The Two Levels of Podocyte Dysfunctions Induced by Apolipoprotein L1 Risk Variants
Abstract
1. Introduction: APOL1 Secretion, a Unique Feature in the APOL Family
2. APOL1 Structure and Interactions
3. Intracellular APOL1 Function: Vesicular Trafficking Control
4. Extracellular APOL1 Function: Specific Innate Immunity
5. Intracellular Variants: Kidney Disease Hit 1
6. Extracellular Variants: Kidney Disease Hit 2
7. Hit 2 Results from Low HDL-C/High APOL1 Ratio
8. Evolutionary Considerations: From Apoptosis Control to Apoptotic Toxin Building
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Duchateau, P.N.; Pullinger, C.R.; Orellana, R.E.; Kunitake, S.T.; Naya-Vigne, J.; O’Connor, P.M.; Malloy, M.J.; Kane, J.P. Apolipoprotein L, a new human high density lipoprotein apolipoprotein expressed by the pancreas. Identification, cloning, characterization, and plasma distribution of apolipoprotein L. J. Biol. Chem. 1997, 272, 25576–25582. [Google Scholar] [CrossRef]
- Pays, E. The function of Apolipoproteins L (APOLs): Relevance for kidney disease, neurotransmission disorders, cancer and viral infection. FEBS J. 2021, 288, 360–381. [Google Scholar] [CrossRef]
- Pays, E. The Janus-faced functions of Apolipoproteins L in membrane dynamics. Cell. Mol. Life Sci. 2024, 81, 134. [Google Scholar] [CrossRef]
- Pays, E.; Vanhollebeke, B.; Uzureau, P.; Lecordier, L.; Pérez-Morga, D. The molecular arms race between African trypanosomes and humans. Nat. Rev. Microbiol. 2014, 12, 575–584. [Google Scholar] [CrossRef]
- Nichols, B.; Jog, P.; Lee, J.H.; Blackler, D.; Wilmot, M.; D’Agati, V.; Markowitz, G.; Kopp, J.B.; Alper, S.L.; Pollak, M.R.; et al. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int. 2015, 87, 332–342. [Google Scholar] [CrossRef]
- Uzureau, S.; Coquerelle, C.; Vermeiren, C.; Uzureau, P.; Van Acker, A.; Pilotte, L.; Monteyne, D.; Acolty, V.; Vanhollebeke, B.; Van den Eynde, B.; et al. Apolipoproteins L control cell death triggered by TLR3/TRIF signaling in dendritic cells. Eur. J. Immunol. 2016, 46, 1854–1866. [Google Scholar] [CrossRef]
- Uzureau, S.; Lecordier, L.; Uzureau, P.; Hennig, D.; Graversen, J.H.; Homblé, F.; Mfutu, P.E.; Oliveira Arcolino, F.; Ramos, A.R.; La Rovere, R.M.; et al. APOL1 C-terminal variants may trigger kidney disease through interference with APOL3 control of actomyosin. Cell Rep. 2020, 30, 3821–3836. [Google Scholar] [CrossRef]
- Vanwalleghem, G.; Fontaine, F.; Lecordier, L.; Tebabi, P.; Klewe, K.; Nolan, D.P.; Yamaryo-Botté, Y.; Botté, C.; Kremer, A.; Burkard, G.S.; et al. Coupling of lysosomal and mitochondrial membrane permeabilization in trypanolysis by APOL1. Nat. Commun. 2015, 6, 8078. [Google Scholar] [CrossRef]
- Xong, H.V.; Vanhamme, L.; Chamekh, M.; Chimfwembe, C.E.; Van Den Abbeele, J.; Pays, A.; Van Meirvenne, N.; Hamers, R.; De Baetselier, P.; Pays, E. A VSG expression site-associated gene confers resistance to human serum in Trypanosoma rhodesiense. Cell 1998, 95, 839–846. [Google Scholar] [CrossRef]
- Vanhamme, L.; Paturiaux-Hanocq, F.; Poelvoorde, P.; Nolan, D.P.; Lins, L.; Van Den Abbeele, J.; Pays, A.; Tebabi, P.; Van Xong, H.; Jacquet, A.; et al. Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature 2003, 422, 83–87. [Google Scholar] [CrossRef]
- Lecordier, L.; Vanhollebeke, B.; Poelvoorde, P.; Tebabi, P.; Paturiaux-Hanocq, F.; Andris, F.; Lins, L.; Pays, E. C-terminal mutants of apolipoprotein L-I efficiently kill both Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense. PLoS Pathog. 2009, 5, e1000685. [Google Scholar] [CrossRef][Green Version]
- Genovese, G.; Friedman, D.J.; Ross, M.D.; Lecordier, L.; Uzureau, P.; Freedman, B.I.; Bowden, D.W.; Langefeld, C.D.; Oleksyk, T.K.; Uscinski Knob, A.L.; et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010, 329, 841–845. [Google Scholar] [CrossRef]
- Beckerman, P.; Bi-Karchin, J.; Park, A.S.; Qiu, C.; Dummer, P.D.; Soomro, I.; Boustany-Kari, C.M.; Pullen, S.S.; Miner, J.H.; Hu, C.A.; et al. Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat. Med. 2017, 23, 429–438. [Google Scholar] [CrossRef]
- Daneshpajouhnejad, P.; Kopp, J.B.; Winkler, C.A.; Rosenberg, A.Z. The evolving story of apolipoprotein L1 nephropathy: The end of the beginning. Nat. Rev. Nephrol. 2022, 18, 307–320. [Google Scholar] [CrossRef]
- Kopp, J.B.; Heymann, J.; Winkler, C.A. APOL1 renal risk variants: Fertile soil for HIV-associated nephropathy. Semin. Nephrol. 2017, 37, 514–519. [Google Scholar] [CrossRef]
- Skorecki, K.L.; Lee, J.H.; Langefeld, C.D.; Rosset, S.; Tzur, S.; Wasser, W.G.; Shemer, R.; Hawkins, G.A.; Divers, J.; Parekh, R.S.; et al. A null variant in the apolipoprotein L3 gene is associated with non-diabetic nephropathy. Nephrol. Dial. Transplant. 2018, 33, 323–330. [Google Scholar] [CrossRef]
- Lecordier, L.; Heo, P.; Graversen, J.H.; Hennig, D.; Skytthe, M.K.; Cornet d’Elzius, A.; Pincet, F.; Pérez-Morga, D.; Pays, E. Apolipoproteins L1 and L3 control mitochondrial membrane dynamics. Cell Rep. 2023, 42, 113528. [Google Scholar] [CrossRef]
- Ultsch, M.; Holliday, M.J.; Gerhardy, S.; Moran, P.; Scales, S.J.; Gupta, N.; Oltrabella, F.; Chiu, C.; Fairbrother, W.; Eigenbrot, C.; et al. Structures of the ApoL1 and ApoL2 N-terminal domains reveal a non-classical four-helix bundle motif. Commun. Biol. 2021, 4, 916. [Google Scholar] [CrossRef]
- Fontaine, F.; Lecordier, L.; Vanwalleghem, G.; Uzureau, P.; Van Reet, N.; Fontaine, M.; Tebabi, P.; Vanhollebeke, B.; Büscher, P.; Pérez-Morga, D.; et al. APOLs with low pH dependence can kill all African trypanosomes. Nat. Microbiol. 2017, 2, 1500–1506. [Google Scholar] [CrossRef]
- Pérez-Morga, D.; Vanhollebeke, B.; Paturiaux-Hanocq, F.; Nolan, D.P.; Lins, L.; Homblé, F.; Vanhamme, L.; Tebabi, P.; Pays, A.; Poelvoorde, P.; et al. Apolipoprotein L-I promotes trypanosome lysis by forming pores in lysosomal membranes. Science 2005, 309, 469–472. [Google Scholar] [CrossRef]
- Kreit, M.; Vertommen, D.; Gillet, L.; Michiels, T. The interferon-inducible mouse Apolipoprotein L9 and prohibitins cooperate to restrict Theiler’s virus replication. PLoS ONE 2015, 10, e0133190. [Google Scholar] [CrossRef]
- Wei, Y.; Chiang, W.C.; Sumpter, R.; Jr Mishra, P.; Levine, B. Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell 2017, 168, 224–238.e101. [Google Scholar] [CrossRef]
- Vanhollebeke, B.; Truc, P.; Poelvoorde, P.; Pays, A.; Joshi, P.P.; Katti, R.; Jannin, J.G.; Pays, E. Human Trypanosoma evansi infection linked to a lack of apolipoprotein L-I. N. Engl. J. Med. 2006, 355, 2752–2756. [Google Scholar] [CrossRef]
- Johnstone, D.B.; Shegokar, V.; Nihalani, D.; Rathore, Y.S.; Mallik, L.; Ashish Zare, V.; Ikizler, H.O.; Powar, R.; Holzman, L.B. APOL1 null alleles from a rural village in India do not correlate with glomerulosclerosis. PLoS ONE 2012, 7, e51546. [Google Scholar] [CrossRef]
- Dippold, H.C.; Ng, M.M.; Farber-Katz, S.E.; Lee, S.K.; Kerr, M.L.; Peterman, M.C.; Sim, R.; Wiharto, P.A.; Galbraith, K.A.; Madhavarapu, S.; et al. GOLPH3 bridges phosphatidylinositol-4-phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell 2009, 139, 337–351. [Google Scholar] [CrossRef]
- Hansen, M.D.; Johnsen, I.B.; Stiberg, K.A.; Sherstova, T.; Wakita, T.; Richard, G.M.; Kandasamy, R.K.; Meurs, E.F.; Anthonsen, M.W. Hepatitis C virus triggers Golgi fragmentation and autophagy through the immunity-related GTPase M. Proc. Natl. Acad. Sci. USA 2017, 114, E3462–E3471. [Google Scholar] [CrossRef]
- Nagashima, S.; Tábara, L.C.; Tilokani, L.; Paupe, V.; Anand, H.; Pogson, J.H.; Zunino, R.; McBride, H.M.; Prudent, J. Golgi-derived PI(4)P-containing vesicles drive late steps of mitochondrial division. Science 2020, 367, 1366–1371. [Google Scholar] [CrossRef]
- Yamashita, S.I.; Jin, X.; Furukawa, K.; Hamasaki, M.; Nezu, A.; Otera, H.; Saigusa, T.; Yoshimori, T.; Sakai, Y.; Mihara, K.; et al. Mitochondrial division occurs concurrently with autophagosome formation but independently of Drp1 during mitophagy. J. Cell Biol. 2016, 215, 649–665. [Google Scholar] [CrossRef]
- Tang, H.W.; Wang, Y.B.; Wang, S.L.; Wu, M.H.; Lin, S.Y.; Chen, G.C. Atg1-mediated myosin II activation regulates autophagosome formation during starvation-induced autophagy. EMBO J. 2011, 30, 636–651. [Google Scholar] [CrossRef]
- Judith, D.; Jefferies HB, J.; Boeing, S.; Frith, D.; Snijders, A.P.; Tooze, S.A. ATG9A shapes the forming autophagosome through Arfaptin 2 and phosphatidylinositol 4-kinase IIIβ. J. Cell Biol. 2019, 218, 1634–1652. [Google Scholar] [CrossRef]
- Thomson, R.; Finkelstein, A. Human trypanolytic factor APOL1 forms pH-gated cation-selective channels in planar lipid bilayers: Relevance to trypanosome lysis. Proc. Natl. Acad. Sci. USA 2015, 112, 2894–2899. [Google Scholar] [CrossRef]
- Bruno, J.; Edwards, J.C. Kidney-disease-associated variants of Apolipoprotein L1 show gain of function in cation channel activity. J. Biol. Chem. 2021, 296, 100238. [Google Scholar] [CrossRef]
- Genes, C.M.; de Lucio, H.; González, V.M.; Sánchez-Murcia, P.A.; Rico, E.; Gago, F.; Fasel, N.; Jiménez-Ruiz, A. A functional BH3 domain in an aquaporin from Leishmania infantum. Cell Death Discov. 2016, 2, 16043. [Google Scholar] [CrossRef]
- Gupta, N.; Waas, B.; Austin, D.; De Mazière, A.M.; Kujala, P.; Stockwell, A.D.; Li, T.; Yaspan, B.L.; Klumperman, J.; Scales, S.J. Apolipoprotein L1 (APOL1) renal risk variant-mediated podocyte cytotoxicity depends on African haplotype and surface expression. Sci. Rep. 2024, 14, 3765. [Google Scholar] [CrossRef]
- Annis, M.G.; Soucie, E.L.; Dlugosz, P.J.; Cruz-Aguado, J.A.; Penn, L.Z.; Leber, B.; Andrews, D.W. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005, 24, 2096–2103. [Google Scholar] [CrossRef]
- Uren, R.T.; Iyer, S.; Kluck, R.M. Pore formation by dimeric Bak and Bax: An unusual pore? Phil. Trans. Royal Soc. London 2017, 72, 20160218. [Google Scholar] [CrossRef]
- Gupta, N.; Wang, X.; Wen, X.; Moran, P.; Paluch, M.; Hass, P.E.; Heidersbach, A.; Haley, B.; Kirchhofer, D.; Brezski, R.J.; et al. Domain-specific antibodies reveal differences in the membrane topologies of apolipoprotein L1 in serum and podocytes. J. Am. Soc. Nephrol. 2020, 31, 2065–2082. [Google Scholar] [CrossRef]
- Fantini, J.; Barrantes, F.J. How cholesterol interacts with membrane proteins: An exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front. Physiol. 2013, 4, 31. [Google Scholar] [CrossRef]
- Vanhollebeke, B.; De Muylder, G.; Nielsen, M.J.; Pays, A.; Tebabi, P.; Dieu, M.; Raes, M.; Moestrup, S.K.; Pays, E. A haptoglobin-hemoglobin receptor conveys innate immunity to Trypanosoma brucei in humans. Science 2008, 320, 677–681. [Google Scholar] [CrossRef]
- Meng, Y.; Luo, Q.; Chen, Q.; Zhu, Y. A noncanonical autophagy function of ATG9A for Golgi integrity and dynamics. Autophagy 2023, 19, 1607–1608. [Google Scholar] [CrossRef]
- Vashi, N.; Andrabi, S.B.; Ghanwat, S.; Suar, M.; Kumar, D. Ca2+-dependent focal exocytosis of Golgi-derived vesicles helps phagocytic uptake in macrophages. J. Biol. Chem. 2017, 292, 5144–5165. [Google Scholar] [CrossRef]
- Fang, R.; Jiang, Q.; Jia, X.; Jiang, Z. ARMH3-mediated recruitment of PI4KB directs Golgi-to-endosome trafficking and activation of the antiviral effector STING. Immunity 2023, 56, 500–515.e6. [Google Scholar] [CrossRef]
- Dickson, E.J.; Jensen, J.B.; Hille, B. Golgi and plasma membrane pools of PI(4)P contribute to plasma membrane PI(4,5)P2 and maintenance of KCNQ2/3 ion channel current. Proc. Natl. Acad. Sci. USA 2014, 111, E2281–E2290. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Balla, T. PI(4,5)P2-dependent and -independent roles of PI(4)P in the control of hormone secretion by pituitary cells. Front. Endocrinol. 2023, 14, 1118744. [Google Scholar] [CrossRef]
- Wills, R.C.; Hammond, G.R.V. PI(4,5)P2: Signaling the plasma membrane. Biochem. J. 2022, 479, 2311–2325. [Google Scholar] [CrossRef]
- Katan, M.; Cockcroft, S. Phosphatidylinositol(4,5)bisphosphate: Diverse functions at the plasma membrane. Essays Biochem. 2020, 64, 513–531. [Google Scholar]
- Itsuki, K.; Imai, Y.; Hase, H.; Okamura, Y.; Inoue, R.; Mori, M.X. PLC-mediated PI(4,5)P2 hydrolysis regulates activation and inactivation of TRPC6/7 channels. J. Gen. Physiol. 2014, 143, 183–201. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, Z.; Meng, X.Y.; Cui, M.; Logothetis, D.E. Structural determinants of phosphatidylinositol 4,5-bisphosphate (PIP2) regulation of BK channel activity through the RCK1 Ca2+ coordination site. J. Biol. Chem. 2014, 289, 18860–18872. [Google Scholar] [CrossRef]
- Kim, E.Y.; Suh, J.M.; Chiu, Y.H.; Dryer, S.E. Regulation of podocyte BK(Ca) channels by synaptopodin, Rho, and actin microfilaments. Am. J. Physiol. Renal Physiol. 2010, 299, F594–F604. [Google Scholar] [CrossRef]
- Ilatovskaya, D.V.; Staruschenko, A. TRPC6 channel as an emerging determinant of the podocyte injury susceptibility in kidney diseases. Am. J. Physiol. Renal Physiol. 2015, 309, F393–F397. [Google Scholar] [CrossRef]
- Tao, J.; Lan, Z.; Wang, Y.; Hei, H.; Tian, L.; Pan, W.; Zhang, X.; Peng, W. Large-conductance calcium-activated potassium channels in glomerulus: From cell signal integration to disease. Front. Physiol. 2016, 7, 248. [Google Scholar] [CrossRef]
- Hu, S.; Han, R.; Chen, L.; Qin, W.; Xu, X.; Shi, J.; Zhu, X.; Zhang, M.; Zeng, C.; Tang, Z.; et al. Upregulated LRRC55 promotes BK channel activation and aggravates cell injury in podocytes. J. Exp. Med. 2021, 218, e20192373. [Google Scholar] [CrossRef]
- Winn, M.P.; Conlon, P.J.; Lynn, K.L.; Farrington, M.K.; Creazzo, T.; Hawkins, A.F.; Daskalakis, N.; Kwan, S.Y.; Ebersviller, S.; Burchette, J.L.; et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 2005, 308, 1801–1804. [Google Scholar] [CrossRef]
- Mukerji, N.; Damodaran, T.V.; Winn, M.P. TRPC6 and FSGS: The latest TRP channelopathy. Bioch. Biophys. Acta 2007, 1772, 859–868. [Google Scholar] [CrossRef]
- Heeringa, S.F.; Möller, C.C.; Du, J.; Yue, L.; Hinkes, B.; Chernin, G.; Vlangos, C.N.; Hoyer, P.F.; Reiser, J.; Hildebrandt, F. A novel TRPC6 mutation that causes childhood FSGS. PLoS ONE 2009, 4, e7771. [Google Scholar] [CrossRef]
- Schlöndorff, J.; Del Camino, D.; Carrasquillo, R.; Lacey, V.; Pollak, M.R. TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am. J. Physiol. Cell Physiol. 2009, 296, C558–C569. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, J.; Wang, M.; Yang, L.; Ning, F.; Xin, H.; Xu, X.; Cai, H.; Zhang, W.; Yu, K.; et al. Mechanism of regulation of big-conductance Ca2+-activated K+ channels by mTOR complex 2 in podocytes. Front. Physiol. 2019, 10, 167. [Google Scholar] [CrossRef]
- Brown, B.J.; Boekell, K.L.; Stotter, B.R.; Talbot, B.E.; Schlondorff, J.S. Gain-of-function, focal segmental glomerulosclerosis Trpc6 mutation minimally affects susceptibility to renal injury in several mouse models. PLoS ONE 2022, 17, e0272313. [Google Scholar]
- Nakamura, T.Y.; Nakao, S.; Wakabayashi, S. Emerging roles of neuronal Ca2+ Sensor-1 in cardiac and neuronal tissues: A mini review. Front. Mol. Neurosci. 2019, 12, 56. [Google Scholar] [CrossRef]
- Guo, W.; Malin, S.A.; Johns, D.C.; Jeromin, A.; Nerbonne, J.M. Modulation of Kv4-encoded K(+) currents in the mammalian myocardium by neuronal calcium sensor-1. J. Biol. Chem. 2002, 277, 26436–26443. [Google Scholar] [CrossRef]
- Pandalaneni, S.; Karuppiah, V.; Saleem, M.; Haynes, L.P.; Burgoyne, R.D.; Mayans, O.; Derrick, J.P.; Lian, L.Y. Neuronal Calcium Sensor-1 Binds the D2 Dopamine Receptor and G-protein-coupled Receptor Kinase 1 (GRK1) Peptides Using Different Modes of Interactions. J. Biol. Chem. 2015, 290, 18744–18756. [Google Scholar] [CrossRef]
- Navarro, G.; Hradsky, J.; Lluís, C.; Casadó, V.; McCormick, P.J.; Kreutz, M.R.; Mikhaylova, M. NCS-1 associates with adenosine A(2A) receptors and modulates receptor function. Front. Mol. Neurosci. 2012, 5, 53. [Google Scholar] [CrossRef]
- Angelats, E.; Requesens, M.; Aguinaga, D.; Kreutz, M.R.; Franco, R.; Navarro, G. Neuronal calcium and cAMP cross-talk mediated by Cannabinoid CB1 Receptor and EF-Hand calcium sensor interactions. Front. Cell Dev. Biol. 2018, 6, 67. [Google Scholar] [CrossRef]
- Muñoz-Reyes, D.; McClelland, L.J.; Arroyo-Urea, S.; Sánchez-Yepes, S.; Sabín, J.; Pérez-Suárez, S.; Menendez, M.; Mansilla, A.; García-Nafría, J.; Sprang, S.; et al. The neuronal calcium sensor NCS-1 regulates the phosphorylation state and activity of the Gα chaperone and GEF Ric-8A. Elife 2023, 12, e86151. [Google Scholar] [CrossRef]
- Schlecker, C.; Boehmerle, W.; Jeromin, A.; DeGray, B.; Varshney, A.; Sharma, Y.; Szigeti-Buck, K.; Ehrlich, B.E. Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium. J. Clin. Investig. 2006, 116, 1668–1674. [Google Scholar] [CrossRef]
- Angebault, C.; Fauconnier, J.; Patergnani, S.; Rieusset, J.; Danese, A.; Affortit, C.A.; Jagodzinska, J.; Mégy, C.; Quiles, M.; Cazevieille, C.; et al. ER-mitochondria cross-talk is regulated by the Ca2+ sensor NCS1 and is impaired in Wolfram syndrome. Sci. Signal 2018, 11, eaaq1380. [Google Scholar] [CrossRef]
- Ma, L.; Chou, J.W.; Snipes, J.A.; Bharadwaj, M.S.; Craddock, A.L.; Cheng, D.; Weckerle, A.; Petrovic, S.; Hicks, P.J.; Hemal, A.K.; et al. Renal-risk variants induce mitochondrial dysfunction. J. Am. Soc. Nephrol. 2017, 28, 1093–1105. [Google Scholar] [CrossRef]
- Wakashin, H.; Heymann, J.; Roshanravan, H.; Daneshpajouhnejad, P.; Rosenberg, A.; Shin, M.K.; Hoek, M.; Kopp, J.B. APOL1 renal risk variants exacerbate podocyte injury by increasing inflammatory stress. BMC Nephrol. 2020, 21, 371. [Google Scholar] [CrossRef]
- Lecordier, L.; Uzureau, S.; Vanwalleghem, G.; Deleu, M.; Crowet, J.M.; Barry, P.; Moran, B.; Voorheis, P.; Dumitru, A.C.; Yamaryo-Botté, Y.; et al. The Trypanosoma brucei KIFC1 kinesin ensures the fast antibody clearance required for parasite infectivity. iScience 2020, 23, 101476. [Google Scholar] [CrossRef]
- Kruzel-Davila, E.; Bavli-Kertselli, I.; Ofir, A.; Cheatham, A.M.; Shemer, R.; Zaknoun, E.; Chornyy, S.; Tabachnikov, O.; Davis, S.E.; Khatua, A.K.; et al. Endoplasmic reticulum-translocation is essential for APOL1 cellular toxicity. iScience 2021, 25, 103717. [Google Scholar] [CrossRef]
- Wu, J.; Raman, A.; Coffey, N.J.; Sheng, X.; Wahba, J.; Seasock, M.J.; Ma, Z.; Beckerman, P.; Laczkó, D.; Palmer, M.B.; et al. The key role of NLRP3 and STING in APOL1-associated podocytopathy. J. Clin. Investig. 2021, 131, e136329. [Google Scholar] [CrossRef]
- Kozlitina, J.; Zhou, H.; Brown, P.N.; Rohm, R.J.; Pan, Y.; Ayanoglu, G.; Du, X.; Rimmer, E.; Reilly, D.F.; Roddy, T.P.; et al. Plasma levels of risk-variant APOL1 do not associate with renal disease in a population-based cohort. J. Am. Soc. Nephrol. 2016, 27, 3204–3219. [Google Scholar] [CrossRef]
- Andrews, M.; Yoshida, T.; Henderson, C.M.; Pflaum, H.; McGregor, A.; Lieberman, J.A.; de Boer, I.H.; Vaisar, T.; Himmelfarb, J.; Kestenbaum, B.; et al. Variant APOL1 protein in plasma associates with larger particles in humans and mouse models of kidney injury. PLoS ONE 2022, 17, e0276649. [Google Scholar]
- Giovinazzo, J.A.; Thomson, R.P.; Khalizova, N.; Zager, P.J.; Malani, N.; Rodriguez-Boulan, E.; Raper, J.; Schreiner, R. Apolipoprotein L-1 renal risk variants form active channels at the plasma membrane driving cytotoxicity. eLife 2020, 9, e51185. [Google Scholar] [CrossRef]
- Olabisi, O.A.; Zhang, J.Y.; VerPlank, L.; Zahler, N.; DiBartolo, S.; 3rd Heneghan, J.F.; Schlöndorff, J.S.; Suh, J.H.; Yan, P.; Alper, S.L.; et al. APOL1 kidney disease risk variants cause cytotoxicity by depleting cellular potassium and inducing stress-activated protein kinases. Proc. Natl. Acad. Sci. USA 2016, 113, 830–837. [Google Scholar] [CrossRef]
- Datta, S.; Kataria, R.; Zhang, J.Y.; Moore, S.; Petitpas, K.; Mohamed, A.; Zahler, N.; Pollak, M.R.; Olabisi, O.A. Kidney disease-associated APOL1 variants have dose-dependent, dominant toxic gain-of-function. J. Am. Soc. Nephrol. 2020, 31, 2083–2096. [Google Scholar] [CrossRef]
- Vandorpe, D.H.; Heneghan, J.F.; Waitzman, J.S.; McCarthy, G.M.; Blasio, A.; Magraner, J.M.; Donovan, O.G.; Schaller, L.B.; Shah, S.S.; Subramanian, B.; et al. Apolipoprotein L1 (APOL1) cation current in HEK-293 cells and in human podocytes. Pflugers Arch. 2023, 475, 323–341. [Google Scholar] [CrossRef]
- Datta, S.; Antonio, B.M.; Zahler, N.H.; Theile, J.W.; Krafte, D.; Zhang, H.; Rosenberg, P.B.; Chaves, A.B.; Muoio, D.M.; Zhang, G.; et al. APOL1-mediated monovalent cation transport contributes to APOL1-mediated podocytopathy in kidney disease. J. Clin. Investig. 2024, 134, e172262. [Google Scholar] [CrossRef]
- Egbuna, O.; Zimmerman, B.; Manos, G.; Fortier, A.; Chirieac, M.C.; Dakin, L.A.; Friedman, D.J.; Bramham, K.; Campbell, K.; Knebelmann, B.; et al. VX19-147-101 Study Group. Inaxaplin for proteinuric kidney disease in persons with two APOL1 variants. N. Engl. J. Med. 2023, 388, 969–979. [Google Scholar] [CrossRef]
- Dart, C. Lipid microdomains and the regulation of ion channel function. J. Physiol. 2010, 588, 3169–3178. [Google Scholar] [CrossRef]
- Vaithianathan, T.; Bukiya, A.; Liu, J.; Liu, P.; Asuncion-Chin, M.; Fan, Z.; Dopico, A. Direct regulation of BK channels by phosphatidylinositol 4,5-bisphosphate as a novel signaling pathway. J. Gen. Physiol. 2008, 132, 13–28. [Google Scholar] [CrossRef]
- Dryer, S.E.; Reiser, J. TRPC6 channels and their binding partners in podocytes: Role in glomerular filtration and pathophysiology. Am. J. Physiol. Renal Physiol. 2010, 299, F689–F701. [Google Scholar] [CrossRef]
- Bukiya, A.N.; Dopico, A.M. Regulation of BK channel activity by cholesterol and its derivatives. Adv. Exp. Med. Biol. 2019, 1115, 53–75. [Google Scholar]
- Bukiya, A.N.; Leo, M.D.; Jaggar, J.H.; Dopico, A.M. Cholesterol activates BK channels by increasing KCNMB1 protein levels in the plasmalemma. J. Biol. Chem. 2021, 296, 100381. [Google Scholar] [CrossRef]
- Vaithianathan, T.; Schneider, E.H.; Bukiya, A.N.; Dopico, A.M. Cholesterol and PIP2 modulation of BKCa channels. Adv. Exp. Med. Biol. 2023, 1422, 217–243. [Google Scholar]
- Lee, Y.; Park, S.; Yuan, F.; Hayden, C.C.; Wang, L.; Lafer, E.M.; Choi, S.Q.; Stachowiak, J.C. Transmembrane coupling of liquid-like protein condensates. Nat. Commun. 2023, 14, 8015. [Google Scholar] [CrossRef]
- Beech, D.J.; Bahnasi, Y.M.; Dedman, A.M.; Al-Shawaf, E. TRPC channel lipid specificity and mechanisms of lipid regulation. Cell Calcium 2009, 45, 583–588. [Google Scholar] [CrossRef]
- Lei, L.; Lu, S.; Wang, Y.; Kim, T.; Mehta, D.; Wang, Y. The role of mechanical tension on lipid raft dependent PDGF-induced TRPC6 activation. Biomaterials 2014, 35, 2868–2877. [Google Scholar] [CrossRef]
- Svobodova, B.; Groschner, K. Mechanisms of lipid regulation and lipid gating in TRPC channels. Cell calcium 2016, 59, 271–279. [Google Scholar] [CrossRef]
- Huber, T.B.; Schermer, B.; Benzing, T. Podocin organizes ion channel-lipid supercomplexes: Implications for mechanosensation at the slit diaphragm. Nephron. Exp. Nephrol. 2007, 106, e27–e31. [Google Scholar] [CrossRef]
- Merscher, S.; Pedigo, C.E.; Mendez, A.J. Metabolism, energetics, and lipid biology in the podocyte—cellular cholesterol-mediated glomerular injury. Front. Endocrinol. 2014, 5, 169. [Google Scholar] [CrossRef]
- Dopico, A.M.; Bukiya, A.N.; Singh, A.K. Large conductance, calcium- and voltage-gated potassium (BK) channels: Regulation by cholesterol. Pharmacol. Ther. 2012, 135, 133–150. [Google Scholar] [CrossRef]
- Naranjo, D.; Moldenhauer, H.; Pincuntureo, M.; Díaz-Franulic, I. Pore size matters for potassium channel conductance. J. Gen. Physiol. 2016, 148, 277–291. [Google Scholar] [CrossRef]
- Hung, A.M.; Assimon, V.A.; Chen, H.C.; Yu, Z.; Vlasschaert, C.; Triozzi, J.L.; Chan, H.; Wheless, L.; Wilson, O.; Shah, S.C.; et al. Genetic inhibition of APOL1 pore-forming function prevents APOL1-mediated kidney disease. J. Am. Soc. Nephrol. 2023, 34, 1889–1899. [Google Scholar] [CrossRef]
- Gupta, Y.; Friedman, D.J.; McNulty, M.T.; Khan, A.; Lane, B.; Wang, C.; Ke, J.; Jin, G.; Wooden, B.; Knob, A.L.; et al. Strong protective effect of the APOL1 p.N264K variant against G2-associated focal segmental glomerulosclerosis and kidney disease. Nat. Commun. 2023, 14, 7836. [Google Scholar]
- Chun, J.; Zhang, J.Y.; Wilkins, M.S.; Subramanian, B.; Riella, C.; Magraner, J.M.; Alper, S.L.; Friedman, D.J.; Pollak, M.R. Recruitment of APOL1 kidney disease risk variants to lipid droplets attenuates cell toxicity. Proc. Natl. Acad. Sci. USA 2019, 116, 3712–3721. [Google Scholar] [CrossRef]
- Ryu, J.H.; Ge, M.; Merscher, S.; Rosenberg, A.Z.; Desante, M.; Roshanravan, H.; Okamoto, K.; Shin, M.K.; Hoek, M.; Fornoni, A.; et al. APOL1 renal risk variants promote cholesterol accumulation in tissues and cultured macrophages from APOL1 transgenic mice. PLoS ONE 2019, 14, e0211559. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Q.; Yang, J.; Ma, Y.; Ding, G. Angiotensin II induces cholesterol accumulation and injury in podocytes. Sci. Rep. 2017, 7, 10672. [Google Scholar] [CrossRef]
- Mitrofanova, A.; Molina, J.; Varona Santos, J.; Guzman, J.; Morales, X.A.; Ducasa, G.M.; Bryn, J.; Sloan, A.; Volosenco, I.; Kim, J.J.; et al. Hydroxypropyl-beta-cyclodextrin protects from kidney disease in experimental Alport syndrome and focal segmental glomerulosclerosis. Kidney Int. 2018, 94, 1151–1159. [Google Scholar] [CrossRef]
- Weaver, A.K.; Olsen, M.L.; McFerrin, M.B.; Sontheimer, H. BK channels are linked to inositol 1,4,5-triphosphate receptors via lipid rafts: A novel mechanism for coupling [Ca(2+)](i) to ion channel activation. J. Biol. Chem. 2007, 282, 31558–31568. [Google Scholar] [CrossRef]
- Feingold, K.R. The bidirectional link between HDL and COVID-19 infections. J. Lipid Res. 2021, 62, 100067. [Google Scholar] [CrossRef]
- Agouridis, A.P.; Pagkali, A.; Zintzaras, E.; Rizos, E.C.; Ntzani, E.E. High-density lipoprotein cholesterol: A marker of COVID-19 infection severity? Atheroscler. Plus 2021, 44, 1–9. [Google Scholar] [CrossRef]
- Fan, J.; Wang, H.; Ye, G.; Cao, X.; Xu, X.; Tan, W.; Zhang, Y. Letter to the Editor: Low-density lipoprotein is a potential predictor of poor prognosis in patients with coronavirus disease 2019. Metab. Clin. Exp. 2020, 107, 154243. [Google Scholar] [CrossRef]
- Kawachi, K.; Kataoka, H.; Manabe, S.; Mochizuki, T.; Nitta, K. Low HDL cholesterol as a predictor of chronic kidney disease progression: A cross-classification approach and matched cohort analysis. Heart Vessel. 2019, 34, 1440–1455. [Google Scholar] [CrossRef]
- Rysz, J.; Gluba-Brzózka, A.; Rysz-Górzyńska, M.; Franczyk, B. The Role and Function of HDL in Patients with Chronic Kidney Disease and the Risk of Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 601. [Google Scholar] [CrossRef]
- You, A.; Li, Y.; Tomlinson, B.; Yue, L.; Zhao, K.; Fan, H.; Liu, Z.; Zhang, Y.; Zheng, L. Association between renal dysfunction and low HDL cholesterol among the elderly in China. Front. Cardiovasc. Med. 2021, 8, 644208. [Google Scholar] [CrossRef]
- Pavanello, C.; Ossoli, A. HDL and chronic kidney disease. Atheroscler. Plus 2023, 52, 9–17. [Google Scholar] [CrossRef]
- Smith, E.E.; Malik, H.S. The apolipoprotein L family of programmed cell death and immunity genes rapidly evolved in primates at discrete sites of host-pathogen interactions. Genome Res. 2009, 19, 850–858. [Google Scholar] [CrossRef]
- Gaudet, R.G.; Zhu, S.; Halder, A.; Kim, B.H.; Bradfield, C.J.; Huang, S.; Xu, D.; Mamiñska, A.; Nguyen, T.N.; Lazarou, M.; et al. A human apolipoprotein L with detergent-like activity kills intracellular pathogens. Science 2021, 373, eabf8113. [Google Scholar] [CrossRef]
- Martínez-Abundis, E.; Correa, F.; Rodríguez, E.; Soria-Castro, E.; Rodríguez-Zavala, J.S.; Pacheco-Alvarez, D.; Zazueta, C. A CRAC-like motif in BAX sequence: Relationship with protein insertion and pore activity in liposomes. Biochim. Biophys. Acta 2011, 1808, 1888–1895. [Google Scholar] [CrossRef]
- Christenson, E.; Merlin, S.; Saito, M.; Schlesinger, P. Cholesterol effects on BAX pore activation. J. Mol. Biol. 2008, 381, 1168–1183. [Google Scholar] [CrossRef]
- Riella, C.V.; McNulty, M.; Ribas, G.T.; Tattersfield, C.F.; Perez-Gill, C.; Eichinger, F.; Kelly, J.; Chun, J.; Subramanian, B.; Guizelini, D.; et al. ADAR regulates APOL1 via A-to-I RNA editing by inhibition of MDA5 activation in a paradoxical biological circuit. Proc. Natl. Acad. Sci. USA 2022, 119, e2210150119. [Google Scholar] [CrossRef]
- Lee, J.G.; Fu, Y.; Zhu, J.Y.; Wen, P.; van de Leemput, J.; Ray, P.E.; Han, Z. A SNARE protective pool antagonizes APOL1 renal toxicity in Drosophila nephrocytes. Cell Biosci. 2023, 13, 199. [Google Scholar] [CrossRef]
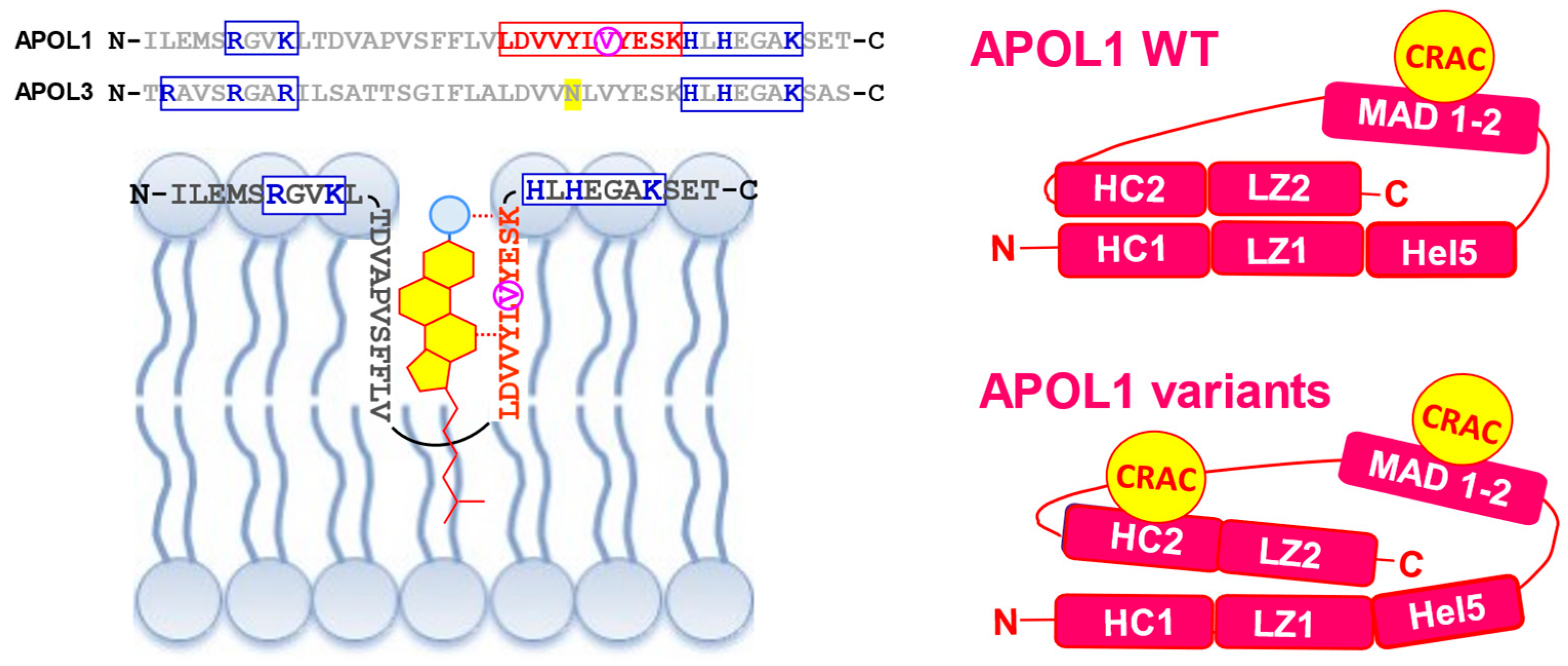
 : in APOL1, TM insertion strictly requires acidic conditions;
: in APOL1, TM insertion strictly requires acidic conditions;  : C-terminal G1 and G2 mutations;
: C-terminal G1 and G2 mutations;  : under calcium-free conditions). For the sake of clarity, each HC-LZ tandem is presented as a linear structure, but these tandems, as well as MAD, are probably folded as double-stranded hairpins. The functions of proteins interacting with APOLs are defined in the text.
: under calcium-free conditions). For the sake of clarity, each HC-LZ tandem is presented as a linear structure, but these tandems, as well as MAD, are probably folded as double-stranded hairpins. The functions of proteins interacting with APOLs are defined in the text.
 : in APOL1, TM insertion strictly requires acidic conditions;
: in APOL1, TM insertion strictly requires acidic conditions;  : C-terminal G1 and G2 mutations;
: C-terminal G1 and G2 mutations;  : under calcium-free conditions). For the sake of clarity, each HC-LZ tandem is presented as a linear structure, but these tandems, as well as MAD, are probably folded as double-stranded hairpins. The functions of proteins interacting with APOLs are defined in the text.
: under calcium-free conditions). For the sake of clarity, each HC-LZ tandem is presented as a linear structure, but these tandems, as well as MAD, are probably folded as double-stranded hairpins. The functions of proteins interacting with APOLs are defined in the text.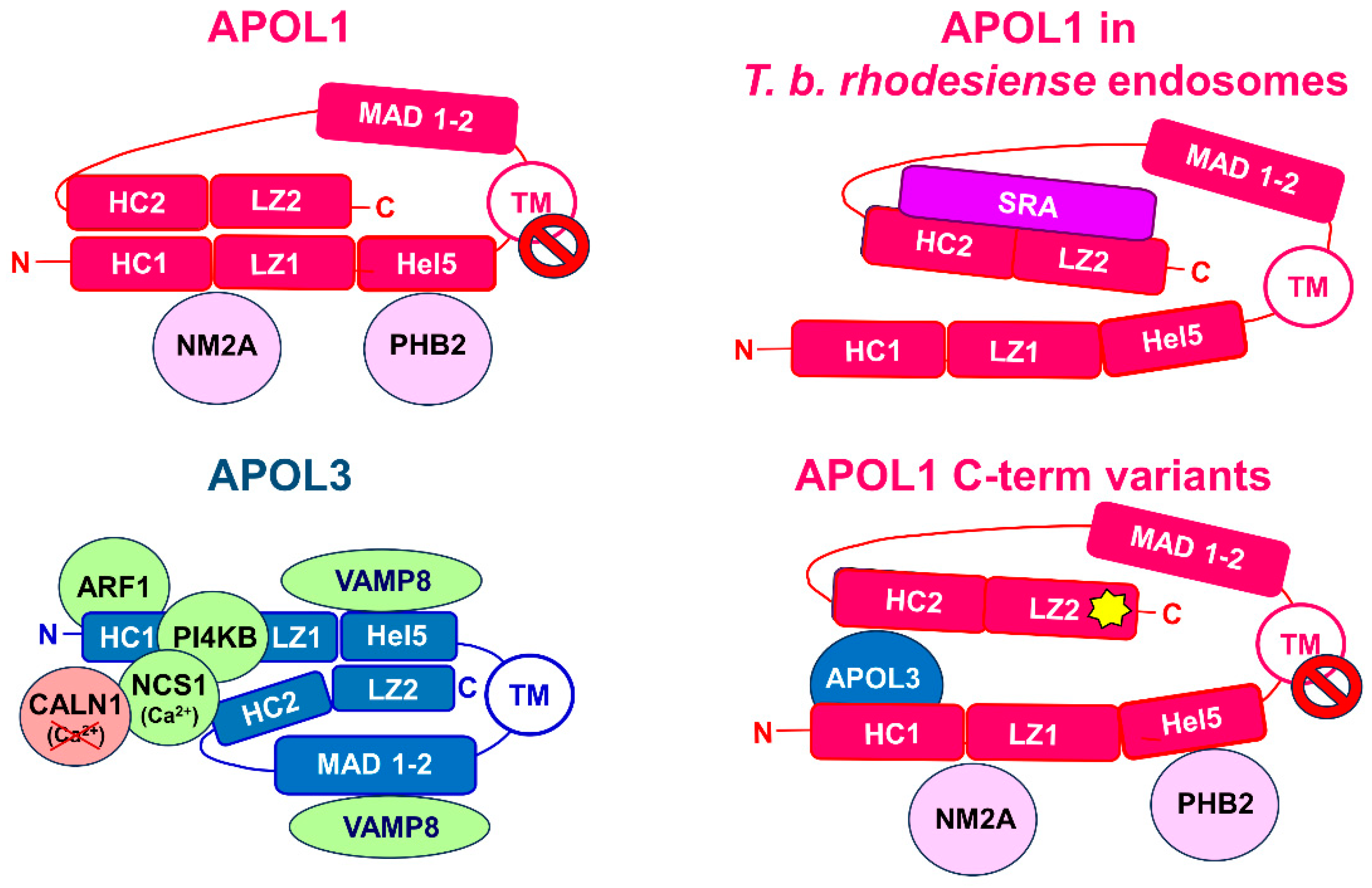

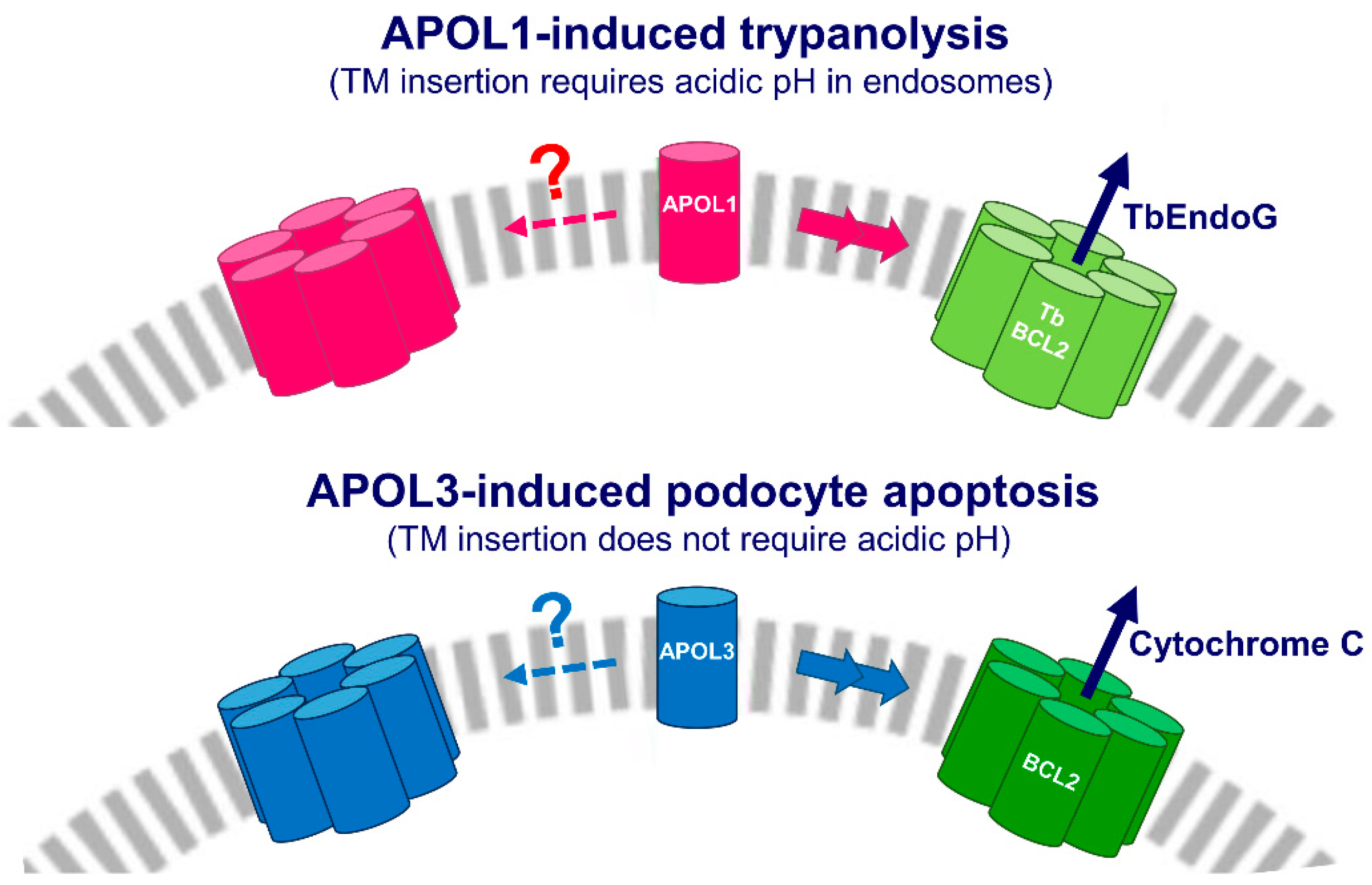
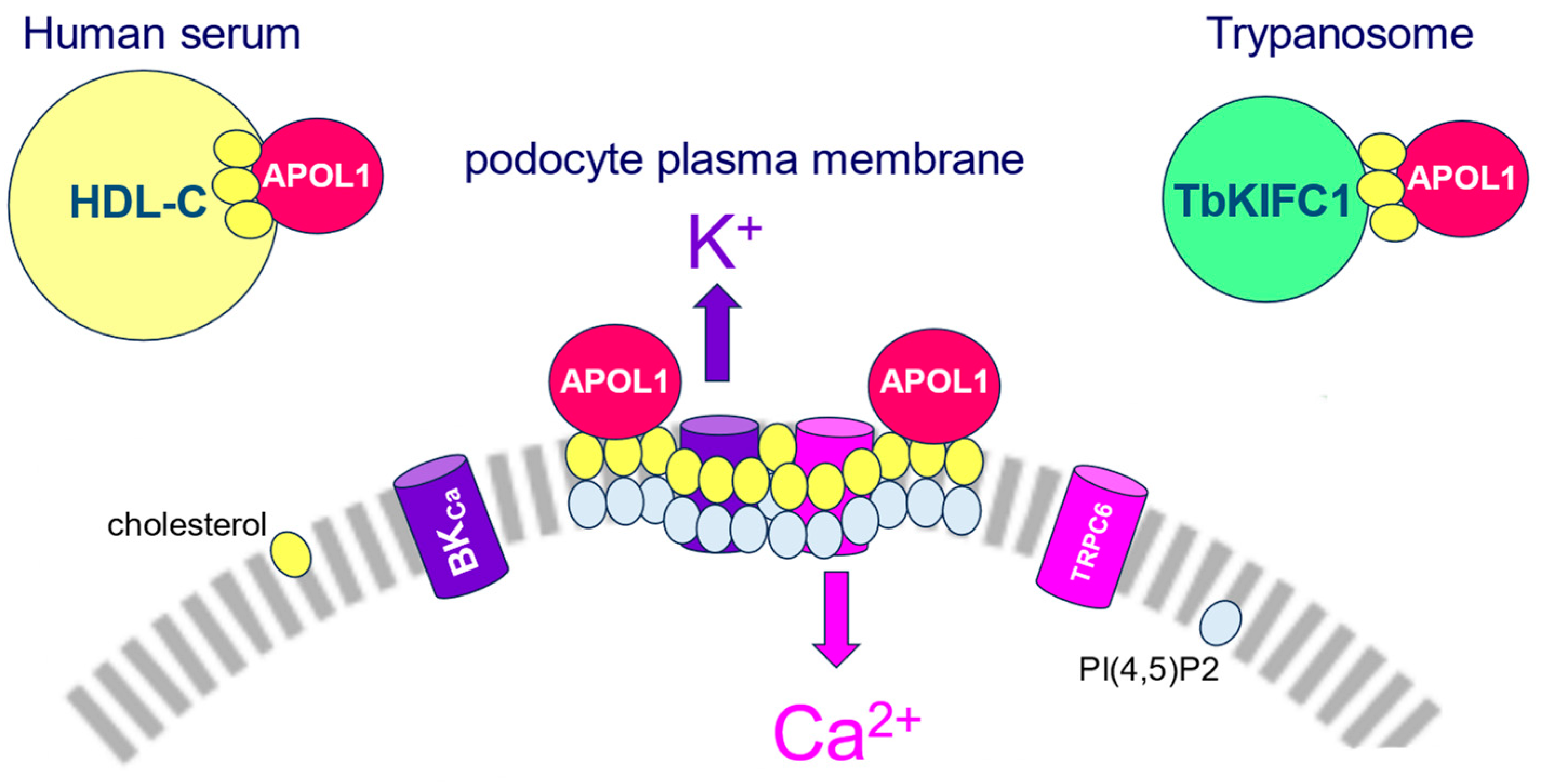
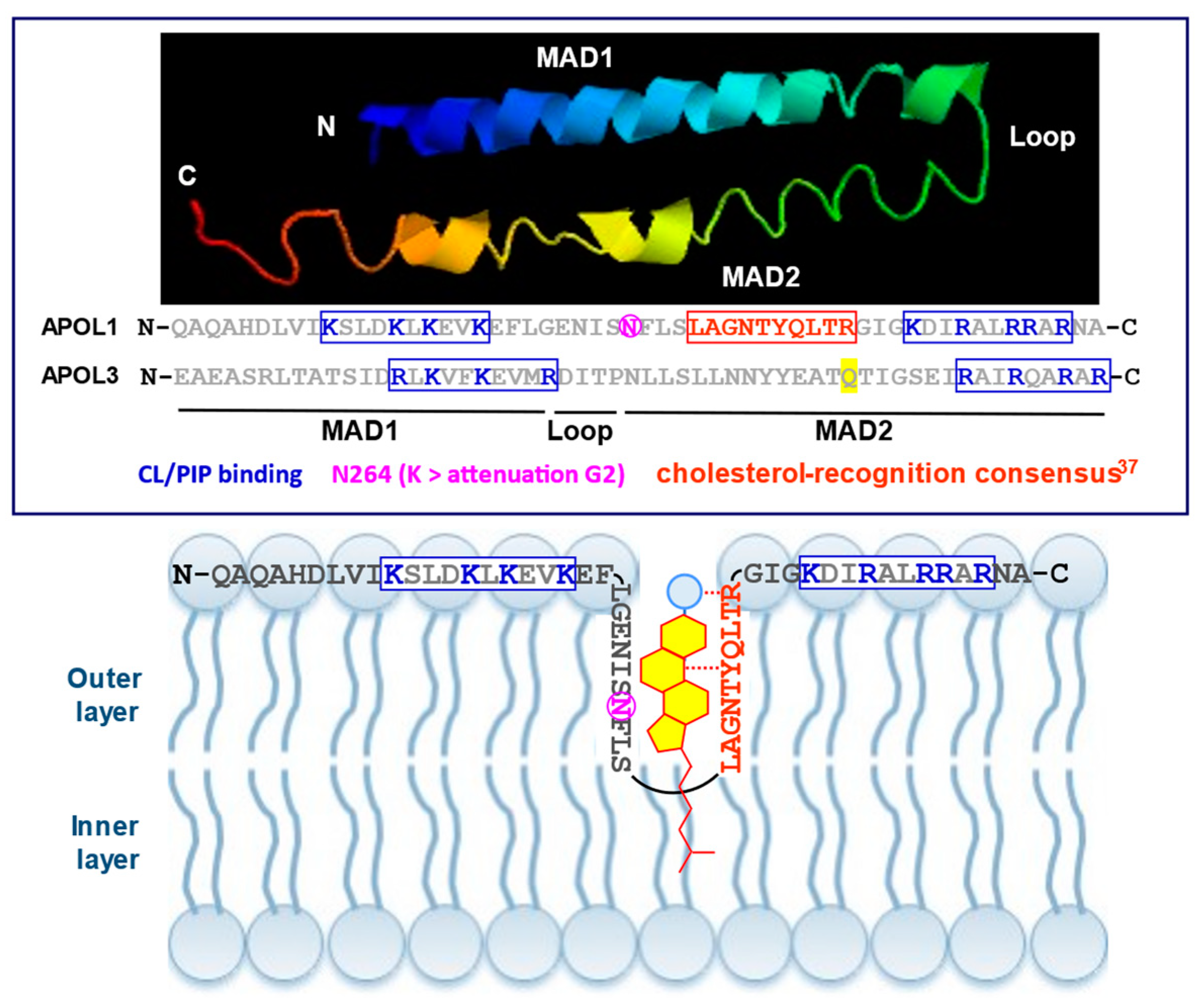
 : see Figure 1).
: see Figure 1).

 : see Figure 1).
: see Figure 1).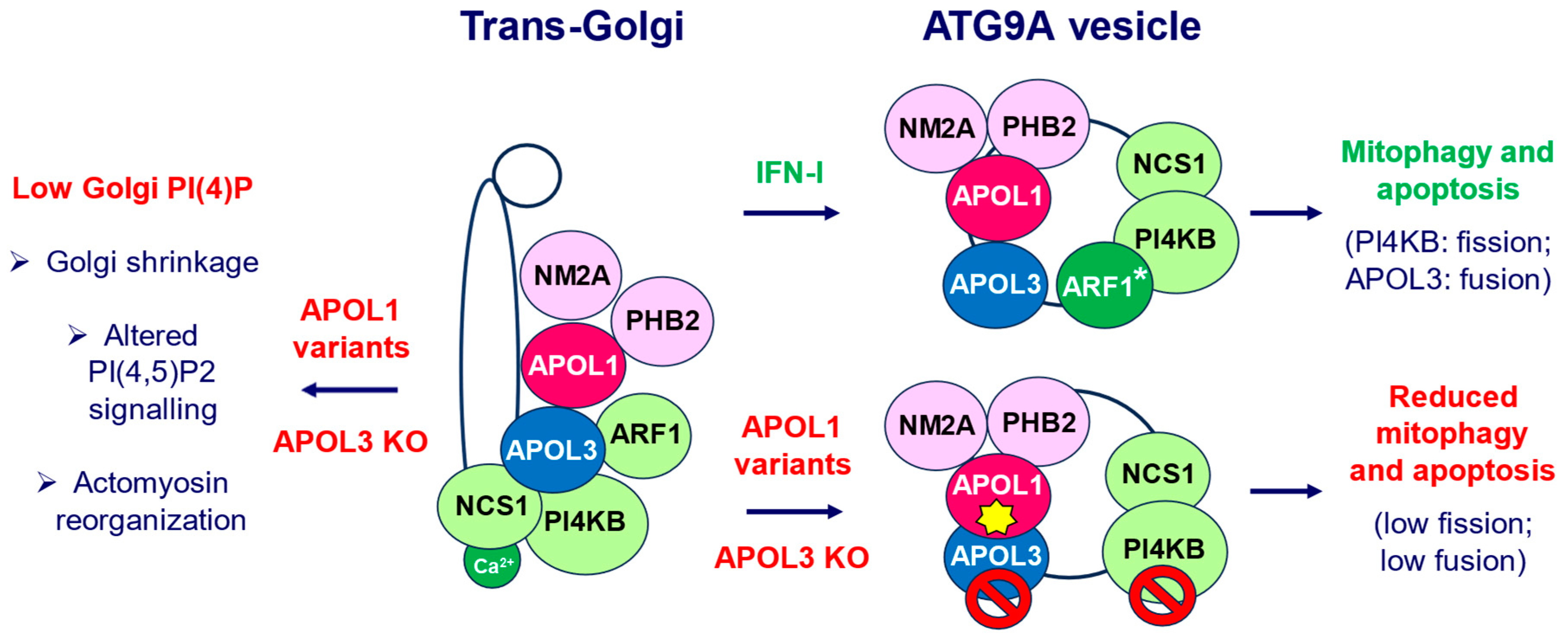
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pays, E. The Two Levels of Podocyte Dysfunctions Induced by Apolipoprotein L1 Risk Variants. Kidney Dial. 2024, 4, 126-143. https://doi.org/10.3390/kidneydial4020010
Pays E. The Two Levels of Podocyte Dysfunctions Induced by Apolipoprotein L1 Risk Variants. Kidney and Dialysis. 2024; 4(2):126-143. https://doi.org/10.3390/kidneydial4020010
Chicago/Turabian StylePays, Etienne. 2024. "The Two Levels of Podocyte Dysfunctions Induced by Apolipoprotein L1 Risk Variants" Kidney and Dialysis 4, no. 2: 126-143. https://doi.org/10.3390/kidneydial4020010
APA StylePays, E. (2024). The Two Levels of Podocyte Dysfunctions Induced by Apolipoprotein L1 Risk Variants. Kidney and Dialysis, 4(2), 126-143. https://doi.org/10.3390/kidneydial4020010





