The Impact of Potassium Binders on Mortality in Patients with Hyperkalemia: A Single-Center Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics and Outcomes
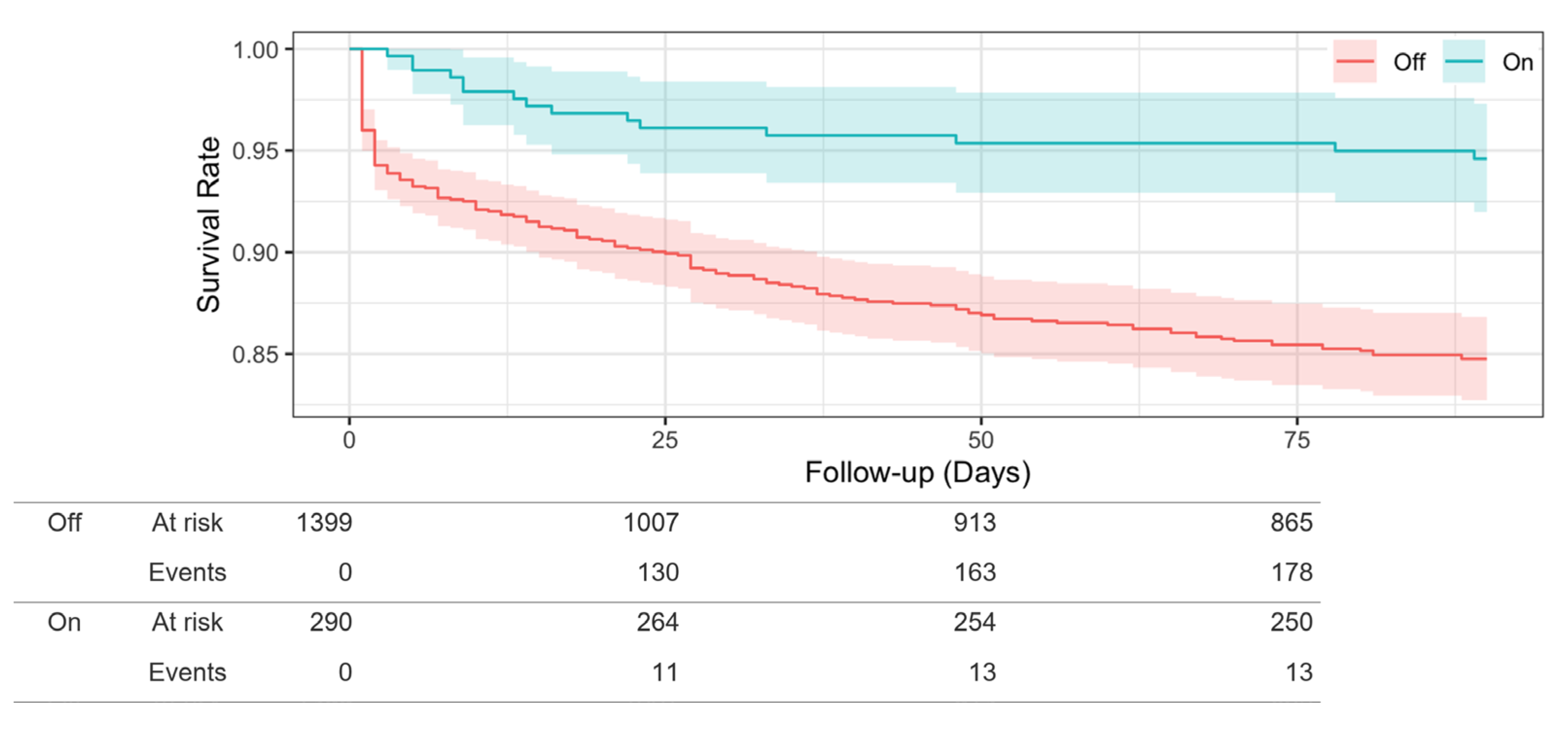
3.2. Relationship between CPS Use and Death
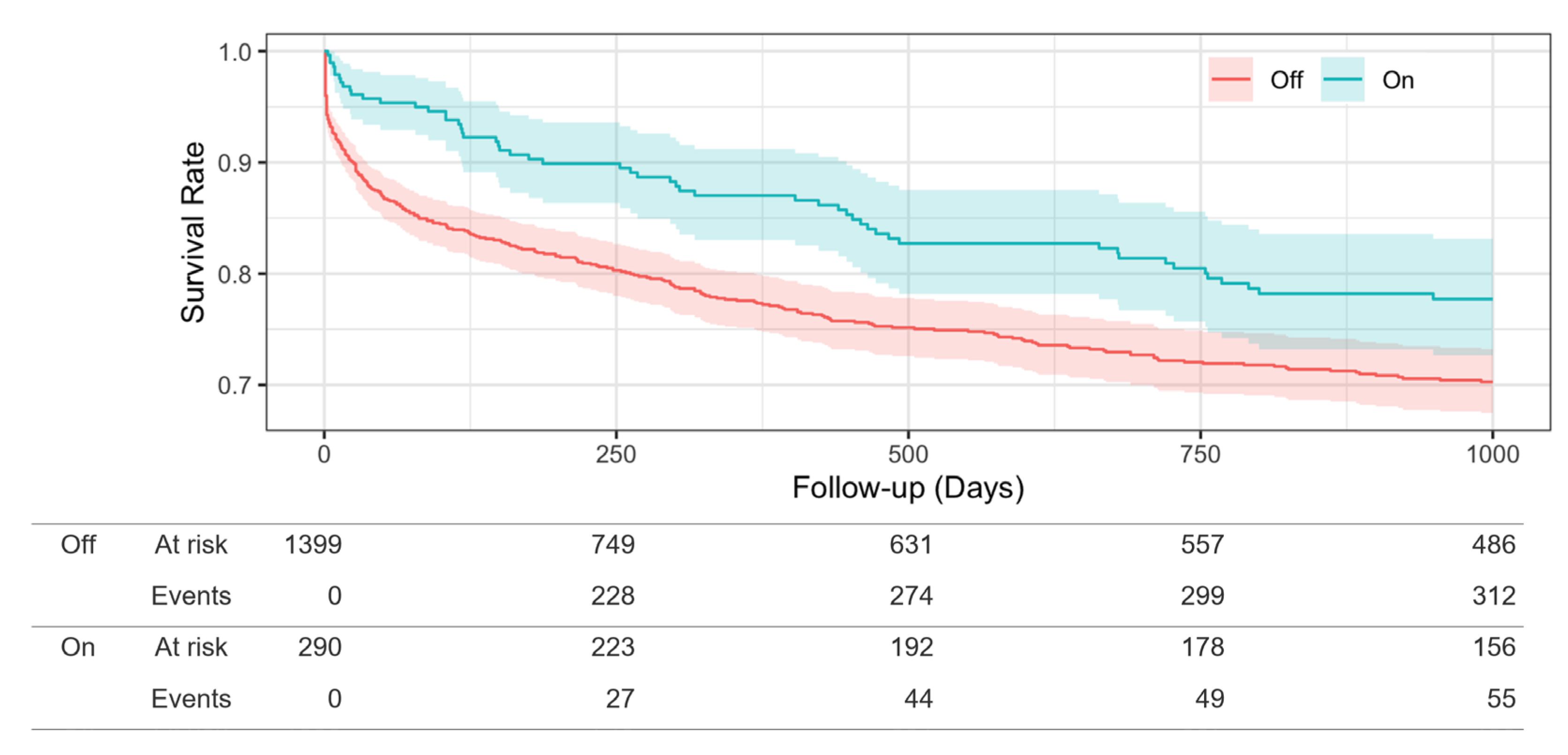
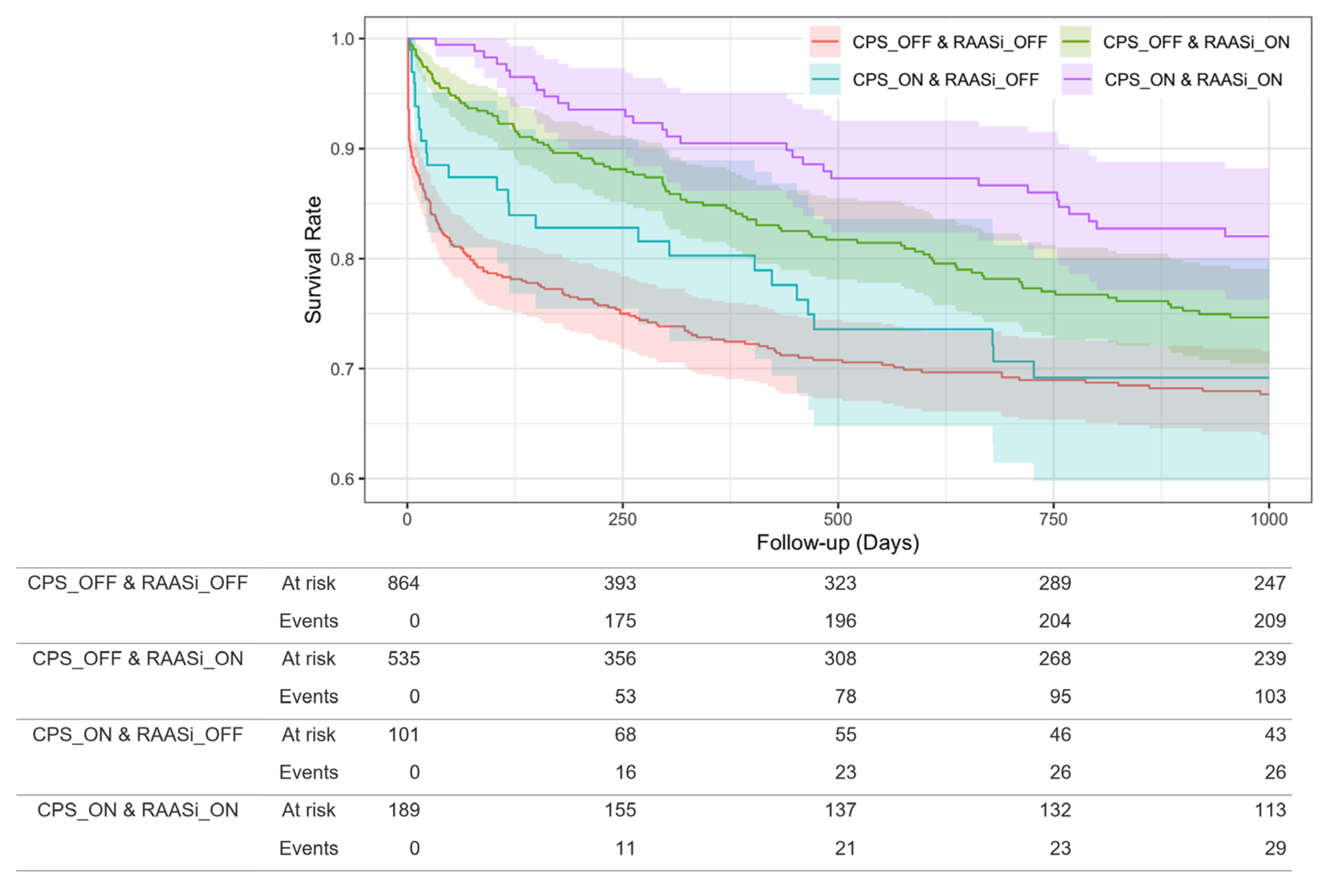
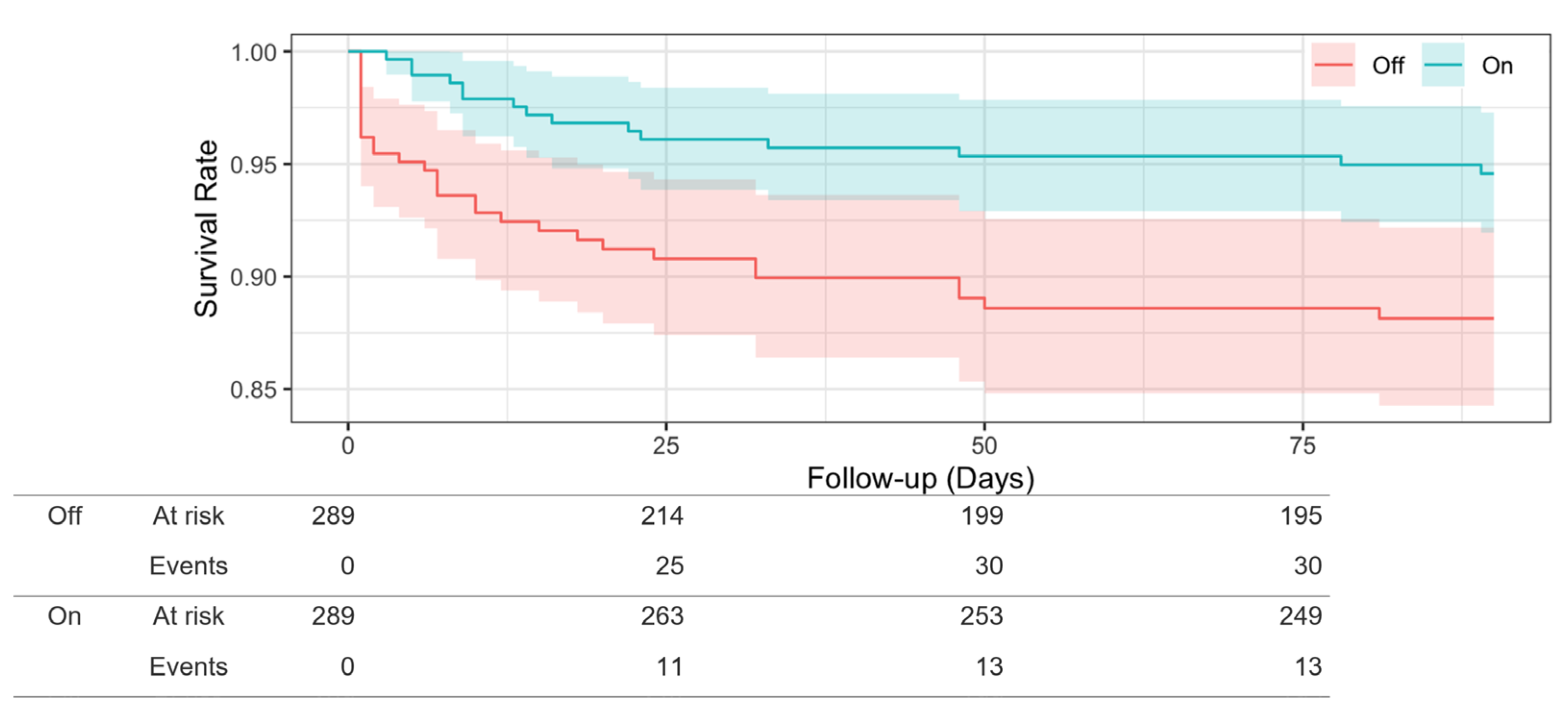
3.3. Propensity Score-Matched Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terzi, N.; Piquilloud, L.; Roze, H.; Mercat, A.; Lofaso, F.; Delisle, S.; Jolliet, P.; Sottiaux, T.; Tassaux, D.; Roesler, J.; et al. Clinical review: Update on neurally adjusted ventilatory assist-report of a round-table conference. Crit. Care 2012, 16, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, B.F. Regulation of Potassium Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1050–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd-Shiwarski, C.R.; Subramanya, A.R. The renal response to potassium stress: Integrating past with present. Curr. Opin. Nephrol. Hypertens. 2017, 26, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N. Engl. J. Med. 2004, 351, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Cohn, J.N.; Tognoni, G.; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N. Engl. J. Med. 2001, 345, 1667–1675. [Google Scholar] [CrossRef]
- Ouwerkerk, W.; Voors, A.A.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; van der Harst, P.; Hillege, H.L.; Lang, C.C.; Ter Maaten, J.M.; et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: A prospective European study. Eur. Heart J. 2017, 38, 1883–1890. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.; Kalantar-Zadeh, K.; Lu, J.L.; Turban, S.; Anderson, J.E.; Kovesdy, C.P. Association of hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: The role of race. Nephron. Clin. Pract. 2012, 120, c8–c16. [Google Scholar] [CrossRef] [Green Version]
- Drawz, P.E.; Babineau, D.C.; Rahman, M. Metabolic complications in elderly adults with chronic kidney disease. J. Am. Geriatr. Soc. 2012, 60, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Sarafidis, P.A.; Blacklock, R.; Wood, E.; Rumjon, A.; Simmonds, S.; Fletcher-Rogers, J.; Ariyanayagam, R.; Al-Yassin, A.; Sharpe, C.; Vinen, K. Prevalence and factors associated with hyperkalemia in predialysis patients followed in a low-clearance clinic. Clin. J. Am. Soc. Nephrol. 2012, 7, 1234–1241. [Google Scholar] [CrossRef] [Green Version]
- Sofue, T.; Nakagawa, N.; Kanda, E.; Nagasu, H.; Matsushita, K.; Nangaku, M.; Maruyama, S.; Wada, T.; Terada, Y.; Yamagata, K.; et al. Prevalences of hyperuricemia and electrolyte abnormalities in patients with chronic kidney disease in Japan: A nationwide, cross-sectional cohort study using data from the Japan Chronic Kidney Disease Database (J-CKD-DB). PLoS ONE 2020, 15, e0240402. [Google Scholar] [CrossRef]
- An, J.N.; Lee, J.P.; Jeon, H.J.; Kim, D.H.; Oh, Y.K.; Kim, Y.S.; Lim, C.S. Severe hyperkalemia requiring hospitalization: Predictors of mortality. Crit. Care 2012, 16, R225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashihara, N.; Kohsaka, S.; Kanda, E.; Okami, S.; Yajima, T. Hyperkalemia in Real-World Patients Under Continuous Medical Care in Japan. Kidney Int. Rep. 2019, 4, 1248–1260. [Google Scholar] [CrossRef] [Green Version]
- Nakhoul, G.N.; Huang, H.; Arrigain, S.; Jolly, S.E.; Schold, J.D.; Nally, J.V., Jr.; Navaneethan, S.D. Serum Potassium, End-Stage Renal Disease and Mortality in Chronic Kidney Disease. Am. J. Nephrol. 2015, 41, 456–463. [Google Scholar] [CrossRef]
- Luo, J.; Brunelli, S.M.; Jensen, D.E.; Yang, A. Association between Serum Potassium and Outcomes in Patients with Reduced Kidney Function. Clin. J. Am. Soc. Nephrol. 2016, 11, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.Y.; Yeo, J.H.; Park, J.S.; Lee, C.H.; Kim, G.H. Long-term efficacy of oral calcium polystyrene sulfonate for hyperkalemia in CKD patients. PLoS ONE 2017, 12, e0173542. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.R.; Sang, Y.; Leddy, J.; Yahya, T.; Kirchner, H.L.; Inker, L.A.; Matsushita, K.; Ballew, S.H.; Coresh, J.; Grams, M.E. Antihypertensive Medications and the Prevalence of Hyperkalemia in a Large Health System. Hypertension 2016, 67, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, L.M.; Zhan, M.; Hsu, V.D.; Walker, L.D.; Moen, M.F.; Seliger, S.L.; Weir, M.R.; Fink, J.C. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch. Intern. Med. 2009, 169, 1156–1162. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, S.; Mehta, S.; Khwaja, A.; Cleland, J.G.F.; Ives, N.; Brettell, E.; Chadburn, M.; Cockwell, P. Renin-Angiotensin System Inhibition in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2022, 387, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Fu, E.L.; Evans, M.; Clase, C.M.; Tomlinson, L.A.; van Diepen, M.; Dekker, F.W.; Carrero, J.J. Stopping Renin-Angiotensin System Inhibitors in Patients with Advanced CKD and Risk of Adverse Outcomes: A Nationwide Study. J. Am. Soc. Nephrol. 2021, 32, 424–435. [Google Scholar] [CrossRef]
- Epstein, M.; Reaven, N.L.; Funk, S.E.; McGaughey, K.J.; Oestreicher, N.; Knispel, J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am. J. Manag. Care 2015, 21 (Suppl. S11), S212–S220. [Google Scholar]
- Desai, N.R.; Rowan, C.G.; Alvarez, P.J.; Fogli, J.; Toto, R.D. Hyperkalemia treatment modalities: A descriptive observational study focused on medication and healthcare resource utilization. PLoS ONE 2020, 15, e0226844. [Google Scholar] [CrossRef] [Green Version]
- Volterrani, M.; Perrone, V.; Sangiorgi, D.; Giacomini, E.; Iellamo, F.; Degli Esposti, L.; on behalf of the LSG. Effects of hyperkalaemia and non-adherence to renin-angiotensin-aldosterone system inhibitor therapy in patients with heart failure in Italy: A propensity-matched study. Eur. J. Heart Fail 2020, 22, 2049–2055. [Google Scholar] [CrossRef]
- Murphy, D.; Ster, I.C.; Kaski, J.C.; Anderson, L.; Banerjee, D. The LIFT trial: Study protocol for a double-blind, randomised, placebo-controlled trial of K(+)-binder Lokelma for maximisation of RAAS inhibition in CKD patients with heart failure. BMC Nephrol. 2021, 22, 254. [Google Scholar] [CrossRef]
- Butler, J.; Anker, S.D.; Siddiqi, T.J.; Coats, A.J.S.; Dorigotti, F.; Filippatos, G.; Friede, T.; Göhring, U.M.; Kosiborod, M.N.; Lund, L.H.; et al. Patiromer for the management of hyperkalaemia in patients receiving renin-angiotensin-aldosterone system inhibitors for heart failure: Design and rationale of the DIAMOND trial. Eur. J. Heart Fail 2022, 24, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [PubMed]
- American Heart Association Nutrition Committee; Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef] [Green Version]
- Zhan, J.; Liu, Y.J.; Cai, L.B.; Xu, F.R.; Xie, T.; He, Q.Q. Fruit and vegetable consumption and risk of cardiovascular disease: A meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 1650–1663. [Google Scholar] [CrossRef]
- Dahabreh, I.J.; Kent, D.M. Can the learning health care system be educated with observational data? JAMA 2014, 312, 129–130. [Google Scholar] [CrossRef]
- Kim, G.H. Pharmacologic Treatment of Chronic Hyperkalemia in Patients with Chronic Kidney Disease. Electrolytes Blood Press. 2019, 17, 1–6. [Google Scholar] [CrossRef] [Green Version]

| All n = 1689 | CPS_OFF n = 1399 | CPS_ON n = 290 | p | |
|---|---|---|---|---|
| Demographic characteristic | ||||
| Age (years) | 77.0 [69.0, 83.0] | 77.0 [69.0, 83.0] | 75.0 [69.0, 81.0] | 0.010 |
| Male (n, %) | 1039 (61.5%) | 852 (60.9%) | 187 (64.5%) | 0.254 |
| Medication | ||||
| CPS (n, %) | 290 (17.2%) | 0 (0.0%) | 100 (100.0%) | – |
| RAASi (n, %) | 724 (42.9%) | 535 (38.2%) | 189 (65.2%) | <0.001 |
| Laboratory measurement | ||||
| K (mEq/L) | 5.1 [5.0, 5.4] | 5.1 [5.0, 5.3] | 5.2 [5.1, 5.5] | <0.001 |
| Hb (g/dL) | 11.6 ± 2.5 | 11.7 ± 2.5 | 11.2 ± 2.2 | <0.001 |
| eGFR (ml/min/1.73 m2) | 41.7 [29.8, 51.1] | 43.2 [31.8, 52.0] | 33.0 [25.7, 44.1] | <0.001 |
| TC (mg/dL) | 168.0 [140.0, 203.0] | 169.0 [140.0, 204.0] | 164.0 [140.0, 195.3] | 0.208 |
| Albumin (g/dL) | 3.7 [3.0, 4.1] | 3.7 [3.0, 4.1] | 3.6 [3.1, 4.0] | 0.182 |
| BG (mg/dL) | 107.0 [94.0, 137.0] | 107.0 [94.0, 137.0] | 107.5 [94.0, 138.0] | 0.984 |
| All | CPS_OFF Group | CPS_ON Group | p | |
|---|---|---|---|---|
| n = 1689 | n = 1399 | n = 290 | ||
| Death, n (%) | 367 (11.8%) | 185 (13.2%) | 15 (5.2%) | <0.001 |
| Observation period, median days (interquartile range) | 90 (26, 90) | 90 (18, 90) | 90 (90, 90) | <0.001 |
| Hazard Ratio (95% CI) | p | |
|---|---|---|
| Age (years) | 1.02 (1.01, 1.03) | <0.001 |
| Male | 1.46 (1.16, 1.84) | 0.002 |
| ln(eGFR) | 0.75 (0.56, 1.02) | 0.063 |
| Albumin (g/dL) | 0.31 (0.27, 0.36) | <0.001 |
| K (mEq/L) | 2.50 (2.16, 2.89) | <0.001 |
| UA (mg/dL) | 1.07 (1.02, 1.13) | 0.008 |
| TC (mg/dL) | 1.00 (1.00, 1.00) | 0.272 |
| BG (mg/dL) | 1.00 (1.00, 1.00) | 0.004 |
| CPS (On:1) | 0.66 (0.49, 0.88) | 0.006 |
| RASSi (On:1) | 0.61 (0.49, 0.76) | <0.001 |
| Na (mEq/L) | 0.98 (0.96, 1.00) | 0.041 |
| Hb (g/dL) | 0.98 (0.93, 1.03) | 0.432 |
| All n = 578 | CPS_OFF n = 289 | CPS_ON n = 289 | p | ASD | |
|---|---|---|---|---|---|
| Demographic characteristic | |||||
| Age (years) | 76 [69.0, 83.0] | 78 [69, 84] | 75 [69, 81] | 0.084 | 0.144 |
| Male | 367 (63.6%) | 181 (31.3%) | 186 (32.1%) | 0.730 | 0.036 |
| Medication | |||||
| CPS | 289 (50.0%) | 0 (0.0%) | 289 (100.0%) | - | - |
| RAASi | 377 (64.3%) | 189 (32.6%) | 188 (32.5%) | 1 | 0.007 |
| Laboratory measurement | |||||
| K (mEq/L) | 5.2 [5, 5.4] | 5.35 [5, 5.4] | 5.2 [5.1, 5.5] | 0.975 | 0.03 |
| Hb (g/dL) | 11.0 ± 2.4 | 11.05 ± 2.47 | 11.15 ± 2.2 | 0.592 | 0.045 |
| eGFR (mL/min/1.73 m2) | 33.8 [25.15, 45.22] | 35.27 [24.61 46.5] | 33.10 [25.8, 43.9] | 0.978 | 0.002 |
| TC (mg/dL) | 166 [141, 199] | 168 [141, 202] | 164 [140.0, 195] | 0.804 | 0.021 |
| Albumin (g/dL) | 3.6 [3.1, 4.0] | 3.7 [3.1,4.1] | 3.6 [3.1, 4.0] | 0.426 | 0.066 |
| BG (mg/dL) | 107 [94, 138] | 107 [138, 94] | 108 [94.0, 138.0] | 0.58 | 0.046 |
| All n = 578 | CPS_OFF Group n = 289 | CPS_ON Group n = 289 | p | |
|---|---|---|---|---|
| Death, n (%) | 119 (20.5) | 64 (11%) | 55 (9.5) | |
| Observation period, median days (interquartile range) | 601.36 (907.75) | 440 (978) | 1000 (715) | <0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagasu, H.; Tokuyama, A.; Kanda, E.; Itano, S.; Kishi, S.; Sasaki, T.; Kashihara, N. The Impact of Potassium Binders on Mortality in Patients with Hyperkalemia: A Single-Center Study. Kidney Dial. 2023, 3, 244-254. https://doi.org/10.3390/kidneydial3030022
Nagasu H, Tokuyama A, Kanda E, Itano S, Kishi S, Sasaki T, Kashihara N. The Impact of Potassium Binders on Mortality in Patients with Hyperkalemia: A Single-Center Study. Kidney and Dialysis. 2023; 3(3):244-254. https://doi.org/10.3390/kidneydial3030022
Chicago/Turabian StyleNagasu, Hajime, Atsuyuki Tokuyama, Eiichiro Kanda, Seiji Itano, Seiji Kishi, Tamaki Sasaki, and Naoki Kashihara. 2023. "The Impact of Potassium Binders on Mortality in Patients with Hyperkalemia: A Single-Center Study" Kidney and Dialysis 3, no. 3: 244-254. https://doi.org/10.3390/kidneydial3030022
APA StyleNagasu, H., Tokuyama, A., Kanda, E., Itano, S., Kishi, S., Sasaki, T., & Kashihara, N. (2023). The Impact of Potassium Binders on Mortality in Patients with Hyperkalemia: A Single-Center Study. Kidney and Dialysis, 3(3), 244-254. https://doi.org/10.3390/kidneydial3030022






