen in nephrocalcinosis grade. Kidney stone event rates remained low [,]. Other registered trials have extended the indication of lumasiran to patients excluded by early studies. The ILLUMINATE-C trial (NCT04152200) evaluates lumasiran in patients of all ages with eGFR ≤ 45 mL/min/1.73 m2 (if ≥12 months of age) or with elevated sCr (if <12 months of age) and those stable in hemodialysis. Lumasiran resulted in substantial reductions in plasma oxalate with acceptable safety in patients with PH1 who have advanced kidney disease, supporting its efficacy and safety in this patient population []. In the few years after its registration, this siRNA, which has been used extensively, has radically revolutionized the prognosis of PH1, in particular its progression to ESRD.
6. Nedosiran in Primary Hyperoxaluria (All Subtypes)
Nedosiran is a therapeutic siRNA that silences the LDHA gene, which encodes the enzyme lactate dehydrogenase A (LDHA). This enzyme is involved in the conversion of glyoxylate to oxalate, a key step in oxalate production. By inhibiting LDHA, nedosiran reduces the conversion of glyoxylate to oxalate, helping to lower oxalate levels in patients with primary hyperoxaluria, including all three types: PH1, PH2, and PH3. After hepatocyte internalization and release into the cytoplasm, nedosiran exploits the endogenous RNAi regulatory mechanism to degrade LDHA mRNA, thereby reducing production of LDHA. Consequently, the activity of hepatic LDHA, the key common enzyme involved in the final conversion of glyoxylate to oxalate in all three known genetic subtypes of PH, is reduced (Figure 2).
Nedosiran was approved in 2023 in the USA to lower urinary oxalate levels in children aged ≥ 9 years and adults with PH1 with an eGFR ≥ 30 mL/min/1.73 m2 with a single monthly subcutaneous administration. The efficacy and safety of this siRNA have been demonstrated by robust human studies. In a phase 1 study (PHYOX1) of a once-monthly subcutaneous nedosiran therapy, the safety, pharmacokinetics, pharmacodynamics and exposure–response of subcutaneous administration have been evaluated. No significant side effects have been reported except for some reactions at the injection site. At day 57 (end of study), a reduction in 24 h urinary oxalate excretion from baseline was observed in nedosiran-treated patients. Mean maximum reduction in urinary oxalate excretion was 55% after single-dose nedosiran, with 33% of participants reaching normal 24 h urinary oxalate excretion []. In a second randomized study (PHYOX2), participants with PH1 receiving nedosiran had clinically meaningful reductions in urinary oxalate, the mediator of kidney damage in PH []. Very satisfactory results are also emerging from studies on PH type 3 [].
In conclusion, while lumasiran reduces oxalate formation in patients with PH1 by decreasing glyoxylate overproduction in peroxisomes at early stages of the metabolic pathway, nedosiran acts downstream by inhibiting hepatic LDHA, thus reducing oxalate formation in all PH subtypes (Figure 2) [].
7. siRNA in Complement-Mediated Diseases
Complement-mediated diseases are disorders caused by abnormal regulation or activation of the complement system, a key part of the immune system. Kidney involvement is common in these conditions and many therapeutic attempts have been aimed at inhibiting one or more components of this system. So far, the best results have been obtained with C5 inhibitors with monoclonal antibodies []. Yet, anti-C5 antibodies require large dosages and frequent intravenous administration because of the high plasma concentration of C5 []. For this and other reasons, alternative ways to block the complement system have been sought. One of these led to a novel siRNA, cemdisiran []. This new investigational N-acetyl-d-galactosamine (GalNAc) siRNA is designed to target and inhibit the production of complement component C5 of the complement system. It acts on messenger RNA (mRNA) that codes for the C5 protein in the liver by degrading it, thereby reducing its production. In patients with paroxysmal nocturnal hemoglobinuria, a C5-mediated condition, C5 suppression by cemdisiran enabled effective inhibition of residual C5 levels, maintained for 6–10 months after the last dose. Thanks to this new therapy, it was possible to reduce the dosage and/or the frequency of administration of eculizumab []. These results were such as to support the continuation of the evaluation of this siRNA in complement-mediated diseases. The delivery of negatively charged large molecules into target cells represents a major challenge in developing such therapeutics []. Lipid nanoparticles’ delivery support (LNPs) have overcome these difficulties and are currently used for three approved siRNA-based medicines []. Experimental kidney disease is the ideal model for testing their efficacy. A small-interfering RNA against complement C5 (C5 siRNA-LNP) in a lipid nanoparticle formulation (C5 siRNA-LNP), was tested in a rat model of antibody-mediated rejection (AMR) of kidney transplantation []. Rats were sensitized by skin grafting and after 4 weeks underwent kidney transplantation. C5 siRNA-LNP was administered daily in association with cyclosporine (CsA) and/or deoxyspergualin (DSG) in the treatment group. Different combinations of immunosuppressive agents were used in control groups. C5 siRNA-LNP in combination with CsA and DSG completely suppressed C5 expression and complement activity (hemolytic activity ≤ 20%) within 7 days from the administration. Immunohistochemical analysis of the grafts revealed that downregulation of C5 expression was associated with a reduction in the membrane attack complex (C5b-9) positive area. Serum creatinine in the siRNA-triple therapy group remained stable, whereas its levels in the control groups were significantly increased. C5 siRNA-triple therapy significantly prolonged the graft survival compared with the combinations used in control groups.
This first use of an siRNA used as a complement blocker, although limited to experimental animals, shows the potential of these agents in the nephrology field. The results in humans were not long in coming. In fact, based on the evidence that IgA nephropathy (IgAN) pathogenesis is related to activation of complement pathways and that 5b–9 membrane attack complex plays a fundamental role in kidney injury, a multinational group has undertaken a phase 2 study on the use of cemdisiran, an siRNA that silences the gene responsible for producing the C5 protein, in this nephropathy []. The trial is a randomized, double-blind, placebo-controlled multicenter study to evaluate the efficacy and safety of cemdisiran in adult patients with IgAN. Thirty-one adult patients (≥18 years and ≤65 years of age and with urine protein ≥1 g/24 h) were randomized (2:1) to subcutaneous cemdisiran 600 mg or placebo every 4 weeks in combination with the standard of care. After a 32-week treatment period, in cemdisiran-treated patients the mean change in serum C5 level from baseline was −98.7% vs. 25.2% in the placebo group. In the treated group, the placebo-adjusted geometric mean change in 24 h urine protein-to-creatinine ratio was −37.4%. The most common adverse event after cemdisiran treatment was injection-site reaction []. The significant reduction in proteinuria was confirmed in the 52-week open-label extension study. Based on these favorable results, the manufacturing company has started the procedures for a phase 3 study. Unexpectedly, in December 2022 the company announced the suspension of this program.
Other siRNAs have been developed that are active against complement components with a role in nephropathies []. Some of these have been tested in experimental animals, others are in the early stages of use in humans. In a mouse model of C3 glomerulopathy, RNA interference-mediated C3 silencing was effective in slowing the formation of mesangial and subendothelial electron-dense deposits, thus paving the way for its use in humans []. In New Zealand, the siRNA drug SGB-9768, targeting the complement C3 component for the treatment of complement-mediated diseases, has recently been approved to conduct a phase 1 clinical trial.
8. siRNA in Acute Kidney Injury [AKI]
In the experimental setting, several approaches to the treatment of AKI have been tried with different siRNAs. The caspase-3 siRNA was first used to protect porcine renal tubular epithelia cells against hydrogen peroxide-induced injury []. Tumor suppressor p53 and chemokine receptor CXCR4 are implicated in AKI pathogenesis []. The combined CXCR4 inhibition and p53 gene silencing with a polymeric CXCR4 antagonist siRNA carrier improves kidney function and decreases renal damage in an experimental AKI model in mice []. These siRNA applications were aimed at treating the kidney by a direct approach by targeting tubular cells. In fact, proximal tubule cells are the primary site for rapid and extensive endocytic uptake of small siRNAs following glomerular filtration. The application of technologies to allow drug delivery to tubular cells from both the luminal and basolateral sides is essential to act on these cells [,]. A naked siRNA has been shown to meet these requirements. Teprasiran (I5NP) is a synthetic siRNA designed to act via the RNA interference pathway to temporarily inhibit expression of the pro-apoptotic protein p53. This agent has been developed to protect cells from acute ischemia/reperfusion injuries such as AKI that can occur during major cardiac surgery or delayed graft function following renal transplantation []. The temporary inhibition of p53 expression by teprasiran affords kidney cells time to repair cellular damage following reperfusion injury and thereby avoid induction of apoptosis []. After animal studies, teprasiran was tested in humans in a phase 1 study with satisfactory results from the safety point of view []. In a phase 2 prospective, multicenter, double-blind, randomized, controlled trial, the effectiveness of this siRNA has been evaluated in reducing the incidence, severity and duration of AKI after cardiac surgery in 341 high-risk patients []. Teprasiran sodium or placebo (isotonic saline) were administered as a single intravenous bolus over 1 to 2 min at 4 h after a cardio-pulmonary bypass or after the last coronary anastomosis. AKI was observed in 49.7% and 36.9% in the placebo and teprasiran groups, respectively (absolute risk reduction −12.8%). The significant reduction in the incidence, severity and duration of early postoperative AKI in high-risk patients in this cardiac surgery setting demonstrated the effectiveness of this siRNA. Teprasiran appeared to be well-tolerated and no safety signal was detected. A Phase 3 trial was started after these results (NCT03510897). The study completed day 90 for the primary endpoint but was terminated early due to results not meeting the efficacy outcome.
9. Anemia in Kidney Disease
Hepcidin, which has been shown to play a role in anemia in chronic kidney disease, has been addressed by early studies with siRNA []. The HAMP gene provides instructions for the production of hepcidin, and siRNAs were developed to silence this gene as a novel therapeutic approach for the treatment of anemia of chronic disease [,]. Despite the exciting expectations, only animal studies, with favorable results, have been reported so far [,,].
10. siRNA to Treat Hypertension
RNA interference as a means of direct and/or indirect renal protection is being studied by blocking hepatic angiotensinogen (AGT) production. Upstream blocking of the renin–angiotensin system (RAS) could circumvent the so-called escape phenomenon, elicited by the compensatory renin elevation upon RAS block. Moreover, studies in rat models of acute kidney injury showed kidney protection by a liver-selective AGT inhibition []. A new siRNA, zilebesiran, targeting liver AGT production has been synthesized that utilizes a N-acetylgalactosamine (GalNAc) conjugate technology. This pharmacological support enables infrequent subcutaneous dosing with increased selectivity. This agent, injected in association with an angiotensin II receptor blocker in spontaneously hypertensive rats, produced a sustained antihypertensive and cardioprotective effect []. Furthermore, anti-AGT has also demonstrated a direct effect on the kidney in the 5/6th nephrectomy model in rat, a hypertensive chronic kidney disease model. With anti-AGT treatment in association with losartan, proteinuria and cardiac hypertrophy were abrogated and glomerulosclerosis was significantly reduced []. These results suggest that targeting hepatic AGT offers new possibilities for the treatment of CKD in humans, especially in non-adherent patients. Shortly after, zilebesiran was tested in hypertensive patients []. A single subcutaneous dose of 200 mg decreased serum angiotensinogen levels and 24 h ambulatory blood pressure for up to 24 weeks. In the following KARDIA-1 study, zilebesiran monotherapy as single 150, 300 or 600 mg doses significantly reduced 24 h mean ambulatory and office systolic blood pressure (SBP) from baseline to months 3 and 6 compared with placebo in patients with mild-to-moderate hypertension. The few adverse effects in <5% of patients were mostly injection-site reactions. Small increases in serum potassium were observed that did not need intervention. Four AKIs were reported in the zilebesiran group []. The subsequent KARDIA-2 randomized study showed that in patients with uncontrolled hypertension, subcutaneous injection of zilebesiran improves 3-month blood pressure []. A new study, KARDIA-3 (NCT06272487), was started to evaluate zilebesiran as add-on therapy in patients who have established CV disease, or high CV risk, and hypertension that is uncontrolled despite stable treatment with two of the standard-of-care antihypertensives []. To gain additional safety and efficacy data in patients with advanced CKD, the study places particular emphasis on the kidney. In fact, there will be a cohort of patients enrolled with eGFR 30 to <45 mL/min/1.73 m2. Among the main benefits of zilebesiran are the improved treatment adherence thanks to the long intervals between administrations and the hepatic storage of siRNA that ensures a lasting target depletion. However, this prolonged blockade of the RAS has caused concern. In fact, the integrity of this system is essential for cardiocirculatory homeostasis in critical situations such as pregnancy, shock and hemorrhage. From this point of view, reassurances have come from two new studies. The first has shown that, in experimental animals, the effects of AGT block by siRNA does not abolish the pressor responsiveness to conventional vasopressors when it is needed []. In the second, the same RNA silencing technology was used. In spontaneously hypertensive rats, the AGT blockade was reversed with an siRNA. Short, synthetic single-stranded oligonucleotides, complementary to the siRNA guide (antisense) strand were loaded in the RNA-induced silencing complex targeting hepatocytes with the same GalNAc delivery approach []. This new use of an siRNA adds additional safety to the use of zilebesiran in arterial hypertension.
11. Conclusions
In the years following their discovery, siRNAs have shown their potential value. Their selectivity in blocking specific proteins in a non-permanent manner and the use of spaced administrations are the most evident advantages. The initial use limited to genetic conditions has progressively extended to chronic clinical conditions. The development of new biotechnologies has extended their delivery from the local to the systemic level. Potentially, siRNAs could eventually replace a large portion of chronic therapies, improving adherence.
As far as the kidney is concerned, there are still few siRNAs that have passed the approval test. This is despite the numerous potential targets that this organ offers and the many studies, in animals and humans, so far performed (Table 2).

Table 2.
Clinical studies on siRNA with renal impact.
This relative delay in development can be attributed to two types of causes. The first lies in the complex structure of the kidney itself and in its physiology. As seen above, it is difficult to reach every single cell of the kidney, of the more than 20 that compose it, without creating problems for the others. The second set of reasons is of a more general nature. The main ones are limitations in the route of administration, overcoming biological barriers, inactivation by nucleases, immunological activation and possible off-target effects []. The latter is perhaps one of the major obstacles for siRNA therapy. This consists in the induction of various side effects caused by unexpected perturbations between RNAi molecules and cellular components.
Finally, last but not least, the enormous costs of the trials required to obtain approval from the regulatory authorities have proven to be a major obstacle and have caused the withdrawal of already registered trials before they even started.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Piatek, M.J.; Werner, A. Endogenous siRNAs: Regulators of internal affairs. Biochem. Soc. Trans. 2014, 42, 1174–1179. [Google Scholar] [CrossRef]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of siRNA. Int. J. Biomed. Sci. 2017, 13, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M.; Bernstein, E.; Beach, D.; Hannon, G.J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000, 404, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Judge, A.D.; Sood, V.; Shaw, J.R.; Fang, D.; McClintock, K.; MacLachlan, I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005, 23, 457–462. [Google Scholar] [CrossRef]
- Kaiser, P.K.; Symons, R.C.; Shah, S.M.; Quinlan, E.J.; Tabandeh, H.; Do, D.V.; Reisen, G.; Lockridge, J.A.; Short, B.; Guerciolini, R.; et al. Sirna-027 Study Investigators. RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am. J. Ophthalmol. 2010, 150, 33–39.e2. [Google Scholar] [CrossRef]
- Coelho, T.; Adams, D.; Silva, A.; Lozeron, P.; Hawkins, P.N.; Mant, T.; Perez, J.; Chiesa, J.; Warrington, S.; Tranter, E.; et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 2013, 369, 819–829. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Gritli, I.; Tolcher, A.; Heidel, J.D.; Lim, D.; Morgan, R.; Chmielowski, B.; Ribas, A.; Davis, M.E.; Yen, Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. USA 2014, 111, 11449–11454. [Google Scholar] [CrossRef]
- Kristen, A.V.; Ajroud-Driss, S.; Conceiçao, I.; Gorevic, P.; Kyriakides, T.; Obici, L. Patisiran, an RNAi therapeutic for the treatment of hereditary transthyretin-mediated amyloidosis. Neurodegener. Dis. Manag. 2018, 9, 5–23. [Google Scholar] [CrossRef]
- Huang, Y.Y. Approval of the first-ever RNAi therapeutics and its technological development history. Prog. Biochem. Biophys. 2019, 46, 313–322. [Google Scholar]
- Schumacher, A.; Rookmaaker, M.B.; Joles, J.A.; Kramann, R.; Nguyen, T.Q.; van Griensven, M.; LaPointe, V.L.S. Defining the variety of cell types in developing and adult human kidneys by single-cell RNA sequencing. NPJ Regen. Med. 2021, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Ahn, I.; Kang, C.; Han, J. Where should siRNAs go: Applicable organs for siRNA drugs. Exp. Mol. Med. 2023, 55, 1283–1292. [Google Scholar] [CrossRef]
- Takabatake, Y.; Isaka, Y.; Imai, E. In vivo transfer of small interfering RNA or small hairpin RNA targeting glomeruli. Methods Mol. Biol. 2009, 466, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Long, K.; Raibi, Y.; Kunniff, J.; Qadir, D.; Lawrence, J. Megalin-mediated siRNA uptake in kidney proximal tubule cells. J. Physiol. 2024, 39, s1. [Google Scholar] [CrossRef]
- Vaidya, A.; Moore, S.; Chatterjee, S.; Guerrero, E.; Kim, M.; Farbiak, L.; Dilliard, S.A.; Siegwart, D.J. Expanding RNAi to Kidneys, Lungs, and Spleen via Selective ORgan Targeting (SORT) siRNA Lipid Nanoparticles. Adv. Mater 2024, 36, 2313791. [Google Scholar] [CrossRef]
- Bondue, T.; van den Heuvel, L.; Levtchenko, E.; Brock, R. The potential of RNA-based therapy for kidney diseases. Pediatr. Nephrol. 2023, 38, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, C.; Zhao, Z.; Zhu, T.; Yang, B. Fighting against kidney diseases with small interfering RNA: Opportunities and challenges. J. Transl. Med. 2015, 1, 39. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, T.; Zhao, Z.; Jia, Y.; Li, L.; Zhang, Y.; Song, M.; Rong, R.; Xu, M.; Nicholson, M.L.; et al. Serum-stabilized naked caspase-3 siRNA protects autotransplant kidneys in a porcine model. Mol. Ther. 2014, 10, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Hori, Y.; Kaname, S.; Yamada, K.; Nishiyama, N.; Matsumoto, S.; Miyata, K.; Oba, M.; Yamada, A.; Kataoka, K.; et al. siRNA-based therapy ameliorates glomerulonephritis. J. Am. Soc. Nephrol. 2010, 21, 622–633. [Google Scholar] [CrossRef]
- Thai, H.B.D.; Kim, K.R.; Hong, K.T.; Voitsitskyi, T.; Lee, J.S.; Mao, C.; Ahn, D.R. Kidney-Targeted Cytosolic Delivery of siRNA Using a Small-Sized Mirror DNA Tetrahedron for Enhanced Potency. ACS Cent. Sci. 2020, 6, 2250–2258. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, A.; Fontana, M.; Gillmore, J.D. Patisiran for the Treatment of Transthyretin-mediated Amyloidosis with Cardiomyopathy. Heart. Int. 2023, 17, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lobato, L.; Rocha, A. Transthyretin amyloidosis and the kidney. Clin. J. Am. Soc. Nephrol. 2012, 7, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; D’ambrosio, V.; Di Paolantonio, A.; Guglielmino, V.; Calabresi, P.; Sabatelli, M.; Luigetti, M. Renal involvement in hereditary transthyretin amyloidosis: An Italian single-centre experience. Brain Sci. 2021, 11, 980. [Google Scholar] [CrossRef]
- Lobato, L. Portuguese-type amyloidosis [transthyretin amyloidosis, ATTR V30M]. J. Nephrol. 2003, 16, 438–442. [Google Scholar] [PubMed]
- Solignac, J.; Delmont, E.; Fortanier, E.; Attarian, S.; Mancini, J.; Daniel, L.; Jourde-Chiche, N. Kidney involvement in hereditary transthyretin amyloidosis: A cohort study of 103 patients. Clin. Kidney J. 2022, 15, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.; Nasr, S.H. 2024 Update on Classification, Etiology, and Typing of Renal Amyloidosis: A Review. Am. J. Kidney Dis. 2024, 84, 361–373. [Google Scholar] [CrossRef]
- Luigetti, M.; Romano, A.; Di Paolantonio, A.; Bisogni, G.; Sabatelli, M. Diagnosis and Treatment of Hereditary Transthyretin Amyloidosis [hATTR] Polyneuropathy: Current Perspectives on Improving Patient Care. Ther. Clin. Risk Manag. 2020, 16, 109–123. [Google Scholar] [CrossRef]
- Luigetti, M.; Guglielmino, V.; Romano, A.; Sciarrone, M.A.; Vitali, F.; D’Ambrosio, V.; Ferraro, P.M. Trajectories of Kidney Function in Patients with ATTRv Treated with Gene Silencers. Genes 2022, 13, 2236. [Google Scholar] [CrossRef]
- Meléndrez-Balcázar, E.; Aranda-Vela, K.; Cervantes-Hernández, A.; López-Cureño, S. Hereditary Transthyretin Amyloidosis: Impact of Classic and New Treatments on Kidney Function. Am. J. Kidney Dis. 2024, 84, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B. An update on primary hyperoxaluria. Nat. Rev. Nephrol. 2012, 8, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, W.; Zhou, J.; Huang, Q.; Zeng, G. Navigating the Evolving Landscape of Primary Hyperoxaluria: Traditional Management Defied by the Rise of Novel Molecular Drugs. Biomolecules 2024, 14, 511. [Google Scholar] [CrossRef] [PubMed]
- Demoulin, N.; Aydin, S.; Gillion, V.; Morelle, J.; Jadoul, M. Pathophysiology and Management of Hyperoxaluria and Oxalate Nephropathy: A Review. Am. J. Kidney Dis. 2022, 79, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Liebow, A.; Li, X.; Racie, T.; Hettinger, J.; Bettencourt, B.R.; Najafian, N.; Haslett, P.; Fitzgerald, K.; Holmes, R.P.; Erbe, D.; et al. An investigational RNAi therapeutic targeting glycolate oxidase reduces oxalate production in models of primary hyperoxaluria. J. Am. Soc. Nephrol. 2017, 28, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Garrelfs, S.F.; Frishberg, Y.; Hulton, S.A.; Koren, M.J.; O’Riordan, W.D.; Cochat, P.; Deschênes, G.; Shasha-Lavsky, H.; Saland, J.M.; Van’t Hoff, W.G.; et al. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. N. Engl. J. Med. 2021, 384, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Hayes, W.; Sas, D.J.; Magen, D.; Shasha-Lavsky, H.; Michael, M.; Sellier-Leclerc, A.L.; Hogan, J.; Ngo, T.; Sweetser, M.T.; Gansner, J.M.; et al. Efficacy and safety of lumasiran for infants and young children with primary hyperoxaluria type 1: 12-month analysis of the phase 3 ILLUMINATE-B trial. Pediatr. Nephrol. 2003, 38, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Sas, D.J.; Magen, D.; Hayes, W.; Shasha-Lavsky, H.; Michael, M.; Schulte, I.; Sellier-Leclerc, A.L.; Lu, J.; Seddighzadeh, A.; Habtemariam, B.; et al. Phase 3 trial of lumasiran for primary hyperoxaluria type 1: A new RNAi therapeutic in infants and young children. Genet. Med. 2022, 24, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Michael, M.; Groothoff, J.W.; Shasha-Lavsky, H.; Lieske, J.C.; Frishberg, Y.; Simkova, E.; Sellier-Leclerc, A.L.; Devresse, A.; Guebre-Egziabher, F.; Bakkaloglu, S.A.; et al. Lumasiran for Advanced Primary Hyperoxaluria Type 1: Phase 3 ILLUMINATE-C Trial. Am. J. Kidney Dis. 2023, 8, 145–155.e1. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.; Koch, A.; Cochat, P.; Garrelfs, S.F.; Baum, M.A.; Groothoff, J.W.; Lipkin, G.; Coenen, M.; Schalk, G.; Amrite, A.; et al. Safety, pharmacodynamics, and exposure-response modeling results from a first in human phase 1 study of nedosiran [PHYOX1] in primary hyperoxaluria. Kidney Int. 2021. [Google Scholar] [CrossRef]
- Baum, M.A.; Langman, C.; Cochat, P.; Lieske, J.C.; Moochhala, S.H.; Hamamoto, S.; Satoh, H.; Mourani, C.; Ariceta, G.; Torres, A.; et al. PHYOX2: A pivotal randomized study of nedosiran in primary hyperoxaluria type 1 or 2. Kidney Int. 2023, 103, 207–217. [Google Scholar] [CrossRef]
- Goldfarb, D.S.; Lieske, J.C.; Groothoff, J.; Schalk, G.; Russell, K.; Yu, S.; Vrhnjak, B. Nedosiran in primary hyperoxaluria subtype 3: Results from a phase I, single-dose study [PHYOX4]. Urolithiasis 2023, 51, 80. [Google Scholar] [CrossRef]
- Bacchetta, J.; Lieske, J.C. Primary hyperoxaluria type 1: Novel therapies at a glance. Clin. Kidney J. 2022, 15, i17–i22. [Google Scholar] [CrossRef]
- Werion, A.; Rondeau, E. Application of C5 inhibitors in glomerular diseases in 2021. Kidney Res. Clin. Pract. 2022. [Google Scholar] [CrossRef]
- Holers, V.M. Complement and its receptors: New insights into human disease. Annu. Rev. Immunol. 2014, 32, 433–459. [Google Scholar] [CrossRef]
- Zimmermann, T.S.; Karsten, V.; Chan, A.; Chiesa, J.; Boyce, M.; Bettencourt, B.R.; Hutabarat, R.; Nochur, S.; Vaishnaw, A.; Gollob, J. Clinical proof of concept for a novel hepatocytetargeting GalNAc-siRNA conjugate. Mol. Ther. 2017, 25, 71–78. [Google Scholar] [CrossRef]
- Badri, P.; Jiang, X.; Borodovsky, A.; Najafian, N.; Kim, J.; Clausen, V.A.; Goel, V.; Habtemariam, B.; Robbie, G.J. Pharmacokinetic and Pharmacodynamic Properties of Cemdisiran, an RNAi Therapeutic Targeting Complement Component 5, in Healthy Subjects and Patients with Paroxysmal Nocturnal Hemoglobinuria. Clin. Pharmacokinet. 2021, 60, 365–378. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug deli-very. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Ishigooka, H.; Katsumata, H.; Saiga, K.; Tokita, D.; Motoi, S.; Matsui, C.; Suzuki, Y.; Tomimatsu, A.; Nakatani, T.; Kuboi, Y.; et al. Novel Complement C5 Small-interfering RNA Lipid Nanoparticle Prolongs Graft Survival in a Hypersensitized Rat Kidney Transplant Model. Transplantation 2022, 106, 2338–2347. [Google Scholar] [CrossRef] [PubMed]
- Poppelaars, F.; Faria, B.; Schwaeble, W.; Daha, M.R. The contribution of complement to the pathogenesis of IgA nephropathy: Are complement-targeted therapies moving from rare disorders to more common diseases? J. Clin. Med. 2021, 10, 4715. [Google Scholar] [CrossRef] [PubMed]
- Barratt, J.; Liew, A.; Yeo, S.C.; Fernström, A.; Barbour, S.J.; Sperati, C.J.; Villanueva, R.; Wu, M.J.; Wang, D.; Borodovsky, A.; et al. Cemdisiran Phase 2 Study Investigators and Collaborators. Phase 2 Trial of Cemdisiran in Adult Patients with IgA Nephropathy: A Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2024, 19, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Sándor, N.; Schneider, A.E.; Matola, A.T.; Barbai, V.H.; Bencze, D.; Hammad, H.H.; Papp, A.; Kövesdi, D.; Uzonyi, B.; Józsi, M. The human factor H protein family—An update. Front. Immunol. 2024, 15, 1135490. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, C.; Locatelli, M.; Cerullo, D.; Aumiller, V.; Corna, D.; Rottoli, D.; Eisermann, M.; Donadelli, R.; Mousavi, M.; Noris, M.; et al. Therapeutic Small Interfering RNA Targeting Complement C3 in a Mouse Model of C3 Glomerulopathy. J. Immunol. 2022, 208, 1772–1781. [Google Scholar] [CrossRef]
- Yang, B.; Elias, J.E.; Bloxham, M.; Nicholson, M.L. Synthetic small interfering RNA down-regulates caspase-3 and affects apoptosis, IL-1 β, and viability of porcine proximal tubular cells. J. Cell Biochem. 2011, 112, 1337–1347. [Google Scholar] [CrossRef]
- Tang, C.; Ma, Z.; Zhu, J.; Liu, Z.; Liu, Y.; Liu, Y.; Cai, J.; Dong, Z. P53 in kidney injury and repair: Mechanism and therapeutic potentials. Pharmacol. Ther. 2019, 195, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, Y.; Jang, H.S.; Hang, Y.; Jogdeo, C.M.; Li, J.; Ding, L.; Zhang, C.; Yu, A.; Yu, F.; et al. Preferential siRNA delivery to injured kidneys for combination treatment of acute kidney injury. J. Control. Release 2022, 341, 300–313. [Google Scholar] [CrossRef]
- Hamar, P.; Song, E.; Kökény, G.; Chen, A.; Ouyang, N.; Lieberman, J. Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc. Natl Acad. Sci. USA 2004, 101, 14883–14888. [Google Scholar] [CrossRef] [PubMed]
- Dolman, M.E.; Harmsen, S.; Storm, G.; Hennink, W.E.; Kok, R.J. Drug targeting to the kidney: Advances in the active targeting of therapeutics to proximal tubular cells. Adv. Drug Deliv. Rev. 2010, 62, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Kornbrust, D.J.; Foy, J.W.; Solano, E.C.; Schneider, D.J.; Feinstein, E.; Molitoris, B.A.; Erlich, S. Toxicological and pharmacokinetic properties of chemically modified siRNAs targeting p53 RNA following intravenous administration. Nucleic Acid Ther. 2012, 22, 255–264. [Google Scholar] [CrossRef]
- Molitoris, B.A.; Dagher, P.C.; Sandoval, R.M.; Campos, S.B.; Ashush, H.; Fridman, E.; Brafman, A.; Faerman, A.; Atkinson, S.J.; Thompson, J.D.; et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J. Am. Soc. Nephrol. 2009, 20, 1754–1764. [Google Scholar] [CrossRef]
- Demirjian, S.; Ailawadi, G.; Polinsky, M.; Bitran, D.; Silberman, S.; Shernan, S.K.; Burnier, M.; Hamilton, M.; Squiers, E.; Erlich, S.; et al. Safety and Tolerability Study of an Intravenously Administered Small Interfering Ribonucleic Acid [siRNA] Post On-Pump Cardiothoracic Surgery in Patients at Risk of Acute Kidney Injury. Kidney Int. Rep. 2017, 2, 836–843. [Google Scholar] [CrossRef]
- Thielmann, M.; Corteville, D.; Szabo, G.; Swaminathan, M.; Lamy, A.; Lehner, L.J.; Brown, C.D.; Noiseux, N.; Atta, M.G.; Squiers, E.C.; et al. Teprasiran, a Small Interfering RNA, for the Prevention of Acute Kidney Injury in High-Risk Patients Undergoing Cardiac Surgery: A Randomized Clinical Study. Circulation 2021, 144, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.; Nemeth, E. Manipulation of the hepcidin pathway for therapeutic purposes. Haematologica 2013, 98, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Chan-Daniels, A.; Sehgal, A.; Foster, D.; Bettencourt, B.R.; Hettinger, J. Targeting the hepcidin pathway with RNAi therapeutics for the treatment of anemia. Blood 2011, 21, 688. [Google Scholar] [CrossRef]
- Hawula, Z.J.; Wallace, D.F.; Subramaniam, V.N.; Rishi, G. Therapeutic Advances in Regulating the Hepcidin/Ferroportin Axis. Pharmaceuticals 2019, 12, 170. [Google Scholar] [CrossRef]
- Rana, S.; Bhatnagar, A.; Singh, S.; Prabhakar, N. Evaluation of liver specific ionizable lipid nanocarrier in the delivery of siRNA. Chem. Phys. Lipids. 2022, 246, 105207. [Google Scholar] [CrossRef]
- Sasu, B.J.; Cooke, K.S.; Arvedson, T.L.; Plewa, C.; Ellison, A.R.; Sheng, J.; Winters, A.; Juan, T.; Li, H.Y.; Begley, C.G.; et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood 2010, 115, 3616–3624. [Google Scholar] [CrossRef] [PubMed]
- Querbes, W.; Bogorad, R.L.; Moslehi, J.; Wong, J.; Chan, A.Y.; Bulgakova, E.; Kuchimanchi, S.; Akinc, A.; Fitzgerald, K.; Koteliansky, V.; et al. Treatment of erythropoietin deficiency in mice with systemically administered siRNA. Blood 2012, 120, 1916–1922. [Google Scholar] [CrossRef][Green Version]
- Mullick, A.E.; Yeh, S.T.; Graham, M.J.; Engelhardt, J.A.; Prakash, T.P.; Crooke, R.M. Blood Pressure Lowering and Safety Improvements With Liver Angiotensinogen Inhibition in Models of Hypertension and Kidney Injury. Hypertension 2017, 70, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Uijl, E.; Mirabito Colafella, K.M.; Sun, Y.; Ren, L.; van Veghel, R.; Garrelds, I.M.; de Vries, R.; Poglitsch, M.; Zlatev, I.; Kim, J.B.; et al. Strong and sustained antihypertensive effect of small interfering RNA targeting liver angiotensinogen. Hypertension 2019, 73, 1249–1257. [Google Scholar] [CrossRef]
- Bovée, D.M.; Ren, L.; Uijl, E.; Clahsen-van Groningen, M.C.; van Veghel, R.; Garrelds, I.M.; Domenig, O.; Poglitsch, M.; Zlatev, I.; Kim, J.B.; et al. Renoprotective effects of small interfering RNA targeting liver angiotensinogen in experimental chronic kidney disease. Hypertension 2021, 77, 1600–1612. [Google Scholar] [CrossRef]
- Desai, A.S.; Webb, D.J.; Taubel, J.; Casey, S.; Cheng, Y.; Robbie, G.J.; Foster, D.; Huang, S.A.; Rhyee, S.; Sweetser, M.T.; et al. Zilebesiran, an RNA Interference Therapeutic Agent for Hypertension. N. Engl. J. Med. 2023, 389, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Saxena, M.; Gupta, A.; Chalhoub, F.; Lee, J.; Stiglitz, D.; Makarova, N.; Goyal, N.; Guo, W.; Zappe, D.; et al. KARDIA-1 Study Group. RNA Interference With Zilebesiran for Mild to Moderate Hypertension: The KARDIA-1 Randomized Clinical Trial. JAMA 2024, 331, 740–749. [Google Scholar] [CrossRef]
- Saxena, M.; Aswad, A.; Badariene, J.; Kazi, F.; Karns, A.; Neutel, J.; Park, W.; Stiglitz, D.; Makarova, N.; Havasi, A.; et al. Zilebesiran as add-on therapy in patients with hypertension inadequately controlled with a standard antihypertensive medication: Efficacy and safety results from the KARDIA-2 STUDY. J. Hypertens. 2024, 42 (Suppl. 1), e115. [Google Scholar] [CrossRef]
- Havasi, A.; Pagidipati, N.; Bakris, G.; Weber, M.; Bengus, M.; Daga, S.; Xiang, Z.; Zee, T.; Bhan, I.; Granger, C.B. KARDIA-3 Study Design: Zilebesiran as Add-On Therapy in Patients with High Cardiovascular Risk and Hypertension Inadequately Controlled by Standard of Care Antihypertensives. J. Hypertens. 2024, 42 (Suppl. 1), e120. [Google Scholar] [CrossRef]
- Uijl, E.; Ye, D.; Ren, L.; Mirabito Colafella, K.M.; van Veghel, R.; Garrelds, I.M.; Lu, H.S.; Daugherty, A.; Hoorn, E.J.; Nioi, P.; et al. Conventional Vasopressor and Vasopressor-Sparing Strategies to Counteract the Blood Pressure-Lowering Effect of Small Interfering RNA Targeting Angiotensinogen. J. Am. Heart Assoc. 2022, 11, e026426. [Google Scholar] [CrossRef]
- Ye, D.; Cruz-López, E.O.; Veghel, R.V.; Garrelds, I.M.; Kasper, A.; Wassarman, K.; Tu, H.C.; Zlatev, I.; Danser, A.H.J. Counteracting Angiotensinogen Small-Interfering RNA-Mediated Antihypertensive Effects with REVERSIR. Hypertension 2024, 81, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Burchard, J.; Schelter, J.; Chau, B.N.; Cleary, M.; Lim, L.; Linsley, P.S. Widespread siRNA “off-target” transcript silencing mediated by seed region sequence complementarity. RNA 2006, 12, 1179–1187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).