Exploring the Cardiorenal Benefits of SGLT2i: A Comprehensive Review
Abstract
1. Introduction
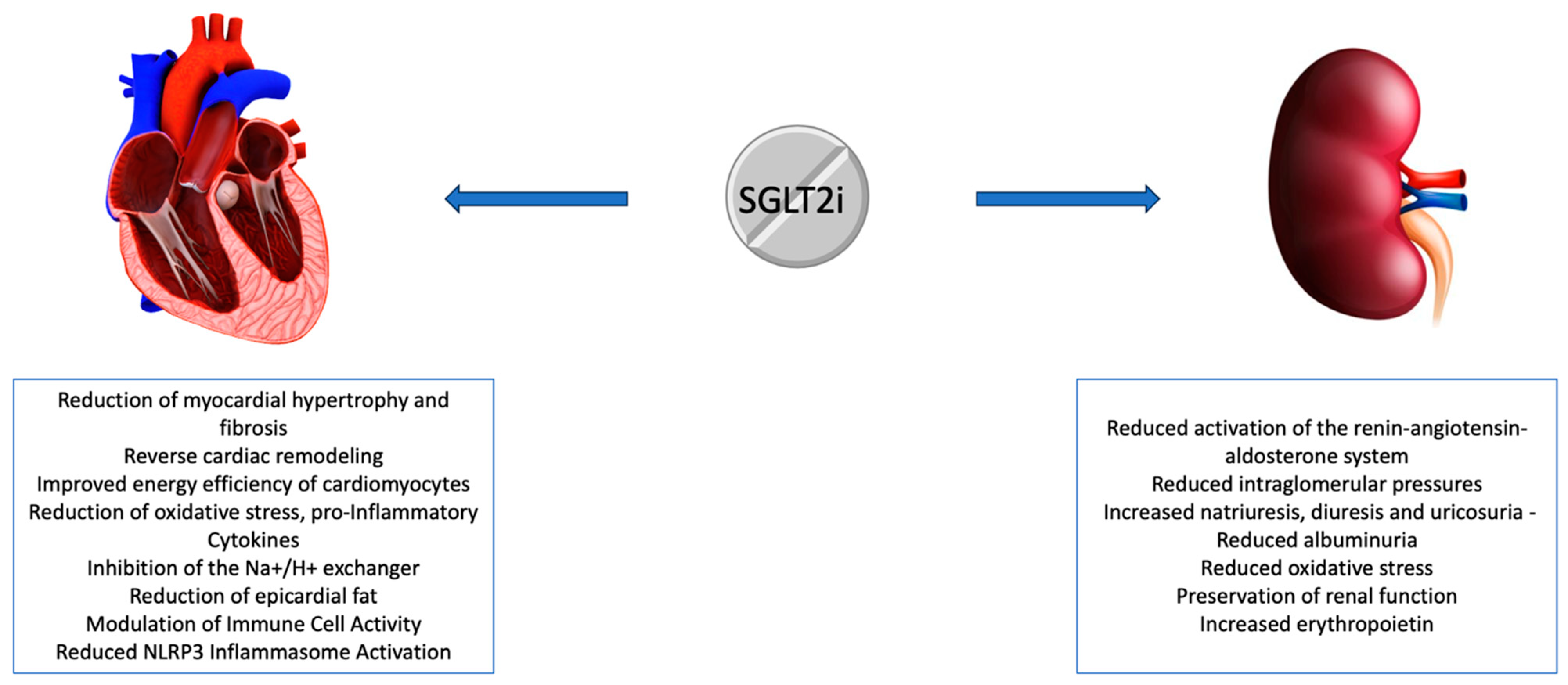
2. Effects of SGLT2i
3. SGLT2i on Heart: Across the Trials
4. SGLT2i on Kidney: Across the Trials
5. Additional Molecular Targets of SGLT2i and Limitations
6. Future Directions in Cardiovascular and Renal Systems and Benefits of Other Anti-Diabetes Drugs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aslam, F.; Haque, A.; Haque, J.; Joseph, J. Heart failure in subjects with chronic kidney disease: Best management practices. World J. Cardiol. 2010, 2, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Zoccali, C. Non-proteinuric rather than proteinuric renal diseases are the leading cause of end-stage kidney disease. Nephrol. Dial. Transplant. 2017, 32 (Suppl. S2), ii194–ii199. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef]
- Ziaeian, B.; Fonarow, G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Emberson, J.; Haynes, R.; Herrington, W.G.; Judge, P.; Landray, M.J.; Mayne, K.J.; Ng, S.Y.; Preiss, D.; Roddick, A.J.; et al. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet 2022, 400, 1788–1801. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar]
- Ghezzi, C.; Loo, D.D.F.; Wright, E.M. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 2018, 61, 2087–2097. [Google Scholar] [CrossRef]
- Song, P.; Onishi, A.; Koepsell, H.; Vallon, V. Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert. Opin. Ther. Targets 2016, 20, 1109–1125. [Google Scholar] [CrossRef]
- Banerjee, S.K.; McGaffin, K.R.; Pastor-Soler, N.M.; Ahmad, F. SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc. Res. 2009, 84, 111–118. [Google Scholar] [CrossRef]
- Ferté, L.; Marino, A.; Battault, S.; Bultot, L.; Van Steenbergen, A.; Bol, A.; Cumps, J.; Ginion, A.; Koepsell, H.; Dumoutier, L.; et al. New insight in understanding the contribution of SGLT1 in cardiac glucose uptake: Evidence for a truncated form in mice and humans. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H838–H853. [Google Scholar] [CrossRef] [PubMed]
- Shaffner, J.; Chen, B.; Malhotra, D.K.; Dworkin, L.D.; Gong, R. Therapeutic Targeting of SGLT2: A New Era in the Treatment of Diabetes and Diabetic Kidney Disease. Front. Endocrinol. 2021, 12, 749010. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Baldi, S.; Frascerra, S.; Astiarraga, B.; Barsotti, E.; Clerico, A.; Muscelli, E. Renal Handling of Ketones in Response to Sodium–Glucose Cotransporter 2 Inhibition in Patients With Type 2 Diabetes. Diabetes Care 2017, 40, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Thomson, S.C. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 317–336. [Google Scholar] [CrossRef]
- Lambers Heerspink, H.J.; De Zeeuw, D.; Wie, L.; Leslie, B.; List, J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 853–862. [Google Scholar] [CrossRef]
- Hallow, K.M.; Helmlinger, G.; Greasley, P.J.; McMurray, J.J.V.; Boulton, D.W. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes. Metab. 2018, 20, 479–487. [Google Scholar] [CrossRef]
- Matthews, V.B.; Elliot, R.H.; Rudnicka, C.; Hricova, J.; Herat, L.; Schlaich, M.P. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J. Hypertens. 2017, 35, 2059–2068. [Google Scholar] [CrossRef]
- Jordan, J.; Tank, J.; Heusser, K.; Heise, T.; Wanner, C.; Heer, M.; Macha, S.; Mattheus, M.; Lund, S.S.; Woerle, H.J.; et al. The effect of empagliflozin on muscle sympathetic nerve activity in patients with type II diabetes mellitus. J. Am. Soc. Hypertens. 2017, 11, 604–612. [Google Scholar] [CrossRef]
- Sano, M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J. Cardiol. 2018, 71, 471–476. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef]
- Ait-Aissa, K.; Blaszak, S.C.; Beutner, G.; Tsaih, S.-W.; Morgan, G.; Santos, J.H.; Flister, M.J.; Joyce, D.L.; Camara, A.K.S.; Gutterman, D.D.; et al. Mitochondrial Oxidative Phosphorylation defect in the Heart of Subjects with Coronary Artery Disease. Sci. Rep. 2019, 9, 7623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jaswal, J.S.; Ussher, J.R.; Sankaralingam, S.; Wagg, C.; Zaugg, M.; Lopaschuk, G.D. Cardiac Insulin-Resistance and Decreased Mitochondrial Energy Production Precede the Development of Systolic Heart Failure After Pressure-Overload Hypertrophy. Circ. Heart Fail. 2013, 6, 1039–1048. [Google Scholar] [CrossRef]
- Mori, J.; Basu, R.; McLean, B.A.; Das, S.K.; Zhang, L.; Patel, V.B.; Wagg, C.S.; Kassiri, Z.; Lopaschuk, G.D.; Oudit, G.Y. Agonist-Induced Hypertrophy and Diastolic Dysfunction Are Associated With Selective Reduction in Glucose Oxidation: A Metabolic Contribution to Heart Failure With Normal Ejection Fraction. Circ. Heart Fail. 2012, 5, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.L.; Zhang, L.; Wagg, C.; Al Batran, R.; Gopal, K.; Levasseur, J.; Leone, T.; Dyck, J.R.B.; Ussher, J.R.; Muoio, D.M.; et al. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc. Res. 2019, 115, 1606–1616. [Google Scholar] [CrossRef]
- Stanciu, G.D.; Rusu, R.N.; Bild, V.; Filipiuc, L.E.; Tamba, B.I.; Ababei, D.C. Systemic Actions of SGLT2 Inhibition on Chronic mTOR Activation as a Shared Pathogenic Mechanism between Alzheimer’s Disease and Diabetes. Biomedicines 2021, 9, 576. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Zhang, C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res. Cardiol. 2008, 103, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.M.; Chang, N.C.; Lin, S.Z. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic. Biol. Med. 2017, 104, 298–310. [Google Scholar] [CrossRef]
- Kang, S.; Verma, S.; Hassanabad, A.F.; Teng, G.; Belke, D.D.; Dundas, J.A.; Guzzardi, D.G.; Svystonyuk, D.A.; Pattar, S.S.; Park, D.S.; et al. Direct Effects of Empagliflozin on Extracellular Matrix Remodelling in Human Cardiac Myofibroblasts: Novel Translational Clues to Explain EMPA-REG OUTCOME Results. Can. J. Cardiol. 2020, 36, 543–553. [Google Scholar] [CrossRef]
- Grubić Rotkvić, P.; Cigrovski Berković, M.; Bulj, N.; Rotkvić, L. Minireview: Are SGLT2 inhibitors heart savers in diabetes? Heart Fail. Rev. 2020, 25, 899–905. [Google Scholar] [CrossRef]
- Benetti, E.; Mastrocola, R.; Vitarelli, G.; Cutrin, J.C.; Nigro, D.; Chiazza, F.; Mayoux, E.; Collino, M.; Fantozzi, R. Empagliflozin Protects against Diet-Induced NLRP-3 Inflammasome Activation and Lipid Accumulation. J. Pharmacol. Exp. Ther. 2016, 359, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Byrne, N.J.; Matsumura, N.; Maayah, Z.H.; Ferdaoussi, M.; Takahara, S.; Darwesh, A.M.; Levasseur, J.L.; Jahng, J.W.S.; Vos, D.; Parajuli, N.; et al. Empagliflozin Blunts Worsening Cardiac Dysfunction Associated With Reduced NLRP3 (Nucleotide-Binding Domain-Like Receptor Protein 3) Inflammasome Activation in Heart Failure. Circ. Heart Fail. 2020, 13, e006277. [Google Scholar] [CrossRef] [PubMed]
- Butts, B.; Gary, R.A.; Dunbar, S.B.; Butler, J. The Importance of NLRP3 Inflammasome in Heart Failure. J. Card. Fail. 2015, 21, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.-D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Lim, V.G.; Bell, R.M.; Arjun, S.; Kolatsi-Joannou, M.; Long, D.A.; Yellon, D.M. SGLT2 Inhibitor, Canagliflozin, Attenuates Myocardial Infarction in the Diabetic and Nondiabetic Heart. JACC Basic Transl. Sci. 2019, 4, 15–26. [Google Scholar] [CrossRef]
- Mustroph, J.; Wagemann, O.; Lücht, C.M.; Trum, M.; Hammer, K.P.; Sag, C.M.; Lebek, S.; Tarnowski, D.; Reinders, J.; Perbellini, F.; et al. Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. ESC Heart Fail. 2018, 5, 642–648. [Google Scholar] [CrossRef]
- Trum, M.; Riechel, J.; Wagner, S. Cardioprotection by SGLT2 Inhibitors—Does It All Come Down to Na+? Int. J. Mol. Sci. 2021, 22, 7976. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y. Glucagon regulates lipolysis and fatty acid oxidation through inositol triphosphate receptor 1 in the liver. J. Diabetes Investig. 2021, 12, 32–34. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. N. Engl. J. Med. 2007, 356, 2457–2471. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Kaul, S.; Smith, R.J. The Cardiovascular Safety of Diabetes Drugs—Insights from the Rosiglitazone Experience. N. Engl. J. Med. 2013, 369, 1285–1287. [Google Scholar] [CrossRef]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Azizogli, A.; Vitti, M.R.; Mishra, R.; Osorno, L.; Heffernan, C.; Kumar, V.A. Comparison of SGLT1, SGLT2, and Dual Inhibitor Biological Activity in Treating Type 2 Diabetes Mellitus. Adv. Ther. 2023, 6, 2300143. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2021, 384, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Fitchett, D.; Zinman, B.; Wanner, C.; Lachin, J.M.; Hantel, S.; Salsali, A.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Inzucchi, S.E. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: Results of the EMPA-REG OUTCOME ® trial. Eur. Heart J. 2016, 37, 1526–1534. [Google Scholar] [CrossRef]
- Kato, E.T.; Silverman, M.G.; Mosenzon, O.; Zelniker, T.A.; Cahn, A.; Furtado, R.H.M.; Kuder, J.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; et al. Effect of Dapagliflozin on Heart Failure and Mortality in Type 2 Diabetes Mellitus. Circulation 2019, 139, 2528–2536. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, D.M.; Oduah, M.-T.; Kiebalo, T.; Nartowicz, S.; Bęben, M.; Pochylski, M.; Ciepłucha, A.; Gwizdała, A.; Lesiak, M.; Straburzyńska-Migaj, E. Heart Failure with Preserved Ejection Fraction-a Concise Review. Curr. Cardiol. Rep. 2020, 22, 82. [Google Scholar] [CrossRef]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Junbo Ge, D.P.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin–Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef]
- Butler, J.; Packer, M.; Filippatos, G.; Ferreira, J.P.; Zeller, C.; Schnee, J.; Brueckmann, M.; Pocock, S.J.; Zannad, F.; Anker, S.D. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur. Heart J. 2022, 43, 416–424. [Google Scholar] [CrossRef]
- Mc Causland, F.R.; Claggett, B.L.; Vaduganathan, M.; Desai, A.S.; Jhund, P.; De Boer, R.A.; Docherty, K.; Fang, J.; Hernandez, A.F.; Inzucchi, S.E.; et al. Dapagliflozin and Kidney Outcomes in Patients with Heart Failure with Mildly Reduced or Preserved Ejection Fraction: A Prespecified Analysis of the DELIVER Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Piek, A.; de Boer, R.A.; Silljé, H.H.W. The fibrosis-cell death axis in heart failure. Heart Fail. Rev. 2016, 21, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.Y.; Brooksbank, K.J.M.; Wetherall, K.; Mangion, K.; Roditi, G.; Campbell, R.T.; Berry, C.; Chong, V.; Coyle, L.; Docherty, K.F.; et al. Effect of Empagliflozin on Left Ventricular Volumes in Patients With Type 2 Diabetes, or Prediabetes, and Heart Failure With Reduced Ejection Fraction (SUGAR-DM-HF). Circulation 2021, 143, 516–525. [Google Scholar] [CrossRef]
- Verma, S.; Rawat, S.; Ho, K.L.; Wagg, C.S.; Zhang, L.; Teoh, H.; Dyck, J.E.; Uddin, G.M.; Oudit, G.Y.; Mayoux, E.; et al. Empagliflozin Increases Cardiac Energy Production in Diabetes. JACC Basic Transl. Sci. 2018, 3, 575–587. [Google Scholar] [CrossRef]
- Brown, A.J.M.; Gandy, S.; McCrimmon, R.; Houston, J.G.; Struthers, A.D.; Lang, C.C. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: The DAPA-LVH trial. Eur. Heart J. 2020, 41, 3421–3432. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Vargas-Delgado, A.P.; Requena-Ibanez, J.A.; Garcia-Ropero, A.; Mancini, D.; Pinney, S.; Macaluso, F.; Sartori, S.; Roque, M.; Sabatel-Perez, F.; et al. Randomized Trial of Empagliflozin in Nondiabetic Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2021, 77, 243–255. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; Antonio, R.S.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Flores, E.; Garcia-Ropero, A.; Sanz, J.; Hajjar, R.J.; et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J. Am. Coll. Cardiol. 2019, 73, 1931–1944. [Google Scholar] [CrossRef]
- Hundertmark, M.J.; Adler, A.; Antoniades, C.; Coleman, R.; Griffin, J.L.; Holman, R.R.; Lamlum, H.; Lee, J.; Massey, D.; Miller, J.J.; et al. Assessment of Cardiac Energy Metabolism, Function, and Physiology in Patients With Heart Failure Taking Empagliflozin: The Randomized, Controlled EMPA-VISION Trial. Circulation 2023, 147, 1654–1669. [Google Scholar] [CrossRef]
- Carluccio, E.; Biagioli, P.; Reboldi, G.; Mengoni, A.; Lauciello, R.; Zuchi, C.; D’addario, S.; Bardelli, G.; Ambrosio, G. Left ventricular remodeling response to SGLT2 inhibitors in heart failure: An updated meta-analysis of randomized controlled studies. Cardiovasc. Diabetol. 2023, 22, 235. [Google Scholar] [CrossRef]
- Sachdeva, P.; Kaur, K.; Fatima, S.; Mahak, F.N.; Noman, M.; Siddenthi, S.M.; Surksha, M.A.; Munir, M.; Fatima, F.N.; Sultana, S.S.; et al. Advancements in myocardial infarction management: Exploring novel approaches and strategies. Cureus 2023, 15, e45578. [Google Scholar] [CrossRef] [PubMed]
- von Lewinski, D.; Kolesnik, E.; Tripolt, N.J.; Pferschy, P.N.; Benedikt, M.; Wallner, M.; Alber, H.; Berger, R.; Lichtenauer, M.; Saely, C.H.; et al. Empagliflozin in acute myocardial infarction: The EMMY trial. Eur. Heart J. 2022, 43, 4421–4432. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Erlinge, D.; Storey, R.F.; McGuire, D.K.; de Belder, M.; Eriksson, N.; Andersen, K.; Austin, D.; Arefalk, G.; Carrick, D.; et al. Dapagliflozin in Myocardial Infarction without Diabetes or Heart Failure. NEJM Evid. 2024, 3, EVIDoa2300286. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Jones, W.S.; Udell, J.A.; Anker, S.D.; Petrie, M.C.; Harrington, J.; Mattheus, M.; Zwiener, I.; Amir, O.; Bahit, M.C.; et al. Empagliflozin after Acute Myocardial Infarction. N. Engl. J. Med. 2024, 390, 1455–1466. [Google Scholar] [CrossRef]
- Myhre, P.L.; Vaduganathan, M.; Claggett, B.L.; Miao, Z.M.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; et al. Influence of NT-proBNP on Efficacy of Dapagliflozin in Heart Failure With Mildly Reduced or Preserved Ejection Fraction. JACC Heart Fail. 2022, 10, 902–913. [Google Scholar] [CrossRef]
- Tamaki, S.; Yamada, T.; Watanabe, T.; Morita, T.; Furukawa, Y.; Kawasaki, M.; Kikuchi, A.; Kawai, T.; Seo, M.; Abe, M.; et al. Effect of Empagliflozin as an Add-On Therapy on Decongestion and Renal Function in Patients With Diabetes Hospitalized for Acute Decompensated Heart Failure: A Prospective Randomized Controlled Study. Circ. Heart Fail. 2021, 14, e007048. [Google Scholar] [CrossRef]
- Pérez-Belmonte, L.M.; Sanz-Cánovas, J.; Millán-Gómez, M.; Osuna-Sánchez, J.; López-Sampalo, A.; Ricci, M.; Jiménez-Navarro, M.; López-Carmona, M.D.; Bernal-López, M.R.; Barbancho, M.A.; et al. Clinical benefits of empagliflozin in very old patients with type 2 diabetes hospitalized for acute heart failure. J. Am. Geriatr. Soc. 2022, 70, 862–871. [Google Scholar] [CrossRef]
- Ueda, T.; Kasama, S.; Yamamoto, M.; Nakano, T.; Ueshima, K.; Morikawa, Y.; Kawata, H.; Yoshihisa, A.; Nakayama, M.; Komatsu, S.; et al. Effect of the Sodium-Glucose Cotransporter 2 Inhibitor Canagliflozin for Heart Failure With Preserved Ejection Fraction in Patients With Type 2 Diabetes. Circ. Rep. 2021, 3, 440–448. [Google Scholar] [CrossRef]
- Phrommintikul, A.; Wongcharoen, W.; Kumfu, S.; Jaiwongkam, T.; Gunaparn, S.; Chattipakorn, S.; Chattipakorn, N. Effects of dapagliflozin vs. vildagliptin on cardiometabolic parameters in diabetic patients with coronary artery disease: A randomised study. Br. J. Clin. Pharmacol. 2019, 85, 1337–1347. [Google Scholar] [CrossRef]
- Griffin, M.; Rao, V.S.; Ivey-Miranda, J.; Fleming, J.; Mahoney, D.; Maulion, C.; Suda, N.; Siwakoti, K.; Ahmad, T.; Jacoby, D.; et al. Empagliflozin in Heart Failure: Diuretic and Cardiorenal Effects. Circulation 2020, 142, 1028–1039. [Google Scholar] [CrossRef]
- Sezai, A.; Sekino, H.; Unosawa, S.; Taoka, M.; Osaka, S.; Tanaka, M. Canagliflozin for Japanese patients with chronic heart failure and type II diabetes. Cardiovasc. Diabetol. 2019, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Uzu, K.; Hashimoto, N.; Onishi, T.; Takaya, T.; Shimane, A.; Taniguchi, Y.; Yasaka, Y.; Ohara, T.; Kawai, H. Empagliflozin’s Ameliorating Effect on Plasma Triglycerides: Association with Endothelial Function Recovery in Diabetic Patients with Coronary Artery Disease. J. Atheroscler. Thromb. 2020, 27, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Sposito, A.C.; Breder, I.; Soares, A.A.S.; Kimura-Medorima, S.T.; Munhoz, D.B.; Cintra, R.M.R.; Bonilha, I.; Oliveira, D.C.; Breder, J.C.; Cavalcante, P.; et al. Dapagliflozin effect on endothelial dysfunction in diabetic patients with atherosclerotic disease: A randomized active-controlled trial. Cardiovasc. Diabetol. 2021, 20, 74. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Mazer, C.D.; Yan, A.T.; Mason, T.; Garg, V.; Teoh, H.; Zuo, F.; Quan, A.; Farkouh, M.E.; Fitchett, D.H.; et al. Effect of Empagliflozin on Left Ventricular Mass in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation 2019, 140, 1693–1702. [Google Scholar] [CrossRef]
- Damman, K.; Beusekamp, J.C.; Boorsma, E.M.; Swart, H.P.; Smilde, T.D.; Elvan, A.; van Eck, J.M.; Heerspink, H.J.; Voors, A.A. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur. J. Heart Fail. 2020, 22, 713–722. [Google Scholar] [CrossRef]
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Singh, J.S.; Mordi, I.R.; Vickneson, K.; Fathi, A.; Donnan, P.T.; Mohan, M.; Choy, A.M.J.; Gandy, S.; George, J.; Khan, F.; et al. Dapagliflozin Versus Placebo on Left Ventricular Remodeling in Patients With Diabetes and Heart Failure: The REFORM Trial. Diabetes Care 2020, 43, 1356–1359. [Google Scholar] [CrossRef]
- Górriz, J.L.; Nieto, J.; Navarro-González, J.F.; Molina, P.; Martínez-Castelao, A.; Pallardó, L.M. Nephroprotection by Hypoglycemic Agents: Do We Have Supporting Data? J. Clin. Med. 2015, 4, 1866–1889. [Google Scholar] [CrossRef]
- Tanaka, A.; Hisauchi, I.; Taguchi, I.; Sezai, A.; Toyoda, S.; Tomiyama, H.; Sata, M.; Ueda, S.; Oyama, J.; Kitakaze, M.; et al. Effects of canagliflozin in patients with type 2 diabetes and chronic heart failure: A randomized trial (CANDLE). ESC Heart Fail. 2020, 7, 1585–1594. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.; Bonaca, M.P.; Mosenzon, O.; Kato, E.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Shiau, C.-H.; Tsau, L.-Y.; Kao, C.-C.; Peng, Y.-C.; Bai, C.-H.; Wu, J.; Hou, W.-H. Efficacy and safety of sodium-glucose cotransporter-2 inhibitors in patients with chronic kidney disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2023, 56, 1359–1381. [Google Scholar] [CrossRef]
- Allegretti, A.S.; Zhang, W.; Zhou, W.; Thurber, T.K.; Rigby, S.P.; Bowman-Stroud, C.; Trescoli, C.; Serusclat, P.; Freeman, M.W.; Halvorsen, Y.-D.C. Safety and Effectiveness of Bexagliflozin in Patients With Type 2 Diabetes Mellitus and Stage 3a/3b CKD. Am. J. Kidney Dis. 2019, 74, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Fioretto, P.; Del Prato, S.; Buse, J.B.; Goldenberg, R.; Giorgino, F.; Reyner, D.; Langkilde, A.M.; Sjöström, C.D.; Sartipy, P.; On Behalf of the DERIVE Study Investigators. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): The DERIVE Study. Diabetes Obes. Metab. 2018, 20, 2532–2540. [Google Scholar] [CrossRef] [PubMed]
- Cherney, D.Z.I.; Dekkers, C.C.J.; Barbour, S.J.; Cattran, D.; Gafor, A.H.A.; Greasley, P.J.; Laverman, G.D.; Lim, S.K.; Di Tanna, G.L.; Reich, H.N.; et al. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): A randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol. 2020, 8, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; Von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Wada, T.; Mori-Anai, K.; Takahashi, A.; Matsui, T.; Inagaki, M.; Iida, M.; Maruyama, K.; Tsuda, H. Effect of canagliflozin on the decline of estimated glomerular filtration rate in chronic kidney disease patients with type 2 diabetes mellitus: A multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase III study in Japan. J. Diabetes Investig. 2022, 13, 1981–1989. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Braunwald, E. Mechanisms of Cardiorenal Effects of Sodium-Glucose Cotransporter 2 Inhibitors. J. Am. Coll. Cardiol. 2020, 75, 422–434. [Google Scholar] [CrossRef]
- Bolinder, J.; Ljunggren, Ö.; Kullberg, J.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sugg, J.; Parikh, S. Effects of Dapagliflozin on Body Weight, Total Fat Mass, and Regional Adipose Tissue Distribution in Patients with Type 2 Diabetes Mellitus with Inadequate Glycemic Control on Metformin. J. Clin. Endocrinol. Metab. 2012, 97, 1020–1031. [Google Scholar] [CrossRef]
- Ansary, T.M.; Nakano, D.; Nishiyama, A. Diuretic Effects of Sodium Glucose Cotransporter 2 Inhibitors and Their Influence on the Renin-Angiotensin System. Int. J. Mol. Sci. 2019, 20, 629. [Google Scholar] [CrossRef]
- Ghanim, H.; Abuaysheh, S.; Hejna, J.; Green, K.; Batra, M.; Makdissi, A.; Chaudhuri, A.; Dandona, P. Dapagliflozin Suppresses Hepcidin And Increases Erythropoiesis. J. Clin. Endocrinol. Metab. 2020, 105, e1056–e1063. [Google Scholar] [CrossRef]
- Aberle, J.; Menzen, M.; Schmid, S.M.; Terkamp, C.; Jaeckel, E.; Rohwedder, K.; Scheerer, M.F.; Xu, J.; Tang, W.; Birkenfeld, A.L. Dapagliflozin effects on haematocrit, red blood cell count and reticulocytes in insulin-treated patients with type 2 diabetes. Sci. Rep. 2020, 10, 22396. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhu, D.; Wang, S.; Jiang, A.; Li, F. Dapagliflozin Attenuates Cardiac Remodeling in Mice Model of Cardiac Pressure Overload. Am. J. Hypertens. 2019, 32, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Khouri, C.; Cracowski, J.; Roustit, M. SGLT-2 inhibitors and the risk of lower-limb amputation: Is this a class effect? Diabetes Obes. Metab. 2018, 20, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Hicks, D.; Patel, D.; Patel, V.; McEwan, P.; Dashora, U. Optimising the Benefits of SGLT2 Inhibitors for Type 1 Diabetes. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2020, 11, 37–52. [Google Scholar]
- Ujjawal, A.; Schreiber, B.; Verma, A. Sodium-glucose cotransporter-2 inhibitors (SGLT2i) in kidney transplant recipients: What is the evidence? Ther. Adv. Endocrinol. Metab. 2022, 13, 20420188221090001. [Google Scholar] [CrossRef]
- Keller, D.M.; Ahmed, N.; Tariq, H.; Walgamage, M.; Walgamage, T.; Mohammed, A.; Chou, J.T.-T.; Kałużna-Oleksy, M.; Lesiak, M.; Straburzyńska-Migaj, E. SGLT2 Inhibitors in Type 2 Diabetes Mellitus and Heart Failure-A Concise Review. J. Clin. Med. 2022, 11, 1470. [Google Scholar] [CrossRef]
- Kolterman, O.G.; Kim, D.D.; Shen, L.; Ruggles, J.A.; Nielsen, L.L.; Fineman, M.S.; Baron, A.D. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am. J. Health Syst. Pharm. 2005, 62, 173–181. [Google Scholar] [CrossRef]
- Rojano Toimil, A.; Ciudin, A. GLP-1 Receptor Agonists in Diabetic Kidney Disease: From Physiology to Clinical Outcomes. J. Clin. Med. 2021, 10, 3955. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Mann, J.F.; Ørsted, D.D.; Brown-Frandsen, K.; Marso, S.P.; Poulter, N.R.; Rasmussen, S.; Tornøe, K.; Zinman, B.; Buse, J.B. Liraglutide and Renal Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 839–848. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Joubert, M.; Jagu, B.; Montaigne, D.; Marechal, X.; Tesse, A.; Ayer, A.; Dollet, L.; Le May, C.; Toumaniantz, G.; Manrique, A.; et al. The Sodium–Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Cardiomyopathy in a Diabetic Lipodystrophic Mouse Model. Diabetes 2017, 66, 1030–1040. [Google Scholar] [CrossRef]
- Apperloo, E.M.; Neuen, B.L.; Fletcher, R.A.; Jongs, N.; Anker, S.D.; Bhatt, D.L.; Butler, J.; Cherney, D.Z.I.; Herrington, W.G.; Inzucchi, S.E.; et al. Efficacy and safety of SGLT2 inhibitors with and without glucagon-like peptide 1 receptor agonists: A SMART-C collaborative meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2024, 12, 545–557. [Google Scholar] [CrossRef]
| Drug | Daily Dose (mg) | Selectivity (SGLT2:SGLT1) | Bioavailability (%) | Half-Life (h) | Excretion |
|---|---|---|---|---|---|
| Empaglifozin | 10 or 25 | 1:2500–2700 | 90–97 (mice) 89 (dogs) 31 (rats) | 13.2 (10 mg) 13.3 (25 mg) | Glucuronidation Excretion fecal (41%) and renal (54%). |
| Dapaglifozin | 10 | 1:1200 | 78 | 12.9 | Glucuronidation Excretion fecal (21%) and renal (75%). |
| Canaglifozin | 100 or 300 | 1:250–414 | 65 | 10.6 (100 mg) 13.1 (300 mg) | Glucuronidation Excretion fecal (42%) and renal (33%). |
| Ertuglifozin | 5 or 15 | 1:2200 | 70–90 | 12.2 | Glucuronidation (86%) Excretion fecal (50%) and renal (41%). |
| Sotaglifozin | 800 | 1:20 | 71 | 35 | Glucuronidation (94%) Excretion fecal (37%) and renal (57%). |
| Ipraglifozin | 50 | 1:360 | 90 | 15–16 | Glucuronidation (92%) Excretion fecal (27%) and renal (73%). |
| Luseoglifozin | 2.5 or 5 | 1:1650 | 35.3 (male rats) 58.2 (female rats) 92.7 (male dogs) | 9.24 | Glucuronidation Excretion fecal (31%) and renal (69%). |
| Tofoglifozin | 10 | 1:2900 | 97.5 | 6.8 | Glucuronidation (90%) Excretion fecal (22%) and renal (78%). |
| Bexagliflozin | 20 | 1:2435 | 93% | 12 | Glucuronidation Excretion fecal (51%) and renal (40.5%). |
| Study | Drug | Dose | Population | Primary CV Outcome | HR(95% CI) |
|---|---|---|---|---|---|
| Canvas [7] | Canaglifozin | 300/100 | DM2 and high CV risk | 14% reduction in 3-point MACE (CV death, non-fatal MI, non-fatal stroke) | 0.86 (0.75–0.97) |
| Credence [10] | Canaglifozin | 300/100 | DM2 and high CV risk | 30% reduction in composite outcome (doubling serum creatinine, ESRD, or renal/CV death) | 0.70 (0.59–0.82) |
| DAPA-HF [14] | Dapaglifozin | 10 | HFrEF | 26% reduction the primary outcome (CV death, HHF) | 0.74 (0.65–0.85) |
| DAPA-MI [73] | Dapaglifozin | 10 | MI and HFpEF | 5%-no reduction in composite outcome (CV death or HHF) | 0.95 (0.64–1.40) |
| DAPA-LVH [66] | Dapaglifozin | 10 | DM2 and LV hypertrophy | Reduction of LV hypertrophy | NA |
| Declare-TIMI [8] | Dapaglifozin | 10 | DM2 and high CV risk | 17% reduction in 3-point MACE (CV death, non-fatal MI, non-fatal stroke) | 0.83 (0.73–0.95) |
| Deliver [61] | Dapaglifozin | 10 | HFpEF | 18% reduction the primary outcome (CV death, HHF) | 0.82 (0.73–0.92) |
| Emmy [72] | Empaglifozin | 10 | MI | Reduction in NT-pro-BNP and LV echocardiographic parameters | NA |
| Empact-MI [74] | Empaglifozin | 10 | MI | 10% reduction of composite outcome (death from any cause or HHF) | 0.90 (0.76–1.06) |
| Empareg-outcome [9] | Empaglifozin | 25/10 | DM2 and high CV risk | 14% reduction in 3-point MACE (CV death, non-fatal MI, non-fatal stroke) | 0.86 (0.74–0.99) |
| Empa-Heart Cardio-link 6 [84] | Empaglifozin | 10 | DM and CAD | Reduction in LV mass index | NA |
| Empa-response AHF [85] | Empaglifozin | 10 | AHF | reduction of composite outcome (progression of kidney disease and CV death) | NA |
| Empa-kidney [86] | Empaglifozin | 10 | CKD | 28% reduction of composite (progression of kidney disease and CV death) | 0.72 (0.64–0.82) |
| Empa tropism [67] | Empaglifozin | 10 | HFrEF | Improvement of LV parameters | NA |
| Emperor-Reduced [11] | Empaglifozin | 10 | HFrEF | 25% Reduction in composite outcome (CV death or HHF); 30% reduction HHF | 0.75 (0.65–0.86) for primary composite outcome 0.70 (0.58–0.85) for HHF |
| Emperor-Preserved [12] | Empaglifozin | 10 | HFpEF | 21% Reduction in composite outcome (CV death or HHF); 30% reduction HHF | 0.79 (0.69–0.90) for primary composite outcome 0.70 (0.59–0.83) for HHF |
| Empulse [62] | Empaglifozin | 10 | AHF | 36% reduction in composite outcome (death from any cause, number of HF events) | 1.75 (1.37–2.23) |
| Reform [87] | Dapaglifozin | 10 | HF | No change in LV CMR parameters | NA |
| Scored [54] | Sotaglifozin | 200/400 | DM2, CKD and high CV risk | 16% reduction in composite (CV death or HHF) | 0.84 (0.72–0.99) |
| Soloist-WHF [53] | Sotaglifozin | 200/400 | DM2 and HF | 33% reduction in composite (CV death or HHF) | 0.67 (0.52–0.85) |
| Sugar-DM [64] | Empaglifozin | 10 | DM2 and HFrEF | Reduction of LV volumes | NA |
| Vertis-CV [51] | Ertuglifozin | 15/5 | DM2 and high CV risk | 13%-No significant difference in 3-point MACE (CV death, non-fatal MI, non-fatal stroke) | 0.87 (0.70–1.1) |
| Study | Drug | Dose | eGFR (mL/min/1.73 m2) | UACR (mg/g) | Primary Renal Outcome | HR (95% CI) |
|---|---|---|---|---|---|---|
| Bexagliflozin study [92] | Bexaglifozin | 20 | 30–59 | >300 | 63% reduction in eGFR decline and UAR | 0.37 (0.20–0.54) for eGFR 30–60 |
| Canvas [7] | Canaglifozin | 300/100 | 30–59 | >300 | 40% reduction in eGFR, renal-replacement therapy, or renal death | 0.73 (0.67–0.79) for albuminuria 0.60 (0.47–0.77) for reduction in eGFR, renal-replacement therapy, or renal death |
| Credence [10] | Canaglifozin | 300/100 | 30–59 | >300 | 30% reduction in composite outcome (doubling of serum creatinine, ESRD, or death) | 0.70 (0.59–0.82) |
| DAPA-CKD [15] | Dapaglifozin | 10 | 25–45 | >1000 | 44% reduction in composite outcome (worsening renal function, transplant, death) | 0.56 (0.45–0.68) |
| DAPA-HF [14] | Dapaglifozin | 10 | 30–59 | NA | 29% reduction in eGFR decline, ESKD, or renal death | 0.71 (0.44–1.16) |
| Declare-TIMI [8] | Dapaglifozin | 10 | <60 | >300 | 40% reduction in eGFR decline, ESKD, or renal death | 0.53 (0.43–0.66) |
| Deliver [61] | Dapaglifozin | 10 | 30–59 | NA | No significant reduction in renal composite outcome | 1.08 (0.79–1-49) |
| Derive [93] | Dapaglifozin | 10 | 30–59 | 30 >1000 | No significant change in UAR or eGFR | NA |
| Diamond [94] | Dapaglifozin | 10 | >25 | 30–3500 | No significant change in 24-h proteinuria and eGFR | NA |
| Empareg-outcome [95] | Empaglifozin | 25/10 | 30–60 | 30–300 | 39% reduction of incident or worsening nephropathy 46% reduction of post hoc renal composite outcome (a doubling of the serum creat-inine level, the initiation of renal-replacement therapy, or death from renal disease) | 0.61 (0.53–0.70) 0.54 (0.40–0.75) |
| Empa-kidney [86] | Empaglifozin | 10 | 20–89 | 30–1000 | 28% reduction in progression of kidney disease or cardiovascular outcomes | 0.72 (0.64–0.82) |
| Emperor-Reduced [11] | Empaglifozin | 10 | 20–59 | >300 | 30% reduction in composite renal outcome (chronic dialysis, RRT, sustained ≥40% eGFR reduction) | 0.70 (0.32–0.77) |
| Emperor-Preserved [12] | Empaglifozin | 10 | 30–60 | >300 | 5%-No significant effect on major renal outcomes | 0.95 (0.73–1.24) |
| Scored [54] | Sotaglifozin | 200/400 | 25–59 | >300 | 29% reduction in composite renal outcome (≥50% GFR decline, ESKD, renal death) | 0.71 (0.46–1.08) |
| Vertis-CV [51] | Ertuglifozin | 15/5 | 30–59 | >300 | 19% reduction in composite renal outcome (doubling of serum creatinine, renal dialysis or transplantation, or renal death, sustained ≥40% GFR reduction, Progression or regression of albuminuria and change) | 0.81 (0.63–1.04) |
| Wada et al. [96] | Canaglifozin | 100 | 30–89 | NA | 40% reduction in eGFR, renal or cardiovascular death | 0.60 (0.23–1.55) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cersosimo, A.; Drera, A.; Adamo, M.; Metra, M.; Vizzardi, E. Exploring the Cardiorenal Benefits of SGLT2i: A Comprehensive Review. Kidney Dial. 2024, 4, 184-202. https://doi.org/10.3390/kidneydial4040016
Cersosimo A, Drera A, Adamo M, Metra M, Vizzardi E. Exploring the Cardiorenal Benefits of SGLT2i: A Comprehensive Review. Kidney and Dialysis. 2024; 4(4):184-202. https://doi.org/10.3390/kidneydial4040016
Chicago/Turabian StyleCersosimo, Angelica, Andrea Drera, Marianna Adamo, Marco Metra, and Enrico Vizzardi. 2024. "Exploring the Cardiorenal Benefits of SGLT2i: A Comprehensive Review" Kidney and Dialysis 4, no. 4: 184-202. https://doi.org/10.3390/kidneydial4040016
APA StyleCersosimo, A., Drera, A., Adamo, M., Metra, M., & Vizzardi, E. (2024). Exploring the Cardiorenal Benefits of SGLT2i: A Comprehensive Review. Kidney and Dialysis, 4(4), 184-202. https://doi.org/10.3390/kidneydial4040016






